Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) are increasingly used in ambulatory breast surgery. The risk of hematoma associated with intraoperative ketorolac is low, but whether concomitant routine discharge with NSAIDs increases the risk of hematoma is unclear.
Methods
We retrospectively identified patients who underwent lumpectomy and sentinel lymph node biopsy (SLNB), and compared the 30-day risk of hematoma between patients discharged with opioids (opioid period: January 2018–August 2018) and patients discharged with NSAIDs with or without opioids (NSAID period: January 2019–April 2020). The association between study period and hematoma risk was assessed using multivariable models. Covariates included intraoperative ketorolac, home aspirin, and race/ethnicity. During the NSAID period, a survey was used to assess analgesic consumption on postoperative days 1–5.
Results
In total, 2724 patients were identified: 858 (31%) in the opioid period and 1866 (69%) in the NSAID period. In the NSAID period, 867 (46%) received NSAIDs and opioids, and 999 (54%) received NSAIDs only. Receipt of intraoperative ketorolac was higher in the NSAID period (78 vs. 64%, P < 0.001). The risks of any hematoma (4.1 vs. 3.6%, P = 0.6) and reoperation for bleeding (0.5 vs. 0.6%, P = 0.8) were similar between groups. Study period was not associated with hematoma risk (odds ratio 0.87, 95% confidence interval 0.56–1.35, P = 0.5). Among survey respondents (41%), nonopioid analgesic consumption did not increase after opioids were removed from the discharge regimen (median, 6 pills/group, P = 0.06).
Conclusions
NSAIDs are associated with a low risk of hematoma after lumpectomy and SLNB, and should be prescribed instead of opioids, unless contraindicated.
As the reduction of opioid use remains a public health priority, nonsteroidal anti-inflammatory drugs (NSAIDs) are increasingly used for perioperative and postdischarge pain control in patients undergoing ambulatory breast surgery. Data suggest that multimodal analgesia—including intravenous and oral NSAIDs, both key components of enhanced recovery after surgery protocols—is associated with reduced perioperative opioid requirement without compromising pain control or patient satisfaction.1–5 NSAIDs have been increasingly used in the postdischarge setting, as efforts to reduce postoperative opioid prescriptions have become more prominent. Recent recommendations from the American Society of Breast Surgeons reflect this paradigm shift, endorsing the use of postdischarge NSAIDs in lieu of routine opioids for pain control after breast surgery.6
As opioid-sparing pain regimens are increasingly adopted, the risk of bleeding complications associated with NSAIDs has gained new relevance. A retrospective cohort study of patients undergoing lumpectomy from the American College of Surgeons National Surgical Quality Improvement Program demonstrated a 0.4% rate of reoperation for bleeding complications in the 30-day postoperative period (28/7167); however, perioperative NSAID exposure could not be assessed from this administrative database.7 Results from two meta-analyses suggest that perioperative NSAID use was not a statistically significant risk factor for bleeding after a wide range of surgical procedures.8,9 Although limited evidence suggests that intraoperative ketorolac is safe in patients undergoing lumpectomy, the safety of ketorolac has been more rigorously studied in patients undergoing mastectomy and reconstruction, with variable results reported for this population.10–14 It remains unclear whether increased use of NSAIDs in both the perioperative and the postdischarge settings concurrently, is associated with a higher risk of hematoma in patients undergoing lumpectomy and sentinel lymph node biopsy (SLNB).
In the present study, we compared the risk of hematoma between patients who received standard discharge prescriptions for opioids without NSAIDs (opioid period) and patients who received prescriptions for NSAIDs with or without opioids (NSAID period) after lumpectomy and SLNB. All patients were treated with a standard multimodal analgesia protocol that included intraoperative ketorolac.
Methods
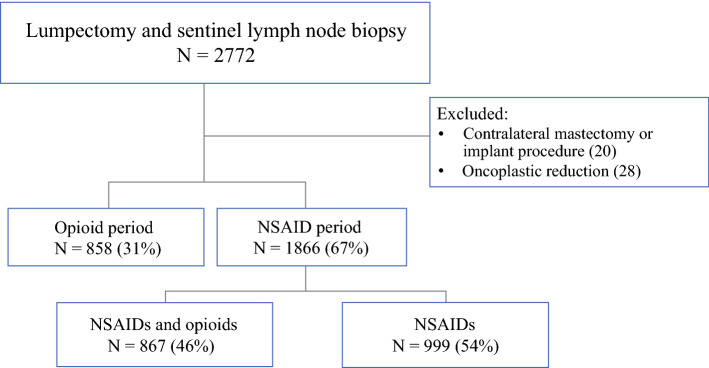
Following approval from the Memorial Sloan Kettering Cancer Center institutional review board, we retrospectively identified consecutive patients who had undergone lumpectomy and SLNB with multimodal analgesia between January 1, 2018 and April 28, 2020. Patients were excluded if they underwent a concurrent contralateral mastectomy, oncoplastic reduction, or implant procedure (Fig. 1).
Fig. 1.
Patients who had lumpectomy and sentinel lymph node biopsy with multimodal analgesia. NSAID nonsteroidal anti-inflammatory drug
All ambulatory breast surgery patients at Memorial Sloan Kettering Cancer Center are treated on a multimodal enhanced recovery after surgery protocol, which includes intraoperative ketorolac, acetaminophen, limited opioids, and injection of local anesthetics at the surgical site. Intravenous acetaminophen 1000 mg is administered at the start of the procedure. At the conclusion of the procedure, 30 mg of ketorolac is recommended (15 mg in patients aged >60 years or with a glomerular filtration rate between 30 and 60 mL/min/1.73 m2), except when there is a documented allergy to NSAIDs or hemostatic concerns are present.
The standard discharge analgesic regimen after lumpectomy and SLNB was changed during the study period in the following manner. From January 2018 to August 2018 (opioid period), 10 tablets of hydrocodone (5 mg) and acetaminophen (325 mg) (Norco) were routinely prescribed. Beginning in September 2018, diclofenac (75 mg) was added to the discharge regimen, unless contraindicated, and was included through the end of the study period in April 2020 (NSAID period). From September 2018 to July 2019, during the NSAID period, diclofenac and 10 opioid tablets were prescribed, and patients were instructed to take diclofenac unless severe pain necessitating the use of an opioid was present. In August 2019, opioid prescriptions were removed from the standard discharge regimen, and patients were routinely prescribed diclofenac only. All discharge regimens included acetaminophen.
Standard data on clinicopathologic characteristics and perioperative administration of medication were collected from an institutional database. Aspirin was continued perioperatively if a cardiovascular indication was present. Anticoagulants and other antiplatelet drugs were held preoperatively for several days, in accordance with guidelines, and were resumed at the discretion of the treating surgeon. Patients who took anticoagulants or antiplatelets were included in the analysis.
The primary outcome was the occurrence of postoperative bleeding or hematoma from postoperative Day (POD) 0 to 30, by study period. Hematomas were classified according to intervention (i.e., reoperation for evacuation, in-office drainage or aspiration, or observation). Hematomas that were aspirated in the outpatient setting were distinguished from seromas on the basis of clinical evaluation of the aspirate. Small, conservatively managed hematomas were documented as a “hematoma” in the postoperative note or in the description of a photo uploaded through an electronic patient portal.
The secondary outcome was patient-reported consumption of nonopioid analgesics, which was collected on PODs 1–5 from a daily electronic survey sent to all patients undergoing ambulatory breast surgery at our institution. On POD 5, patients were asked to report the number of NSAIDs and/or acetominophen pills taken since discharge.
Statistical Analysis
Clinicopathologic and treatment characteristics were compared between groups using Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for continuous variables. We tested for an association between study period and risk of hematoma using multivariable logistic regression adjusted for home aspirin use, intraoperative ketorolac, and variables significant on univariable analyses. The type I error rate (α) was set to 0.05. All statistical analyses were conducted using R software (version 3.6.1, R Core Development Team, Vienna, Austria).
Results
Patient and Perioperative Treatment Characteristics
From January 1, 2018 to April 28, 2020, a total of 2724 patients underwent lumpectomy and SLNB, including 858 patients (31%) in the opioid period and 1866 patients (69%) during the NSAID period. During the NSAID period, 867 patients (46%) received discharge prescriptions for NSAIDs and opioids, and 999 (54%) received prescriptions for NSAIDS only (Fig. 1). Patient and perioperative treatment characteristics are listed in Table 1.
Table 1.
Patient and perioperative treatment characteristics by study period
| Variable | Opioid period (n = 858) | NSAID period (n = 1866) | P |
|---|---|---|---|
| Age at surgery, yr | 59 (50–67) | 59 (51–67) | 0.9 |
| BMI, kg/m2 | 27.1 (23.4–31.9) | 27.3 (23.8–32.1) | 0.2 |
| Race/ethnicity | 0.03 | ||
| White | 616 (72) | 1182 (63) | |
| Black/African American | 59 (6.9) | 168 (9.0) | |
| Hispanic/Latino | 64 (7.5) | 142 (7.6) | |
| Asian/Pacific Islander | 73 (8.5) | 180 (9.6) | |
| Unknown | 46 | 194 | |
| ASA class | 0.4 | ||
| 1 | 10 (1.2) | 26 (1.4) | |
| 2 | 558 (65) | 1148 (62) | |
| 3 | 289 (34) | 690 (37) | |
| 4 | 1 (0.1) | 2 (0.1) | |
| Home medications | |||
| Aspirin | 108 (13) | 206 (11) | 0.2 |
| Anticoagulant or other antiplatelet | 22 (2.6) | 56 (3.0) | 0.6 |
| Intraoperative ketorolac | 549 (64) | 1463 (78) | < 0.001 |
| Intraoperative acetaminophen | 830 (97) | 1823 (98) | 0.2 |
| Intraoperative opioids, MME | 20 (10–20) | 20 (10–20) | 0.7 |
Data are expressed as medians with interquartile range in parentheses or numbers with percentages in parentheses unless otherwise indicated
ASA American Society of Anesthesiologists; BMI body mass index; MME morphine milligram equivalent; NSAID nonsteroidal anti-inflammatory drug
Overall, 2012 patients (74%) received intraoperative ketorolac; intraoperative ketorolac was more frequently administered in the NSAID period than in the opioid period (78 vs. 64%, P < 0.001). Patients who were non-white/non-Hispanic or Latino were more frequently represented in the NSAID period than in the opioid period (P = 0.03). There were no other statistically significant differences in patient or perioperative characteristics between study periods.
Thirty-Day Postoperative Risk of Hematoma
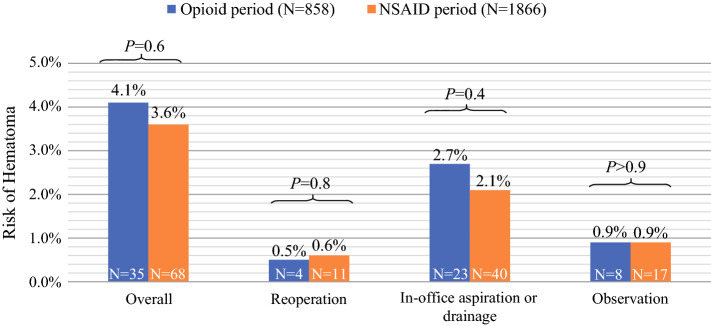
During the opioid period, the 30-day risk of any hematoma was 4.1% (n = 35); 4 patients (0.5%) required reoperation for hemostasis, 23 patients (2.7%) patients had undergone an in-office aspiration or drainage procedure, and 8 patients (0.9%) were conservatively managed. During the NSAID period, the 30-day risk of any hematoma was 3.6% (n = 68); 11 patients (0.6%) required reoperation, 40 patients (2.1%) had undergone an in-office procedure, and 17 patients (0.9%) were observed. There were no statistically significant differences in the risk of any hematoma, regardless of definitive management, between the study periods (Fig. 2).
Fig. 2.
Risk of hematoma by study period. NSAID, nonsteroidal anti-inflammatory drug
Among patients who received intraoperative ketorolac regardless of study period, the risk of reoperation for bleeding was 0.7% (14/1998) compared with 0.1% (1/712) among patients who did not receive intraoperative ketorolac (P = 0.14). Among patients in the opioid period who did not receive intraoperative ketorolac and who did not receive postdischarge NSAIDs, the 30-day risk of any hematoma was 4.5% (14/313) and no patients required reoperation for bleeding complications, compared with 3.7% (89/2411) and 0.6% (15/2411), respectfully, among all other patients. There were no statistically significant differences in the risk of any hematoma (4.5 vs. 3.7%, P = 0.5) or risk or reoperation (0 vs. 0.6%, P = 0.2) between groups.
On multivariable analysis, the overall risk of hematoma was not statistically significantly associated with the NSAID study period, after adjustment for receipt of intraoperative ketorolac, home aspirin use, and race/ethnicity (odds ratio 0.87, 95% confidence interval 0.56–1.35, P = 0.5; Table 2). Because a low number of patients took such medications, anticoagulation and other antiplatelet medications could not be included as a covariate.
Table 2.
Multivariable logistic regression to predict the risk of hematoma
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| NSAID study period | 0.87 | 0.56–1.35 | 0.5 |
| Intraoperative ketorolac | 0.91 | 0.58–1.47 | 0.7 |
| Home aspirin use | 1.22 | 0.64–2.15 | 0.5 |
| Race/ethnicity | |||
| White (ref) | – | – | – |
| Black/African American | 0.45 | 0.14–1.10 | 0.12 |
| Hispanic/Latino | 1.41 | 0.69–2.59 | 0.3 |
| Asian/Pacific Islander | 0.82 | 0.36–1.63 | 0.6 |
NSAID nonsteroidal anti-inflammatory drug
Patient-Reported Consumption of Nonopioid Analgesics During the NSAID Period
During the NSAID period, the overall postdischarge survey response rate was 41%, and was similar before and after elimination of opioids from the standard discharge regimen (39 vs. 42%, P = 0.3). The number of NSAIDs and/or acetaminophen tablets taken by POD 5 did not increase after routine opioid prescriptions were discontinued. The median (interquartile range) number of pills taken was 6 (3–9) among patients prescribed NSAIDs and opioids, and 6 (3–10) among patients prescribed NSAIDs only (P = 0.06).
Discussion
For patients undergoing lumpectomy and SLNB, the risk of hematoma was 3.6% and the risk of reoperation was 0.6% after implementation of a standard discharge regimen, including NSAIDs. Neither intraoperative ketorolac exposure nor treatment during the NSAID study period was statistically significantly associated with risk of hematoma.
As multimodal analgesia is increasingly used in breast surgery, the analgesic efficacy of NSAIDs must be weighed against the associated risk of bleeding complications. In both the opioid and the NSAID study periods, most hematomas were definitively managed with an in-office drainage or aspiration procedure. The overall incidence of these subacute hematomas was low (3.6%) and was not statistically significantly higher after NSAIDs were included in the standard discharge regimen. Similar to our findings, a double-blind, randomized, controlled trial of 145 patients undergoing ambulatory breast surgery (lumpectomy or mastectomy with or without axillary surgery) demonstrated a low risk of hematoma by POD 7 in both patients receiving NSAIDs and acetaminophen (2.8%) and patients receiving acetaminophen, codeine, and caffeine (Tylenol 3) (4.3%; P = 0.56). Only 1.4% of patients in each group required reoperation.15 However, this study excluded patients who received intraoperative ketorolac, limiting generalizability of the data with respect to current multimodal anesthetic regimens. Taken in context with published results, our findings suggest that, in the current era of increasing use of intraoperative ketorolac, discharge prescriptions, including NSAIDs, are not a risk factor for significant bleeding events after lumpectomy.
During the NSAID period, patient-reported consumption of nonopioid drugs (NSAIDs and acetaminophen) was not statistically significantly different before and after opioids were removed from the standard discharge regimen. This finding indicates that patients did not take a higher number of NSAIDs or acetaminophen in the absence of prescribed opioids, supporting previously published findings that nonopioid analgesia is adequate for pain control after ambulatory breast surgery.4,15
In our study, receipt of intraoperative ketorolac was statistically significantly higher among patients during the NSAID period than during the opioid period, likely reflecting an increasing comfort among surgeons and anesthesiologists with its use over time. After adjustment for multiple potential confounders, intraoperative ketorolac was not statistically significantly associated with hematoma. Furthermore, the rate of reoperation for bleeding was low (<1%) with or without intraoperative ketorolac. Similarly, Rojas et al. assessed the risk of hematoma among 157 patients undergoing lumpectomy and 57 patients undergoing mastectomy, and found no statistically significant difference between patients who did and did not receive intraoperative ketorolac (0.9 vs. 2.0%, P = 0.6), and no patients undergoing lumpectomy experienced hematoma.10 Our findings suggest that, in patients undergoing lumpectomy and SLNB, receipt of intraoperative ketorolac is not a significant risk factor for postoperative bleeding events, including those requiring reoperation.
To our knowledge, our study is the largest to retrospectively examine the associations between routine intraoperative and postdischarge administration of NSAIDs and bleeding complications after lumpectomy and SLNB. The 30-day follow-up period permitted a comprehensive evaluation of the incidence of acute hematomas requiring reoperation and subacute hematomas beyond the immediate postoperative period both before and after NSAIDs were routinely included in the discharge analgesic regimen.
Our study has several limitations, including its retrospective nature. Although the incidence of hematomas requiring reoperation or in-office intervention was reliably captured, it is possible that some small hematomas not requiring intervention were not documented in the medical records. However, this problem should be equally frequent between the NSAID and opioid periods, and is unlikely to have changed the conclusions of our study. In addition, there is the potential for responder bias pertaining to patient-reported data on analgesic consumption. We could not discern NSAID versus acetaminophen consumption from the postdischarge surveys, as patients were asked to report intake of both medications combined, limiting our ability to draw conclusions specifically regarding postdischarge NSAID use. Lastly, there is an overlap of approximately 1 month between the end of the study period and pausing of most breast oncologic surgery because of the COVID-19 pandemic.16 It is possible that subacute hematomas were underestimated during this period, when postoperative visits were routinely conducted via telemedicine. However, because this would likely bias our estimate of conservatively managed hematomas only, we can reasonably conclude that our estimation of clinically significant bleeding was accurate despite this temporary practice change.
Conclusions
Among patients undergoing lumpectomy and SLNB with multimodal analgesia, the risk of hematoma in the 30-day postoperative period, including hematoma requiring reoperation and in-office aspiration or drainage, was low overall and not statistically significantly higher despite increased use of intraoperative ketorolac and implementation of a standard discharge regimen of NSAIDs in lieu of opioids. Due to their efficacy and safety in the perioperative and postdischarge settings, NSAIDs should be included in discharge regimens, unless contraindicated.
Funding
This research was funded by the National Institutes of Health/National Cancer Institute (Cancer Center Support Grant P30 CA008748).
Disclosures
Dr. Monica Morrow has received honoraria from Exact Sciences and Roche. The remaining authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel AR, Vuong B, Kuehner GE, et al. Adoption of opioid-sparing and non-opioid regimens after breast surgery in a large, integrated health care delivery system. Ann Surg Oncol. 2020;27(12):4835–4843. doi: 10.1245/s10434-020-08897-6. [DOI] [PubMed] [Google Scholar]
- 2.Hartford LB, Van Koughnett JAM, Murphy PB, et al. The Standardization of Outpatient Procedure (STOP) narcotics: a prospective health systems intervention to reduce opioid use in ambulatory breast surgery. Ann Surg Oncol. 2019;26(10):3295–3304. doi: 10.1245/s10434-019-07539-w. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy GT, Hill CM, Huang Y, et al. Enhanced recovery after surgery (ERAS) protocol reduces perioperative narcotic requirement and length of stay in patients undergoing mastectomy with implant-based reconstruction. Am J Surg. 2020;220(1):147–152. doi: 10.1016/j.amjsurg.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Moo TA, Pawloski KR, Sevilimedu V, et al. Changing the default: a prospective study of reducing discharge opioid prescription after lumpectomy and sentinel node biopsy. Ann Surg Oncol. 2020;27(12):4637–4642. doi: 10.1245/s10434-020-08886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M, Hallway A, Brummett C, Waljee J, Englesbe M, Howard R. Patient-reported outcomes after opioid-sparing surgery compared with standard of care. JAMA Surg. 2021;156(3):286–287. doi: 10.1001/jamasurg.2020.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenin DR, Dietz JR, Baima J, et al. Pain management in breast surgery: recommendations of a multidisciplinary expert panel-The American Society of Breast Surgeons. Ann Surg Oncol. 2020;27(12):4588–4602. doi: 10.1245/s10434-020-08892-x. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hilli Z, Thomsen KM, Habermann EB, Jakub JW, Boughey JC. Reoperation for complications after lumpectomy and mastectomy for breast cancer from the 2012 National Surgical Quality Improvement Program (ACS-NSQIP) Ann Surg Oncol. 2015;22(Suppl 3):S459–S469. doi: 10.1245/s10434-015-4741-7. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni T, Lancaster E, Ledesma Y, et al. Systematic review and meta-analysis of the association between non-steroidal anti-inflammatory drugs and operative bleeding in the perioperative period. J Am Coll Surg. 2021;3:1072–7515. doi: 10.1016/j.jamcollsurg.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker NJ, Jones VM, Kratky L, Chen H, Runyan CM. Hematoma risks of nonsteroidal anti-inflammatory drugs used in plastic surgery procedures: a systematic review and meta-analysis. Ann Plast Surg. 2019;82((6S Suppl 5)):S437–S445. doi: 10.1097/SAP.0000000000001898. [DOI] [PubMed] [Google Scholar]
- 10.Rojas KE, Fortes TA, Flom P, Manasseh DM, Andaz C, Borgen P. Intraoperative ketorolac use does not increase the risk of bleeding in breast surgery. Ann Surg Oncol. 2019;26(10):3368–3373. doi: 10.1245/s10434-019-07557-8. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen BN, Barta RJ, Stewart CE, Heinrich CA. Toradol following breast surgery: is there an increased risk of hematoma? Plast Reconstr Surg. 2018;141(6):814e–e817. doi: 10.1097/PRS.0000000000004361. [DOI] [PubMed] [Google Scholar]
- 12.Mikhaylov Y, Weinstein B, Schrank TP, et al. Ketorolac and hematoma incidence in postmastectomy implant-based breast reconstruction. Ann Plast Surg. 2018;80(5):472–474. doi: 10.1097/SAP.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 13.Firriolo JM, Nuzzi LC, Schmidtberg LC, Labow BI. Perioperative ketorolac use and postoperative hematoma formation in reduction mammaplasty: a single-surgeon experience of 500 consecutive cases. Plast Reconstr Surg. 2018;142(5):632e–e638. doi: 10.1097/PRS.0000000000004828. [DOI] [PubMed] [Google Scholar]
- 14.McCormick PJ, Assel M, Van Zee KJ, et al. Intraoperative ketorolac is associated with risk of reoperation after mastectomy: a single-center examination. Ann Surg Oncol. 2021. 10.1245/s10434-021-09722-4. [DOI] [PMC free article] [PubMed]
- 15.Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19(12):3792–3800. doi: 10.1245/s10434-012-2447-7. [DOI] [PubMed] [Google Scholar]
- 16.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 Pandemic Breast Cancer Consortium. Breast Cancer Res Treat. 2020;181(3):487–97. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]