Abstract
Background
The recent lockdown due to the COVID-19 pandemic has been linked to a higher incidence of psychiatric manifestations and substance abuse. The recreative use of nitrous oxide is more and more widespread and neurological complications are frequent.
Methods
We report clinical characteristics and biological findings of five consecutive patients presenting to our tertiary care center between April 2020 and February 2021 with various neurological symptoms occurring after recent nitrous oxide abuse.
Results
Our patients presented with subacute combined degeneration of the spinal cord (4/5 patients) or with acute inflammatory demyelinating polyneuropathy (1/5 patients). No patient had reduced vitamin B-12 titer, but all had elevated blood levels of homocysteine and methylmalonic acid. This reflects the functional deficit in vitamin B-12 that can be linked to nitrous oxide consumption. After vitamin B-12 supplementation, clinical signs regressed at least partially in all 5 patients.
Conclusion
We report an elevated incidence of neurological complications of nitrous oxide abuse occurring during the recent COVID-19 lockdown. Nitrous oxide abuse should be tracked down in patients presenting with compatible neurological symptoms and elevated homocysteinemia. Vitamin B-12 should be supplemented as soon as the diagnosis is made.
Keywords: Toxicology, Substance-related disorders, Nitrous oxide, Vitamin B-12, COVID-19
Introduction
Nitrous oxide (NO) is a non-inflammable, odourless and colourless gas used mainly as an anaesthetic agent. It is well known for its euphoric properties if inhaled. Being used by the food industry in aerosol sprays like in whipped cream canisters, it is easily and widely available, at a relatively low cost [1].
Therefore it is susceptible to be misused for recreational purposes. In the past years, this phenomenon became more and more widespread [2].
During the recent COVID-19 pandemic and especially during the recent lockdown, psychiatric manifestations became more frequent, which can lead to a higher prevalence of substance abuse [3].
The toxicity of inhaled NO has been reported only for a few years [1]. Described cases are mainly neurological and psychiatric presentations. Frequently patients present with a subacute combined degeneration of the spinal cord (SACD). In most reported cases, especially neurological presentations, symptoms have been linked to a vitamin B12 deficiency, with reduced vitamin B12 blood levels [4]. We aim to discuss the clinical variability of these presentations and the variable biological findings, especially the frequent absence of decrease in vitamin B12, through five consecutive cases.
Methods
We report a total of five consecutive patients admitted to our tertiary care centre in France, between April 2020 and February 2021, presenting with rapidly progressive neurologic symptoms following NO abuse. Collected date were clinical manifestations, biological, radiological and electromyographic findings.
Case reports (Table 1)
Table 1.
Patient characteristics: clinical, radiological, and biological findings
| Patient | Age | Gender | Clinical signs | Initial presentation | B-12 (µg/L) (N 0.22–0.91) | Homocysteinemia (µmol/L) (N 3.7–13.9) | MMA (µmol/L) (N < 0.5) | MRI | EMG | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | Male | Progressive distal symmetric paraesthesia, hypopallesthesia, hypoesthesia, right Babinski sign, proprioceptive ataxia of the four limbs | SACD | High (11.63) | High (69.8) | High (1.9) | T2- and STIR hyperintensities of the posterior cervical spinal cord (C2-C6) | / | Vit. B-12 supplementation | At 3 months: regression of clinical anomalies and MRI signs |
| 2 | 20 | Male | Progressive distal symmetric paraesthesia, hypopallesthesia | SACD | Normal (0.33) | High (91.6) | High (1.7) | T2- and STIR hyperintensities of the posterior cervical spinal cord (C1-C2) | Normal | Vit. B-12 supplementation | / |
| 3 | 29 | Male | Ascending progressive distal symmetric paraesthesia; hypopallesthesia, progressive motor deficit of the four limbs, abolition of OTR | AIDP | Normal (0.39) | High (49.5) | High (0.8) | Normal | Demyelinating process of the four limbs | Vit. B-12 supplementation and IV IgG 2 g/kg | At 5 months: regression of clinical anomalies, regression of acute demyelination signs on EMG |
| 4 | 23 | Female | Asymmetric progressive hypoesthesia (Left > Right), Lhermitte sign, dysmetria of the left inferior limb aggravated by eye closures | SACD | Normal (0.38) | High (34.2) | / | Normal | / | Vit. B-12 supplementation | / |
| 5 | 27 | Female | Progressive distal symmetric paraesthesia, hypoesthesia, Lhermitte sign, proprioceptive ataxia, paroxysmal dystonia of the hands and fingers, abolition of OTR | SACD | Normal (0.31) | High (32.0) | High (0.6) | T2 hyperintensities of the cervical spinal cord (C3-C5) with discrete contrast-enhancement | Moderate sensitive amplitude diminution of the inferior limbs | Vit. B-12 supplementation + Carbamazepine | At 1 month: persistence of proprioceptive ataxia, normalisation of OTR, regression of paraesthesia |
Clinical manifestations and laboratory findings are summarised in Table 1
Clinical manifestations
Our patients were between 19 and 29 years old (median 23 years old) and had no prior medical history. They all had a recent increase in their consumption (up to 50 capsules a day) of inhaled NO for its euphoric properties.
Neurological signs had a rapidly progressing onset and installed over 48–96 h (median 72 h hours).
Sensitive signs were the main neurological manifestations, with 4 patients presenting because of progressive symmetric ascending paraesthesia. Hypoesthesia was noted in 3 patients, as was reduced vibration sense. Two patients described Lhermitte’s sign. One patient presented paroxysmal dystonia affecting both hands.
Proprioceptive ataxia was also prevalent and responsible for walking difficulties in 2 of our patients. Those two patients had the highest weekly consumption of NO in our cohort (300 cartridges a week).
We linked these clinical presentations to a subacute degeneration of the spinal cord (SACD).
The clinical presentation of patient 3 was different, as he rapidly developed a progressive motor deficit, predominating on the legs, with abolished tendon reflexes. Walking was severely impaired. His clinical examination was compatible with an acute inflammatory demyelinating polyneuropathy (AIDP).
Biological findings
Blood samples showed normal or even elevated vitamin B12 (mean: 2.6 ± 5.04 µg/L; N 0.22–0.91) in all our patients. However, homocysteinemia (mean: 55.42 ± 25.2 µmol/L; N 3.7–13.9) and methylmalonic acid (mean: 1.25 ± 0.64 µmol/L; N < 0.5) were elevated in all patients. One patient had moderated macrocytic anaemia (Patient 1, MCV = 109, 7 fL, N: 78–98 fL).
Radiological findings
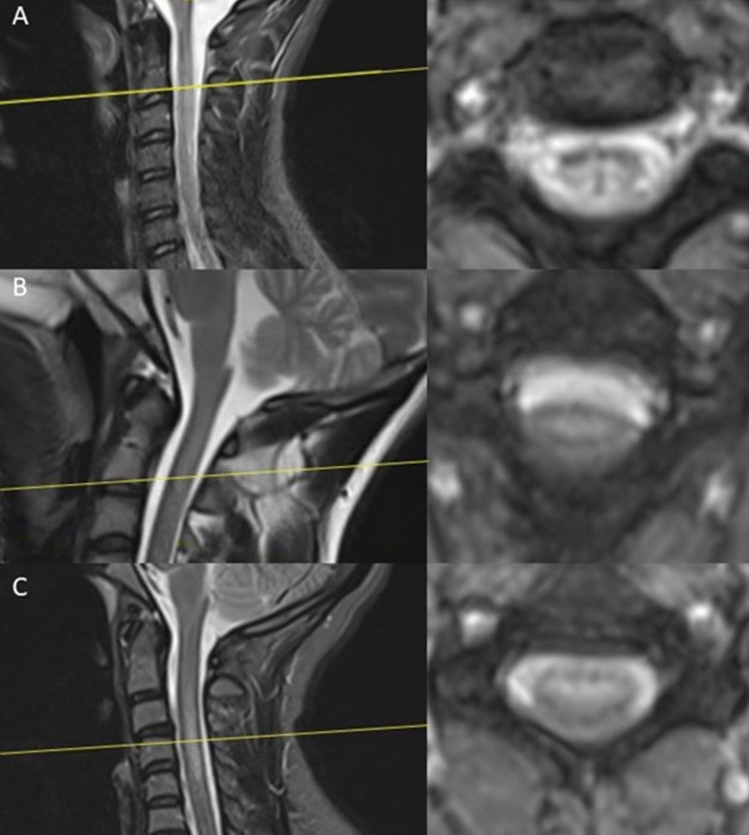
Medullar MRI was performed in all patients. In two patients (Patient 3 and 4) no abnormalities were found. Three patients had a T2- and STIR-hypersignal of the cervical spinal cord; in one of them, a discrete contrast-enhancement was described (Fig. 1).
Fig.1.
Spine MRI at admission. Left: sagittal T2-sequence of the cervical spinal cord, Right: axial View of the pathological segment, A = Patient 1: hyper-intensities of the posterior cervical spinal cord (C2-C6), B = Patient 2: hyper-intensities of the posterior cervical spinal cord (C1-C2) and C = Patient 5: hyper-intensities of the posterior cervical spinal cord (C3-C5)
Electroneuromyography (EMG)
EMG was performed in three patients. Patient 3 had diffuse demyelination compatible with acute inflammatory demyelinating polyneuropathy. At 5 months, a control-EMG showed regression of acute signs of demyelination and appearance of moderate sensitive and motor amplitude diminution compatible with sequels of the earlier demyelinating process. Patient 5 had sensitive axonal neuropathy. In patient 2, EMG showed no anomalies.
Differential diagnosis
We conducted extensive assessment of differential diagnosis to eliminate the possibility of other toxic causes or of infectious, diabetic, or autoimmune myeloneuropathy. Potential infectious causes like HIV, Syphilis or COVID-19 could be excluded in all our patients. None of our patients had a history of professional exposure to industrial solvents nor to toxic components like lead, arsenic, or mercury. Two of our patients reported occasional alcohol consumption. One was an active tobacco smoker. No other substance use was reported.
None of our patients had a history of diabetes and fasting blood glucose was normal in all our patients. We conducted an extensive autoimmune assessment in 4 of our patients. Antinuclear antibodies, Anti-Ro, Anti-La and ANCA (anti-neutrophil cytoplasmic antibodies) and ATA (anti-transglutaminase antibodies) were negative in all these patients.
Vitamin B9 level was normal in 4 of our patients, and only slightly reduced (4 µg/L, N: < 5 µg/L) in one patient. Furthermore, none of our patients had associated megaloblastic anaemia and only one had an isolated elevation of mean corpuscular volume (109 fL, N: 80–100).
Treatment
Vitamin-B12 supplementation was initiated (1000 µg/day the first week, then 1000 µg/week for a month and finally 1000 µg/month for 3 months). Supplementation was intramuscular during the hospital stay and was continued orally. A folate supplementation was associated.
In all patients, clinical improvement was observed after initiation of supplementation therapy.
In one patient, where a follow-up was reached, clinical anomalies regressed significatively, as did biological anomalies. (Patient 1, at 3 months, Homocysteinemia: 8, 1 µmol/L; MMA: 0.5 µmol/L).
As another aetiology could not be entirely eliminated at symptom onset for patient 3, an intravenous immunoglobulins therapy at 2 g/kg was associated.
In addition to vitamin-B12 supplementation, patient 5 was successfully treated with carbamazepine 200 mg per day as a symptomatic treatment for the paroxysmal dystonia of the hand.
Previous similar cases admitted to our neurological clinic
A retrospective analysis of admissions to our neurological clinic from 2010 to 2019 with myelopathy of unknown origin and/or vitamin B12-deficiency showed no similar cases of nitrous oxide intoxication.
Discussion
We reported the cases of five consecutive patients with neurological complications of NO abuse.
In all our cases, symptoms were preceded by a recent increase in consumption of inhaled NO. Four patients had a diagnosis of SACD, confirmed in three patients by medullar MRI. A fifth patient presented with AIDP and electromyographic examination was compatible with the diagnosis.
Neurological complications of NO abuse are quite rare, and the sudden rise in cases presenting to our neurological clinic since 2020 could suggest an implication of the recent COVID-19 pandemic. Conditions like anxiety, fear, or anger, undoubtedly favoured by the pandemic and its consequences, could explain a rise in substance abuse [3]. To the best of our knowledge, no previous cases with similar presentation had been admitted to our neurological clinic prior to 2020. The use of NO was not necessarily asked for at admission in past years, but all our patients spontaneously reported the substance abuse at admission.
Our cases highlight the danger of neurological complications following NO abuse. As previously reported, the main clinical presentation was that of SACD. Other neurological presentations, notably Guillain–Barré syndrome and even toxic leukoencephalopathy, are less common but have also been reported [4–6]. Our patient with AIDP adds to these previous reports. Although a differential diagnosis cannot be eliminated concerning this patient, hence the treatment with IV immunoglobulins, the clear temporal association with the substance abuse, previously published similar cases and the compatible biological mechanism plead in favour of a direct implication of NO abuse in the symptomatology.
All these complications may occur with acute or chronic intake and are usually tied to a vitamin B12 deficiency [7]. Interestingly, none of our patients had reduced vitamin B12 levels. Homocysteinemia was markedly elevated in all cases, as was MMA when analysed (4 patients). These findings indicate a functional deficit of vitamin B12, which can be linked to an excessive NO consumption.
Several mechanisms might be involved in the neurologic toxicity of NO. Mainly, NO affects vitamin-B12 by creating an irreversible oxidation of cobalt ions, inactivating the function of the vitamin, without decreasing vitamin-B12 itself [8]. Vitamin B12 is a cofactor in the transformations of homocysteine into methionine and MMA into succinyl-CoA. Elevated homocysteine and MMA, as seen in our patients, can therefore be interpreted as a sign of functional loss of vitamin B12.
As methionine is essential for methylation of myelin protein, NO abuse can lead to demyelination [9]. This could explain that various clinical presentations can be linked to NO abuse.
Treatment is based on B12 supplementation, but strategies are highly variable among case reports. Prognosis is inconstant but early and intramuscular rather than oral supplementation seems to be associated with better outcomes [7]. A folate supplementation must be associated [10]. In our patients, biological anomalies quickly normalized after intramuscular supplementation therapy, suggesting a recovery of vitamin B12 function.
In conclusion, neurologists need to be aware of the clinical presentations compatible with NO abuse, as fast B12 supplementation is crucial for recovery. As vitamin B12 itself is not necessarily reduced, we suggest a systematic testing of homocysteine levels on admission in compatible clinical presentations. If homocysteine is elevated, NO consumption should be tracked down. Supplementation treatment with vitamin B12 and folates should be initiated as early as possible in a patient with progressing neurological symptoms, a history of recent NO consumption and elevated homocysteinemia.
Funding
No funding was received for this study.
Compliance with ethical standards
Conflict of interest
ME, PV, SD, PK, TB, LG, CR, AN, JDS, LK, IS, KB report no conflicts of interest relevant to the manuscript.
Ethical approval
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants involved in the study.
References
- 1.Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358–369. doi: 10.1111/ajad.12372. [DOI] [PubMed] [Google Scholar]
- 2.van Amsterdam J, Nabben T, van den Brink W. Recreational nitrous oxide use: prevalence and risks. Regul Toxicol Pharmacol RTP. 2015;73(3):790–796. doi: 10.1016/j.yrtph.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal JC, Cheng Y, Merchant S, Zarnegar R. An acute, severe axonal sensorimotor polyneuropathy in the setting of nitrous oxide abuse. Neurohospitalist. 2020;10(4):293–296. doi: 10.1177/1941874420910648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algahtani H, Shirah B, Abdelghaffar N, Abuhawi O, Alqahtani A. Nitrous oxide recreational abuse presenting with myeloneuropathy and mimicking Guillain-Barre syndrome. Intractable Rare Dis Res. 2020;9(1):54–57. doi: 10.5582/irdr.2020.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assaf R, Michael PG, Langford N. Nitrous oxide-induced toxic leukoencephalopathy. BMJ Case Rep. 2020;13(12):e238315. doi: 10.1136/bcr-2020-238315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noh T, Osman G, Chedid M, Hefzy H. Nitrous oxide-induced demyelination: clinical presentation, diagnosis and treatment recommendations. J Neurol Sci. 2020;414:116817. doi: 10.1016/j.jns.2020.116817. [DOI] [PubMed] [Google Scholar]
- 8.Keddie S, Adams A, Kelso ARC, et al. No laughing matter: subacute degeneration of the spinal cord due to nitrous oxide inhalation. J Neurol. 2018;265(5):1089–1095. doi: 10.1007/s00415-018-8801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SE, Kinney HC, Swoboda KJ, Levy HL. Subacute combined degeneration of the spinal cord in cblC disorder despite treatment with B12. Mol Genet Metab. 2006;88(2):138–145. doi: 10.1016/j.ymgme.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Marotta DA, Kesserwani H. Nitrous oxide induced posterior cord myelopathy: beware of the methyl folate trap. Cureus. 2020;12(7):e9319. doi: 10.7759/cureus.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]