Abstract
Ghost cells (GCs) have been a curious topic since a great deal of time. Extensive research has been done to deduce the true characteristics and formation of these cells. GCs are balloon-shaped, elliptical, pale eosinophilic epithelial cells with pyknotic nuclei, leaving only a faint outline. In routine H and E staining, these cells give shadowy appearance and hence are also called shadow cells or translucent cells. The present article is an attempt to describe in detail about the origin, microscopic appearance, staining property, immunohistochemistry profile and diagnostic importance of GCs.
Keywords: ghost cell, hematoxylin and eosin, Masson's trichrome stain, shadow cell, translucent cell, van Gieson stain
HISTORY
The ghost cells (GCs) were first reported by Highman and Ogden (1936) in cutaneous calcifying epithelioma of Malherbe (pilomatricomas). They are described as swollen, pale, eosinophilic epithelial cells with pyknotic nuclei and faint cellular outline. In H- and E- stained [Figure 1] sections, these cells give a shadowy appearance, hence the name “GC.”[1] They are also called shadow cells or translucent cells. GCs in odontogenic lesions were demonstrated in 1962 by Gorlin et al. in calcifying odontogenic cyst (COC).[2] The GCs are of diagnostic importance and seen in numerous odontogenic and nonodontogenic lesions [Table 1].
Figure 1.
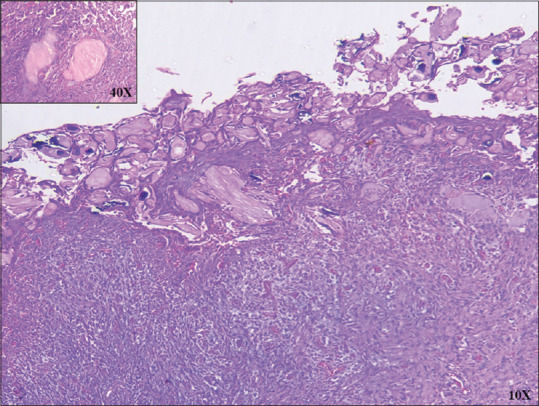
H&E-stained ghost cells are swollen, pale, with pyknotic nuclei and faint cellular outline (×10). Inset magnified (×40) H and E view of ghost cells
Table 1.
Ghost cell-associated lesions
| Odontogenic lesions | Nonodontogenic lesions |
|---|---|
| Inner enamel epithelium of developing teeth | Cutaneous calcifying epithelioma of Malherbe/pilomatrixoma (in skin) |
| Eruption cyst | Craniopharyngioma (in pituitary gland) |
| Glandular odontogenic cyst | |
| Calcifying epithelial odontogenic cyst | |
| Ameloblastoma (granular cell type) | |
| Ameloblastic fibroma | |
| Ameloblastic fibro-odontoma | |
| Odontoameloblastoma/dentinoameloblastoma | |
| Odontoma (complex and compound) | |
| Dentinogenic ghost cell tumor | |
| Ghost cell odontogenic carcinoma |
GHOST CELL HISTOGENESIS AND ITS FATE
GCs are always epithelial in origin and are believed to originate from any layer of epithelium i.e., basal, intermediate, or superficial and lack intercellular junctions.[3] Form of true keratinization, prekeratin, stages in the process of ortho, para and aberrant keratin formation, abnormal/aberrant keratinization, highly keratinized epithelial cells and cells which have lost their developmental and inductive effect are the various confusing terminologies used to describe the illusional nature of GCs. Following are the different theories put forward regarding the histogenesis of GC.[4]
Degenerative changes: local anoxia as a cause for degeneration
Coagulative necrosis: altered or absence of cytokeratin expression by GCs was probably due to this coagulative necrosis
Metaplastic transformation of odontogenic epithelium: ischemia as a reason for squamous metaplasia of odontogenic epithelium
Aberrant keratinization and/or accumulation of hard keratin
Abortive formation of enamel matrix: Several investigators have found positive results for enamel matrix proteins in GCs of odontogenic lesions
Terminal differentiation and apoptosis appear to supplement the pathologic progression of the odontogenic/nonodontogenic epithelium to ghost/shadow cells
Role of Wnt-β-catenin-Lef pathway and Notch signaling partially explains the link between tumorigenesis of these lesions and ghost/shadow cell formation and/or calcification
Mucin-induced GC transformation.
ULTRA-STRUCTURAL FINDINGS
Fejerskov and Krogh[5] explained the ultrastructure of GCs in COCs, where they found coarse, thick tonofilament bundles in their cytoplasm, thus distinguishing it from the keratin pattern of the epidermis or oral epithelium which exhibits evenly distributed, fine tonofilaments in the cytoplasm. Endoplasmic reticulum, mitochondria, Golgi apparatus and ribosomes could not be identified in GC. These findings were supported by Regezi et al.,[6] who suggested that GCs represent an unusual or aberrant form of keratin and not the true keratin. In light microscopy, GC contains granular cytoplasm and pale nuclear zone. The electron microscopy shows bundles of tonofilaments of 60–400 nm in diameter, arranged in various directions in the cytoplasm of the GCs. Under TEM, the cytoplasmic fibrils in GCs have been recognized as tonofilaments, sometimes wrongly considered as keratin.[7] Lucchese et al.[8] analyzed the GCs using confocal laser scanning microscope. Based on the different fluorescent effects, they divided GCs into three types: (1) Scarcely detectable, (2) well-resolved and (3) cells with excellent resolution.
STAINING CHARACTERISTICS
To differentiate GCs from similarly stained cornified areas (e.g., poorly decalcified osteodentine, dentinoid, amyloid-like material) various stains were employed like Taenzer-Unna orcein, peracetic acid, azure A-eosin B, periodic acid Schiff with or without diastase digestion, Bensley's modification of Mallory's stain and the DDD stain for sulfhydryl and disulfide of Barnett and Seligman. To differentiate GCs from true dentinoid phloxin-tartrazine stain can be used, it stains both but to a different degree.
GCs showed nonfluorescent to frankly positive yellow fluorescence when observed with the rhodamine B method, dull orange-brown to red with the Mallory's aniline blue reaction and light brown to bright yellow with van Gieson stain [Figure 2]. GCs exhibit various degrees of chromophilia with Heidenhain's iron hematoxylin, negative staining with Alcian blue but some were Periodic Acid-Schiff positive. Masson's trichrome stained GC dull brown, orange-brown or red[4] [Figures 3, 4 and Table 2].
Figure 2.
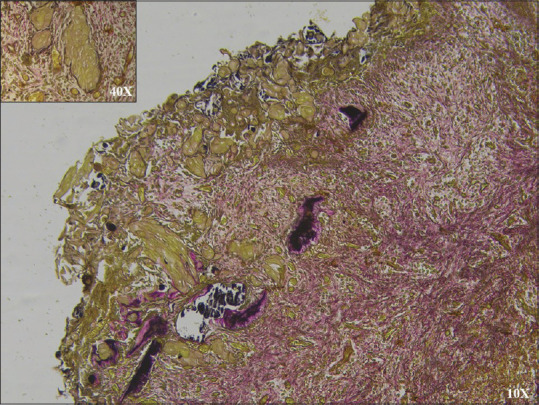
Light brown ghost cells in van Gieson stain (×10). Inset magnified (×40) view of ghost cells
Figure 3.
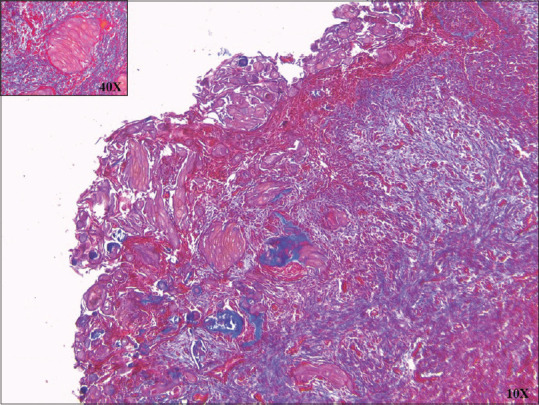
Dark red stained ghost cells in Masson's Trichrome stain (×10). Inset magnified (×40) view of ghost cells
Figure 4.
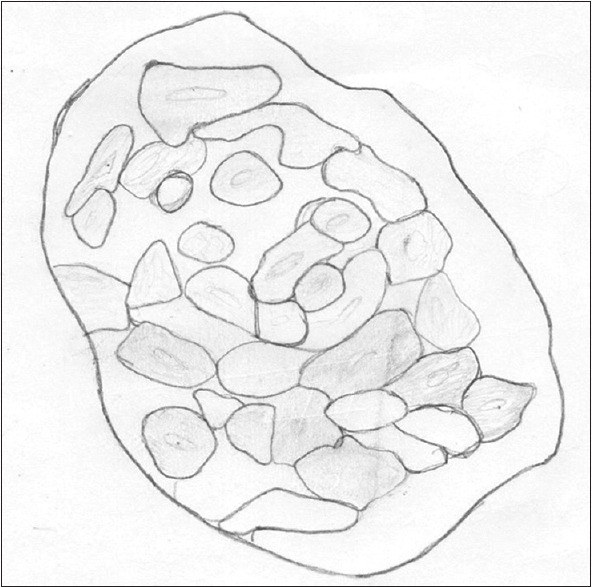
Corresponding hand draw illustration of ghost cells
Table 2.
Various stains for ghost cells
| Stains used | Reaction of ghost cell |
|---|---|
| H&E | Pale pink |
| Goldner stain | Pale red |
| Masson’s trichrome | Dull brown, orange-brown, or red |
| Mallory’s | Orange-brown to red |
| Van Gieson | Light brown to bright yellow |
| Rhodamine B | Yellow fluorescence |
| PAS | Magenta |
PAS: Periodic acid-Schiff
IMMUNOHISTOCHEMICAL CHARACTERISTICS
GC s show positive immunoexpression for cytokeratins AE1/AE3 and 34 βE12. Takata et al.[9] examined the immunoreactivity of GCs in COCs and dermal calcifying epitheliomas, with antibodies against amelogenin, enamelin, sheath protein (sheathlin) and enamelysin in the cytoplasm of GCs. They found a distinct immunolocalization of the enamel-related proteins within GCs of COC, while similar areas in the calcifying epitheliomas of the skin showed a negative reaction. These findings strengthen the belief that GCs seen associated with odontogenic lesions are different from those seen in the lesions of skin origin.[10]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the patient who participated in the report.
REFERENCES
- 1.Yadav G, Shirol P, Koshya A, Ladke V. Ghost cells - still a mystery: A short review. Universal Research Journal of Dentistry. 2016;6:15. [Google Scholar]
- 2.Gorlin RJ, Pindborc JJ, Clausen FP, Vickers RA. Calcifying odontogenic cyst-possible analogue of cutaneous calcifying epithelioma of Malherbe. Oral Surg. 1962;15:1235–43. doi: 10.1016/0030-4220(62)90159-7. [DOI] [PubMed] [Google Scholar]
- 3.Mehendiratta M, Bishen KA, Boaz K, Mathias Y. Ghost cells: A journey in the dark…. Dent Res J (Isfahan) 2012;9:S1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Mankapure PK. Ghost cells and its histogenesis: A narrative review. IJSS Case Rep Rev. 2015;6:35–9. [Google Scholar]
- 5.Fejerskov O, Krogh J. The calcifying ghost cell odontogenic tumor-or the calcifying odontogenic cyst. J Oral Pathol. 1972;1:273–87. doi: 10.1111/j.1600-0714.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 6.Regezi JA, Courtney RM, Kerr DA. Keratinization in odontogenic tumors. Oral Surg Oral Med Oral Pathol. 1975;39:447–55. doi: 10.1016/0030-4220(75)90088-2. [DOI] [PubMed] [Google Scholar]
- 7.Bajpai M. Ghost cells – An overview. IJCPD. 2011;7:205–8. [Google Scholar]
- 8.Lucchese A, Scivetti M, Pilolli GP, Favia G. Analysis of ghost cells in calcifying cystic odontogenic tumors by confocal laser scanning microscopy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:391–4. doi: 10.1016/j.tripleo.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Takata T, Zhao M, Nikai H, Uchida T, Wang T. Ghost cells in calcifying odontogenic cyst express enamel-related proteins. Histochem J. 2000;32:223–9. doi: 10.1023/a:1004051017425. [DOI] [PubMed] [Google Scholar]
- 10.Halappa TS, George J, Shukla A. Odontogenic ghost cells: Realities behind the shadow…. J Oral Res Rev. 2014;6:40–3. [Google Scholar]


