Abstract
Chronic undernutrition is a type of metabolic stress that impairs reproduction in multiple species. Although energy balance and female reproductive capacity is recognized as tightly coupled, the neuroendocrine loci and molecular mechanisms that mediate ovarian cycle dysfunction during chronic undernutrition in adult females remain poorly understood. Here, we present a series of studies in which we tested the hypothesis that inhibition of kisspeptin (Kiss1) neurons, which are critical for controlling luteinizing hormone (LH) pulses and the preovulatory LH surge in females, underlies the impairment of the ovarian cycle by undernutrition. We first investigated the effect of chronic undernutrition (70% of unrestricted feed intake) on estrous cyclicity in intact female c57bl6 mice. Undernutrition caused a rapid cessation of ovarian cyclicity during the 2-week treatment, suppressing ovarian steroidogenesis and inhibiting ovulation. Using 2 well-defined estradiol-replacement paradigms, we directly tested the hypothesis that undernutrition inhibits Kiss1 neurons in the arcuate nucleus (ARCKiss1), which are required for LH pulses and in the anteroventral periventricular nucleus (AVPVKiss1), which are necessary for LH surge secretion. Undernutrition prevented LH pulses and impaired ARCKiss1 neuronal activation, using c-Fos as a marker, in ovariectomized females subcutaneously implanted with a pellet containing a diestrus-like level of estradiol. In addition, undernutrition completely blocked the estradiol-induced LH surge and diminished Kiss1 messenger RNA abundance, without decreasing estradiol receptor α (Erα), in micropunches of the AVPV. Collectively, these studies demonstrate that undernutrition disrupts ovarian cyclicity in females via impairment both of ARCKiss1 control of LH pulses and AVPVKiss1 induction of the LH surge.
Keywords: kisspeptin, luteinizing hormone, gonadotropin-releasing hormone, feed restriction, stress, metabolism
Chronic undernutrition is a type of metabolic stress that exists worldwide (1) and has wide-reaching implications for female reproductive health and offspring development (2, 3). In adult females, undernutrition is associated with ovarian cycle disorders and reduced fertility across species (4-8). Given the energetic requirements for reproduction (9), females are especially susceptible to the disruptive effects of undernutrition; however, the neuroendocrine loci and molecular mechanisms that mediate ovarian cycle dysfunction during chronic undernutrition remain poorly understood.
Various models have been used to evaluate reproductive neuroendocrine function in adult females during states of low energy balance, including acute fasting or food deprivation (10), insulin-induced hypoglycemia (11) or glycoprivation (12); however, few studies have investigated the neural mechanisms activated by chronic undernutrition within the context of ovarian cycle suppression. In women, undernutrition elicits impairment of the menstrual cycle (13) and is considered a leading cause of functional hypothalamic amenorrhea, an anovulatory condition that results from decreased gonadotropin-releasing hormone (GnRH) and subsequently reduced pulsatile luteinizing hormone (LH) secretion (14, 15). In the few studies that have evaluated the ovarian cycle in animal models of undernutrition, decreased GnRH signaling and gonadotropin secretion is also implicated. For example, exogenous GnRH reinstated ovulation in musk shrews (16) and exogenous gonadotropins ameliorated atrophy of the ovaries and uterus in rats (17).
Two populations of kisspeptin (Kiss1)-synthesizing neurons in the hypothalamus have essential roles in maintaining ovarian cyclicity in females and may facilitate the inhibitory effect of undernutrition on impaired ovarian function. The Kiss1 cell population in the arcuate nucleus (ARC, infundibular nucleus in primates) is the GnRH pulse generator (18–23) and coexpresses the neuropeptides neurokinin B (NKB, encoded by Tac2) and dynorphin (encoded by pDyn), and referred to as KNDy neurons (24). Kiss1 is an output of KNDy neurons that signals via Kiss1r (formally termed GPR54) on GnRH neurons to elicit pulsatile GnRH and LH release in both sexes, which supports gamete development and steroidogenesis (18-23). In female rodents, another population of Kiss1 cells in the anteroventral periventricular nucleus and neighboring periventricular nucleus (AVPV/PeN) is critical for generation of the GnRH/LH surge (25).
Only a handful of studies have investigated the effects of undernutrition on pulsatile or surge LH secretion in adult females. In reproductive-age women, chronic undernutrition inhibited the frequency of LH pulses (7, 13). Undernutrition suppressed LH pulses in ovariectomized (OVX) female rats via a pathway enhanced by physiologic levels of estradiol (26). In OVX ewes, the ability of undernutrition to suppress pulsatile LH (27, 28) was associated with a reduction in the number of Kiss1-expressing cells and lower Kiss1 levels per cell within the ARC and preoptic area (analogous to AVPV/PeN) (29). Furthermore, messenger RNA (mRNA) levels for all 3 KNDy peptides were reduced in OVX mice following chronic undernutrition (30). With regard to the GnRH/LH surge, one study has shown that undernutrition impaired the positive-feedback response to estradiol in a nonhuman primate model (31). Collectively, these studies support the hypothesis, which was comprehensively tested for the first time within the present study, that undernutrition impairs ovarian cyclicity via the inhibition of central pathways controlling surge-type LH secretion as well as pulsatile gonadotropin secretion.
Food scarcity and undernutrition represent global health crises in humans and have been exacerbated by the COVID-19 pandemic. In adult females, the impairment of gonadotropin secretion during undernutrition would be expected to not only diminish reproductive capacity because of reduced gonadotropin secretion, but also compromise the protective effect of ovarian estradiol on cardiovascular, bone, and mental health (32); thus, regulation of LH secretion is profoundly important for human and animal health. In the present study, we used a mouse model of chronic undernutrition to investigate the neural mechanism(s) conveying ovarian cycle dysfunction. Based on our initial findings, which demonstrated impaired ovarian function and anovulation within days of undernutrition onset, we conducted 2 sets of experiments using well-defined estradiol-replacement paradigms to test the central mechanisms controlling LH pulses and induction of the preovulatory LH surge during chronic undernutrition.
Materials and Methods
Animals
Adult (age > 10 weeks) female C57BL/6 (Envigo) or Kiss1hrGFP (33) mice, weighing 17 to 24 grams, were typically housed 2 per cage under standard conditions under a 12-hour light, 12-hour dark cycle with lights on at 0600 hours. Animals had ad libitum access to water and were fed Harlan irradiated chow number 2920X according to the experimental details described later. Mice were handled daily for 5 weeks prior to blood collection to acclimatize animals to tail-bleed sampling required for LH measurement (34, 35). All surgeries and pulse blood sampling periods were performed between 0800 and 1200 hours. All animal procedures were performed at a University of California, San Diego vivarium in accordance with the National Institutes of Health guidelines for the care and use of research animals. The University of California, San Diego Institutional Animal Care and Use Committee (IACUC) reviewed and approved the protocol of this study, as well as the animal protocol detailing all laboratory procedures performed.
Chronic Undernutrition Model
Daily feed intake was determined for each cage of mice (2 mice/cage) by weighing chow offered and remaining each day. The feeder allowed 2 animals to eat at once; thus, access to allotted feed was unhindered. After a 2-week period of monitoring daily feed intake (animals had free access to feed), animals were randomly assigned by cage into 2 treatment groups: 1) control (ad libitum) animals had free access to feed, or 2) feed-restricted animals received a daily feed allotment equal to 70% of the daily feed intake measured during the second week of the intake monitoring period. The following tasks occurred on a daily basis between 1400 and 1600 hours throughout each experiment: measurement of individual body weight and feed intake, followed by administration of daily feed. This undernutrition paradigm was based on previous mouse studies that documented that a 30% reduction in feed intake resulted in an approximately 20% decrease in body weight (80% of age-matched controls) over 2 weeks of treatment (36, 37). A preliminary study was conducted to confirm this prior finding (37) during exposure to 8 weeks of feed restriction (70% of ad libitum levels). Specifically, we observed that a 30% reduction in feed induced a decrease in body weight, which stabilized after 2 weeks for a period of 6 weeks, at approximately 20% below age-matched controls.
Evaluation of Estrous Cyclicity
Estrous cyclicity was confirmed by classifying vaginal smears prior to experimentation in all animals, and throughout the 4-week period of observation in Experiment 1. Vaginal lavage was performed daily with distilled H2O (between 1400 and 1600 hours), and the recovered fluid was mounted on glass slides for microscopic examination of cell type (38). Smears were classified into 1 of 4 phases of the estrous cycle, diestrus, proestrus, estrus, or metestrus, by an investigator blinded to treatment groups. An estrous cycle was defined as positive classification of, at least, an estrus to diestrus to estrus transition. Only female mice exhibiting a 4- to 8-day estrous cycle, prior to treatment, were used in animal experiments. Estrous cycle length was calculated as the number of days between the first day of estrus of each cycle. In feed-restricted animals, when only one estrus phase occurred during the treatment period, the cycle length was calculated from estrus to the end of the observation period. If no estrus was identified, the cycle length was determined to be the total time of the treatment period (ie, 14 days). The time spent in each cycle stage was calculated as the percentage of time (days) classified in each cycle stage relative to the length of the observation period.
Estradiol-Replacement Models
Ovariectomized and low-estradiol, luteinizing hormone pulse paradigm
Mice were OVX aseptically under isoflurane anesthesia and implanted subcutaneously with a silastic capsule (1.98 mm inner diameter × 3.18 mm outer diameter) containing 100 ng 17-β estradiol (MilliporeSigma) dissolved in sesame oil (OVX + LowE), as described previously (39). This OVX + LowE paradigm approximates a diestrus-like level of estradiol, based on uterine mass measured 10 days after surgery (40), and establishes an LH pulse frequency of approximately 1 pulse per 30 minutes (39, 41).
Luteinizing hormone pulse analysis
Detection of an LH pulse was based on 3 criteria, proposed by Goodman and Karsch (42): 1) the pulse peak must be within 3 points from the preceding nadir; 2) the amplitude must be greater than the sensitivity of the assay; and 3) the amplitude must also be 2 SDs above the variability of the assay. Average values for pulse frequency, mean LH, and pulse amplitude were calculated across the pre and post periods. Pulse frequency was defined as the number of pulses in each 88-minute period. Mean LH was calculated by averaging all LH values in each sampling period. Pulse amplitude was calculated as the difference from the pulse peak to the preceding nadir, and an average pulse amplitude value for each animal during the presampling and postsampling period was determined.
Ovariectomized and high-estradiol, luteinizing hormone surge paradigm
Mice were OVX and implanted subcutaneously with a silastic capsule (1.98 mm inner diameter × 3.18 mm outer diameter) filled with 1.0 µg 17-β estradiol (OVX + HighE) dissolved in sesame oil (43). Under this hormonal milieu, female mice will produce a daily circadian-timed LH surge, occurring each evening exclusively around the time of lights off (44, 45). Tail blood samples were collected in the morning (1000-1100 hours) and evening within 30 minutes of lights off (1800 hours) for determination of LH surge occurrence. An LH surge was defined as an evening LH concentration exceeding 4 times the morning LH concentration for each animal.
Experimental Details
Experiment 1: Effect of Chronic Undernutrition on the Ovarian Cycle of Intact Female Mice
This experiment tested whether chronic undernutrition would disrupt estrous cyclicity and ovarian function in intact C57BL/6 female mice. Estrous cyclicity, body weight, and feed intake were evaluated daily during the 4-week experiment (see Fig. 1A for experimental details). After a 2-week control period, in which all animals received ad libitum feed, cages containing animal pairs were randomly assigned to 2 treatment groups (n = 8-11/group): 1) feed-restricted (70% of feed consumed during the baseline period) or 2) ad libitum–fed controls. On diestrus, approximately 2 weeks following the initiation of treatment (range, 14-19 days), animals were euthanized under isoflurane via rapid decapitation. The brain, pituitary gland, and one ovary (which was dissected of fat and weighed) were each quickly frozen on dry ice and stored at –80 °C for RNA extraction and gene expression analysis. The second ovary and uterus (which was collected and weighed) were each placed in fixative (60% ethanol, 30% formalin, and 10% acetic acid) for overnight storage and then transferred to 70% ethanol.
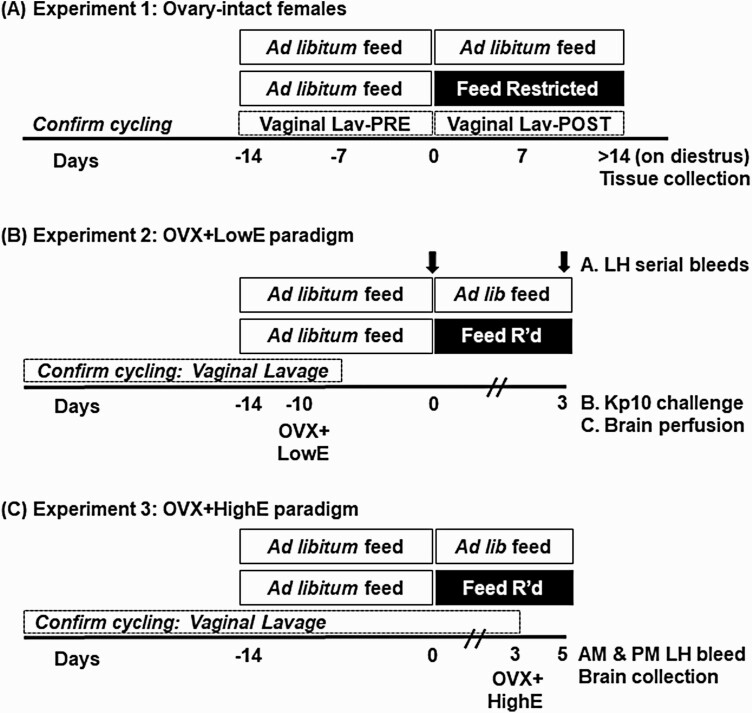
Figure 1.
Experimental details. Time is depicted as days relative to the initiation of feed restriction or ad libitum feeding in controls, indicated by the horizontal bars. A, In experiment 1, vaginal lavage (Lav) was performed to assess estrous cyclicity in intact females receiving ad libitum feed or feed restriction; animals were euthanized for tissue collection the first day of diestrus that occurred after 14 days of feed restriction or ad libitum (control) feeding. B, Experiment 2 consisted of 3 subexperiments in which females were ovariectomized (OVX) and received a low, diestrus-like, estradiol implant (OVX + LowE) 10 days prior to receiving ad libitum feed or feed restriction. In experiment 2A, frequent blood samples were collected prior to (–1.5 to 0 hours) or following (72-73.5 hours) the onset of treatment, as indicated by the arrows. (Experiment 2B) Blood to evaluate Kp10-induced luteinizing hormone (LH) secretion or (experiment 2C) fixed neural tissue were collected 3 days following onset of ad libitum feed or feed restriction. C, In experiment 3, females were OVX and received a high, LH surge-inducing, estradiol implant (OVX + HighE) and blood and tissues were collected 2 days later.
Experiment 2: Effect of Undernutrition on Luteinizing Hormone Pulses
Based on the results of experiment 1, in which mice entered diestrus 2.2 ± 0.5 days from the onset of feed restriction, we conducted 2 sets of experiments to focus on the neural pathways that initiate impairment of cyclicity during chronic undernutrition. Therefore, the period of feed restriction was reduced to 3 days (experiment 2: LH pulses) or 5 days (experiment 3: LH surge). An average body weight reduction of 6.4% on day 2 (experiment 2: LH pulses) and 8.2% on day 4 (Experiment 3: LH surge), in feed-restricted vs ad libitum animals, was determined the day before blood collection to minimize manipulations the day of the experiment.
Experiment 2 determined the effect of undernutrition on: 1) LH pulses; 2) the LH response to exogenous Kiss1 (Kp10); and 3) ARCKiss1 neuronal activity. For this set of studies, the OVX + LowE paradigm was initiated prior to feed restriction (or control feeding). Timing of surgery and treatment is detailed in Fig. 1B. The advantage of this model is 2-fold: Estradiol levels are normalized across females, and other factors influenced by the ovarian cycle are removed.
Serial blood samples (3.2 µL) were collected from the tail vein with a pipette every 8 minutes during two 88-minute observation periods. The first serial sampling period occurred during ad libitum feeding in all animals, corresponding to 10 days after OVX + LowE. The second serial sampling period occurred approximately 72 hours following initiation of feed restriction or ad libitum feeding (n = 5/group). The feed-restricted group consisted of 4 Kiss1hrGFP females and 1 C57BL/6 female; all controls were Kiss1hrGFP females. We have not observed a difference in the LH response to undernutrition across these genotypes.
Tail blood for measurement of LH (6.4 µL) was collected prior to and 10 minutes following Kp10 (2 µg/g, intraperitoneally; Tocris Bioscience [46]) in OVX + LowE feed-restricted or control C57BL/6 females following 3 days of treatment (n = 5/group). The interval from OVX + LowE to LH sampling varied from 10 or 13 days (n = 2/group or 3/group, respectively).
Feed-restricted or control OVX + LowE Kiss1hrGFP females (n = 4-5/group) were euthanized following the pulse bleed detailed in experiment 2A with an overdose of pentobarbitol (Fatal Plus, MWI Animal Health) and perfused by cardiac puncture with 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed and stored overnight in 4% paraformaldehyde and then transferred to 30% sucrose in phosphate buffer (PB) for at least 1 day.
Experiment 3: Effect of Undernutrition on the Luteinizing Hormone Surge in
Females were OVX and implanted with a surge-inducing estradiol implant on day 3 of feed restriction or ad libitum feeding (n = 5-6/group). Timing of surgery and treatment is detailed in Fig. 1C. am and pm tail blood samples (6.4 µL) were collected 2 days later, according to the OVX + HighE LH surge paradigm. After the pm blood sample, the animals were euthanized under isoflurane via rapid decapitation and brains were collected and immediately frozen on dry ice and stored at –80 °C for RNA extraction and gene expression analysis.
Mouse Luteinizing Hormone Enzyme-Linked Immunosorbent Assay
Individual whole blood (3.2 or 6.4 μL) samples for measurement of LH were immediately diluted (1:20 or 1:10, respectively) into assay buffer, mixed, and placed on ice until storage at –20 °C. LH was measured in singleton by an enzyme-linked immunosorbent assay, based on a validated method by Steyn et al (47), and verified for use in our laboratory using capture monoclonal antibovine LH antibody (Dr Janet Roser, Department of Animal Science, University of California, Davis; catalog No. 518B7 [48], RRID:AB_2665514), detection polyclonal rabbit anti-LH antibody (A.F. Parlow National Hormone and Peptide Program [NHPP]; catalog No. AFP240580Rb, RRID:AB_2665533), goat antirabbit HRP-conjugate (Bio-RAD; catalog No. 170-6515, RRID:AB_11125142), and mouse LH reference prep AFP5306A (A.F. Parlow, NHPP) as the assay standard. Accuracy was calculated as percentage recovery across a range of spiked concentrations and determined to be 94.7%. Precision was determined by intraassay coefficient of variation (%CV) of a high and low pool of LH, as measured in duplicate. The limit of quantitation (functional sensitivity) is defined as the lowest concentration that demonstrates accuracy within 20% of expected values, with intra-assay and interassay %CV less than 20%, and was determined by serial dilutions of a defined sample pool. Functional sensitivity was 0.2 ng/mL for 3.2 µL samples and 0.1 ng/mL for 6.4 µL samples. Intra-assay and interassay %CVs were 3.8% and 5.1%, respectively.
RNA Isolation and Gene Expression Analysis
RNA was extracted from individual pituitaries and ovaries via Trizol reagent (Life Technologies–Ambion), per the manufacturer’s directions. To isolate RNA from the ARC or AVPV regions, brains were sectioned coronally (250 μm) on a cryostat at –10 °C. Sections were collected from –1.22 to –2.54 mm (ARC) or 0.62 to –0.22 mm (AVPV) from bregma, according to a mouse brain atlas (49). Either 2-mm semicircular or circular micropunches (ARC or AVPV, respectively) were removed for each region and stored at –80 °C. RNA was isolated using the RNAqueous Micro Kit (Life Technologies–Ambion), per the manufacturer’s instructions.
Following isolation, DNA contamination was removed with DNA-free DNase treatment (Life Technologies–Ambion) and RNA was quantified by nanodrop. RNA (0.5-1 μg, brain or pituitary; 2 μg, ovary) was reverse transcribed using the iScript Complementary DNA (cDNA) synthesis kit (Bio-RAD). For quantitative polymerase chain reaction, cDNA and gene-specific primers were loaded according to Table 1, along with SYBR green (Bio-RAD) and equivalent amounts of cDNA for each primer set. Data were analyzed using the comparative cycle threshold (ΔΔCt) method using glyceraldehyde-3-phosphate dehydrogenase (Gapdh; brain and pituitary) or Ppia (ovary) as a reference gene (50). Reference gene abundance was confirmed to be stable for each tissue across treatments.
Table 1.
Primer sequences for quantitative polymerase chain reaction
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Ppia | AAGTTCCAAAGACAGCAGAAAAC | CTCAAATTTCTCTCCGTAGG |
| Cyp11a1 | GATTCCAGCCAAGACTTTGGTAC | TCACGGAGATTTTGAACTTCAAT |
| Cyp17a1 | GAGTTTGCCATCCCGAAGGA | CCAGCTCCGAAGGGCAAATA |
| Cyp19a1 | TTTCGCTGAGAGACGTGGAG | AGGATTGCTGCTTCGACCTC |
| Lhr | GCCATGCATTCAATGGGACG | GGCCTGCAATTTGGTGGAAG |
| Fshr | GTGCATTCAACGGAACCCAG | TCTAAGCCATGGTTGGGCAG |
| Gapdh | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
| Kiss1 | CCCTTCCTCCCAGAATGATC | TCCCAGGCATTAACGAGTTC |
| Tac2 | CGTGACATGCACGACTTC | CCAACAGGAGGACCTTAC |
| pDyn | AGCAGTAAGCAGGTCATTCATCC | CACGCCATTCTGACTCACTTGT |
| Erα | GCGCAAGTGTTACGAAGTG | TTCGGCCTTCCAAGTCATC |
| Lhβ | CTGTCAACGCAACTCTGG | ACAGGAGGCAAAGCAGC |
| Fshβ | CCGTCTGCTAGGTAGATCATCC | CCGTCTGCTAGGTAGATCATCC |
| αGsu | CCGTCTGCTAGGTAGATCATCC | CCGTCTGCTAGGTAGATCATCC |
| GnRHr | GCCCCTTGCTGTACAAAGC | CCGTCTGCTAGGTAGATCATCC |
Abbreviations: αGsu, glycoprotein hormone α-subunit; Cyp11a1, Cytochrome P450 Family 11 Subfamily A Member 1; Cyp117a1, Cytochrome P450 Family 17 Subfamily A Member 1; Cyp19a1, Cytochrome P450 Family 19 Subfamily A Member 1; Erα, estradiol receptor α; Fshβ, follicle-stimulating hormone β; Fshr, follicle-stimulating hormone receptor; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Gnrhr, gonadotropin-releasing hormone receptor; Kiss1, kisspeptin; Lhβ, luteinizing hormone β; Lhr, luteinizing hormone receptor; pDyn, prodynorphin; Ppia, Peptidyl-prolyl cis-trans isomerase A; Tac2, tachykinin 2.
Immunohistochemistry for Kisspeptin and c-Fos
Fixed neural tissue was sectioned (40 μm) on a cryostat in 3 series and stored in cryoprotectant solution (30% ethylene glycol, 30% sucrose, 1% polyvinyl pyrrolidone in PB) at –20 °C until processed for immunohistochemistry, as previously described (39). One series of 40-μm sections, encompassing the entire ARC, was processed for each animal. The following steps were performed at room temperature with gentile agitation, unless noted otherwise. Tissue was rinsed in PB, 12 times for 15 minutes, and incubated overnight at 4 °C. The next day, tissue was rinsed 6 times in PB and then 6 times in PBS, for 5 minutes each (the following rinsing steps were performed in PBS). Antigen retrieval was performed by incubating tissue in boiling Citra Buffer (Thermo Fisher Scientific) for 10 minutes, twice. Next, tissue was rinsed and then incubated in blocking solution, containing PBS with 0.4% Triton X-100, and 4% NGS, for 1 hour. Tissue was then incubated in rabbit anti–c-Fos (1:1000, Cell Signaling Technology, catalog No. 2250, RRID:AB_2247211) in blocking solution for 18 hours at 4 °C. The next day tissue was rinsed and then incubated with Alexa Fluor 555 goat antirabbit (1:200, Thermo Fisher Scientific, catalog No. A27039, RRID:AB_2536100). Tissue was rinsed and incubated in blocking solution for 1 hour and incubated in rabbit anti–green fluorescent protein (anti-GFP) conjugated to Alexa 488 (1:1000, Thermo Fisher Scientific, catalog No. A-21311, RRID:AB_221477) in blocking solution for 18 hours at 4 °C. Finally, tissue was rinsed, mounted on SuperFrost slides (Thermo Fisher Scientific), coverslipped with gelvatol solution per published recipe (51), and stored at 4 °C until microscopy.
Histology
Fixed ovaries were embedded in paraffin wax, sectioned at 16 µm onto slides in 3 series, and heated to adhere tissue to slides for 24 hours at 37 °C. One series of tissue (spaced 48 μm) was stained with hematoxylin and eosin and quantified by follicle stage: primary, secondary, antral and corpora lutea (52), using a bright field microscope (Evos Xi Core; Life Technologies). Counts were performed by an observer blinded to treatment group, and care was taken not to count structures that appeared in multiple sections.
Imaging
Imaging of neural tissue was performed with a Nikon Ti2-E inverted epifluorescent microscope with DS-Qi2 monochrome CMOS camera controlled with NIS Elements. At least 2 sections from each of 3 arcuate regions were selected for analysis (ie, rostral, middle, and caudal). The total number of Kiss1 cells and the percentage of Kiss1 cells that contained c-Fos were determined in each hemisection and averaged per region. Kiss1-GFP cell intensity was determined by measuring the relative fluorescence unit of each individual cell within the section and averaged per section per region. Comparisons were then made between treatment groups (within region) with the animal as the experimental unit. All cell counting was performed by an observer blinded to treatment group using ImageJ software (53). Minor adjustments to brightness and contrast were made to the images presented in the figures in this manuscript (independent of intensity analysis).
Data Analysis
Two-way analysis of variance (ANOVA) followed by Tukey honestly significant difference test was used to determine significant differences across time (pre vs post, am vs pm) and treatments (ad libitum vs feed restriction), to identify significant interactions between variables: body weight and feed intake, mean LH or LH pulse frequency, estrous cycle characteristics (cycles, cycle length, cycle phases), am/pm LH, and LH response to Kp10. One-way analysis of variance (ad libitum vs feed restriction) was used to determine significant differences in uterine and ovarian weight, ovarian follicle, and corpora lutea numbers, ΔΔCt relative quantity, Kiss1 cells, GFP(Kiss1) relative fluorescence, and colocalization of c-Fos/Kiss1 values. Values are expressed as mean ± SEM. All statistical analyses were performed using JMP 14.0.0 (SAS Institute) and statistical significance was defined as P less than .05.
Results
Experiment 1: Effect of Chronic Undernutrition on the Ovarian Cycle of Intact Female Mice
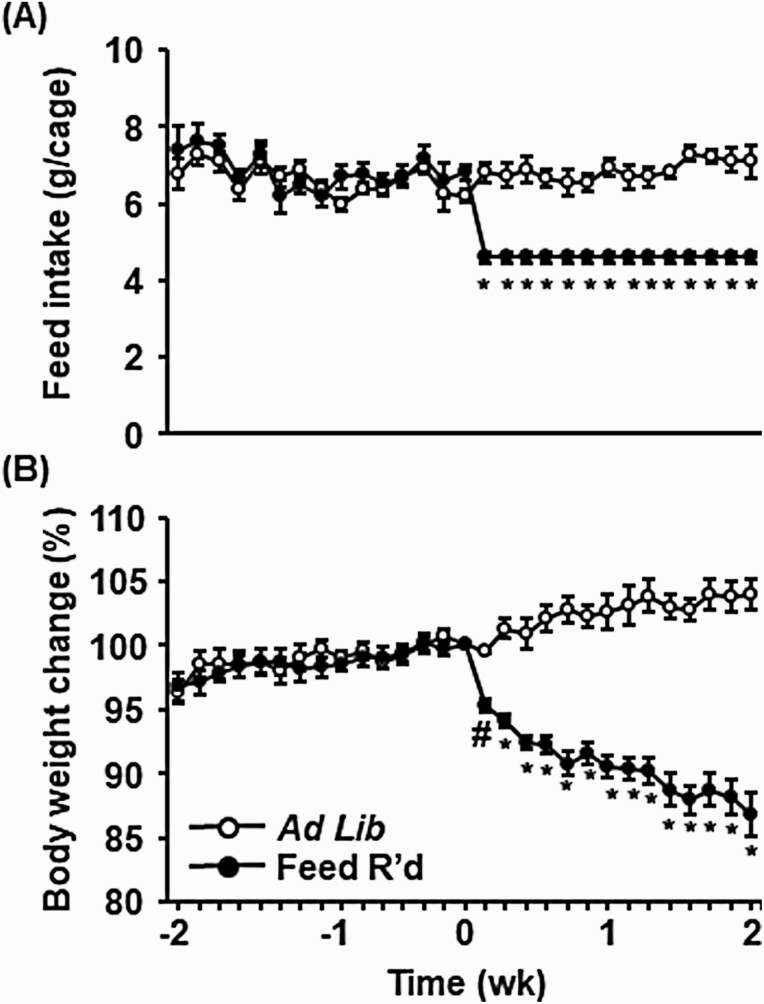
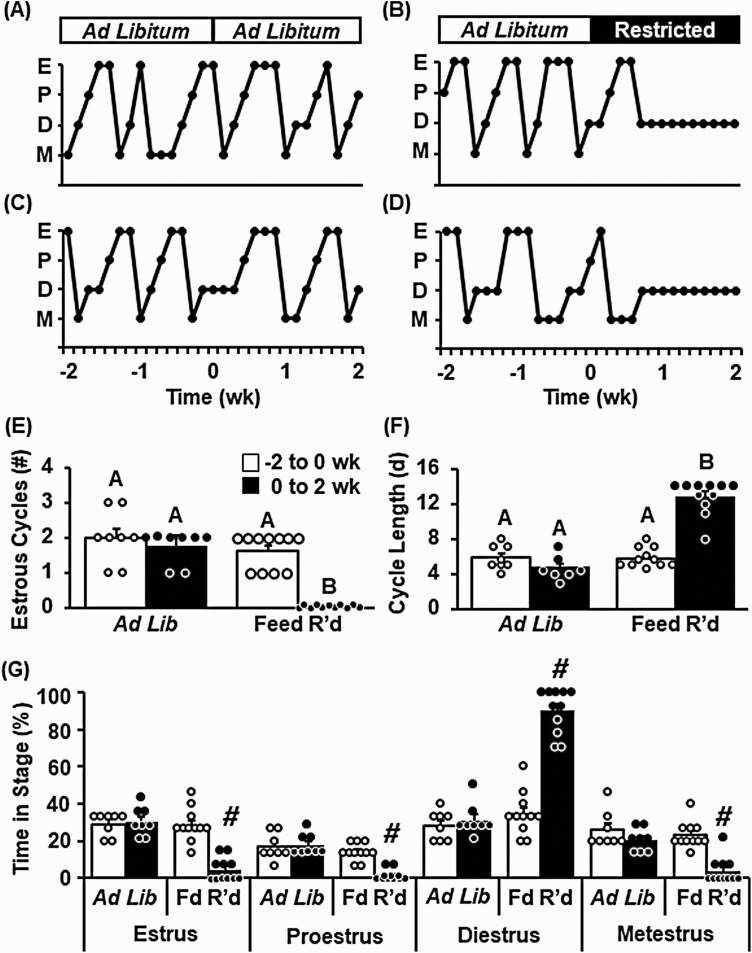
Measurements of feed intake and body weight were collected daily throughout the 4-week observation period in control ad libitum–fed or feed-restricted animals. Feed was reduced to 70% of ad libitum levels the afternoon of day 0 (Fig. 2A). Feed restriction caused a progressive decrease in body weight of underfed animals averaging 9.5% after 7 days and 13.2% after 14 days, as compared to day 0 values. This reduction in body weight was equivalent to a loss of 12.1% and 17.2%, after 7 and 14 days, respectively, compared to control animals that received ad libitum feed (Fig. 2B). Control animals displayed clear and regular estrous cycles throughout the 4-week observation period (Fig. 3A and 3C). In contrast, feed restriction caused a robust cessation of ovarian cyclicity (Fig 3B and 3D), significantly reducing the number of estrous cycles that occurred during the 2-week treatment period (Fig. 3E, P < .05). Feed restriction increased the length of the estrous cycle (Fig. 3F, P < .05), eliciting an increased amount of time in diestrus and a decrease in time spent in proestrus, estrus, and metestrus compared to control animals (Fig. 3G, P < .05).
Figure 2.
Chronic undernutrition reduces body weight of intact female mice. A, Mean (± SEM) feed intake per female pair depicted as grams/cage, and B, average individual body weight depicted as the percentage change relative to time 0, in females that received control (ad libitum) feed or feed restriction (70% of ad libitum feed intake). Values (mean ± SEM) were analyzed by 2-way analysis of variance with time and treatment as factors; n = 8-11 animals/group. #Significance compared to day 0 in feed-restricted animals (P < .05). *Significance compared to day 0 in feed-restricted animals, as well as significance compared to control animals the same day (P < .05).
Figure 3.
Chronic undernutrition impairs estrous cyclicity in intact female mice. Representative profiles depicting estrous cyclicity in intact female mice, as measured by vaginal cytology, prior to and during, A and C, ad libitum feeding, or B and D, feed restriction (Restricted/Fd R’d). Number of estrous cycles, E, defined by an estrus-diestrus-estrus transition, and F, average estrous cycle length prior to and during ad libitum feeding or feed restriction. G, Average time spent in each stage of the cycle prior to and during treatment. Values (mean ± SEM) were analyzed by 2-way analysis of variance with time and treatment as factors; n = 8-11 animals/group. Unique letters signify significant differences between values (P < .05). #Time × treatment interaction (P < .05). E, estrus, P, proestrus, D, diestrus, M, metestrus.
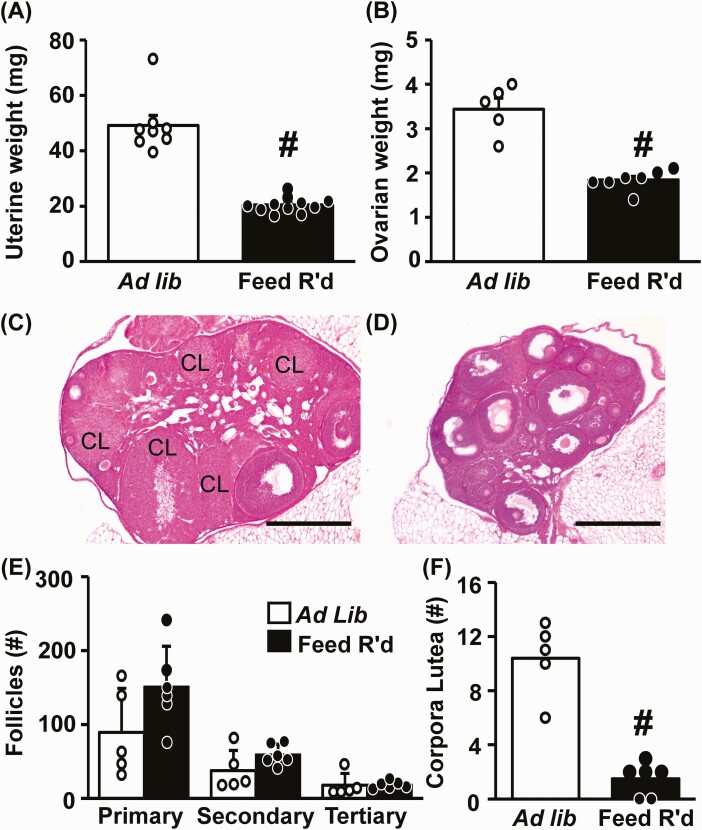
Feed restriction caused a significant reduction in the weight of the uterus, as well as the ovary, compared to diestrus-staged control females (Fig. 4A and 4B, P < .05); the reduced ovarian size was also evident on hematoxylin and eosin staining (Fig. 4C control vs 4D feed restricted). Quantification of ovarian structures revealed no change in the number of primary, secondary, or tertiary follicles (Fig. 4E, P > .05), but a significant decrease in the number of corpora lutea (Fig. 4F P < .05). Ovarian mRNA expression levels of LH receptor (Lhr), P450scc (Cyp11a1), 17α-hydroxylase (Cyp17a1), and aromatase (Cyp19a1) were significantly reduced following feed restriction (P < .05); follicle-stimulating hormone (FSH) receptor levels (Fshr) were not different from controls (Table 2, P > .05).
Figure 4.
Diminished ovarian function and anovulation induced by chronic undernutrition. Mean (± SEM) A, uterine weight, and B, ovarian weight collected from diestrus females following 2 weeks of ad libitum feeding or feed restriction (Feed R’d); n = 8-11 (uterine weight) or n = 5-7 (ovarian weight) animals per group. Representative photomicrographs of hematoxylin and eosin–stained fixed ovarian tissue collected from females following C, ad libitum feeding, or D, feed restriction. Scale bar equals 500 μm; CL, corpora lutea. Values (mean ± SEM) for E, primary, secondary, and tertiary follicles, and F, corpora lutea, were quantified in each ovary and analyzed by one-way analysis of variance; n = 5-7 animals/group. #Effect of treatment (P < .05).
Table 2.
Ovarian gene expression in intact females
| Relative quantitya | ||
|---|---|---|
| Ad libitum | 14-d Feed R’d | |
| Cyp11a1 | 1.00 ± 0.55 | 0.10 ± 0.17b |
| Cyp17a1 | 1.00 ± 0.19 | 0.23 ± 0.09b |
| Cyp19a1 | 1.00 ± 0.45 | 0.27 ± 0.04b |
| Lhr | 1.00 ± 0.09 | 0.15 ± 0.04b |
| Fshr | 1.00 ± 0.26 | 0.74 ± 0.19 |
Abbreviation: Cyp11a1, Cytochrome P450 Family 11 Subfamily A Member 1; Cyp117a1, Cytochrome P450 Family 17 Subfamily A Member 1; Cyp19a1, Cytochrome P450 Family 19 Subfamily A Member 1; Fshr, follicle-stimulating hormone receptor; Lhr, luteinizing hormone receptor; R’d, restricted.
aValues are mean ± SEM.
b Treatment effect as determined by analysis of variance (P < .05).
Reproductive neuroendocrine gene expression within brains and pituitary glands were compared between diestrus-staged controls and feed-restricted females (Table 3). Although Kiss1 and NKB (Tac2) mRNA levels were downregulated by feed restriction (P < .05), dynorphin (pDyn) was unchanged in ARC micropunches. In the AVPV, Kiss1 was reduced by feed restriction (P < .05), but estradiol receptor α (Erα) was similar to controls. In the pituitary, mRNA expression of Lhβ, glycoprotein hormone alpha-subunit (αGsu), and GnRH receptor (Gnrhr) were significantly suppressed (P < .05), but Fshβ was not affected by feed restriction.
Table 3.
Arcuate nucleus, anteroventral periventricular nucleus, and pituitary gene expression in intact females
| Relative quantitya | ||
|---|---|---|
| Ad libitum | 14-d Feed R’d | |
| ARC | ||
| Kiss1 | 1.00 ± 0.11 | 0.71 ± 0.07b |
| Tac2 | 1.00 ± 0.23 | 0.39 ± 0.11b |
| pDyn | 1.00 ± 0.13 | 0.89 ± 0.09 |
| AVPV | ||
| Kiss1 | 1.00 ± 0.17 | 0.21 ± 0.07b |
| Erα | 1.00 ± 0.22 | 1.07 ± 0.31 |
| Pituitary | ||
| Lhβ | 1.00 ± 0.08 | 0.56 ± 0.05b |
| Fshβ | 1.00 ± 0.19 | 1.20 ± 0.37 |
| αGsu | 1.00 ± 0.06 | 0.67 ± 0.08b |
| Gnrhr | 1.00 ± 0.09 | 0.42 ± 0.14b |
Abbreviations: αGsu, glycoprotein hormone α-subunit; ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; Erα, estradiol receptor α; Fshβ, follicle-stimulating hormone β; Gnrhr, gonadotropin-releasing hormone receptor; Kiss1, kisspeptin; Lhβ, luteinizing hormone β; pDyn, prodynorphin; R’d, restricted; Tac2, tachykinin 2.
aValues are mean ± SEM.
b Treatment effect as determined by analysis of variance (P < .05).
Experiment 2: Effect of Undernutrition on Luteinizing Hormone Pulses
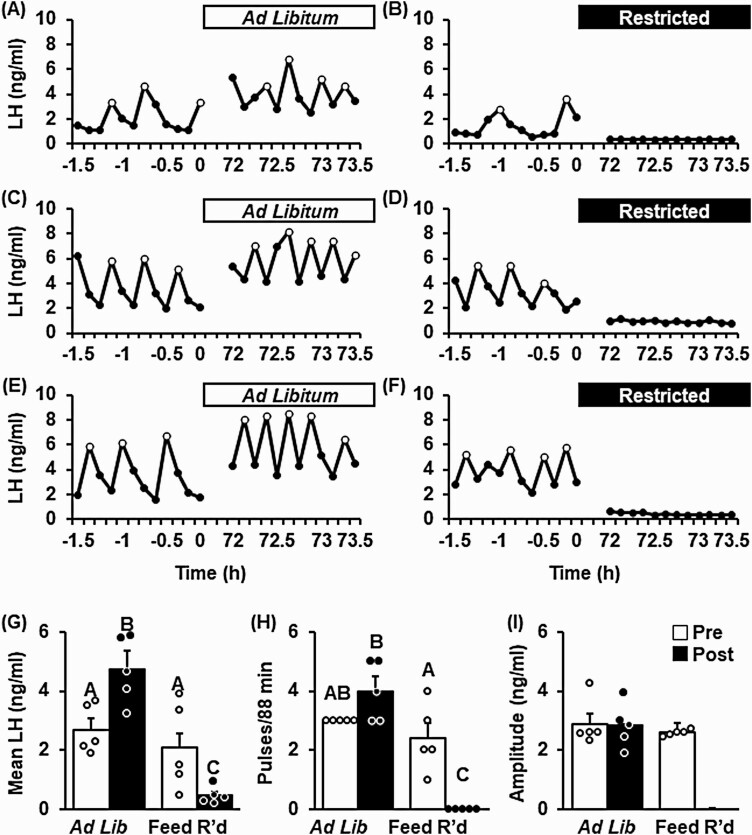
Fig. 5 shows representative LH pulse profiles collected from OVX + LowE mice prior to and following 3 days of control feed (ad libitum, Fig. 5A, 5C, and 5E) or feed restriction (Fig. 5B, 5D, and 5F). Pulsatile LH patterns were similar during the pre and post periods in control females, demonstrating a significant increase in mean LH, trending increase in pulse frequency, and stable pulse amplitude across time (Fig. 5G-5I). In contrast, feed restriction elicited a robust suppression of pulsatile LH secretion. Mean LH was near assay detectability in all feed-restricted animals during the treatment window (see Fig. 5G) and no LH pulses were detected (see Fig. 5H), preventing assessment of LH pulse amplitude (see Fig. 5I).
Figure 5.
Undernutrition disrupts luteinizing hormone (LH) pulses in female mice ovariectomized and implanted with a, diestrus-like, estradiol-implant (OVX + LowE). Pattern of pulsatile LH secretion, measured in serial tail-tip blood samples, in 3 representative OVX + LowE mice prior to or following 3 days of A, C, and E, ad libitum feed, or B, D, and F, feed restriction (Feed R’d). LH pulses are identified by open circles. Values (mean ± SEM) for G, mean LH; H, pulse frequency; and I, pulse amplitude were calculated across time (pre [–1.5 to 0 hours], white bars vs post [72-73.5 hours], black bars) and between treatment groups (n = 5 mice/group) by 2-way analysis of variance with time and treatment as factors. Unique letters signify significant differences between values (P < .05). Note, pulse amplitude was not calculated following feed restriction because no pulses were identified during the post period.
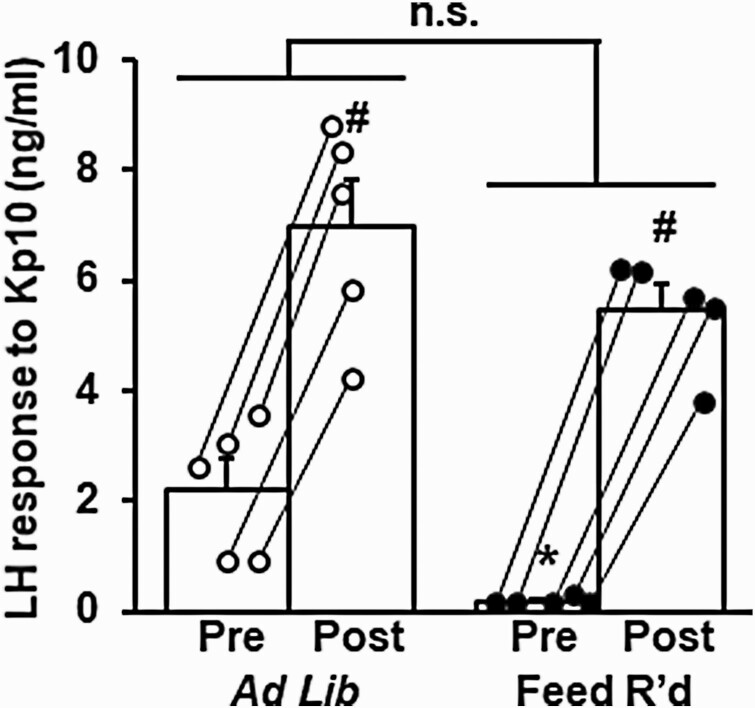
Circulating LH was measured prior to and following an injection of Kp10 in OVX + LowE mice following 3 days of control feeding or feed restriction (Fig. 6). Prior to injection, LH levels were significantly reduced by feed restriction (2.23 ± 0.55 vs 0.18 ± 0.03 ng/mL, control vs feed restriction, P < .05). LH levels were significantly increased both in controls and feed-restricted females in response to Kp10 (P < .05), and the amplitude of the LH response was not significantly different between treatments (4.3 ± 0.5 vs 5.3 ± 0.4 ng/mL, control vs feed restriction, P > .05).
Figure 6.
Undernutrition does not alter the luteinizing hormone (LH) response to exogenous kisspeptin (Kp10). Mean (± SEM) LH measured 10 minutes prior to and following Kp10 (2 µg/g, intraperitoneally) administered to female mice ovariectomized and implanted with a, diestrus-like, estradiol-implant (OVX + LowE) following 3 days of ad libitum feeding (white circles) or feed restriction (black circles). Lines connect the pre and post sample for each animal. *Effect of treatment (P < .05). #Effect of time (Pre/Post) (P < .05). No significant treatment × time interaction was identified (P > .05).
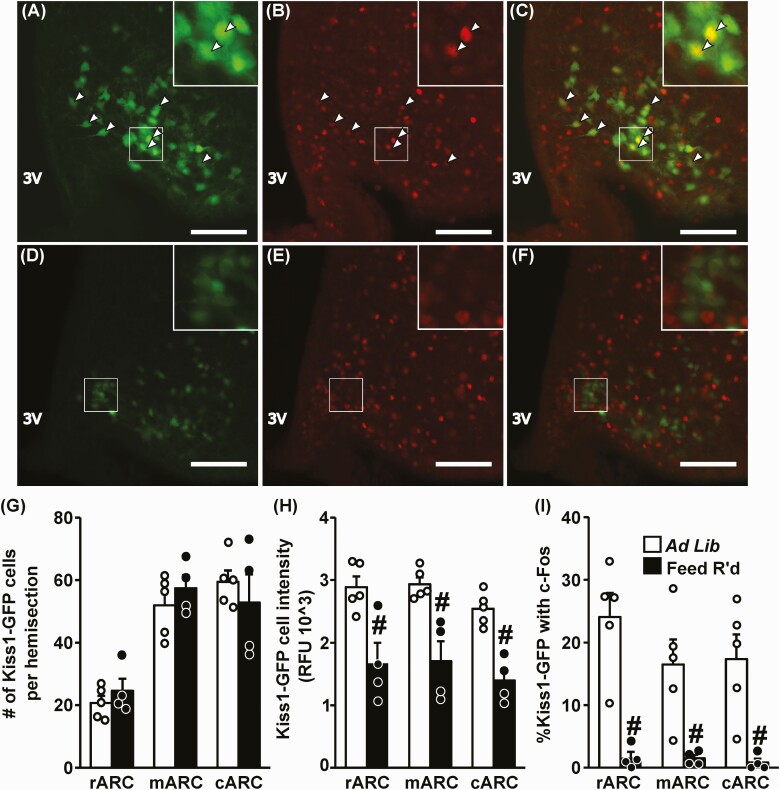
We investigated the effect of feed restriction on ARC Kiss1 protein expression and neuronal activation using a Kiss1hrGFP mouse model, in which GFP expression is regulated by the Kiss1 promoter. Representative photomicrographs demonstrate nuclear c-Fos within GFP-labeled Kiss1 cells in a control OVX + LowE female (Fig. 7A-7C) and absent coexpression with c-Fos in a feed-restricted female, following 3 days of treatment (Fig. 7D-7F). Although an equivalent number of Kiss1 cells were identified in the rostral, middle, and caudal regions of the ARC between controls and feed-restricted females (Fig. 7G), feed restriction caused a robust suppression in the fluorescence intensity (ie, Kiss1 expression) across the rostral-caudal ARCKiss1 populations (Fig. 7H, P < .05). In addition, feed restriction elicited a significant reduction in the percentage of ARCKiss1 cells expressing c-Fos in OVX + LowE females compared to controls (Fig. 7I, P < .05, 93% suppression); the suppression was present in all 3 regions of the ARC.
Figure 7.
Undernutrition reduces Kiss1 expression and neuronal activation in the arcuate nucleus. Representative photomicrographs depicting staining for A and D, Kiss1 (green); B and E, c-Fos (red); and C and F, dual-labeled Kiss1/c-Fos cells in the middle ARC of OVX + LowE Kiss1hrGFP mice following A to C, ad libitum feeding, or D to F, 3 days of feed restriction (Feed R’d). White box indicates location of zoomed panels. White arrowheads indicate dual-labeled Kiss1/c-Fos cells. Scale bar equals 100 μm. Brightness and contrast were adjusted similarly in images from control and feed-restricted animals. G, Number of Kiss1 cells per hemisection; H, Kiss1 cell intensity per section; I, percentage of Kiss1 cells with c-Fos per hemisection following ad libitum feeding (white bars) or feed restriction (black bars). Regions of the ARC are referred to as rostral (r), middle (m), and caudal (c); 3V, third ventricle. Values (mean ± SEM) were analyzed by one-way analysis of variance. #Effect of treatment (P < .05).
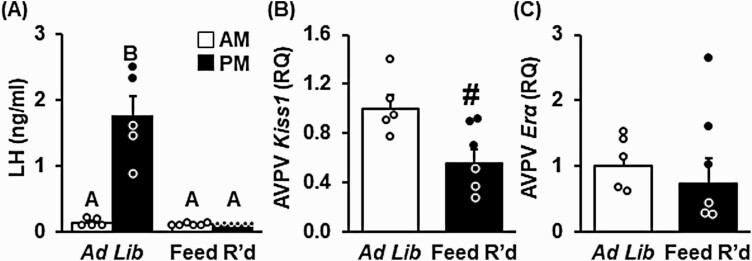
Experiment 3: Effect of Undernutrition on the Luteinizing Hormone Surge
LH was measured in the morning and evening the day of the anticipated LH surge in control and feed-restricted females during the OVX + HighE surge paradigm. As anticipated, LH levels were low in the morning in both treatment groups (Fig. 8A). In control females, LH was significantly elevated in the evening, with all females expressing an LH surge (P < .05). In contrast, 5 days of feed restriction completely blocked the estradiol-induced LH surge. Expression of Kiss1 mRNA abundance in micropunches of the AVPV, collected at the time of the anticipated LH surge, was significantly diminished by feed restriction (Fig. 8B, P < .05); Erα was not changed (Fig. 8C, P > .05).
Figure 8.
Undernutrition lowers Kiss1 neurons in the anteroventral periventricular nucleus (AVPVKiss1) expression and prevents the estradiol-induced luteinizing hormone (LH) surge. A, Blood was collected in the morning (am, 1000 hours) and evening (pm, 1800 hours) of the expected LH surge in female mice following 5 days of ad libitum feeding or feed restriction (Feed R’d) and analyzed by 2-way analysis of variance (ANOVA) with time and treatment as factors. Unique letters signify significant differences between values (P < .05). Kiss1 and estradiol receptor α (Erα) gene expression in AVPV micropunches, collected following ad libitum feeding (white bars) or feed restriction (black bars), were analyzed by 1-way ANOVA. #Effect of treatment (P < .05); n = 5-6/group. Abbreviation: RQ, Relative Quantity.
Discussion
The goal of the present study was to test the hypothesis that chronic undernutrition impairs ovarian cyclicity in females by disrupting the neural pathways that generate LH pulses and the preovulatory LH surge. We observed that chronic feed restriction prevented ovulatory cyclicity in intact female mice and elicited reduced expression of key reproductive genes at the neuroendocrine and ovarian levels. Based on the observation that reproductive suppression, as assessed by constant diestrus, occurred within a few days of the start of the undernutrition, we used 2 estradiol-replacement paradigms to understand the inhibitory effects of feed restriction that initiate cycle disruption. We demonstrated that feed restriction greatly diminished activation of ARCKiss1 neurons and completely suppressed pulsatile LH secretion, measured in OVX females replaced with a diestrus-like level of estradiol. Using a hormonal paradigm to generate the estradiol-induced LH surge in OVX female mice, we observed that feed restriction prevented the positive feedback response to estradiol and prevented enhanced AVPVKiss1 expression at the time of the anticipated LH surge. Collectively, these experiments indicate that impairment of both modes of gonadotropin secretion occurs during undernutrition and results in disruption of ovulatory cyclicity in female mice.
The robust suppression across hypothalamic, pituitary, and gonadal tissues in intact females supports the hypothesis that central suppression of neuroendocrine function contributes to the disruption of the ovarian cycle during undernutrition. Although persistent diestrus and lowered uterine weight (a well-used proxy for circulating estradiol level [40]) support the conclusion that estradiol production was impaired during feed restriction, this is presumably due to the effect of deficient pulsatile LH secretion to support ovarian steroidogenesis. We observed decreased expression of key steroidogenic enzymes involved in estradiol biosynthesis, including P450scc (Cyp11a1), 17a-hydroxylase (Cyp17a1), and aromatase (Cyp19a1), which is not surprising because these genes are transcriptionally regulated by LH (54). In addition, we observed lowered Lhr expression, which may be a result of diminished estradiol signaling within the ovary (55). Surprisingly, we did not observe a change in Fshβ which is transcriptionally regulated by GnRH (56). Although these findings may stem from a primary deficit of reduced pulsatile LH, the absence of corpora lutea suggests failure to ovulate, presumably due to LH surge blockade. Furthermore, reduced Kiss1 expression both in the ARC and AVPV in intact diestrus females during undernutrition suggests a diminishment in the central circuits controlling both LH pulses and the LH surge. Taken together, these observations support the interpretation that both ARCKiss1 control of LH pulses and AVPVKiss1 induction of the LH surge are impaired in this model of nutritional anovulation and led us to use estradiol-replaced models, which allowed positive and negative feedback states to be dissected.
We used an OVX + LowE model to directly test the effect of undernutrition on the neural control of LH pulses to avoid the confounding effects of altered estradiol in intact females. Although the LH pulse frequency in this model is not as low as shown in intact females (57), the pulse frequency is significantly lower than in OVX mice (39, 41), suggesting an effect of estradiol negative feedback. After 3 days of feed restriction, we observed robust suppression in ARCKiss1 cell activation as well as a diminishment in ARCKiss1 intensity (ie, Kiss1 expression), which is consistent with the reduction in Kiss1 expression in ARC micropunches in intact females during chronic undernutrition. The LH response to exogenous Kiss1 was not altered, suggesting signaling downstream of ARCKiss1-Kiss1r activation was unaffected by undernutrition. We interpret these data to imply that the initial suppression of LH pulses in adult females occurs within ARCKiss1 neurons, or upstream of this cell population, as proposed in a recent review of findings in sheep (58). Evidence that exogenous administration of chronic Kiss1 was shown to reinstate puberty in underfed female rats supports the idea that KNDy cells are the neuroendocrine center mediating suppression (59). A similar effect was observed in response to repeated administration of the NKB agonist senktide, which reinstated puberty in female rats with pubertal arrest due to chronic undernutrition (60). The reduction in Tac2 (observed in intact feed restricted females) supports the idea that reduced NKB signaling may underlie slowing of the pulse generator, as supported by evidence in chronically underfed OVX mice (30). However, we cannot exclude the possibility that changes in KNDy gene expression represent a downstream inhibitory effect of other neural pathways in response to chronic undernutrition. Similarly, it is logical to speculate that as the duration of feed restriction continues, hypothalamic, pituitary, and ovarian cell function downstream of ARCKiss1 signaling are impaired because of the diminishment in the trophic functions of upstream secretagogues, that is, Kiss1. Indeed, the reduction in Lhb, Gnrhr, and aGSU mRNA levels and ovarian genes in intact underfed animals collected after 2 weeks of treatment support this idea. Using the OVX + LowE paradigm, we conclude that the suppression of pulsatile LH secretion observed following 3 days of feed restriction was not due to downstream GnRH and gonadotrope cell dysfunction; yet, future studies would be required to determine a direct effect on either cell type, or the ovary itself, as the duration of feed restriction is prolonged.
Our data support 2 mechanisms that could underly suppression of the LH surge. First, our findings in intact animals suggest undernutrition could reduce estradiol production, which is required to initiate the LH surge. Indeed, the disrupted pattern of vaginal cytology (ie, no cornification) and diminished uterine weight both support the premise that estradiol concentrations were insufficient to induce the LH surge and ovulation, implicating ARCKiss1 control of pulsatile LH section as a mechanism underlying impairment of the LH surge. Second, our data also support the premise that undernutrition directly inhibits the central mechanisms responsible for LH surge secretion. Using an OVX + HighE model to directly test the positive feedback response to estradiol, we found that feed restriction prevented the LH surge and reduced Kiss1 within AVPVKiss1 cells at the time of the expected LH surge. The effect of undernutrition to prevent the estradiol-induced LH surge confirms a previous report in rhesus macaques (31), expanding our understanding by implicating AVPVKiss1 cells in the mechanism of surge suppression. Future studies investigating the impaired positive feedback response are required to understand the broad impact of this mechanism as species differences exist in generation of the GnRH/LH surge. For example, generation of the LH surge in female rodents requires circadian-driven neural inputs to AVPVKiss1 cells (61), which are not necessary in nonhuman primates, raising a potential species-specific mechanism underlying LH surge suppression. Although many questions remain, these data clearly demonstrate that either ARCKiss1 or AVPVKiss1 neuronal centers are potential sites of LH surge suppression during undernutrition.
This study raises multiple interesting questions, the most compelling we imagine is how Kiss1 neurons in either the ARC or AVPV become inhibited during undernutrition. One candidate cell type is the agouti-related peptide (AgRP)-containing cell population in the ARC that coexpresses the inhibitory neuropeptides AgRP, neuropeptide Y, and γ-aminobutyric acid (62-64). This population is activated in fasted mice or in mutant anorexia (anx/anx) mice (62, 63) and were recently shown to directly inhibit Kiss1 neurons in either the ARC or AVPV when activated using chemogenetics (65). In addition to AgRP, a host of metabolic factors (eg, leptin, insulin, proopiomelanocortin, α-melanocyte-stimulating hormone, and ghrelin) have been proposed to contribute to reproductive suppression during negative energy balance (3, 66). Clearly, future studies are required to identify the neural pathway(s) upstream of Kiss1 cells in the ARC, as well as AVPV, that mediate responsiveness to undernutrition and result in anovulation in the female.
Another intriguing question is regarding the effect of feed restriction on sex behavior and receptivity, which require neural and hormonal inputs (67, 68). Regarding hormonal requirements, estradiol production is a necessary factor for sexual behavior in the rodent (69), which we speculate is deficient in feed-restricted females. In addition, a recent study has demonstrated that AVPVKiss1 neurons are an essential part of the neural circuit conveying motivation and receptivity (70). Although we speculate that undernutrition would greatly diminish sex behavior and fertility because of impairment of both hormonal and neural circuits dependent on Kiss1 signaling, our finding that the number of developing follicles in feed-restricted mice is maintained supports the idea that reproductive function could resume following refeeding (71).
Finally, a remaining question is regarding the role of psychosocial stress vs metabolic stress in the present paradigm. Studies in nonhuman primates indicate that nutritional/metabolic signals, rather than distress caused by missing a meal, is responsible for suppression of LH pulses (72, 73). It is worth mentioning that these studies show that LH secretion is often stimulated very rapidly (ie, within 20-40 minutes) after either a refeed meal, or via intragastric infusion of nutrients, suggesting that a signal arising from the gastrointestinal tract may play a role in LH suppression during feed restriction (73). One difference between these studies and our present study is the type (fasted vs underfed) and duration (24 hours vs 3 days) of undernutrition. Certainly, the experimental paradigm and animal model used in the present study will provide an excellent opportunity for elucidating the cellular sites and signals by which nutrition supports neuroendocrine function.
In summary, we demonstrated that feed restriction rapidly and robustly diminishes hypothalamic, pituitary, and ovarian function in female mice. A similar relationship between negative energy balance and clinical or pathological menstrual cycle disorders is observed in female athletes, during lactation, and in individuals with anorexia nervosa (74–77). Both ARCKiss1 and AVPVKiss1 populations are impaired during undernutrition and mediate diminished pulsatile LH secretion and impairment of the LH surge. This mouse model expresses the hallmarks observed in a nonhuman primate model of hypothalamic amenorrhea (31, 71) and may be useful for identifying the neural pathways underlying ovarian and menstrual cycle disorders associated with undernutrition.
Acknowledgments
The authors wish to thank Dr Carol Elias (University of Michigan) for her generous gift of the Kiss1hrGFP mouse line. We also thank Drs Dan Bernard (McGill University), Luisina Ongaro (McGill University), and Rona Carroll (Brigham and Women’s Hospital) for assistance with setting up the LH assay within our laboratory. LH assay reagents were graciously provided by Dr Janet Roser (University of California) and Dr Al Parlow and the National Hormone and Peptide Program (NHPP), and we acknowledge Quidel CORP (San Diego, California, USA) for initial assay development. We wish to thank Dr Karen Tonsfeldt for guidance with ovarian processing and appreciate the Nikon Imaging Center at UC San Diego for access to microscopes and technical assistance with imaging.
Financial Support: This work was supported by the National Institutes of Health (NIH grant Nos. R01 HD086100 and P50 HD012303 Pilot Funds to K.M.B.) and the UCSD Academic Senate. R.B.M. was supported by NIH grant F32 HD096811 and T32 HD007203. K.S.T. was partially supported by UCSD Undergraduate Research Scholarships including the Ledell Family Research Scholarship and Doris A. Howell Research Scholarship.
Glossary
Abbreviations
- AgRP
agouti-related peptide
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- AVPV/PeN
anteroventral periventricular nucleus and neighboring periventricular nucleus
- cDNA
complementary DNA
- CV
coefficient of variation
- FSH
follicle-stimulating hormone
- GFP
green fluorescent protein
- GnRH
gonadotropin-releasing hormone
- Kiss1
kisspeptin
- Kp10
exogenous Kiss1
- LH
luteinizing hormone
- mRNA
messenger RNA
- NKB
neurokinin B
- OVX
ovariectomized
- PB
phosphate buffer
- PBS
phosphate-buffered saline.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Knorr D, Khoo CH. COVID-19 and food: challenges and research needs. Front Nutr. 2020;7:598913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun J, Shen X, Liu H, Lu S, Peng J, Kuang H. Caloric restriction in female reproduction: is it beneficial or detrimental? Reprod Biol Endocrinol. 2021;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iwasa T, Matsuzaki T, Yano K, et al. Effects of low energy availability on reproductive functions and their underlying neuroendocrine mechanisms. J Clin Med. 2018;7(7):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biol Reprod. 1985;33(3):660-667. [DOI] [PubMed] [Google Scholar]
- 5. Foster DL, Ebling FJ, Micka AF, et al. Metabolic interfaces between growth and reproduction. I. Nutritional modulation of gonadotropin, prolactin, and growth hormone secretion in the growth-limited female lamb. Endocrinology. 1989;125(1):342-350. [DOI] [PubMed] [Google Scholar]
- 6. Tropp J, Markus EJ. Effects of mild food deprivation on the estrous cycle of rats. Physiol Behav. 2001;73(4):553-559. [DOI] [PubMed] [Google Scholar]
- 7. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297-311. [DOI] [PubMed] [Google Scholar]
- 8. Hill JR Jr, Lamond DR, Henricks DM, Dickey JF, Niswender GD. The effects of undernutrition on ovarian function and fertility in beef heifers. Biol Reprod. 1970;2(1):78-84. [DOI] [PubMed] [Google Scholar]
- 9. Hayward A, Gillooly JF. The cost of sex: quantifying energetic investment in gamete production by males and females. PLoS One. 2011;6(1):e16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill JW, Levine JE. Abnormal response of the neuropeptide Y-deficient mouse reproductive axis to food deprivation but not lactation. Endocrinology. 2003;144(5):1780-1786. [DOI] [PubMed] [Google Scholar]
- 11. McCosh RB, Kreisman MJ, Tian K, Ho BS, Thackray VG, Breen KM. Insulin-induced hypoglycaemia suppresses pulsatile luteinising hormone secretion and arcuate Kiss1 cell activation in female mice. J Neuroendocrinol. 2019;31(12):e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology. 2003;144(10):4325-4331. [DOI] [PubMed] [Google Scholar]
- 13. Koltun KJ, De Souza MJ, Scheid JL, Williams NI. Energy availability is associated with luteinizing hormone pulse frequency and induction of luteal phase defects. J Clin Endocrinol Metab. 2020;105(1):185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon CM, Ackerman KE, Berga SL, et al. Functional hypothalamic amenorrhea: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(5):1413-1439. [DOI] [PubMed] [Google Scholar]
- 15. Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea—an update. J Clin Endocrinol Metab. 2015;100(3):812-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Temple JL, Rissman EF. Acute re-feeding reverses food restriction-induced hypothalamic-pituitary-gonadal axis deficits. Biol Reprod. 2000;63(6):1721-1726. [DOI] [PubMed] [Google Scholar]
- 17. Mulinos MG, Pomerantz L. Pituitary replacement therapy in pseudo-hypophysectomy. Effect of pituitary implants upon organ weights of starved or underfed rats. Endocrinology. 1941;29(4):558-563. [Google Scholar]
- 18. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073-4077. [DOI] [PubMed] [Google Scholar]
- 20. Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324(1-2):51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy cells revisited. Endocrinology. 2018;159(9):3219-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723-3736. [DOI] [PubMed] [Google Scholar]
- 24. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752-5760. [DOI] [PubMed] [Google Scholar]
- 25. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774-1783. [DOI] [PubMed] [Google Scholar]
- 26. Spillar PA, Piacsek BE. Underfeeding alters the effect of low levels of estradiol on luteinizing hormone pulsatility in ovariectomized female rats. Neuroendocrinology. 1991;53(3):253-260. [DOI] [PubMed] [Google Scholar]
- 27. Thomas GB, Mercer JE, Karalis T, Rao A, Cummins JT, Clarke IJ. Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology. 1990;126(3):1361-1367. [DOI] [PubMed] [Google Scholar]
- 28. Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Clarke IJ. Intracerebroventricular infusion of leptin elevates the secretion of luteinising hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of bodyweight. J Endocrinol. 2001;168(1):67-77. [DOI] [PubMed] [Google Scholar]
- 29. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151(5):2233-2243. [DOI] [PubMed] [Google Scholar]
- 30. Yang JA, Yasrebi A, Snyder M, Roepke TA. The interaction of fasting, caloric restriction, and diet-induced obesity with 17β-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol Cell Endocrinol. 2016;437:35-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lujan ME, Krzemien AA, Reid RL, Van Vugt DA. Caloric restriction inhibits steroid-induced gonadotropin surges in ovariectomized rhesus monkeys. Endocrine. 2005;27(1):25-31. [DOI] [PubMed] [Google Scholar]
- 32. Shufelt CL, Torbati T, Dutra E. Hypothalamic amenorrhea and the long-term health consequences. Semin Reprod Med. 2017;35(3):256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cravo RM, Frazao R, Perello M, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8(3):e58698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCosh RB, Kreisman MJ, Breen KM. Frequent tail-tip blood sampling in mice for the assessment of pulsatile luteinizing hormone secretion. J Vis Exp. 2018;(137):57894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang JA, Song CI, Hughes JK, et al. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology. 2017;158(11):3716-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzales C, Voirol MJ, Giacomini M, Gaillard RC, Pedrazzini T, Pralong FP. The neuropeptide Y Y1 receptor mediates NPY-induced inhibition of the gonadotrope axis under poor metabolic conditions. FASEB J. 2004;18(1):137-139. [DOI] [PubMed] [Google Scholar]
- 37. Pan F, Zhang L, Li M, et al. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome. 2018;6(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. 2nd ed. Lippincott; 1995. [Google Scholar]
- 39. Kreisman MJ, McCosh RB, Tian K, Song CI, Breen KM. Estradiol enables chronic corticosterone to inhibit pulsatile luteinizing hormone secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2020;110(6):501-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shim WS, Conaway M, Masamura S, et al. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology. 2000;141(1):396-405. [DOI] [PubMed] [Google Scholar]
- 41. Makowski KN, Kreisman MJ, McCosh RB, Raad AA, Breen KM. Peripheral interleukin-1β inhibits arcuate Kiss1 cells and LH pulses in female mice. J Endocrinol. 2020;246(2):149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286-1290. [DOI] [PubMed] [Google Scholar]
- 43. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104(5):1247-1255. [DOI] [PubMed] [Google Scholar]
- 44. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stephens SBZ, Tolson KP, Rouse ML Jr, et al. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology. 2015;156(9):3091-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tonsfeldt KJ, Cui LJ, Mellon PL. The contribution of Bmal1 expression in the suprachiasmatic nucleus to female fertility. J Endocr Soc. 2019;3(Suppl 1):SUN-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4(3):157-165. [DOI] [PubMed] [Google Scholar]
- 49. Franklin KBJ, Paxinos G.. The Mouse Brain in Stereotaxic Coordinates. 4th ed. Academic Press, an imprint of Elsevier; 2013. [Google Scholar]
- 50. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 51. Harlow E, Lane D. Mounting samples in Gelvatol or Mowiol. CSH Protoc. 2006;2006(1):pdb.prot4461. [DOI] [PubMed] [Google Scholar]
- 52. Kauffman AS, Thackray VG, Ryan GE, et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod. 2015;93(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004;101(49):17294-17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Menon KMJ, Clouser CL, Nair AK. Gonadotropin receptors: role of post-translational modifications and post-transcriptional regulation. Endocrine. 2005;26(3):249-257. [DOI] [PubMed] [Google Scholar]
- 56. Coss D, Jacobs SBR, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279(1):152-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794-4802. [DOI] [PubMed] [Google Scholar]
- 58. Merkley CM, Shuping SL, Nestor CC. Neuronal networks that regulate gonadotropin-releasing hormone/luteinizing hormone secretion during undernutrition: evidence from sheep. Domest Anim Endocrinol. 2020;73:106469. [DOI] [PubMed] [Google Scholar]
- 59. Castellano JM, Navarro VM, Fernández-Fernández R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146(9):3917-3925. [DOI] [PubMed] [Google Scholar]
- 60. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32(7):2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95(25):15043-15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271-272. [DOI] [PubMed] [Google Scholar]
- 64. Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756(1-2):283-286. [DOI] [PubMed] [Google Scholar]
- 65. Padilla SL, Qiu J, Nestor CC, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A. 2017;114(9):2413-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Castellano JM, Tena-Sempere M. Metabolic control of female puberty: potential therapeutic targets. Expert Opin Ther Targets. 2016;20(10):1181-1193. [DOI] [PubMed] [Google Scholar]
- 67. Micevych PE, Meisel RL. Integrating neural circuits controlling female sexual behavior. Front Syst Neurosci. 2017;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jennings KJ, de Lecea L. Neural and hormonal control of sexual behavior. Endocrinology. 2020;161(10):bqaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boling JL, Blandau RJ. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology. 1939;25(3):359-364. [Google Scholar]
- 70. Hellier V, Brock O, Candlish M, et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun. 2018;9(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lujan ME, Krzemien AA, Reid RL, Van Vugt DA. Developing a model of nutritional amenorrhea in rhesus monkeys. Endocrinology. 2006;147(1):483-492. [DOI] [PubMed] [Google Scholar]
- 72. Schreihofer DA, Parfitt DB, Cameron JL. Suppression of luteinizing hormone secretion during short-term fasting in male rhesus monkeys: the role of metabolic versus stress signals. Endocrinology. 1993;132(5):1881-1889. [DOI] [PubMed] [Google Scholar]
- 73. Schreihofer DA, Amico JA, Cameron JL. Reversal of fasting-induced suppression of luteinizing hormone (LH) secretion in male rhesus monkeys by intragastric nutrient infusion: evidence for rapid stimulation of LH by nutritional signals. Endocrinology. 1993;132(5):1890-1897. [DOI] [PubMed] [Google Scholar]
- 74. Loucks AB, Mortola JF, Girton L, Yen SS. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402-411. [DOI] [PubMed] [Google Scholar]
- 75. Gold PW, Gwirtsman H, Avgerinos PC, et al. Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med. 1986;314(21):1335-1342. [DOI] [PubMed] [Google Scholar]
- 76. Allaway HC, Southmayd EA, De Souza MJ. The physiology of functional hypothalamic amenorrhea associated with energy deficiency in exercising women and in women with anorexia nervosa. Horm Mol Biol Clin Investig. 2016;25(2):91-119. [DOI] [PubMed] [Google Scholar]
- 77. Brown RSE, Herbison AE, Grattan DR. Prolactin regulation of kisspeptin neurones in the mouse brain and its role in the lactation-induced suppression of kisspeptin expression. J Neuroendocrinol. 2014;26(12):898-908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.