Abstract
Objective:
Various histological grading of oral squamous cell carcinoma has been contributed to the literature at different periods, but the reliability of such grading systems is controversial. This study attempted to measure the efficacy of Bryne's parameters on the full thickness of incisional biopsies which are representative of the original lesion with the proven molecular malignancy markers P53 and Ki67.
Materials and Methods:
One hundred incisional biopsy specimens of oral squamous cell carcinoma were obtained and histologically graded according to Broder's grading system. The same was graded using Bryne's parameters on full thickness of obtained incision sample. Immunohistochemistry was carried out for both p53 and Ki67.
Results:
We found a high discrepancy in the grading of lesions with Broader as well as Bryne grading parameters within the same lesion. When compared with the molecular expression percentages of p53 and Ki67, highly significant correlation was found in Bryne's parameters (Pearson Chi-square, P value 0.000) in incisional biopsies than Broder's.
Conclusion:
The use of parameters by Bryne on invasive front of excision biopsies can provide significant grading on incisional biopsies which are more comparable to the molecular behavior of tumor given by the p53 and Ki67 expression.
Keywords: Broder's grading, Bryne's grading, Incisional biopsies, Ki67, malignancy grading, p53
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most frequent malignancy in the mouth, accounting for 95% of all oral malignant lesions.[1] In clinical practice, the treatment plan and prognosis of OSCC are mainly based on the tumor node metastasis (TNM) (primary tumor, regional lymph node metastasis, and distant metastasis) staging system. However, the TNM system does not provide any information on the biological characteristics and clinical behavior of the tumor.[2,3] Histological grading of OSCC represents major challenge as patients with similar clinical staging can present different outcomes. The histological features of OSCC may differ from area to area within the same tumor thus requiring a careful evaluation for its proper diagnosis. A precise histological grading of OSCC is of prime importance as it gives the idea of the severity of the lesion in addition to the adoption of therapeutic management and to predict the possible clinical course of the disease. Several histological grading systems are in practice designed to predict the clinical behavior of OSCC.[2]
Various histological grading parameters are adopted by authors in literature focusing on the best histological behavior of lesion. Few such parameters are the degree of differentiation, degree of keratinization, nuclear pleomorphism and mitoses, amount of inflammatory infiltrate, tumor thickness, depth of tumor invasion, the pattern of invasion, perineural and vascular invasion. Among these, all or few parameters were considered by various authors for their proposed grading system namely Broder's, (1920) Anneroth's et al. on full-thickness biopsy specimens and later Bryne's et al. (1992) on invasive front of excised tumors.[4,5,6] Even though biopsy remains the gold standard in diagnosis no single histological grading system till date is compared with its molecular behavior to explain the severity of the lesion. Excision and Incision biopsies hold its pros and cons as excision biopsy can provide the invasive front of the lesion which is now considered the most reliable site for tumor grading but it may result in over treatment. On the other hand, incision biopsies can provide early information on tumor behavior with minimum invasiveness preventing over treatment. The aim of this study is to compare the parameters by Bryne's et al. (1992) (invasive front of excision biopsies) on the full thickness of incision biopsies with the expression of proliferation markers p53 and Ki67.
MATERIALS AND METHODS
Study sample
Three 5 μm thick sections were cut from 100 formalin-fixed paraffin-embedded (FFPE) incision biopsy specimens of OSCC and one from each case were subjected to H and E (hematoxylin-eosin) staining.
Grading criteria
All cases were graded according to Broder's grading system [Table 1] by three independent oral pathologists. The same tissue sections were evaluated for Bryne's parameters [Table 2] on full thickness of the specimen. Each case was subjected to immunohistochemical (IHC) staining by p53 and Ki67 to access the proliferation potential of tumor.
Table 1.
Broder’s (1920) grading system
| Grade | Type | Degree of differentiation (%) |
|---|---|---|
| Grade 1 | Well | 75-100 |
| Grade 2 | Moderate | 50-75 |
| Grade 3 | Poor | 25-50 |
| Grade 4 | Anaplastic | 0-25 |
Table 2.
Bryne’s criteria (1992)
| Morphologic features | Score 1 | Score 2 | Score 3 | Score 4 |
|---|---|---|---|---|
| Degree of keratinisation | High | Moderate | Minimal | No |
| Nuclear polymorphism | Little | Moderate | Abundant | Extreme |
| Pattern of invasion | Pushing infiltrating borders | Infiltrating solid, cord or band | Small group or cord of cells | Marked and wide cellular dissociation |
| Host response | Marked | Moderate | Slight | None |
Bryne's criteria:
Grade 1: total score 4–8 (well differentiated)
Grade 2: total score 9–12 (moderate differentiated)
Grade 3: total score 13–16 (poorly differentiated)
Immunohistochemical analysis
From FFPE blocks of 100 OSCC specimens, 5 micrometer thick sections on poly L lysine coated slides were subjected to IHC analysis of p53 (RTU (Ready To Use), Primary antihuman rabbit antibody, Leica Biosystems, Japan), and Ki67 (RTU, Primary antihuman rabbit monoclonal antibody, Leika). Tissue sections were deparaffinized in xylene (twice), treated with a graded series of alcohol (100%, 95%, 85%, and 75% ethanol), and then incubated in phosphate-buffered saline (PBS, pH 7.4) for 5 min. Heat-induced antigen retrieval was done by immersion in 10 mM Tris-ethylenediaminetetraacetic acid with pH 9 at 600W in pressure boiler until two whistles. Endogenous peroxidase was inactivated by 3% hydrogen peroxide for 10 min. The tissue sections were incubated with primary antibodies against p53 and Ki67 for 40 min in humidifying chambers followed by incubation with secondary polyclonal conjugate (Dako, Glostrup, Denmark) for 30 min. Lastly, tissue sections were treated with diaminobenzidine as a substrate chromogen and counterstained with hematoxylin. As negative controls, tissue sections were treated with PBS instead of the primary antibody. Skin sections and lung SCC were taken as positive controls for Ki67 and p53 respectively. The slides were then mounted, observed, and evaluated using research microscope (Nikon Eclipse Ni-U) using NIS Basic research software.
Immunohistochemical evaluation
The positivity for p53 and Ki-67 were evaluated using research microscope (Nikon Eclipse Ni-U). The expression was quantitatively assessed on five randomly selected fields under 400X by grid aided image analysis using NIS Basic research software. Positivity for both markers was observed in the nucleus of cell. The percentage positivity for both markers was calculated by the number of positive cells/1000 cells in the specimen. Ki67 labeling index was assigned from the percentage expression.
p53 evaluation:
<10%= negative
11%–50%= score1
51%–70%=score2
>70%=score 3
Ki-67 labelling index:
0 = negative for IHC staining
1 = 1%–25% cells were positive
2 = 26%–50% cells were positive
3 = more than 50% cells were positive
Statistical analysis
Results were statistically analyzed by entering the findings into Microsoft excel worksheet and compared for statistical significance using SPSS (Statistical Package for the Social Sciences) version 25 (IBM, US). The percentage expression of p53 and Ki67 was compared with the frequency of cases under different grades by both Broder's and Bryne's criteria using the Pearson Chi-square test. The P ≤ 0.05 was considered significant and <0.001 as highly significant.
RESULTS
One hundred hematoxylin and eosin attained sections of OSCC specimens were graded on basis of Broder's criteria [Table 1[ by three oral pathologists. The same sections were assessed using four parameters of Bryne's grading system [Table 2 and Figures 1–3]. On the case of discrepancy, final grading was done by re-evaluation with inter-observer agreement. The mean score was obtained by the sum of scores attributed to each morphological feature by each independent observer, to prevent possible bias. The final score for each case was tabulated and graded. The samples were subjected to IHC staining using P53 and Ki67 [Figure 4].
Figure 1.
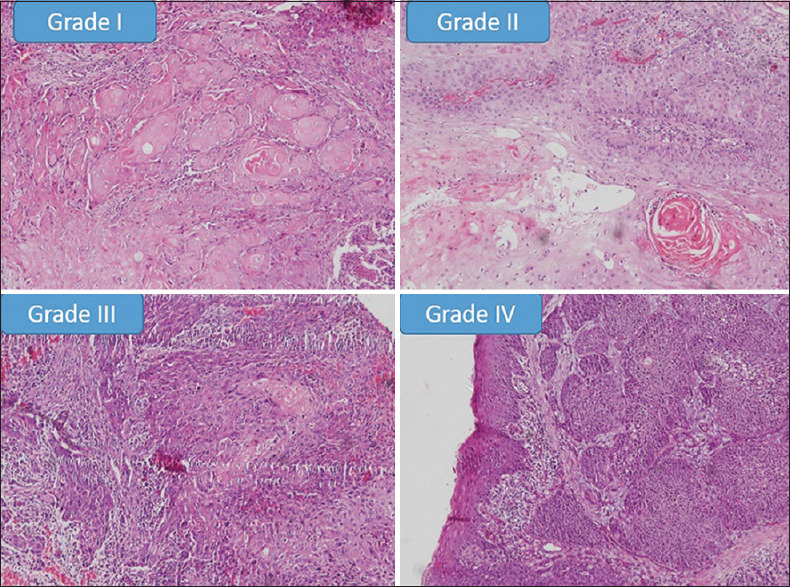
Showing histopathological photomicrographs of different grades of degree of keratinisation in OSCC samples in comparison with Anneroth's criteria (×100)
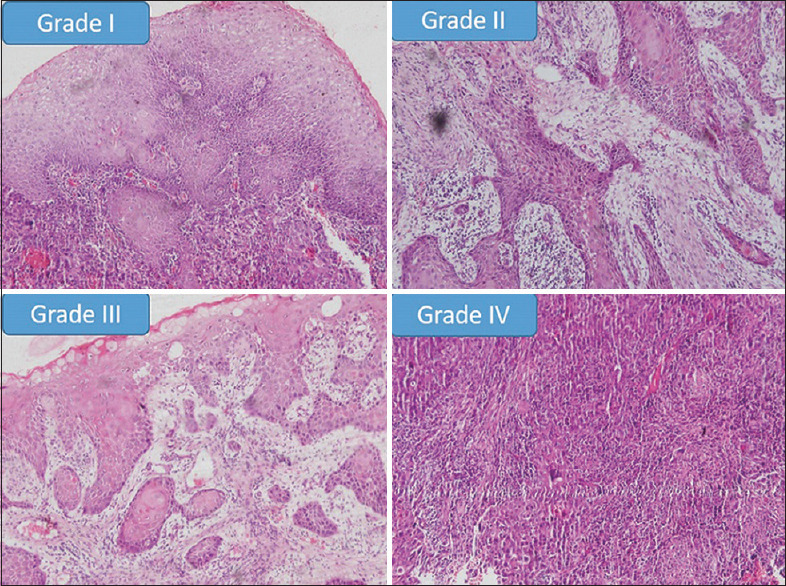
Figure 3.
Showing histopathological photomicrographs of different grades of host response in OSCC samples in comparison with Anneroth's criteria (×100)
Figure 4.
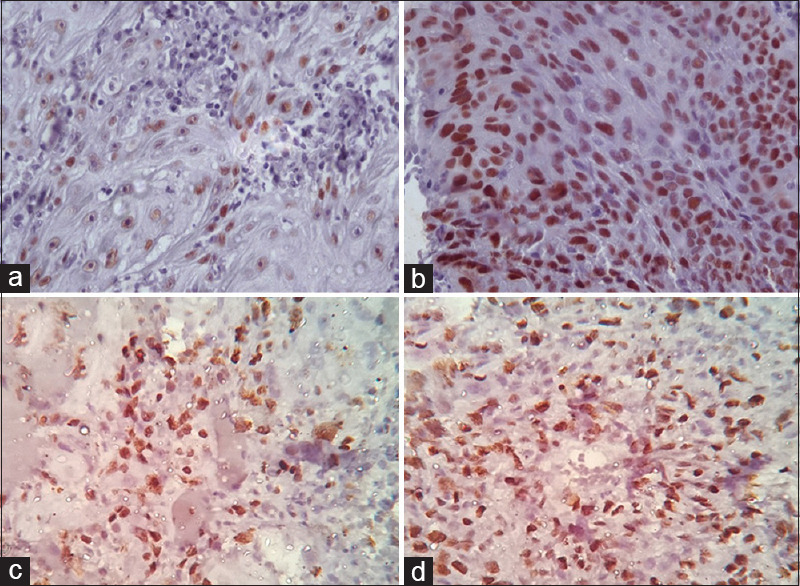
Showing immunohistochemical expression of P53 and Ki67 in OSCC samples graded by Broder's criteria (×400). (a) P53 expression in well differentiated OSCC. (b) P53 expression in Moderately differentiated OSCC. (c) Ki67 expression in well differentiated OSCC. (d) Ki67 expression in Moderately differentiated OSCC
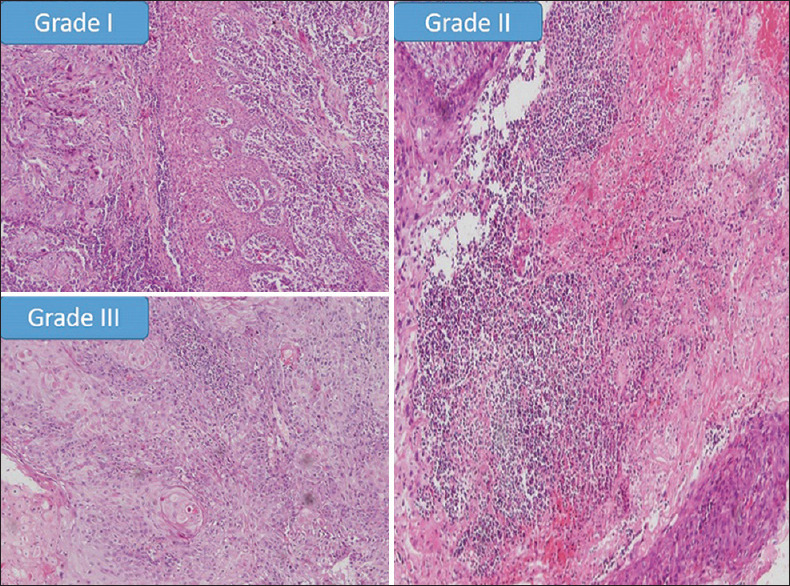
Figure 2.
Showing histopathological photomicrographs of different grades of pattern of invasion in OSCC samples in comparison with Anneroth's criteria (×100)
Histological evaluation
According to Broder's criteria = Out of 100 cases– 63 cases were well-differentiated squamous cell carcinoma (WDSCC) and 37 cases were moderately differentiated squamous cell carcinoma (MDSCC)
According to Bryne's criteria = 47 cases scored 7 (Grade 1-well differentiated), 52 cases scored 10 (Grade 2-moderately differentiated) and one case scored 13 (Grade 3-poorly differentiated) (excluded from the study for comparison)
Discrepancy in Broder and Bryne grading
In this study, 63/100 cases were graded as WDSCC on basis of Broder's system while 47/100 were under Grade 1 (well differentiated) on grading using Bryne's criteria. Similarly, 37/100 cases were of MDSCC on basis of Broder's system whereas it was 52/100 on Bryne's criteria. One case of Bryne Grade 3 was omitted from the study for ease of comparison. In comparison in the 63 WDSCC cases by Broder, 29 cases were Grade 2 (moderate) and 34 were Grade 1 (well) according to Bryne's criteria. Similarly, out of 37 MDSCC cases by Broder, 13 cases were of Grade 1 (well), 23 cases were Grade 2 (moderate) and one was Grade 3 (poor) [Chart 1].
Chart 1.
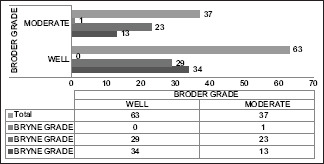
Comparison of cases according to Broder's and Bryne's grading criteria
P53 expression and Broder grading
On comparison of p53 expression with Broder's grading, in WDSCC 8/63 cases had low p53 expression but 47/63 had expression above 50% and 8/63 showed very high p53 expression(>70%) [Chart 2]. Similarly, in 36 cases of MDSCC, no cases had low p53 while 24/36 had expression above 50% and 12/36 cases had very high p53 expresion (>70%). Statsitical analysis by Pearson Chi-square test showed significant comparison (P = 0.008).
Chart 2.
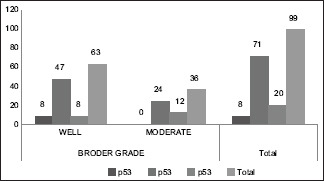
Comparison of p53 expression with Broder grading
P53 expression and Bryne's grading
In 47 cases of Grade 1 SCC 27/47 cases had expression above 50% and 20/47 showed very high p53 expression(>70%). In 52 cases of Grade 2 SCC 8/52 cases had low p53 while 44/52 cases had expression above 50% and no cases had high p53 expresion(>70%) [Chart 3]. Statsitical analysis by Pearson Chi square test showed higher significant relation than that of Broder's (P = 0.000).
Chart 3.
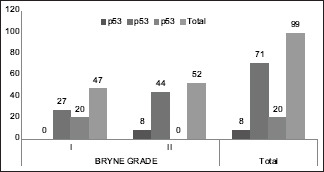
Comparison of p53 expression with Bryne grading
Ki67 expression and Broder's grading
On comparison of Ki67 expression with Broder's grading, in WDSCC 14/63 cases had low Ki67 expression but 36/63 had expression (26%–50%) and 13/63 showed very high p53 expression (>50%). Similarly, in 36 cases of MDSCC no cases had low Ki67 expression while 23/36 had expression of 26%–50% and 13/36 cases had very high Ki67 expression (>50%). Statistical analysis by Pearson Chi-square test showed significant comparison (P = 0.006) [Chart 4].
Chart 4.
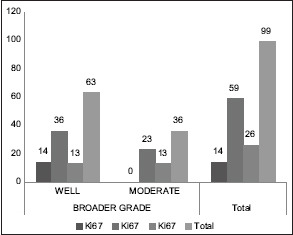
Comparison of Ki67 expression with Broder's grading
Ki67 expression and Bryne's grading
In 47 cases of Grade 1 SCC 14/47 cases had low expression of Ki67, 23/47 showed expression from 26 to 50% and 10/47 showed high expression(>50%). In 52 cases of Grade 2 SCC no cases showed low expression for Ki67,36/52 cases had 26%–50% positivity while 16/52 cases had high Ki67 expression (>50%) [Chart 5]. Statsitical analysis by Pearson Chi square test showed higher significant relation than that of Broder's (P = 0.000).
Chart 5.
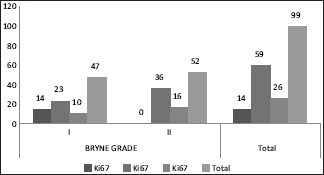
Comparison of Ki67 expression with Bryne grading
DISCUSSION
Oral cancer is the commonest cancer in India, accounting for 50%–70% of total cancer mortality. In our study on 100 OSCC cases with a mean age of 57.03 years, males comprised 79% of cases. The high proportion of cases among males may be due to high prevalence of tobacco consumption.
AC Broders' in 1920 suggested a quantitative grading system for the cancer of the lip. Broders' concept was grading of the tumors according to the differentiation of tumor cells.[2,3] Despite the widespread use of this system even in the present time, the outcome of treatment and survival of the patient is still not convincing. The poor correlation with the grading and prognosis is probably the heterogeneity of the cell population present within the tumors. Later in literature, many parameters for grading of tumor were suggested. Jacobsson et al. (1973) multifactorial grading system was based on structure, differentiation, nuclear pleomorphism, mitosis, mode of invasion, stage of invasion, vascular invasion and lymphoplasmacytic infiltration. Many other researchers modified or developed a new system based on the Jacobssons' grading system. These include Fisher (1975), Lund (1975), Willen (1975), Anneroth and Hansen (1984), Crissman (1980 and 1984).[2,3]
Anneroth et al. (1987) modified the existing multifactorial grading systems in use and proposed a new grading system. In this system, the number of parameters studied was the degree of keratinization, nuclear pleomorphism, mitoses, pattern of invasion, stage of invasion, and lymphoplasmacytic infiltration.[4,5,6] Bryne et al. in 1992 modified the grading system used by Anneroth considering the concept that the heterogeneous tumor cell populations in the invasive front of tumor is less differentiated than the cells in the superficial part of the tumor. Thus in Bryne's system, only the cells at the deep invasive margin of the tumor were graded.[4,5]
Grading of routine OSCC specimens using Bryne's requires proper excision biopsy along with safe margins to identify the true invasive front. Obtaining an invasive front in incision biopsy is unreliable as incision specimens may be representative of tumor but may not be the invasive front. The discrepancy in invasive front reliability and inter-observer bias is a matter of concern as studied by Faleh et al.[7] Hence in our study, we assessed the parameters of Bryne's criteria of the invasive front on full thickness of an incision specimen to compare with Broder's grading. Both histological gradings were then compared with the molecular behavior of tumor using the malignancy markers p53 and Ki67. P53 in proven to be involved in apoptosis and cell-cycle control making it a plausible biomarker of malignant potential.[8,9,10] Ki-67 can be detected in phases G1, S, G2 and M of the cell cycle, but not on G0 phase, exclusively in the nuclei of cycling cells making it a reliable marker of proliferating cells.[2]
On comparing Broder's grading with Bryne's criteria on the same incisional biopsy specimens discrepancy was observed in the number of cases of both well and moderate differentiation [Chart 1]. Studies with such comparisons were scarce in literature. In our study 29 of 63 Broder's WDSCC cases turned out to be Grade 2 (moderately differentiated) under Bryne's grading. Similarly, 13 cases under MDSCC by Broder's were Grade 1 (well differentiated) by Bryne's. This shows a scenario of upgraded diagnosis of 29 cases in first and downgraded diagnosis of 13 cases in second. A comparison of this histopathological grading was done with the proven molecular markers of OSCC, p53 and Ki67 with an aim to find the grading parameter more relatable to molecular behaviour of tumor.
In the present study 91/99 cases which showed a p53 expression >50% [Chart 2], 55 cases were under WDSCC and 36 were under MDSCC by Broder's criteria. The same cases under Bryne's criteria showed 47 cases under Grade 1 and 44 cases under Grade 2 [Chart 3]. On statistical comparison both grading was significant for p53 expression but a higher significance was observed for Bryne's grading (P = 0.000) than Broder's (P = 0.008). The Ki67 expression in Broder's grading [Chart 4], similar expression percentage were observed for both well and moderately differentiated cases. In Bryne's grading 14/47 cases of Grade 1 but no cases of Grade 2 showed Ki67 expression <25%. Most number of cases under Grade 1 Bryne's criteria showed high KI67 expression [Chart 5]. On statistical comparison both grading was significant for Ki67 expression but a higher significance was observed for Bryne's grading (P = 0.000) than Broder's (P = 0.006).
A study by Verma et al. 66.7% of cases of OSCC showed p53 positivity while 70% cases showed Ki67 positivity.[11] The correlation of Anneroth's parameters with the expression of P53 was studied by Dave et al. in which 65% expression of p53 was found.[9] Their study showed significant correlation of grading parameters like the degree of keratinization, nuclear pleomorphism, number of mitosis and lymphoproliferative infiltrate with p53 expression. The increasing P53 expression along the increasing grade of tumor severity is proven in many studies.[9] The accuracy of Bryne's grading over Broder's grading was studied by Wanger et al. and suggested the use of Bryne.'s grading for histopathological grading of OSCC.[2] In a study by Jayade et al. the positive correlation between the grading parameters and p53 expression is observed. The present study results were similar to that of study by Hideo Kurokawa et al. in which they have opined that the p53 and ki67 expression on the invasive front is associated with proper grading of tumor.[12] Hence, those parameters on invasive front when evaluated on full-thickness incision biopsies and compared with the same markers showed significant results in the present study.
CONCLUSION
The present study was an attempt to compare two different histologic grading systems and its molecular behavior with p53 and Ki-67 expression on incisional biopsy which was found lacking in previous literature. Incisional biopsy specimens when histopathologically graded with Bryne's parameters gave significant results on comparison with the corresponding molecular markers of malignancy, the p53 and ki67. Hence the tumor grading on incisional biopsy using the parameters of Bryne is more reliable in grading the tumor than Broders considering the molecular behavior of tumor. Furthermore, multicentric studies should be encouraged for establishing the most accurate classification for diagnosing OSCC cases on incisional biopsy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pereira MC, Oliveira DT, Landman G, Kowalski LP. Histologic subtypes of oral squamous cell carcinoma: Prognostic relevance. J Can Dent Assoc. 2007;73:339–44. [PubMed] [Google Scholar]
- 2.Wagner VP, Webber LP, Curra M, Klein IP, Meurer L, Carrad VC, et al. Bryne's grading system predicts poor disease-specific survival of oral squamous cell carcinoma: A comparative study among different histologic grading systems. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:688–96. doi: 10.1016/j.oooo.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Doshi NP, Shah SA, Patel KB, Jhabuawala MF. Histological grading of oral cancer: A comparison of different systems and their relation to lymph node metastasis. Natl J Community Med. 2011;2:136–42. [Google Scholar]
- 4.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders' grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 5.Bryne M, Jenssen N, Boysen M. Histological grading in the deep invasive front of T1 and T2 glottic squamous cell carcinomas has high prognostic value. Virchows Arch. 1995;427:277–81. doi: 10.1007/BF00203395. [DOI] [PubMed] [Google Scholar]
- 6.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 7.Sawair FA, Irwin CR, Gordon DJ, Leonard AG, Stephenson M, Napier SS. Invasive front grading: Reliability and usefulness in the management of oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:1–9. doi: 10.1034/j.1600-0714.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Tang S, Xu D, Zhou B. Analysis of P53 mutation and invasion front grading in oral squamous cell carcinomas. J Huazhong Univ Sci Technolog Med Sci. 2010;30:525–9. doi: 10.1007/s11596-010-0462-0. [DOI] [PubMed] [Google Scholar]
- 9.Dave KV, Chalishazar M, Dave VR, Panja P, Singh M, Modi TG. Immunohistochemical expression of p53 and its clinicopathological correlation with modified Anneroth's histological grading system. J Oral Maxillofac Pathol. 2016;20:29–35. doi: 10.4103/0973-029X.180922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atarbashi Moghadam S, Atarbashi Moghadam F, Mokhtari S, Eini E. Immunohistochemical analysis of P63 expression in odontogenic lesions. Biomed Res Int. 2013;2013:624176. doi: 10.1155/2013/624176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma R, Singh A, Jaiswal R, Chandra A, Verma R, Tak J. Association of Ki-67 antigen and p53 protein at invasive tumor front of oral squamous cell carcinoma. Indian J Pathol Microbiol. 2014;57:553–7. doi: 10.4103/0377-4929.142660. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tanaka T, Tomoyose T, et al. The relationship of the histologic grade at the deep invasive front and the expression of Ki-67 antigen and p53 protein in oral squamous cell carcinoma. J Oral Pathol Med. 2005;34:602–7. doi: 10.1111/j.1600-0714.2005.00358.x. [DOI] [PubMed] [Google Scholar]


