The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) defines right heart failure (RHF) as signs and symptoms of persistent right ventricular (RV) dysfunction after LVAD implantation. This is further stratified by duration of inotrope requirement as mild (≤ 7 days), moderate (8–14 days), severe (>14 days), or severe acute (Right Ventricular Assist Device (RVAD) implantation).1 Inotrope use for at least fourteen days or RVAD implant has been frequently used to define RHF after LVAD implant in published studies.2–4 Severe RV failure has been associated with a threefold increase in the risk of death at 6 months and is associated with significantly longer hospitalizations.3,4 While RV failure after LVAD is known to portend worse clinical outcomes, the degree to which this relates to post-implantation inotropic support duration is unknown. Further, this stratification approach may be confounded by heterogeneity in inotrope weaning protocols amongst different institutions. With this in mind, we sought to evaluate clinical outcomes associated with the INTERMACS RHF stratification system.
Patients at least 18 years old who underwent implantation of a HeartMate II® (Thoratec Corp., Pleasanton, CA) or HVAD® (HeartWare Corp., Framingham, MA) at our center between 1/2005 and 9/2014 were identified. Cohorts were defined based on post-VAD implant inotrope requirement of ≤7, 8–14, 15–21, or >21 days, or implantation of an RVAD. Although our institution does not utilize a strictly defined post-implant inotrope weaning protocol, the inotropic agent (milrinone is generally favored, though dobutamine is also used) is weaned to maintain an ScVO2 >60% while the pulmonary artery catheter remains in place, and thereafter weaning is clinically driven. All clinically relevant data was extracted by review of the medical record with last available hemodynamic, clinical, and laboratory data recorded prior to the CF-LVAD implant date. The primary outcome for analysis was time to death from any cause. A secondary analysis evaluated time to the combined end-point of all-cause death, HF, or gastrointestinal (GI) bleeding. Time until death and time until the combined end-point were evaluated through Kaplan Meier (KM) analysis and curves were compared with the log-rank test. Patient characteristics were compared between less than severe right heart failure and severe right heart failure using Student’s two-sample t-test for continuous variables and Fisher’s exact test or Pearson’s chi-square test for categorical. Non-normal and ordinal data were shown as median (1st quartile, 3rd quartile) and compared using the Mann-Whitney U-test. All data were collected and managed in REDCap®, an electronic data capture tool hosted by our institution. The study was approved by the Institutional Review Board at Washington University School of Medicine. A two-sided P-value of < 0.05 was considered significant. All data analysis was conducted in SAS v9.4 (SAS Institute Inc., Cary, NC).
The majority of patients were male (81%) with a median INTERMACS profile of 2 at the time of LVAD implant (Table 1). Of the 445 patients analyzed, 376 patients received a HeartMate II ® and the remaining 69 received an HVAD®. Bridge to transplant (BTT) strategy accounted for 281 implants, destination therapy (DT) 153 implants, and 11 bridge to decision implants.
Table 1:
Patient Data
| Overall (n=445) | Less than Severe RV failure (n=306) | Severe RV failure (n=139) | p-value | |
|---|---|---|---|---|
| Age (years) | 56.7 ± 11.9 | 56.3 ± 11.9 | 57.6 ± 11.8 | 0.29 |
| Male, No. (%) | 359 (81%) | 243 (79.4%) | 116 (83.5%) | 0.37 |
| Type of LVAD | ||||
| HeartMate II | 376 (84.5%) | 264 (86.3%) | 112 (80.6%) | 0.15 |
| HVAD | 69 (15.5%) | 42 (13.7%) | 27 (19.4%) | |
| INTERMACs profile | 2 (1, 2) | 2 (1, 2) | 2 (1, 2) | 0.14 |
| Bridge to Transplant | 281 (63%) | 195 (63.7%) | 86 (61.9%) | 0.75 |
| BMI | 29.2 (±) 6.1 | 28.8 ± 6 | 30.1 ± 6.2 | 0.04 |
| Duration of f/u (months) | 10.7 (4.2, 21.9) | 13.2 (6, 25.5) | 5.1 (1.3, 14.7) | <0.001 |
| Prior sternotomy | 129 (29%) | 77 (25.2%) | 52 (37.4%) | 0.01 |
| CrCl | 53.9 (39, 79.7) | 58.9 (40.4, 85.7) | 49.6 (35.6, 67.6) | <0.001 |
| AST | 39 (27, 66.8) | 36.5 (26, 59) | 44.5 (31.8, 100.3) | <0.001 |
| Albumin | 3.7 (±) 2.8 | 3.8 (±) 3.4 | 3.51 (±) 0.53 | 0.3 |
| INR | 1.47 (±) 0.38 | 1.44 (±) 0.33 | 1.55 (±) 0.46 | 0.05 |
| RA pressure (baseline) | 15 (10, 20) | 14 (9, 19) | 17 (13, 21) | <0.001 |
| RA pressure (72 hours after implant) | 12 (9, 15) | 11 (9, 15) | 13 (10, 16) | 0.04 |
| Pre-VAD support | ||||
| Inotropes | 389 (87.4%) | 269 (87.9%) | 120 (86.3%) | 0.65 |
| IABP | 110 (25%) | 67 (21.9%) | 43 (30.9%) | 0.04 |
| Impella | 20 (4%) | 11 (3.6%) | 9 (6.5%) | 0.22 |
| Days on inotrope post-VAD | 8 (6, 15) | 7 (5, 9) | 19 (16, 27) | <0.001 |
| Pump speed on discharge (rpm) (HM II only) | 9356 (±) 327 | 9346 (±) 320 | 9390 (±) 350 | 0.35 |
Values are shown as absolute numbers (percentages), mean ± SD, or median (Q1, Q3). BMI = body mass index; CrCl = creatinine clearance; AST = aspartate aminotransferase; INR = international normalized ratio; RA = right atrial; IABP = intraortic balloon pump
Patients were supported with inotropes for a median of 8 days (IQR 6, 15) after implantation. Milrinone was used preferentially early post LVAD implantation (65%), while dobutamine predominated in those requiring 21 days or more (68%) (Supplementary Table S1). A total of 270 patients (60.7%) required inotropes for >7 days (8–14 days n=131, 15–21 days n=66, >21 days n=38) and 35 required RVAD implantation, while only 175 (39.3%) patients were weaned off inotropes in >7 days. 10 of the 35 RVADs were planned at the time of LVAD implantation to manage known RV dysfunction.
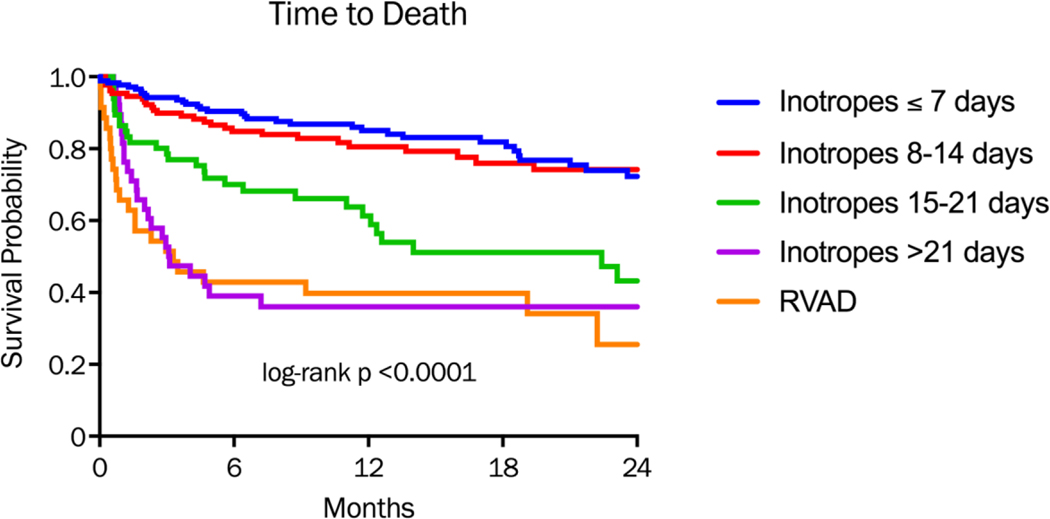
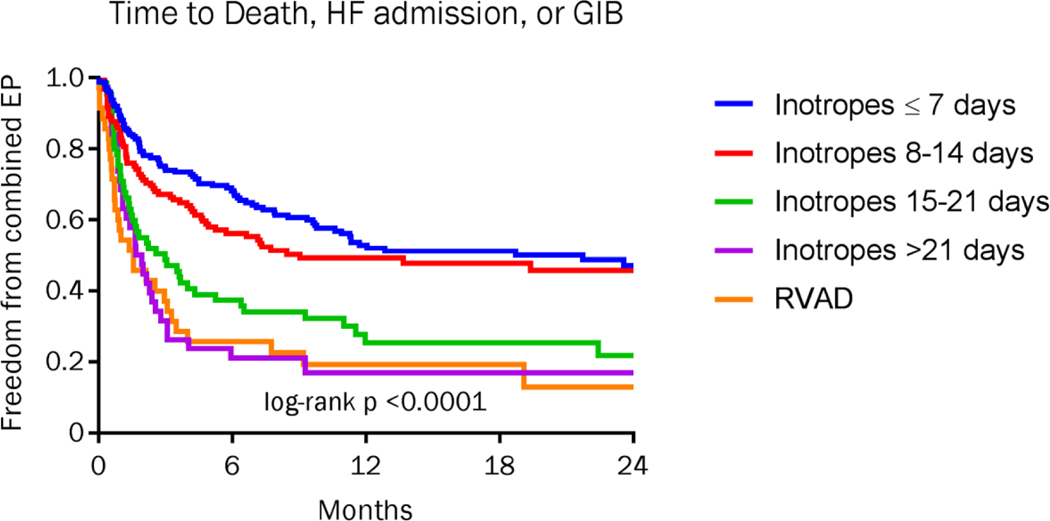
Time to death was similar in those supported >7 days and 8–14 days, but was statistically higher in the groups supported >14 days or RVAD cohort (p<0.0001)(Figure 1a). Two-year mortality was 22.3% in the group on inotropes for >7 days and 26.7% in those supported 8–14 days, compared with 47% (15–21 days), 63% (> 21 days) and 68.6% in the RVAD cohort. The combined endpoint of time to death, heart failure readmission, or GIB were also similar in those supported ≤7 days and 8–14 days, and again were statistically higher in the groups supported >14 days and the RVAD cohort (Figure 1b).
Figure 1a:
Kaplan-Meier analysis of freedom from death divided by duration of inotropic support or RVAD requirement after LVAD implantation. P-value for comparison across all groups.
Figure 1b:
Kaplan-Meier analysis of freedom from death, HF rehospitalization, or gastrointestinal bleed divided by duration of inotropic support or RVAD requirement after LVAD implantation. P-value for comparison across all groups.
Comparison of baseline characteristics between the two groups defined by less than severe (n=306) and severe or greater (n=139) RV failure demonstrates similar age, gender, LVAD type, implant strategy, and INTERMACs profile between groups (Table 1). Median right atrial pressure at baseline (17 v 14, P<0.001) and 72 hours post implant (13 v 11, p=0.04) (Supplementary Table S2) were higher in the severe RV failure group, likely representing greater RV dysfunction. The severe RV failure group had a statistically significantly higher BMI (30.1 v 28.8, p=0.04), lower CrCl (49.6 v 58.9, p<0.001), and a greater proportion of patients with a prior sternotomy (37% v 25%, p=0.01).
In this single-center, retrospective analysis, INTERMACS defined Severe or Severe Acute RHF (inotrope use >14 days or RVAD implantation) was associated with significantly higher mortality (2.6 fold at two years post-implant) than INTERMACS defined mild, moderate, or no RV failure. Further, there was no significant difference in mortality between those requiring inotropic therapy for ≤7 days and 8–14 days. Our data suggest a paradigm of mild/no RV failure (inotropes ≤ 14 days), moderate RV failure (inotropes 15–21 days), and severe RV failure (inotropes > 21 days or RVAD) would be more clinically relevant. Considerable effort is currently being undertaken to successfully predict, avoid, and manage RV failure after LVAD implantation5. This effort has been substantiated by the critical importance RV failure holds in determining the post-LVAD course. 6 However, efforts to do so should be grounded in a consistent and clinically meaningful definition of RV failure, with a particular desire not to include lesser degrees of RV failure in risk prediction models if those lesser degrees of RV failure do not ultimately lead to morbid or mortal outcomes.
Though additional study is warranted to further investigate the outcomes associated with various definitions of RV failure, our study suggests that inotrope use greater than 14 days is most clinically meaningful and lesser durations of inotropic support should not be categorized as RV failure for the purpose of risk prediction and the ultimate impact of RV failure upon longer term clinical outcomes.
Supplementary Material
Acknowledgments
Funding Sources
This study was supported in part by research funds from the National Institutes of Health (NIH grant U10 HL110309, Heart Failure Network), Grant # UL1 TR000448, and the Barnes Jewish Hospital Foundation.
Footnotes
Disclosures
Shane J. LaRue – None
Michael E. Nassif – None
David S. Raymer – None
Brian R. Pierce – None
Christopher T. Sparrow - None
Justin M. Vader – None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Appendix_A_INTERMACS_AE_Definitions__05152013.
- 2.Slaughter MS, Rogers JG, Milano C a, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N. Engl. J. Med 2009;361(23):2241–51. [DOI] [PubMed] [Google Scholar]
- 3.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg 2016;139(5):1316–1324. [DOI] [PubMed] [Google Scholar]
- 4.Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am. J. Cardiol 2010;105(7):1030–5. [DOI] [PubMed] [Google Scholar]
- 5.Kalogeropoulos AP, Al-Anbari R, Pekarek A, et al. The Right Ventricular Function After Left Ventricular Assist Device (RVF-LVAD) study: rationale and preliminary results. Eur. Heart J. Cardiovasc. Imaging 2016;17(4):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: a 10,000-patientdatabase. J. Heart Lung Transplant 2014;33(6):555–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.