Abstract
N-Ethylhexedrone [2-(ethyloamino)-1-phenylhexan-1-one; α-ethylaminohexanophenone (NEH)] is one of the most recent synthetic cathinones that appeared on the illegal market in late 2015. The majority of information concerning the model of consumption of NEH and its impact on the body originates only from self-reports from gray literature websites and drug forums. There are only limited data associated with the concentrations of NEH in blood samples available in the literature. This article presents a case of fatal NEH intoxication and a method for the determination of this substance in whole blood. A 21-year-old man without any diagnosed diseases was admitted to the hospital due to disorientation, aggression and finally loss of consciousness. Hyperthermia (>41°C), tachycardia (>160 beats per minute), tachypnea (20 breaths per minute), blood pressure (110/60 mmHg) and acute kidney failure were diagnosed. After a few hours of hospitalization, the patient died. A plastic bag with a white powder was found in his underwear. Analysis of the powder by another laboratory revealed cocaine hydrochloride; however, no cocaine or its metabolites were found in the biological material upon testing in our laboratory. Therefore, re-analysis of the powder was performed, and NEH was identified. Liquid–liquid extraction followed by liquid chromatography-triple quadrupole-mass spectrometry (LC-MS/MS) analysis were used for the determination of NEH in blood. The validation parameters were as follows: calibration range 1–250 ng/mL, accuracy 106.5–109.9%, precision 3.5–6.3%, recovery 90.1–96.9%, limit of detection 0.07 ng/mL and limit of quantification 1 ng/mL. NEH was quantified in the blood at a concentration of 145 ng/mL. Additionally, amphetamine at low concentrations and 11-nor-9-karboksy-Δ9-tetrahydrokannabinol (THC-COOH) were detected.
Our study provided information on the possible lethal concentration and toxidrome that clinicians can observe for NEH-intoxicated patients and can be helpful during the preparation of toxicology analysis reports for a court of law for proper data interpretation.
Introduction
N-Ethylhexedrone [2-(ethyloamino)-1-phenylhexan-1-one; α-ethylaminohexanophenone], also known as hexen, HEX-EN or NEH, is an ethyl group-substituted derivative of hexedrone that is a synthetic cathinone (Figure 1) (1). Although NEH was synthesized for the first time in 1964 by Boehringer Ingelheim, it has spread: it appeared in Belgium in late 2015, and in 2017, it was one of the most frequently seized cathinones (2, 3). In March 2020, the Commission on Narcotic Drugs decided to include NEH in Schedule II of the Convention on Psychotropic Substances of 1971 (4).
Figure 1.
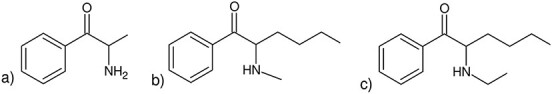
Structures of (a) cathinone, (b) hexedrone and (c) N-ethylhexedrone.
NEH is sold via the Internet in the form of white, yellow or gray powder as well as a fine crystalline solid (1). Currently, the majority of information concerning the model of NEH consumption and its impact on the body originates from self-reports from gray literature websites and drug forums. Similar to other synthetic cathinones, the strongest effects are observed when NEH is snorted. It may also be ingested orally, administered rectally or intravenously or smoked in e-cigarettes. Depending on the route of administration and the tolerance of the user, the dose of NEH resulting in the desired effects ranges from 10 to 250 mg. In addition, depending on the route of admission, NEH effects appear within 2 or 20–25 min after intranasal or oral administration, respectively (1, 5).
Due to the novelty of the drug, there are few data in the scientific literature considering the pharmacokinetics, pharmacological and toxicological effects, dependence and abuse potentials as well as the potential of acute overdose effects. Only a few toxicological case reports on NEH have been published so far. Authors have reported non-fatal mixed intoxication, driving under the influence and—in fatalities—included traffic accidents and overdose in which both psychoactive substances and health conditions were co-factors in the fatal outcome (1, 5, 6). Moreover, in studies available in the scientific literature, there are no procedures describing the quantification of NEH by liquid chromatography-triple quadrupole-mass spectrometry (LC–MS-MS). Therefore, the aim of this study was to present the results of an analysis of a healthy young man’s death due to overdose from a new psychoactive substance in which NEH was detected and quantified in blood by LC–MS-MS. The results of our study can assist in the recognition of possible effects caused by this drug and can be helpful during the preparation and analysis of toxicology reports for a court of law for proper data interpretation. Validation of the methodology for NEH determination in blood samples by LC–MS-MS is also presented.
Case History
A 21-year-old man with a history of drug and alcohol abuse was taken from a place of residence to the hospital due to disorientation, aggression and, finally, loss of consciousness. Upon arrival at the emergency department (ED), no logical verbal contact was found, and the patient had a dry mouth. He was agitated and aggressive; therefore, direct coercion was applied. Non-reactive hyperthermia (>40°C) and wide pupils with a preserved reaction to light were found during the examination. After administration of benzodiazepine (diazepam), the patient was transferred to the intensive care unit. The patient was generally in severe condition with deep disorders of consciousness. Hyperthermia (>41°C), tachycardia (>160 beats per minute), tachypnea (20 breaths per minute), blood pressure (110/60 mmHg) and anuria were present. Intensive liquid therapy, passive oxygen therapy and physical cooling were continued. After obtaining relative stabilization of circulatory parameters, a decrease in body temperature (<38°C) and collecting 200 mL of urine, toxicological screening was performed. The urine test showed only the presence of benzodiazepines. His biochemical blood results 5 h after presentation to the ED are shown in Table I. Due to increasing renal parameters and oliguria, the decision to perform hemodialysis was made. Approximately 30 min after the patient was transferred to the dialysis center, sudden cardiac arrest occurred in the asystole mechanism. Despite nearly 1 h of resuscitation, the patient died. Medical personnel after the man’s death discovered a plastic bag with white powder and a rolled banknote in his underwear. Tests conducted by one of the laboratories cooperating with the police indicated the presence of cocaine hydrochloride in the powder.
Table I.
Blood Investigation 5 Hours after Presentation to the ED
| Parameters | Level | Normal range |
|---|---|---|
| pH | 7.268 | 7.350–7.450 |
| Bicarbonate | 14.3 | 22.0–26.0 mmol/L |
| Base excess | –11.4 | −2 to 2 mmol/L |
| Urea | 81 | 19–44 mg/dL |
| Creatinine | 3.70 | 0.70–1.20 mg/dL |
| eGFR | 20.8 | adults: >120 mL/min |
Medico-legal autopsy showed no obvious pathological changes in the organs apart from congestion of the lungs, mild focal pulmonary edema, swelling and congestion of the brain, left ventricular hypertrophy and focal liver steatosis.
Femoral blood specimens with the addition of potassium fluoride collected during autopsy were submitted to the Department of Forensic Medicine (DFM), Medical University of Gdańsk, Poland for toxicological analysis. No biological material collected ante-mortem was available. The results of the blood analysis were negative for alcohol. Routine screening analyses for common drugs of abuse (including cocaine and its metabolites) and medicines conducted by an enzyme-linked immunosorbent assay and—in the next step—by gas chromatography–mass spectrometry (GC–MS) and LC–MS-MS revealed positive results for illicit drugs: amphetamine and cannabinoids. Furthermore, cocaine and its metabolites in the biological material were excluded. Therefore, the laboratory requested the secured material (white powder), which was sent to the DFM for re-identification.
Materials and Methods
Reagents and materials
The certified standard of N-ethylhexedrone was manufactured by Cayman Chemical Company (Ann Arbor, MI, USA) as a powder with a mass of 1 mg. A methanolic solution of racemic-methamphetamine-d5 at a concentration of 0.1 mg/mL, used as an internal standard (IS), was manufactured by LGC Standards (London, UK).
All solvents used were HPLC-grade purity (for high-performance liquid chromatography). 1-Chlorobutane (n-butyl chloride), acetonitrile (ACN), methanol (MeOH) and formic acid (FA) were supplied by Sigma-Aldrich (St. Louis, MO, USA), while hydrochloric acid (HCl, analytical grade purity) at a concentration of 35–38% was purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland). An HCl solution in MeOH was prepared by mixing both chemicals in a volume ratio of 1:9. Ultrapure water was produced by a Millipore Synergy UV and Elix 10 UV water system (Millipore, Warsaw, Poland).
The blood used as blank samples for the development and validation of the method was obtained from a regional blood donation center (Gdańsk, Poland) and was stored at −20°C prior to analysis. Blank blood was screened to be negative for drugs of abuse (including NEH).
GC–MS analysis of evidence material
Because the evidence material supplied to DFM for re-identification was in the form of a string bag with traces of white powder, only qualitative analysis was performed. The powder residue was dissolved in MeOH, and the solution was analyzed by GC–MS using a 7890A GC System equipped with a G4567A autosampler and a split/splitless injection port and connected with a 5975C single quadrupole mass spectrometer with an electron ionization (EI) ion source (all from Agilent Technologies, Santa Clara, CA, USA). Separation of the analytes was carried out on a Phenomenex ZB-5 MS capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness; Phenomenex, Izabelin, Poland) with helium at a purity of 99.999% as the carrier gas at a constant flow rate of 1 mL/min. Split injection mode (10:1) was used. The oven temperature was programmed at 50°C for 1 min, then increased to 200°C at a rate of 20°C/min and finally ramped up to 285°C at a rate of 10°C/min and held for 7 min. The temperatures of the injection port, MS transfer line, ion source and detector were set at 250°C, 285°C, 280°C and 200°C, respectively. The injection volume was 2 µL. The mass spectrometer was operated in positive electron impact mode (electron beam energy 70 eV). Full-scan acquisition was performed with the mass detection range set at m/z 40–450. The target compound, NEH, was identified by matching the spectra against a reference library (Cayman Spectral Library; primary ions m/z 114, 77, 58). In the next step, the retention time (RT = 9.61 min), target and qualifier ions and their ratio were compared with the certified standard. Data acquisition and analysis were accomplished with MSD ChemStation software by Agilent Technologies (version E.02.02.1431).
Analysis of NEH in the biological material
Standard, calibrators and quality control sample preparation
Stock solutions of NEH were prepared in MeOH by diluting the standard solution to concentrations of 0.5 and 10 µg/mL. An IS stock solution was prepared in MeOH at a concentration of 2 µg/mL. All stock solutions were stored at −20°C. The calibrators (n = 3) were prepared by spiking 0.2 mL of drug-free blood samples with an appropriate amount of the analyte stock solution to obtain concentrations of 1, 2.5, 5, 10, 25, 50, 100 and 250 ng/mL. The concentration of the IS in each sample was maintained at 100 ng/mL by adding 10 µL of the IS stock solution. Quality control (QC) samples were prepared in a similar manner as the calibrators at three concentration levels within the linear range of the assay (n = 3): low, 2.5 ng/mL (LQC); medium, 50 ng/mL (MQC); and high 200 ng/mL (HQC).
Extraction
The samples were extracted using modified method published by Adamowicz et al. (7). A 0.2-mL aliquot of blood or QC sample was mixed with 10 µL of the IS stock solution and 200 μL of carbonate buffer (pH 12) in a 2 mL Eppendorf vial. Subsequently, 1 mL of 1-chlorobutane was added, and the sample was vortexed for 1 min followed by centrifugation at 14,500 rpm (14,100×g) for 2 min. The organic layer (800 µL) was transferred to a glass tube, and 50 µL of HCl solution in MeOH (1:9, v/v) was added to the extract. Next, the solution was evaporated under a gentle stream of nitrogen at 40°C. The residue was dissolved in 100 µL of a mixture of ACN and water (1:9, v/v) and transferred to a glass vial with a polypropylene insert. The sample was injected into the LC–MS/MS system. The injection volume was 10 μL.
LC–MS-MS conditions
Analyses were performed using an Agilent Technologies 1260 Infinity liquid chromatograph coupled to a 6420 triple quad mass spectrometer (Santa Clara, CA, USA). Chromatographic separation of the analytes was achieved on an Agilent Technologies InfinityLab Poroshell 120 EC-C18 column (3.0 mm × 100 mm; 2.7 μm) equipped with an InfinityLab Poroshell 120 EC-C18 Fast Guard (3.0 mm × 5 mm; 2.7 μm) pre-column. The column was thermostatted at 30°C. Data acquisition, result analyses and processing were performed using Mass Hunter software by Agilent Technologies (version B.08.00). The mobile phase consisted of a mixture of 0.05% FA in water (v/v, component A) and 0.05% FA in ACN (v/v, component B). The flow rate was set to 0.5 mL/min. The following gradient elution program was applied (shown in relation to component A): 0 min—90%, 4.5 min—65%, 6 min—10%, 12 min—10%, 12.01 min—90%. The total analytical run time was 16 min. The RT for the IS was 3.90 min, and for NEH, it was 6.62 min. During the procedure, multiple reaction monitoring (MRM) mode was applied. The following MRM transitions (m/z) were monitored: 220.2→202.2 and 220.2→91.1 for NEH and 155.2→92.0 for the IS (bolded transitions were used for quantification). Fragmentor voltages were set at 110 V and 80 V for NEH and the IS, respectively. In the order of the above-mentioned transitions, the collision energies were 9 V, 9 V, and 21 V, and the collision cell acceleration voltages were 4 V, 4 V, and 5 V, respectively. The relative area quantifier/qualifier transition was 100/75. Electrospray ionization (ESI) source parameters were set as follows: positive ionization mode (ESI+), gas flow (nitrogen): 10 L/min, gas temperature 350°C, nebulizer pressure: 40 psi, and capillary voltage: 3,500 V. These parameters were optimized by the use of Mass Hunter Optimizer and Source Optimizer software (Agilent Technologies).
Method validation
The method for NEH determination in blood samples was validated according to international guidelines in the field of our study (8, 9) in terms of selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), repeatability (accuracy and precision), recovery rate, and matrix effects (ME).
Selectivity:
The selectivity was verified by analyzing 20 blood samples (both ante- and postmortem in origin) that were negative for NPS during preliminary screening analyses.
Linearity:
The linearity was investigated as the correlation coefficient (r) of calibration curve.
LOD and LOQ:
The LOD of the method was calculated by a signal-to-noise ratio (S/N) equal to 3 (peak-to-peak noise definition), and the LOQ was assumed to be the lowest linear point from the calibration curve.
Repeatability (accuracy and precision):
The repeatability test was performed as intra- and inter-day assay accuracy and precision (in terms of coefficients of variation; CVs [%]). Intra-day measurements were carried out by analysis of drug-free blank blood samples fortified with the analyte and IS (QC samples). Then, the test was repeated over the following three consecutive days to obtain the inter-day assay repeatability as between-day averages. The accuracy was calculated as the mean ratio of the measured and nominal concentrations.
Recovery:
The recovery was tested at three concentration levels (similar to the QC samples) by comparing the analyte-to-IS peak area ratios of the spiked and extracted drug-free blank blood samples with the corresponding analyte-to-IS peak area ratios of the appropriate matrix extracts fortified with standard. IS was added post-extraction to avoid its loss during the extraction step. Each experiment was performed three times.
ME:
Due to the potential variability between matrix sources, ME were investigated using three blank samples ante- and three blank samples postmortem origin. ME studies were performed using post-extraction addition technique at three concentration levels (n = 3) as during testing of the repeatability.
Results and Discussion
Qualitative toxicological analysis of the residue of the seized material identified NEH. The EI-MS spectrum of NEH is shown in Figure 2. No traces of cocaine hydrochloride that were identified in another laboratory were found.
Figure 2.
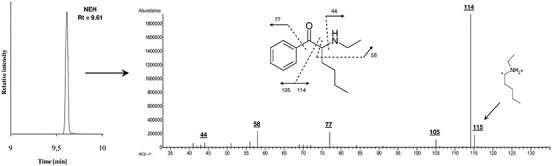
GC–EI-MS-SCAN chromatogram of the analyzed seized material (only one peak was observed) and EI mass spectrum of the identified compound (N-ethylhexedrone) with its fragmentation pattern (only main fragments are marked).
No naturally occurring substances were observed in the blood that could affect and interfere with the quantitative and qualitative determination of NEH using the developed analytical procedure (no coelution at the same RT as the analyte). There was also no peak for the reagents used in the sample preparation step on the chromatogram obtained for analysis of the blank sample. A blank blood sample chromatogram and a sample spiked with NEH and the IS at a concentration of 10 ng/mL is shown in Figure 3.
Figure 3.
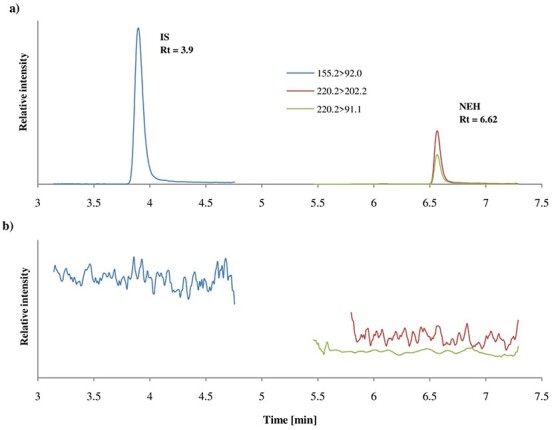
MRM chromatogram of blank blood sample spiked with NEH and the IS (a) at a concentration of 10 ng/mL and (b) a blank blood sample.
The validation data are summarized in Table II. An eight-point calibration curve was constructed using the peak area ratio (NEH vs. IS) against concentration. In order to increase the accuracy, a weighing factor of 1/x was applied to the calibration curve. The linearity was tested in the concentration range of 1–250 ng/mL. The calibration curve equation was y = 0.46244x−0.0251, and the r was equal to 0.9966. The LOD and LOQ of the method were 0.07 and 1 ng/mL, respectively. Thus, the method was determined to be sensitive and enables the quantification of NEH in blood even at low concentration levels. The accuracy and precision of the developed method were in the ranges of 98.9–113.3% and 0.7–8.3% for the intra-day assay test and in the ranges of 106.5–109.9% and 3.5–6.5% for the inter-day assay test, respectively. The recoveries over the studied concentration ranged from 90.1 to 96.9%. All validation parameters fulfilled the established international criteria for bioanalytical methods used in forensic toxicology (mean CVs less than 20% and accuracy between 80% and 120%). ME were between 105.2 and 141.1% while the variability (CVs) between different sources of blank matrix did not exceed 15%. Validation of the developed procedure proved that the method is characterized by high sensitivity, selectivity and precision and can also be used for the analysis of real samples.
Table II.
Validation Parameters of the Method: Accuracy (Precision), Recovery Rates, and ME for the Quantification of NEH
| Intra-day assay (%) | |||||||
|---|---|---|---|---|---|---|---|
| Concentration [ng/mL] | Day 1 | Day 2 | Day 3 | Inter-day assay [%] | Recovery [%] (mean ± SD) | ME (CV) [%] | |
| 2.5 | 98.3 (5.7) | 113.5 (1.6) | 108.0 (1.0) | 107.7 (6.3) | 93.2 ± 2.8 | 118.1 (5.4) | |
| 50 | 104.5 (8.3) | 104.4 (3.5) | 110.0 (2.9) | 106.5 (4.8) | 96.9 ± 2.5 | 141.1 (4.7) | |
| 200 | 110.9 (1.5) | 109.8 (5.8) | 109.1 (3.2) | 109.9 (3.5) | 90.1 ± 1.6 | 105.2 (4.1) | |
In the postmortem blood sample, 145 ng/mL NEH was quantified. In addition, other drugs like amphetamine (12 ng/mL) and 11-nor-9-karboksy-Δ9-tetrahydrokannabinol (THC-COOH) (<LOQ; <5 ng/mL) were found.
Eshleman et al. (10) stated that NEH has a high affinity for noradrenaline, serotonin and dopamine receptors compared with other cathinones. This means that NEH intake may result in sympathomimetic or serotonergic syndromes. We confirmed that the toxidrome reported in the patient involved disorientation, a confusional state, aggression, wide pupils, hyperthermia, tachycardia, tachypnea and acute kidney failure, which is characteristic of both sympathomimetic syndrome and serotonergic syndrome. Additionally, we think that the low concentration of amphetamine may not have had a significant impact on the development of sympathomimetic syndrome resulting in death.
To date, only few papers have been published in the literature presenting cases in which N-ethylhexedrone was detected. Two of them describe non-fatal acute toxicity related to the use of N-ethylhexedrone. Dunlop et al. (6) presented a case of mixed intoxication (NEH, 3-HO-PCP and clephedrone); however, there were no determined concentrations of these drugs because only qualitative analysis was performed. Moreover, toxidrome presented by the patient resembled the effects of hallucinogenic substances, i.e., as is observed in acute PCP intoxication. In Wagmann et al. (11) studies, a case of 41-year-old man associated with the use of four drugs (4-CEC, 4F-PHP, ephylone, NEH) was described. However, NEH blood concentration was low (1 ng/mL) and information on dosage and time of intake was not presented.
The other two papers have been published presenting the concentrations of NEH in blood. Mikołajczyk et al. (1) presented three cases (Driving under the influence DUI; however, one man died as a result of injuries from a road accident) in which NEH was determined at concentrations of 8 ng/mL, 34 ng/mL and 37 ng/mL. Kovacs et al. (5) reported that in 14 blood samples of living subjects, the mean concentration of NEH in the blood was 28.3 ng/mL (range: 10.2–83.9 ng/mL). Furthermore, Kovacs reported that in 50% of the cases in which NEH was detected, other psychoactive substances were also present. This regularity was confirmed in paper published by Mikołajczyk et al. (1) and our other studies (12). Additionally, in these papers limited data associated with symptoms and detailed cases’ description were presented because in many cases medical or police documentation was not available (in contrast to this paper in which such documents were supplied).
In the case of sudden death, a young man described in the other paper (5), 285 ng/mL NEH and 0.08 ng/mL ADB-FUBINACA were determined in the blood. The man was not hospitalized directly before his death; he died at home a few hours after returning from a party. The estimated time of consumption of designer drugs based on the testimony of his mother was 5–12 h before death. The determined concentration of NEH in this case was unusually high; nevertheless, his deteriorating health conditions were very likely a co-factor in the fatal outcome.
In our case, the deceased was generally a healthy young man. He was not diagnosed with any disease. He was taken by medical services from a place of residence after the occurrence of disquieting symptoms. The period of his hospitalization was approximately 12 h, which during this time medical staff implemented symptomatic treatment that was unsuccessful. Given the above, we know that in this case, it should be presumed that the maximum concentration of NEH in the man’s blood was probably higher than was determined. The issue of postmortem decomposition could also be significant in the context of the determined concentration of NEH as well as the postmortem redistribution. Redistribution of NEH in various biological materials (urine, bile, brain and lungs) was also described in the literature (13). The knowledge associated with drugs’ redistribution may facilitate data interpretation when compounds of interest are not detected in blood. However, in our case, only blood samples were available for analysis. Additionally, we assume that the low concentration of amphetamine, which was below the toxic level at the time of death, probably did not significantly contribute to death. This assumption is also supported by the fact that amphetamine was not detected in the urine screening during hospitalization. The conclusion regarding the detected THC-COOH is similar.
Conclusion
In this study, we present the case of a fatal outcome related to N-ethylhexedrone. The developed LC–MS-MS method for the determination of NEH was successfully applied for analysis. Our study provided information on the possible lethal concentration and toxidrome that clinicians can observe for NEH-intoxicated patients. In this case, it should be presumed that the maximum concentration of NEH in the man’s blood was higher than it was determined because of hospitalization for several hours, including life-saving procedures and possible postmortem distribution of the compound. In addition, it should be noted that the original analysis of the seized material showed the presence of cocaine hydrochloride, which was not confirmed in studies conducted by our institution. Considering the above, it should be strived for that both the biological samples and the evidence secured in the same case be examined in the same laboratory.
Contributor Information
Ewa Domagalska, Department of Forensic Medicine, Faculty of Medicine, Medical University of Gdańsk, 3A Marii Skłodowskiej-Curie Str., Gdańsk 80-210, Pomeranian Voivodeship, Poland.
Laura Banaszkiewicz, Department of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, 11/12 Narutowicza Str., Gdańsk 80-233, Pomeranian Voivodeship, Poland.
Mateusz Kacper Woźniak, Department of Analytical Chemistry, Faculty of Chemistry, Gdańsk University of Technology, 11/12 Narutowicza Str., Gdańsk 80-233, Pomeranian Voivodeship, Poland.
Marzena Kata, Department of Forensic Medicine, Faculty of Medicine, Medical University of Gdańsk, 3A Marii Skłodowskiej-Curie Str., Gdańsk 80-210, Pomeranian Voivodeship, Poland.
Beata Szpiech, Department of Forensic Medicine, Faculty of Medicine, Medical University of Gdańsk, 3A Marii Skłodowskiej-Curie Str., Gdańsk 80-210, Pomeranian Voivodeship, Poland.
Michał Kaliszan, Department of Forensic Medicine, Faculty of Medicine, Medical University of Gdańsk, 3A Marii Skłodowskiej-Curie Str., Gdańsk 80-210, Pomeranian Voivodeship, Poland.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1. Mikołajczyk A., Adamowicz P., Tokarczyk B., Sekuła K., Gieroń J., Wrzesień W., et al. (2017) Determination of N-ethylhexedrone, a new cathinone derivative, in blood collected from drivers–analysis of three cases. Probelms of Forensic Science, 109, 53–63. [Google Scholar]
- 2.(2016) EMCDDA–Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA . European Monitoring for Drugs and Drug Addiction. https://www.emcdda.europa.eu/system/files/publications/4724/TDAN17001ENN_PDFWEB.pdf (accessed May 24, 2020).
- 3.(2020) Update on emerging substances of abuse. United Nations Office on Drugs and Crime (UNODC), Tettey J.. https://www.unodc.org/documents/commissions/CND/CND_Sessions/CND_61Reconvened/UNODC_CND_Justice_Tettey_Reconvened_Session_final.pdf (accessed May 24, 2020).
- 4.(2020) Report on the sixty-third session. Commission on Narcotic Drugs, Economic and Social Council. https://www.unodc.org/documents/commissions/CND/CND_Sessions/CND_63/E2020_28_e_V2001956_Advance_Version.pdf (accessed May 24, 2020).
- 5. Kovács K., Kereszty É., Berkecz R., Tiszlavicz L., Sija É., Körmöczi T., et al. (2019) Fatal intoxication of a regular drug user following N-ethyl-hexedrone and ADB-FUBINACA consumption. Journal of Forensic and Legal Medicine, 65, 92–100. doi: 10.1016/j.jflm.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 6. Dunlop L.C., Wood D., Archer J., Hudson S., Dargan P. (2020) Severe toxicity to the new psychoactive substances 3-hydroxyphencyclidine and N-ethylhexedrone: an analytically confirmed case report. Journal of Medical Toxicology, 16, 67–70. doi: 10.1007/s13181-019-00734-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adamowicz P., Gieroń J., Gil D., Lechowicz W., Skulska A., Tokarczyk B., et al. (2016) Blood concentrations of α-pyrrolidinovalerophenone (α-PVP) determined in 66 forensic samples. Forensic Toxicology, 34, 227–234. doi: 10.1007/s11419-016-0306-0 [DOI] [Google Scholar]
- 8. Scientific Working Group for Forensic Toxicology (SWGTOX) . (2013) Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. Journal of Analytical Toxicology, 37, 452–474. doi: 10.1093/jat/bkt054 [DOI] [PubMed] [Google Scholar]
- 9. Wille S.M.R., Coucke W., De Baere T., Peters F.T. (2017) Update of standard practices for new method validation in forensic toxicology. Current Pharmaceutical Design, 23, 5442–5454. [DOI] [PubMed] [Google Scholar]
- 10. Eshleman A.J., Nagarajan S., Wolfrum K.M., Reed J.F., Swanson T.L., Nilsen A., et al. (2019) Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology (Berl), 236, 939–952. doi: 10.1007/s00213-018-5059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagmann L., Manier S.K., Eckstein N., Maurer H.H., Meyer M.R. (2020) Toxicokinetic studies of the four new psychoactive substances 4-chloroethcathinone, N-ethylnorpentylone, N-ethylhexedrone, and 4-fluoro-alpha-pyrrolidinohexiophenone. Forensic Toxicology, 38, 59–69. doi: 10.1007/s11419-019-00487-w [DOI] [Google Scholar]
- 12. Woźniak M.K., Banaszkiewicz L., Wiergowski M., Tomczak E., Kata M., Szpiech B., et al. (2020) Development and validation of a GC–MS/MS method for the determination of 11 amphetamines and 34 synthetic cathinones in whole blood. Forensic Toxicology, 38, 42–58. doi: 10.1007/s11419-019-00485-y [DOI] [Google Scholar]
- 13. Adamowicz P., Jurczyk A., Gil D., Szustowski S. (2020) A case of intoxication with a new cathinone derivative α-PiHP—a presentation of concentrations in biological specimens. Legal Medicine, 42, 101626. doi: 10.1016/j.legalmed.2019.101626 [DOI] [PubMed] [Google Scholar]


