Abstract
Background:
Unfavorable modifications of tooth and its surrounding structures result in periodontal complications. Viruses, in specific herpes virus, are known to increase disease severity in periodontal patients. Periodontitis is known to be more established in type 2 diabetes mellitus (DM2) patients. Hence, the detection of the viral load, its effect on the prevalence of periodontitis and the glycemic control status of patients are to be evidenced. The study aimed to reveal the association of herpes virus with periodontal parameters and its prevalence in DM2 patients.
Materials and Methods:
The cross-sectional study involved a total of 120 patients falling into three groups; Group I (healthy), Group II (periodontitis without DM2) and Group III (periodontitis with DM2) were subjected for sampling. Subgingival samples of periodontitis patients were tested for clinical parameters, and DNA extraction was performed. The presence of herpes virus (Epstein–Barr virus [EBV-1] and human Cytomegalovirus [HCMV]) was detected using multiplex polymerase chain reaction primers. Glycemic status of patients was recorded as glycosylated hemoglobin and scored accordingly. Chi-square test was performed to analyze the association between the categorical variables, and t-test/Mann–Whitney U-test/analysis of variance/Kruskal–Wallis test was used for continuous data.
Results:
Significant levels of EBV-1 were detected in Group III (n = 21, 52.5%), followed by Group II (n = 16, 40%) and Group I (n = 2, 5%) (P < 0.0001). HCMV was not detected. A significant association of EBV-1 to periodontal site-specific parameters was observed in Group II patients (P < 0.05). EBV-1 was predominant with poor glycemic status patients.
Conclusion:
This study revealed that the incidence of herpes virus infection in periodontal patients was higher in diabetic patients and the examined patients were prone to EBV-1 infections.
Keywords: Diabetes mellitus, Epstein–Barr virus, multiplex polymerase chain reaction, periodontitis
INTRODUCTION
The most common complex disease that affects the oral cavity is periodontitis. It affects about 30%–50% of adults, with 7%–15% suffering from its severity.[1] Periodontitis is a deregulated inflammatory response that results in damage of tooth-supporting tissues. Collagen fiber breakdown in periodontal ligament results in periodontal pocket formation between gingiva and tooth. As this destruction progresses, it leads to deepening of pockets resulting in loss of attachment. Parallel to this progressing attachment loss, there is alveolar resorption leading to tooth mobility and tooth loss. The microbial species, host immune responses and ecosystem-based aspects are accountable for its development making it a multifactorial disease.[2] The risk factors also have an important role in patients with periodontal infection, which can be modifiable behavioral or nonmodifiable intrinsic factors.[3] According to the European Federation of Periodontology and the International Diabetes Federation report (2017), periodontitis and diabetes are chronic nontransmissible diseases that are independently allied with the mortality and are known to have a bidirectional relationship.[4] Type 2 diabetes mellitus (DM2) is a well-established risk factor for periodontitis.[5] The possible links between these two diseases are elevation in levels of oxidative stress, interleukin (IL)-1-β, tumor necrosis factor-α, IL-6, receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio and expression of Toll-like receptor 2/4.[4]
Diabetic patients with uncontrolled diabetes and high levels of glycosylated hemoglobin (HbA1c) are subjected to high degree of attachment loss, alveolar destruction and elevated local inflammatory cytokines, compared to controlled diabetic patients.[6] This influences the subgingival atmosphere, thereby controlling the microbial profile of the individual. The evidences suggest that the occurrence of specific periodontal pathogens and the levels of HbA1c in DM2 patients with periodontitis are significantly associated.[7] Hence, in such cases, detection of the specific microorganisms subgingivally plays an important role in its local and systemic propositions. Although bacterial plaque is the most common causative factor, it is unable to explain various facts such as site specificity of the disease, variations in progression of gingivitis into periodontitis and the destruction severity among the same and different individuals with similar bacterial load. Hence, it cannot be a solitary modulator of this complex disease. These facts have also spurred the efforts to uncover a supplementary etiologic factor for periodontitis.
Viruses explain the sporadic activity of the disease in specific sites. Many bacterial infections are known to occur as superinfection to viral diseases. Herpesviruses, such as herpes simplex virus-1, Epstein–Bar virus (EBV) and Cytomegalovirus (CMV) alone or in alliance with other subgingival pathogens, influences not only the development but also the progression of periodontitis.[8] These viruses were found in progressive periodontitis, aggressive periodontitis, HIV-associated periodontitis, necrotizing ulcerative gingivitis, periodontal abscesses and some types of medical disorders but at varying levels.[9,10,11,12] Several studies have investigated what role, inflammatory mechanisms play in the link between periodontitis and diabetes. However, barely a handful have evaluated, related or compared the prevalence of herpes virus in chronic periodontitis patients with diabetes mellitus.
MATERIALS AND METHODS
The cross-sectional study was undertaken in a tertiary care hospital at Karad, Maharashtra, India, after the due approval of the Ethics Committee. We included a total of 120 patients satisfying the inclusion (age: 35–75 years; patients with chronic moderate periodontitis based on the criteria of American Academy of Periodontology; minimum of 15 teeth present and patients with diagnosed DM2 before 4 years of this study with good, moderate or poor glycemic control based on the HbA1c test) criteria. Individuals not meeting these criteria (patients with any other systemic condition such as cardiac diseases, nutritional deficiency and immunocompromised patients; smokers and tobacco chewers; patients with symptomatic viral infection and having received periodontal treatment or antibiotics before 6 months of study and pregnant and lactating mothers) were excluded. The study procedure was explained, and due consent was obtained before commencing. The basic demographic data, socioeconomic status, oral hygiene habits, education levels, past medical and dental history and glycemic status were recorded in a predesigned pro forma.
Forty patients periodontally healthy without any systemic diseases were categorized as Group I, Group II included forty patients with chronic moderate periodontitis without DM2 and Group III comprised forty chronic moderate periodontitis patients with DM2 [Table 1]. The periodontal status was evaluated using plaque index (PI), gingival index (GI), probing pocket depth (PPD) and clinical attachment loss as per Sillness and Loe method.[13]
Table 1.
Characteristics of participants
| Group I | Group II | Group III | Total | P | |
|---|---|---|---|---|---|
| Mean age±SD | 47.30±8.47 | 48.05±9.86 | 47.88±9.37 | 47.74±9.18 | 0.9306 |
| Male:female | 20:20 | 18:22 | 17:23 | 55:65 | 0.7906 |
*No significance was observed with Chi-square test and ANOVA. SD: Standard deviation
Supragingival plaque was removed gently using sterile cotton pellets. It was randomly collected from Group I, and from the deepest periodontal pocket site in Group II, and Group III, with a single stroke of a sterile curette. The samples were immediately transferred into microcentrifuge tubes containing storage medium (TE buffer) for DNA extraction and multiplex polymerase chain reaction (PCR) studies. DNA extraction was performed using Qiagen QIAamp DNA Mini Kit (51,304, Setlab, India) as per manufacturer's specifications.
PCR was performed in a thermal cycler (Himedia, Prima-DUO™, Mumbai, India), with the constituents mixed and kept at 94°C for 30s, 60°C for 40s and 72°C for 50s. The samples were further incubated for 15 min at 78°C for primer extension. Further, the amplicons were run on a 5% agarose gel and bands visualized under UV transilluminator (Bio-Imaging Systems, NY, USA). The primers used in multiplex PCR were as follows, EP5: 5'-AACAATGGCAGCAGGTAAGC-3' and EM3: 5'-ACTTACCAAGTGTCCATAGGAGC-3' for EBV-1; CP16: 5'-GTACACGCACGCTGGTTACC-3' and CM3: 5'-GTAGAAAGCCTCGACATCGC-3' synthesized from Macrogen.
R i386.3.5.1 statistical software was used for data analysis. Continuous data are represented as mean ± standard deviation and the categorical variable are represented by the frequency table. An association between categorical variables is studied using Chi-square test. Continuous data were compared using t-test/Mann–Whitney U-test/analysis of variance/Kruskal–Wallis test.
RESULTS
The mean age of three different groups was not significantly different, although Group II had the highest age (48.05 ± 9.86 years), followed by Group III (47.88 ± 9.37 years) and finally Group I (47.30 ± 8.47 years). In Group I, gender was equally distributed, whereas in Group I, gender was predominantly male (45%) [Table 1]. No significant difference was observed between the average and site-specific parameters PPD and clinical attachment level (CAL) at P ≥ 0.05 in moderate chronic periodontitis patients (Groups II and III). The significance was observed between Groups I and II (P ≤ 0.001) and Groups I and III (P ≤ 0.001) in terms of site-specific and average PII, GI and PPD. Fair PI was observed in Group III, followed by Groups II and I (P ≤ 0.001); similar results were obtained for GI (P ≤ 0.001), as shown in Table 2.
Table 2.
Distribution of clinical parameters in three different groups (I, II and III)
| Factors | Group I | Group II | Group III | P | Post hoc analysis | ||
|---|---|---|---|---|---|---|---|
| I versus II | I versus III | II versus III | |||||
| SS PPD | 2.55±0.75 | 6.80±1.45 | 7.00±1.47 | <0.0001K | <0.0001 | <0.0001 | 0.6944 |
| SS CAL | 0.20±0.88 | 7.23±1.40 | 7.53±1.55 | <0.0001K | <0.0001 | <0.0001 | 0.6404 |
| SS PII | 1.23±1.10 | 1.93±0.73 | 1.88±0.69 | 0.0020K | 0.0046 | 0.0051 | 0.8100 |
| SS GI | 0.60±0.71 | 2.10±0.67 | 2.10±0.63 | <0.0001K | <0.0001 | <0.0001 | 0.9892 |
| PPD | 2.55±0.75 | 3.68±0.46 | 3.61±0.42 | <0.0001A | <0.0001 | <0.0001 | 0.8116 |
| CAL | 0.20±0.88 | 3.70±0.53 | 4.01±0.51 | <0.0001A | <0.0001 | <0.0001 | 0.1093 |
| PII | 0.77±0.56 | 1.94±0.50 | 1.74±0.43 | <0.0001A | <0.0001 | <0.0001 | 0.1900 |
| GI | 0.64±0.54 | 1.78±0.69 | 1.68±0.49 | <0.0001A | <0.0001 | <0.0001 | 0.7434 |
KKruskal- Wallis test, AOne-way ANOVA. SS: Site specific, PPD: Probing pocket depth, CAL: Clinical attachment level, PII: Plaque index, GI: Gingival index
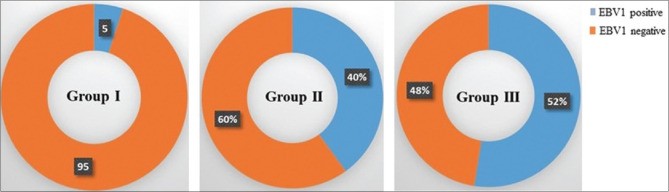
Only two samples were positive for EBV-1 in Group I. Sixteen samples were positive for EBV-1 in Group II (chronic periodontitis patients without type 2 DM) and 21 samples positive for EBV-1 in Group III (chronic periodontitis patients with type 2 DM), as shown in Figure 1, while none of the samples from all the groups showed positive for human CMV (HCMV).
Figure 1.
Prevalence of EBV1 in different groups of participants
Group II patients showed a significant association (P < 0.05) of EBV-1 to periodontal site-specific parameters (PII, GI, PPD and CAL), and Group III displayed a significant association (P < 0.05) of EBV-1 with PII, PPD and CAL [Table 3]. Hence, it is evident that the presence of EBV-1 is significantly associated with increasing severity of periodontitis. The glycemic status of Group II patients indicated that out of forty samples, only ten had good glycemic control. The rest fell in streams of moderate and poor with 15 participants each [Figure 2]. The incidence of EBV-1 was highest in poor glycemic control and least in good control participants, thus indicating the prevalence of EBV-1 having statistical significance (P < 0.0001) with glycemic status of the participants.
Table 3.
Distribution of clinical parameters with respect to the prevalence of herpes virus in all the three groups (I, II and III)
| Factors | EBV-1 | Group I | Group II | Group III | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SD | P | n | Mean±SD | P | n | Mean±SD | P | ||
| SS PII | + | 2 | 1.50±0.71 | - | 16 | 2.19±0.66 | 0.0600U | 21 | 2.10±0.62 | 0.0294U |
| − | 38 | 1.21±1.12 | 24 | 1.75±0.74 | 19 | 1.63±0.68 | ||||
| SS GI | + | 2 | 2.00±0.00 | - | 16 | 2.63±0.50 | <0.0001U | 21 | 2.29±0.64 | 0.0476U |
| − | 38 | 0.53±0.65 | 24 | 1.75±0.53 | 19 | 1.89±0.57 | ||||
| SS PPD | + | 2 | 4.00±0.00 | - | 16 | 7.94±1.39 | <0.0001U | 21 | 8.05±1.12 | <0.0001U |
| − | 38 | 2.47±0.69 | 24 | 6.04±0.91 | 19 | 5.84±0.76 | ||||
| SS CAL | + | 2 | 4.00±0.00 | - | 16 | 8.44±1.15 | <0.0001U | 21 | 8.57±1.33 | <0.0001U |
| − | 38 | 0.00±0.00 | 24 | 6.42±0.88 | 19 | 6.37±0.76 | ||||
| PII | + | 2 | 1.65±0.21 | - | 16 | 2.23±0.36 | 0.0012t | 21 | 1.83±0.37 | 0.1692t |
| − | 38 | 0.72±0.54 | 24 | 1.74±0.48 | 19 | 1.64±0.47 | ||||
| GI | + | 2 | 2.45±0.07 | - | 16 | 2.34±0.50 | <0.0001t | 21 | 1.93±0.42 | 0.0002t |
| − | 38 | 0.54±0.35 | 24 | 1.40±0.51 | 19 | 1.40±0.41 | ||||
| PPD | + | 2 | 4.00±0.00 | - | 16 | 3.99±0.50 | 0.0003t | 21 | 3.88±0.39 | <0.0001W |
| − | 38 | 2.47±0.69 | 24 | 3.48±0.31 | 19 | 3.31±0.16 | ||||
| CAL | + | 2 | 4.00±0.00 | - | 16 | 4.27±0.29 | <0.0001t | 21 | 4.39±0.15 | <0.0001W |
| − | 38 | 0.00±0.00 | 24 | 3.33±0.24 | 19 | 3.58±0.44 | ||||
UMann- Whitney U-test; tt-test; WWelch t-test. SS: Site specific, PPD: Probing pocket depth, CAL: Clinical attachment level, PII: Plaque index, GI: Gingival index, SD: Standard deviation
Figure 2.
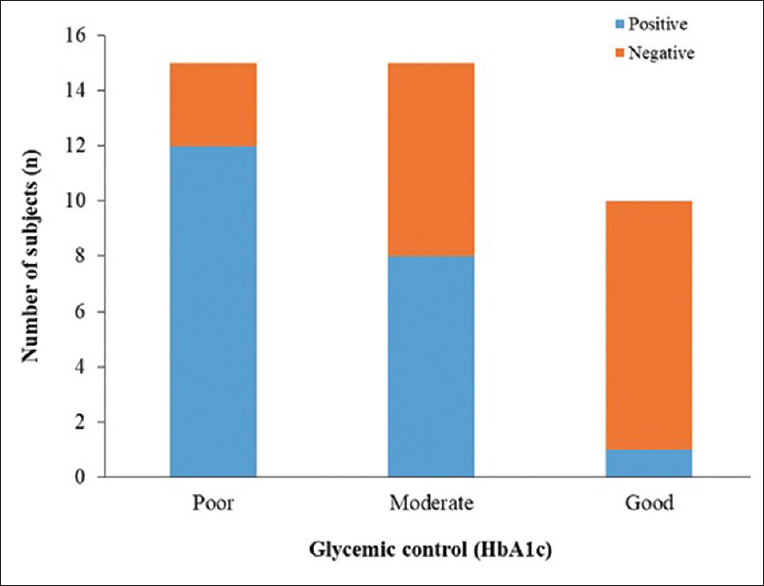
Glycemic status of the patients
DISCUSSION
Periodontitis, a critical periodontal disease, results in damage to the dentition and the cost incurred on treatment of the same to the patients.[14] High prevalence of periodontitis has been reported in 72%–85% of the general population which is alarming.[15,16] Thus, for ideal management, it is mandatory to have proper understanding of its etiology and risk factors influencing it. With an increase in acquaintance of periodontitis, the focus from bacterial plaque as the primary causative factor has shifted to an additional cause by the viruses (HCMV and EBV) having a significant impact on the periodontium.
Demographic studies from early reports suggest that the prevalence of chronic periodontitis was more in the third to seventh decade of life.[10,17] Here, the cumulative mean age was 47.74 ± 9.18 years. The study also excluded the patients with systemic disease or conditions such as cardiovascular diseases, hypertension and pregnancy, except for DM2, which are known to influence periodontitis.[18] Patients with smoking history were excluded as it imparts unfavorable effect on the occurrence and progress of periodontitis.[19]
The precise and quick method of viral detection by PCR was performed. EBV-1 was highly detected subgingivally of Group III patients, followed by Groups II and I. Similarly, Contreras et al. found EBV-1 in 79% of patients with chronic periodontitis in the American population.[20] Similarly, EBV-1 was found in 44% of cases by Konstantinidis et al. in chronic periodontitis patients in Greece population,[21] and Kubar et al. also found 56% of prevalence of EBV-1 in periodontitis patients.[22] This difference in detection is attributed to the difference in geographical distribution and ethnicity of the population as evaluated by Haffajee et al.[23]
Here, HbA1c values were utilized for categorizing (good, moderate and poor control) patients according to their glycemic control in Group III based on ADA 2009 guidelines.[24,25] Casarin et al. evaluated the influence of glycemic control on EBV-1 and HCMV infection in periodontal pocket of DM2 patients. EBV-1 was found more commonly present in diabetic patients as compared to HCMV, which is in line with our findings. The reason for this can be that the CMV infections are more predominant in the gingival region and also more common in sites undergoing active periodontitis.[10] In our study the sample was obtained from subgingival plaque region and the stage of periodontitis was pronounced. Poor glycemic control harbors higher levels of EBV-1 (81%) compared to those with good (42.9%) or moderate (61.1%) glycemic control.[26] Thus, the results of our study concur with previous findings where the prevalence of EBV-1 was highest in chronic periodontitis patients with DM 2 (Group III) and least in healthy patients (Group I). It was also observed that the prevalence of EBV-1 was highest in participants with poor glycemic control (n = 12) and was least in participants with good control (n = 1). Therefore, DM2 modifies the subgingival flora as this alters the neutrophil levels which are accorded for their role in chemotactic, phagocytic and microbicidal actions. This altered immune response ultimately results in accumulation of high concentration of viral and bacterial substrate.[27,28]
Subgingival samples were collected from the deepest pocket site, as the destruction and progression of periodontitis are active at the supra- and subgingival region.[29] Further, the site-specific and average PII, GI and PPD values of the healthy group (Group I) were highly significant to the chronic periodontitis patients (Groups II and III). It is indicative that the presence of EBV-1 is significantly associated with increasing severity of periodontitis and has a strong association with clinical parameters (probing depth and attachment loss).[30] It is in accordance with the reports by Chalabi et al., where EBV-1 was more prevalent in pockets with PD >6 mm as compared to PD <3 mm.[31] Various reports suggest that the incidence of chronic periodontitis is more in the third to seventh decade of life.[17,32] Therefore, in this study, the age considered was 35–75 years, the mean age being 47.74 ± 9.18 with a total of 120 patients. The current study also confirms the higher pocket depth in EBV-1-positive sites than in virus-negative sites.[33] Shah et al. also found a positive association of EBV with increased GI, PPD and CAL, which is also reported in the present study.[10]
Thus, viral proteins or substrates that are expressed on cell membrane act as a bacterial receptor, further promoting the colonization of bacteria. As reported by Shah et al., the bacterial plaque increases the gingival inflammation, it further smoothens the entry of herpes virus (EBV-1) into the periodontium, thus aggravating the periodontal disease.[10] There is an increase in the hard- and soft-tissue destruction due to the release of cytokines and chemokines. Moreover, there is a decrease in tissue turnover rate. Hence, the occurrence of EBV increases in individuals with poor glycemic status and sites with greater clinical attachment loss.
One of the limitations of this study was that the results were qualitative (showing presence or absences of EBV-1 and HCMV) and not quantitative (viral load). Hence, quantitative results from real-time PCR will prove to be helpful. Although the virus (like EBV) and diabetes with poor glycemic status have an important role in development and progression of periodontitis, the present treatment modalities are still, primarily focused on the removal of bacterial plaque. A substantial amount of work is required to develop an effective antiviral agent, maybe in the form of vaccines along with proper guidelines for using it in routine practice. In addition, as the current study was a cross-sectional study and established a relation, further studies required to establish causation.
CONCLUSION
A higher incidence of herpes virus infection was visible in moderate chronic periodontitis patients than healthy individuals. Between the evaluated viral strains, EBV-1 was predominant herpes virus detected in periodontitis patients and it was seen to be associated with the glycemic status of type 2 DM patients. The prevalence of other herpes viruses in other chronic diseases, specifically type 2 DM, has to be fostered, thereby reporting the role of glycemic diet in viral infections under chronic diseases can be accorded.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We extend our thanks to Dr Girish Suragimath, Dr Sameer Zope and Dr Apurva Kale (Dept. of Periodontology,SDS, KIMSDU) for their invaluable guidance. We also highly appreciate the extended support from Dr Kailas D Datkhile and Miss Madhavi N Patil (Dept. of Molecular biology and Genetics, KIMSDU, Karad) in processing of the samples.
REFERENCES
- 1.Azodo CC, Erhabor P. The roles of viruses in periodontal disease. J Dent Res Rev. 2015;2:37–41. [Google Scholar]
- 2.Solomon SM, Filioreanu AM, Stelea CG, Grigoras SI, Sufaru IG, Maftei GA, et al. The assessment of the association between herpesviruses and subgingival bacterial plaque by Real-time PCR analysis. Rev Chim (Bucharest) 2018;69:507–10. [Google Scholar]
- 3.Van Dyke TE, Dave S. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European federation of periodontology. Diabetes Res Clin Pract. 2018;45:138–49. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 5.Negrato CA, Tarzia O, Jovanovic L, Chinellato LE. Periodontal disease and diabetes mellitus. J Appl Oral Sci. 2013;21:1–12. doi: 10.1590/1678-7757201302106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, Grbic JT, et al. Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and Type 2 diabetes. J Periodontol. 2004;75:1203–8. doi: 10.1902/jop.2004.75.9.1203. [DOI] [PubMed] [Google Scholar]
- 7.Makiura N, Ojima M, Kou Y, Furuta N, Okahashi N, Shizukuishi S, Amano A. Relationship of Porphyromonas gingivalis with glycemic level in patients with Type 2 diabetes following periodontal treatment. Oral Microbiol Immunol. 2008;23:348–51. doi: 10.1111/j.1399-302X.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 8.Slots J. Human viruses in periodontitis. Periodontol. 2000;2010(53):89–110. doi: 10.1111/j.1600-0757.2009.00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Kazi MM, Bharadwaj R, Bhat K, Happy D. Association of herpes viruses in mild, moderate and sever chronic periodontitis. J Clin Diagn Res. 2015;9:DC05–8. doi: 10.7860/JCDR/2015/13781.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah R, Mehta D. Prevalence of herpesviruses in gingivitis and chronic periodontitis: Relationship to clinical parameters and effect of treatment. J Indian Soc Periodontol. 2016;20:279–85. doi: 10.4103/0972-124X.179896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava AK, Shukla S, Srivastava P, Dhole TN, Nayak MT, Nayak A, et al. Real time detection and quantification of Epstein Barr virus in different grades of oral gingivitis and periodontitis patients. J Exp Ther Oncol. 2019;13:9–14. [PubMed] [Google Scholar]
- 12.Chatzopoulou E, Fanourakis G, Trianti M, Dereka X. Herpes simplex virus 1 and 2 in chronic periodontitis: Prevalence and association with clinical parameters. Dent Health Curr Res. 2018;4:2–4. [Google Scholar]
- 13.Silness P, Löe H. Periodontal disease in pregnancy II. Acta Odontol Scand. 1964;22:121–6. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 14.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. 11th ed. Vol. 1. Amsterdam, Netherlands: Elsevier; 2011. [Google Scholar]
- 15.Peter KP, Mute BR, Pitale UM, Shetty S, Shashikiran HC, Satpute PS. Prevalence of periodontal disease and characterization of its extent and severity in an adult population an observational study. J Clin Diagn Res. 2014;8:4–7. doi: 10.7860/JCDR/2014/8684.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shewale AH, Gattani DR, Bhatia N, Mahajan R, Saravanan SP. Prevalence of periodontal disease in the general population of India a systematic review. J Clin Diagn Res. 2016;6:4–7. doi: 10.7860/JCDR/2016/17958.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaji S K, Lavu V, Rao S. Chronic periodontitis prevalence and the inflammatory burden in a sample population from South India. Indian J Dent Res. 2018;29:254–9. doi: 10.4103/ijdr.IJDR_335_17. [DOI] [PubMed] [Google Scholar]
- 18.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim) 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CH, Han ML, Teng NC, Lee CY, Huang WT, Lin CT, et al. Cigarette smoking aggravates the activity of periodontal disease by disrupting redox homeostasis an observational study. Sci Rep. 2018;8:110–55. doi: 10.1038/s41598-018-29163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras A, Umeda M, Chen C, Bakker I, Morrison JL, Slots J. Relationship between herpesviruses and adult periodontitis and periodontopathic bacteria. J Periodontol. 1999;70:478–84. doi: 10.1902/jop.1999.70.5.478. [DOI] [PubMed] [Google Scholar]
- 21.Konstantinidis A, Sakellari D, Papa A, Antoniadis A. Realtime polymerase chain reaction quantification of Epstein-Barr virus in chronic periodontitis patients. J Periodontal Res. 2005;40:294–8. doi: 10.1111/j.1600-0765.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 22.Kubar A, Saygun I, Ozdemir A, Yapar M, Slots J. Realtime polymerase chain reaction quantification of human cytomegalovirus and Epstein-Barr virus in periodontal pockets and the adjacent gingiva of periodontitis lesions. J Periodontal Res. 2005;40:97–104. doi: 10.1111/j.1600-0765.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 23.Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol. 2004;31:996–1002. doi: 10.1111/j.1600-051X.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 24.International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 26.Shi M, Wei Y, Hu W, Nie Y, Wu X, Lu R. The subgingival microbiome of periodontal pockets with different probing depths in chronic and aggressive periodontitis: A pilot study. Front Cell Infect Microbiol. 2018;8:124. doi: 10.3389/fcimb.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casarin RC, Duarte PM, Santos VR, Lima JA, Gagnon G, Casati MZ, et al. Influence of glycemic control on Epstein-Bar and Cytomegalovirus infection in periodontal pocket of Type 2 diabetic subjects. Arch Oral Biol. 2010;55:902–6. doi: 10.1016/j.archoralbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Dıaz-Romero RM, Casanova-Roman G, Beltran-Zuniga M, Padilla JB, Mendez JD, Avila-Rosas H. Oral infections and glycemic control in pregnant Type 2 diabetics. Arch Med Res. 2005;36:42–8. doi: 10.1016/j.arcmed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Beikler T, Schnitzer S, Abdeen G, Ehmke B, Eisenacher M, Flemmig TF. Sampling strategy for intraoral detection of periodontal pathogens before and following periodontal therapy. J Periodontol. 2006;77:1323–32. doi: 10.1902/jop.2006.050204. [DOI] [PubMed] [Google Scholar]
- 30.Saygun I, Şahin S, Özdemir A, Kurtiş B, Yapar M, Kubar A, Ozcan G. Detection of human viruses in patients with chronic periodontitis and the relationship between viruses and clinical parameters. J Periodontol. 2002;73:1437–43. doi: 10.1902/jop.2002.73.12.1437. [DOI] [PubMed] [Google Scholar]
- 31.Chalabi M, Moghim S, Mogharehabed A, Najafi F, Rezaie F. EBV and CMV in chronic periodontitis: A prevalence study. Arch Virol. 2008;153:17–9. doi: 10.1007/s00705-008-0186-7. [DOI] [PubMed] [Google Scholar]
- 32.Stanko P, Holla LI. Bidirectional association between diabetes mellitus and inflammatory periodontal disease. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:35–8. doi: 10.5507/bp.2014.005. [DOI] [PubMed] [Google Scholar]
- 33.Sharma R, Padmalatha O, Kaarthikeyan G, Jayakumar ND, Varghese S, Sherif K. Comparative analysis of presence of Cytomegalovirus (CMV) and Epsteinbarr virus 1 (EBV-1) in cases of chronic periodontitis and aggressive periodontitis with controls. Indian J Dent Res. 2012;23:454–8. doi: 10.4103/0970-9290.104948. [DOI] [PubMed] [Google Scholar]