The phenomenon of osteochondral loose bodies within a joint was termed osteochondritis dissecans (OCD) by König in 18871,2. OCD is conventionally defined as a focal idiopathic alteration of subchondral bone with risk of instability and disruption of adjacent articular cartilage that may result in premature osteoarthritis3. The etiology is generally believed to be multifactorial, but in-depth understanding is lacking partially because of the difficulty of investigating OCD before the development of overt clinical symptoms. Treatment is largely based on skeletal maturity and lesion stability, defined by the presence or absence of articular cartilage fracture and subchondral bone separation, with the goals of symptomatic relief and joint preservation4. The literature has grown substantially since the 1996 Current Concepts Review article by Schenck and Goodnight5. A multicenter group, Research in OsteoChondritis of the Knee (ROCK), was formed in 2010 to investigate the etiology and diagnostic criteria, optimize prevention and treatment strategies, reduce variation in care, and improve quality of life6. The present Current Concepts Review article focuses on OCD of the knee, elbow, and talus, recognizing them as a common entity while highlighting important distinctions.
Epidemiology
OCD is divided into juvenile and adult forms, depending on skeletal maturity at diagnosis5,7. Most adult lesions are likely unresolved juvenile lesions, but de novo adult OCD has been described8. Adult OCD has an incidence rate of 3.42 per 100,000 person-years and predominantly affects ankles, followed by knees9. Juvenile OCD is more common, affecting 9.5 to 29 of 100,000 knees, 2.2 of 100,000 elbows, and 2 to 4.6 of 100,000 ankles10–15. Clinically apparent OCD is rare in patients who are <10 or ≥50 years old10,11,13,14. The risk of juvenile OCD is higher in patients who are ≥12 years old than in those between 6 and 11 years old and is 3 times higher in knees, 22 times higher in elbows, and 7 times higher in ankles in the older children than in the younger group9,11,13,14. Boys have more risk of developing OCD in the knee (4 times) and elbow (7 times), whereas girls have more risk of OCD developing in the talus (1.5 times)11,13,14.
OCD predilection sites differ for each joint. The classic site of knee OCD is the posterior-central aspect of the medial condyle (63.6% to 85%) compared with inferior-central aspect of the lateral condyle (15% to 32.5%), inferomedial aspect of the patella (1.5% to 10%), and trochlea (2%)11,16–20. Elbow OCD typically affects the anterolateral or central aspect of the capitellum (97.5%) rather than the trochlea (2.5%)14. Most OCD lesions of the talus are posteromedial (71.8%) and are less frequently anterolateral (22.4%) or central (3.5%)13. OCD usually involves a single site but can be multifocal within one or multiple joints with lesions in different stages21. Bilateralism occurs in knees (7.3% to 29%) and elbows (8.1%), and is rare in ankles11,13,14,22,23.
OCD tends to affect active children and young adults8,19. Participation in high-level athletics is associated with the development of OCD in the knee (55% to 60%), elbow (84%), and talus (67.4%)13,14,16,20. Knee and talar OCD cases are commonly associated with soccer, football, and basketball, whereas elbow OCD is often seen in throwing athletes and gymnasts13,14,24,25. Interestingly, children with extreme obesity have more risk for the development of elbow and talar OCD, whereas moderate obesity increases the risk of knee OCD26. An observed trend of younger onset of OCD may be related to earlier sports participation or specialization and increasing childhood obesity11,13,14,19,27–29.
Etiology
The etiology of OCD is incompletely understood. Despite nomenclature implying an inflammatory process (osteochondritis), minimal supporting evidence exists, suggesting that the condition would be more appropriately named “osteochondrosis,” as it is in veterinary medicine. Growing evidence from the veterinary literature has favored ischemic necrosis of epiphyseal cartilage as a key event30–32. Indeed, histochemical studies of human specimens have found subchondral bone and epiphyseal cartilage necrosis, matrix metalloproteinase expression consistent with chondrocyte apoptosis, and cartilage degradation reminiscent of osteoarthritis6,33,34. Understanding has been confounded by similarities to osteochondral fractures and osteonecrosis associated with hemoglobinopathy, steroids, radiation, and chemotherapy18,35,36. A recent systematic review concluded that the etiopathogenesis of OCD is likely a convergence of biological and mechanical factors37.
Vascular Factors
The function and survival of subarticular epiphyseal cartilage, which determines adult joint geometry, depend on blood supply from cartilage canal vessels. These vessels regress during development as the secondary ossification center expands38,39. However, premature interruption of these vessels in animals, naturally or surgically, causes epiphyseal cartilage necrosis, termed osteochondrosis latens32,40,41. This lesion is initially confined to epiphyseal cartilage without articular cartilage or subchondral bone involvement. When the ossification front reaches the lesion, failure of endochondral ossification occurs, and an island of necrotic epiphyseal cartilage is retained within subchondral bone, termed osteochondrosis manifesta, which provides poor mechanical support to overlying cartilage42. Animal studies have demonstrated that these subclinical lesions usually heal without intervention; however, exposure to biomechanical trauma in a single event or repeated events may create osteochondral lesions, termed osteochondrosis dissecans (Fig. 1)43.
Fig. 1.
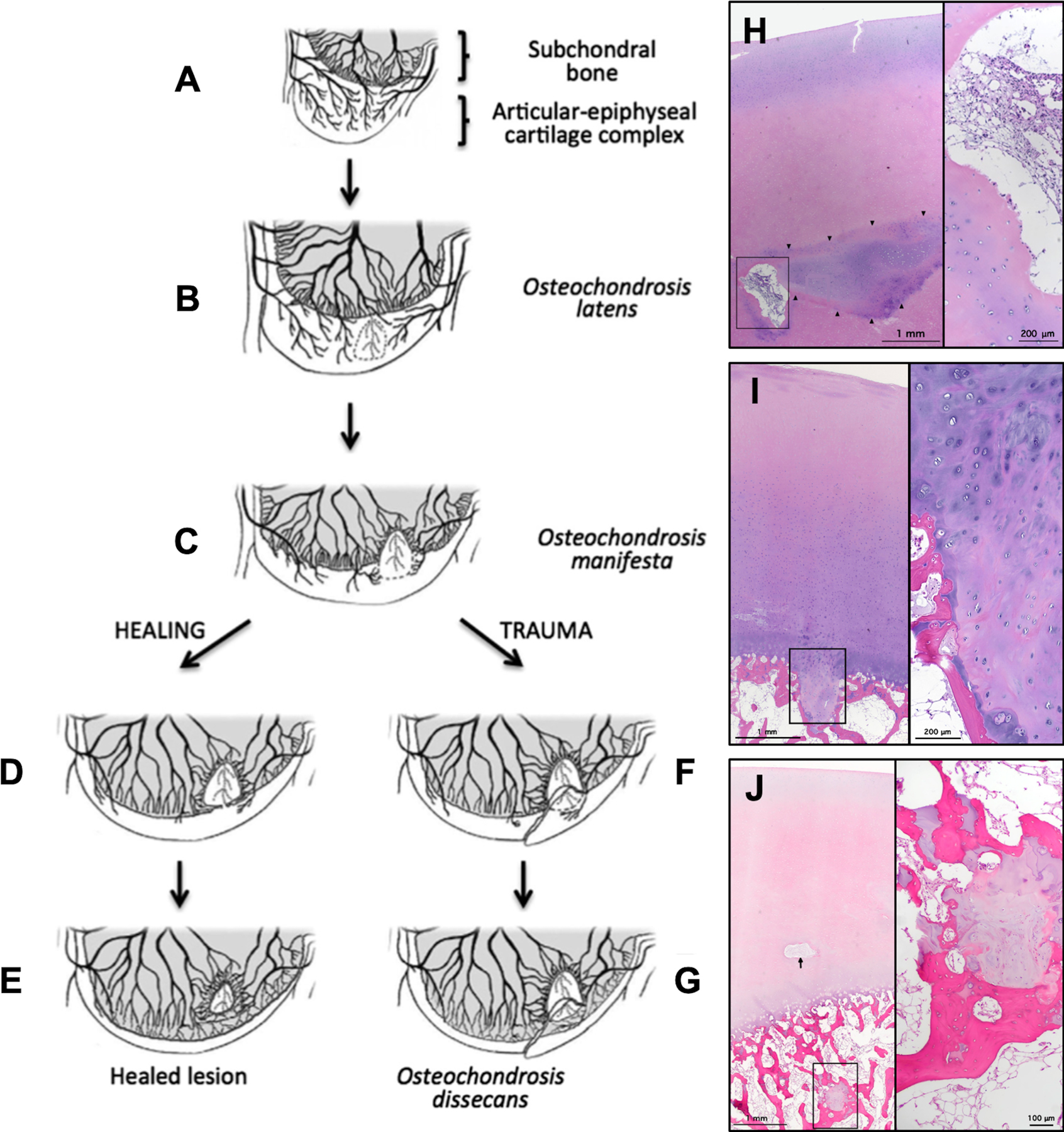
Figs. 1-A through 1-J Diagrams and photomicrographs demonstrating the pathogenesis of osteochondrosis in animals (Figs. 1-A through 1-G) and OCD in human pediatric cadavers (Figs. 1-H, 1-I, and 1-J). (Figures 1-A through 1-G modified, with permission, from: McCoy AM, Toth F, Dolvik NI, Ekman S, Ellermann J, Olstad K, Ytrehus B, Carlson CS. Articular osteochondrosis: a comparison of naturally-occurring human and animal disease. Osteoarthritis Cartilage. 2013 Nov;21[11]:1638–47; Ytrehus B, Carlson CS, Ekman S. Etiology and Pathogenesis of Osteochondrosis. Vet Pathol. 2007 Jul;44[4]:429–48; and Ekman S, Carlson C. The pathophysiology of osteochondrosis. Vet Clin North Am Small Anim Pract. 1998 Jan;28[1]:17–32; and Figures 1-H, 1-I, and 1-J modified from: Tóth F, Tompkins MA, Shea KG, Ellermann JM, Carlson CS. Identification of areas of epiphyseal cartilage necrosis at predilection sites of juvenile osteochondritis dissecans in pediatric cadavers. J Bone Joint Surg Am. 2018 Dec 19;100[24]:2132–39.) Fig. 1-A Normal endochondral ossification. Fig. 1-B Development of osteochondrosis latens because of failure of the cartilage canal blood supply, resulting in epiphyseal cartilage necrosis. Fig. 1-C Osteochondrosis manifesta lesion develops as a delay in the progression of the ossification front. Figs. 1-D and 1-E Healing of an osteochondrosis manifesta lesion by incorporation into subchondral bone. Figs. 1-F and 1-G Development of an OCD lesion as a result of trauma, causing collapse of articular cartilage overlying areas of necrotic epiphyseal cartilage. Fig. 1-H Hematoxylin and eosin staining of articular and epiphyseal cartilage from the central aspect of the medial femoral condyle from a 4-year-old female donor containing an osteochondrosis latens lesion. Arrowheads demarcate necrotic epiphyseal cartilage. Right: High-magnification image demonstrates cartilage canal containing multiple degenerate vessels. Fig. 1-I Hematoxylin and eosin staining of articular and epiphyseal cartilage from the central aspect of the medial femoral condyle from a 9-year-old male donor containing an osteochondrosis manifesta lesion. Right: High-magnification image demonstrates necrotic epiphyseal cartilage partially surrounded by bone. Fig. 1-J Hematoxylin and eosin staining of articular and epiphyseal cartilage from the central aspect of the lateral femoral condyle from a 4-year-old female donor containing a healing osteochondrosis manifesta lesion. Arrow points to a degenerate cartilage canal. Right: High-magnification image demonstrates necrotic epiphyseal cartilage completely surrounded by bone.
Histologic studies have identified lesions identical to osteochondrosis latens and manifesta at predilection sites in human cadaveric specimens42. Furthermore, 3-dimensional quantitative magnetic resonance imaging (MRI) demonstrating vascular regression and delayed secondary ossification at predilection sites of OCD in pediatric cadaveric knees supports a vascular etiology (Fig. 2, Video 1)39. Indeed, susceptibility-weighted MRI revealed common vascular patterns in developing distal femora of humans and pigs, species that share identical predilection sites38. Interestingly, goats, which have not been reported to develop distal femoral osteochondrosis, have dense vascular networks that seemingly protect against the condition38. Tóth et al. demonstrated that, even after surgical interruption of distal femoral vasculature, most goats only develop subclinical osteochondrosis40. Tenuous epiphyseal cartilage vasculature is a shared feature among predilection sites across species susceptible to OCD44–46.
Fig. 2.
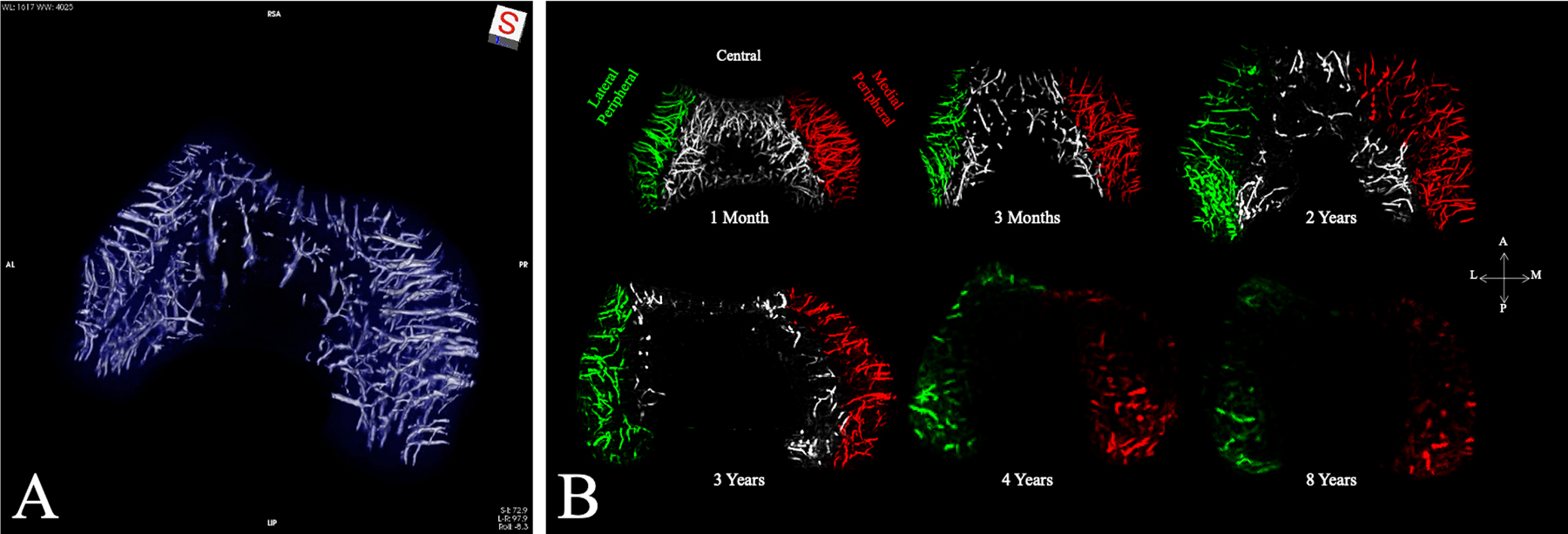
Figs. 2-A and 2-B Three-dimensional quantitative MRI demonstrating vascular watershed zones and regression. Fig. 2-A Three-dimensional reconstruction of susceptibility-weighted MRI data obtained from a distal femoral specimen from a 3-month-old human cadaveric donor demonstrating axial (central) and abaxial (peripheral) vascular beds with an avascular region in the midsagittal plane. Fig. 2-B Visualizations and density changes in vascular network with age. Maximum-intensity projections of the quantitative susceptibility mapping-post-processed vessel-enhanced (vesselness) maps from 6 selected cadaveric specimens with increasing age are shown. The vasculature is color-coded according to the vascular network (lateral peripheral = green vessels, central = gray, and medial peripheral = red). No central network was observed in the specimens from donors who were ≥4 years old. By 8 years of age, there was a small, residual peripheral vascular network. At 10 years of age (not shown), no substantial epiphyseal cartilage vascularity could be detected. A = anterior, L = lateral, M = medial, and P = posterior. (Modified, with permission, from: Ellermann JM, Ludwig KD, Nissi MJ, Johnson CP, Strupp JP, Wang L, Zbýň Š, Tóth F, Arendt E, Tompkins M, Shea K, Carlson CS. Three-dimensional quantitative magnetic resonance imaging of epiphyseal cartilage vascularity using vessel image features: new insights into juvenile osteochondritis dissecans. JBJS Open Access. 2019 Dec 5;4[4]:e0031. Reproduced with permission.)
Endochondral Ossification-Related Factors
Ribbing initially described separation of an accessory epiphyseal ossification center that progressed to OCD after subsequent trauma47. It was later proposed that insult to the secondary subarticular physis, which fails to heal or forms irregular subchondral tissue, predisposes to OCD5,6,48,49. Laor et al. described chondro-epiphyseal widening and subchondral edema on MRI studies of children with knee OCD, implicating an ossification abnormality50. Similarly, an advanced MRI study of juvenile OCD demonstrated an epiphyseal cartilage etiology with delayed endochondral ossification51. Tóth et al. identified epiphyseal cartilage necrosis adjacent to failed endochondral ossification at OCD predilection sites in pediatric cadaveric knees42. In animal models, surgically induced ischemic necrosis of epiphyseal cartilage is associated with delayed endochondral ossification, creating a vulnerable area where articular cartilage is only supported by necrotic tissue40.
Biomechanical Factors
A history of mild trauma is commonly reported by patients with OCD in the knee (40%), capitellum (33%), and talus (47% to 80%)7,13,36,52. OCD secondary to acute trauma, such as ankle sprains frequently associated with lateral talar osteochondral lesions, is likely a different entity although treatment may be similar52–54. Repetitive loading triggering vascular compromise is a widely proposed theory as there is often an association between OCD and athletic activities (55% to 60%)8,10,16,20. Capitellar OCD predominantly occurs in the dominant arm of throwing athletes and gymnasts subject to valgus or axial stress36. Baseball catchers tend to develop knee OCD at a younger age and in a more posterior location in the knee compared with noncatchers, likely from loading in hyperflexion55. Juvenile OCD of the femoral trochlea is most common in basketball, football, and soccer players exposed to forces across the patellofemoral joint24,56. Correlation with anatomic variations has also been suggested, including narrower intercondylar width, a larger tibial spine, greater posterior-medial tibial slope, dysmorphic femoral condyles, and more distal femoral attachment of the posterior cruciate ligament57–62. Studies have associated meniscal injury with the development of OCD in the less common lateral aspect of the femoral condyle20,63,64. Moreover, correlation has been reported between knee OCD and alignment, in which genu varum predisposed to medial condylar lesions and genu valgum predisposed to lateral condylar lesions, and the mechanical axis deviation has been found to significantly coincide with unstable knee lesions65–67.
Genetic Factors
Genetic predisposition was initially proposed after Mubarak and Carroll reported the development of OCD in 12 family members over 4 generations68. It was disputed by Petrie in his study of 34 patients, in which only 1.2% (1) of 86 first-degree relatives was found to have OCD69. Multiple case reports have since described monozygotic and fraternal twins with OCD of the knee, capitellum, and talus70–77. A recent study found that 14% (14) of 103 children treated for knee OCD had positive family histories78. OCD is found in various inherited conditions, including dwarfism, tibia vara, Legg-Calvé-Perthes disease, and Stickler syndrome35,79–81. It has been considered a subgroup of epiphyseal dysplasia with abnormal accessory ossification nuclei47,82. Familial OCD is an autosomal dominant syndrome involving the aggrecan gene that manifests as multiple joint OCD, disproportionate short stature, and early osteoarthritis83–85. Recently, a genome-wide association study in humans identified genetic loci related to juvenile OCD86,87.
Diagnostic Strategies
History and Physical Examination
Patients with OCD may not recall trauma. Symptoms depend on the lesion location and stage. Stable lesions can cause nonspecific symptoms, including vague or intermittent pain, whereas unstable lesions or loose bodies can cause mechanical symptoms, including catching or locking. For medial femoral condylar lesions, the Wilson test reproduces pain with tibial internal rotation during knee extension from 90° to 30° that is relieved with tibial external rotation88. For capitellar lesions, the radiocapitellar compression test reproduces pain with active pronation-supination in full elbow extension46. However, these provocative maneuvers have limited diagnostic value89. Restricted range of motion and joint effusion may signify unstable lesions or loose bodies. Limb alignment and joint stability should be evaluated as they influence biomechanical stress on joints.
Imaging
Radiography
Radiography is the first-line workup for OCD and for monitoring treatment response7,18,22,90. Comprehensive views are recommended for knees (anteroposterior, lateral, notch or tunnel, and skyline or sunrise radiographs), elbows (anteroposterior in extension and 45° of flexion, lateral, and external oblique radiographs), and ankles (anteroposterior, lateral, and mortise radiographs) to examine predilection sites7,90–93. Bilateral and standing alignment radiographs should be made with a low threshold to evaluate suspected bilateral disease and malalignment. Characteristic findings in early lesions are contour abnormality and radiolucency at the articular surface. More advanced lesions display a well-circumscribed, variably ossified fragment (progeny) separated from underlying bone (parent) by a crescent-shaped radiolucent line that may later ossify with healing. Normal variations of accessory epiphyseal ossification nuclei should be noted19. Radiography can reliably identify lesion location and size but is inaccurate for determining stability and identifying subtle lesions16,94–99.
MRI
MRI is the standard of care for diagnosing and characterizing OCD18,100. T1-weighted sequences allow lesion size measurement. T2-weighted sequences provide information on articular cartilage integrity, reactive marrow edema in the parent bone, and fluid or cystic changes at the parent-progeny interface. However, high signal intensity around the lesion may ambiguously represent fluid or granulation tissue101,102. Postgadolinium fat-suppressed T1-weighted sequences can distinguish fluid from granulation tissue but may not correlate with healing100,103–105. Gadolinium is controversial in children because of observable deposition in the brain, and no data showing long-term clinical consequences are available106,107. Ellermann et al. described a noncontrast 3-T MRI protocol, called T2* mapping, using the shortest clinical echo time to provide additional information on soft-tissue composition, including osseus versus cartilaginous progeny lesions, fluid versus a fibrous interface, and normal versus sclerotic parent bone (Fig. 3)51. This protocol also generates a bone window that can obviate the need for computed tomography (CT).
Fig. 3.
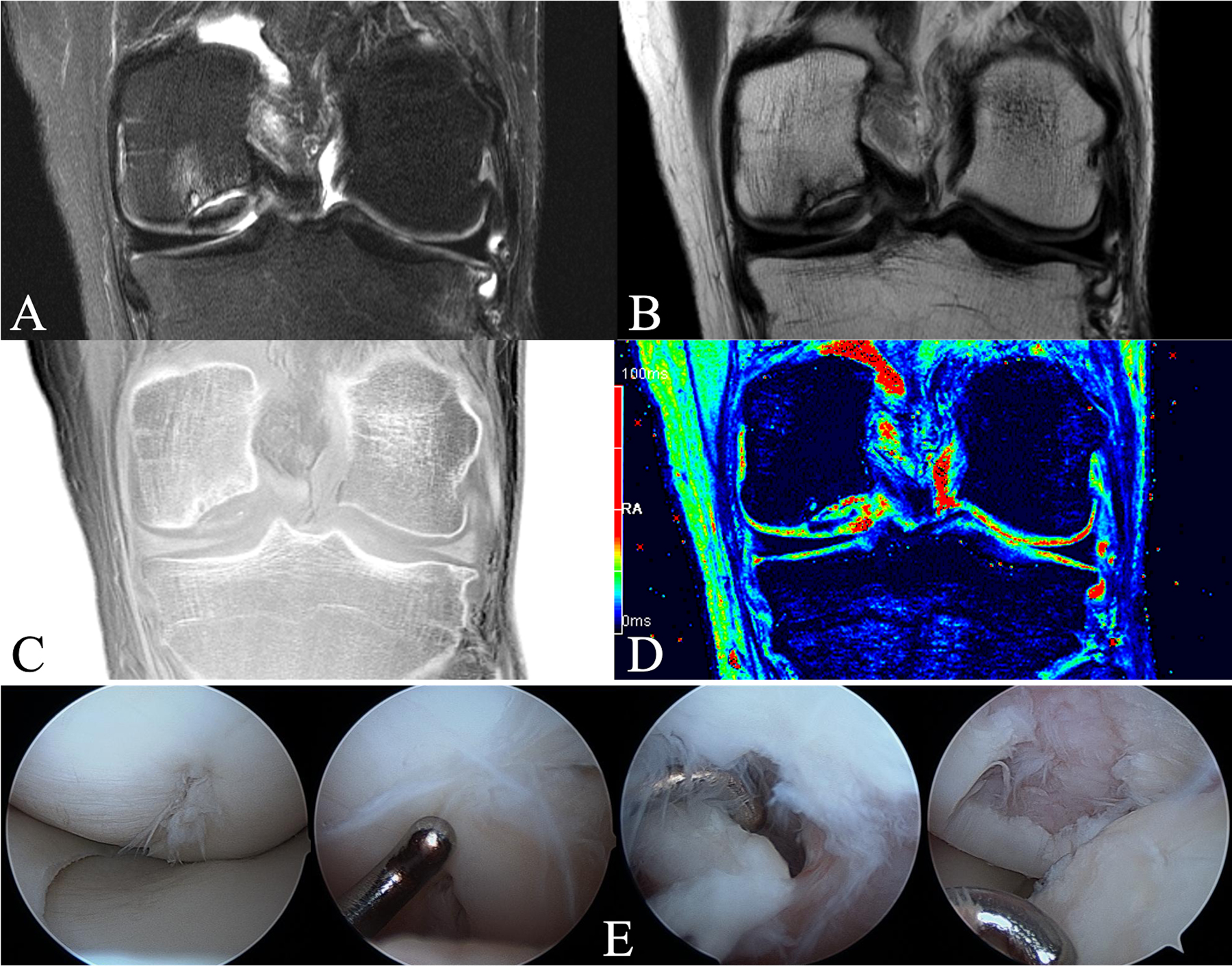
Figs. 3-A through 3-E A 23-year-old man with an OCD lesion on the central aspect of the medial femoral condyle evaluated with an advanced clinical 3-T MRI protocol and arthroscopy. Fig. 3-A T2-weighted fat-saturated coronal TSE (turbo-spin-echo) image (TR [repetition time] = 3,600 ms, and TE [echo time] = 59 ms) demonstrating cystic changes and marrow edema of the parent bone. Substantial fluid at the parent-progeny interface is indicative of an unstable lesion. Overlying articular cartilage appears grossly intact. Fig. 3-B Proton density-weighted coronal TSE image (TR = 3,400 ms, and TE = 27 ms), demonstrating a dark progeny fragment suggesting osseous tissue. Fig. 3-C CT-like GRE (gradient-recalled echo) image (TR = 1,000 ms, and TE = 3.15 ms) with inverted contrast (“bone window”), demonstrating parent bone with cystic changes, sclerosis, and marginal mineralization and/or ossification surrounding the cartilaginous progeny lesion. Fig. 3-D T2* map (TR = 1,000 ms and TE = 3.15, 7.5, 11.9, 22.2, and 34.5 ms). Color-coding signal intensities: blue to black = bone and mineralization, green to red = epiphyseal and articular cartilage, and red = fluid at the parent-progeny lesion interface. T2* mapping affirms a cartilaginous OCD lesion (green centrally) with marginal ossification (blue), deemed unstable because of fluid (red) at the interface. Of note is mild overlying articular cartilage edema (red). Fig. 3-E Arthroscopic photographs confirming an unstable lesion. Arthroscopy was unable to determine the presence of bone on the fragment; thus, the advanced clinical 3-T MRI protocol helped to guide intraoperative decision-making. The patient underwent screw fixation, went on to heal, and returned to all activities.
MRI has excellent sensitivity and specificity for detecting OCD but uncertain accuracy in determining stability108–110. De Smet et al. defined 4 MRI criteria based on T2-weighted sequences that are used to predict stability (Table I)111. Evaluating these criteria for knee OCD, Kijowski et al. found 100% sensitivity but only 11% specificity in 36 juvenile lesions compared with 100% sensitivity and specificity in 33 adult lesions112. After implementing 3 secondary MRI criteria (Table I), the sensitivity and specificity of predicting stability improved to 100%.
TABLE I.
Classification Systems for OCD of the Knee*
| Scintigraphy | Radiography | MRI | Lesion Stability | Arthroscopy |
|---|---|---|---|---|
| Cahill and Berg250 (1983) Stage 0: Normal scintigraphy and radiography Stage I: Normal scintigraphy but radiographic findings of OCD Stage II: Focal avidity at site of OCD defect, and radiographic findings of OCD Stage III: Focal avidity at site of OCD defect and increased activity in the femoral condyle harboring the OCD lesion, as well as radiographic findings of OCD Stage IV: Stage III and increased activity of the juxta-articular tibial plateau, and radiographic findings of OCD |
Berndt and Harty251 (1959) Stage 1: Small subchondral compression Stage 2: Partially detached osteochondral fragment Stage 3: Completely detached but nondisplaced fragment Stage 4: Completely detached and displaced fragment (loose body) Scranton and McDermott modification252 (2001) Stage 5: Subchondral cyst |
Dipaola et al.253 (1991) Stage I: Thickening of articular cartilage and low-signal changes Stage II: Articular cartilage breached and low-signal rim behind fragment indicating fibrous attachment Stage III: Articular cartilage breached and high-signal changes behind fragment indicating synovial fluid between fragment and underlying subchondral bone Stage IV: Loose body Hefti et al.16 (1999) Stage I: Small change of signal without clear margins of fragment Stage II: Osteochondral fragment with clear margins but without fluid between fragment and underlying bone Stage III: Fluid is visible partially between fragment and underlying bone Stage IV: Fluid is completely surrounding the fragment, but the fragment is still in situ Stage V: Fragment is completely detached and displaced (loose body) Ellermann et al.51 (2017) Type I: Epiphyseal cartilage lesion with necrotic center (no cleft at the interface) Type II: Epiphyseal cartilage lesion with complete or incomplete rim calcification (cleft at the interface) Type III: Partially or completely ossified lesion (varying degrees of osseous bridging and/or clefting at the interface) Type IV: Healed osseous lesion with a linear osseous scar (no cleft at the interface) Type V: Unhealed detached osseous lesion (sequestrum) |
De Smet et al.111 (1996) (T2-weighted MRI) Sign 1: A thin line of high-signal-intensity ≥5 mm in length at the interface between the lesion and underlying bone (fibrovascular granulation tissue) Sign 2: A round area of homogeneous high-signal-intensity ≥5 mm in diameter beneath the lesion (cysts) Sign 3: A focal defect with a width of ≥5 mm in the articular surface of the lesion (displacement of the lesion into the joint) Sign 4: A high-signal-intensity line traversing articular cartilage into the lesion (articular fracture) O’Connor et al. modification102 (2002) Instability determined only when accompanied by cartilage breach on T1-weighted MRI Kijowski et al. modification112 (2008) A high T2-signal-intensity rim or cysts surrounding an adult OCD lesion are unequivocal signs of instability A high T2-signal-intensity rim surrounding a juvenile OCD lesion indicates instability only if it has the same signal intensity as adjacent joint fluid, is surrounded by a second outer rim of low T2 signal intensity, or is accompanied by multiple breaks in the subchondral bone plate on T2-weighted MRI Cysts surrounding a juvenile OCD lesion indicate instability only if they are multiple in number or large in size |
Guhl254,255 (1982) Stage 1: Intact lesions Stage 2: Lesions showing signs of early separation Stage 3: Partially detached lesions Stage 4: Craters with loose bodies (salvageable or unsalvageable) Brittberg and Winalski256 (2003) ICRS OCD I: Stable lesions with a continuous but softened area covered by intact cartilage ICRS OCD II: Lesions with partial discontinuity that are stable when probed ICRS OCD III: Lesions with a complete discontinuity that are not yet dislocated (“dead in situ”) ICRS OCD IV: Empty defects and defects with a dislocated fragment or loose fragment within the bed ROCK Group (Carey et al.130; 2013) Immobile lesions: Cue ball: No abnormality detectable arthroscopically Shadow: Cartilage is intact and subtly demarcated (possibly under low light) Wrinkle in the rug: Cartilage is demarcated with a fissure, buckle, and/or wrinkle Mobile lesions: Locked door: Cartilage fissuring at periphery and unable to hinge open Trap door: Cartilage fissuring at periphery and able to hinge open Crater: Exposed subchondral bone defect Other classification systems: Ewing and Voto257 (1988) Johnson et al.258 (1990) Dipaola et al.253 (1991) O’Connor et al.102 (2002) Chen et al.259 (2013) |
OCD = osteochondritis dissecans, ICRS = International Cartilage Repair Society, MRI = magnetic resonance imaging, and ROCK = Research in OsteoChondritis of the Knee.
CT
CT is useful for evaluating lesion size and location, loose bodies, and particularly osseous healing after fixation1–5. It is commonly used for capitellar and talar OCD. A study evaluating CT and MRI for osteochondral lesions of the talus in 103 patients found comparable sensitivities and specificities113. Limitations of CT include radiation exposure and inability to obtain soft-tissue contrast18,114.
Ultrasonography
Ultrasound is most commonly used for capitellar OCD91,93,115–120. Harada et al. tested on-the-field utility of ultrasound for detecting elbow injuries among 153 youth baseball players and found 35 with abnormalities, of which 2 were confirmed capitellar OCD116. Takahara et al. found ultrasound comparable with radiography (85%; 23 of 27 patients), MRI (90%; 9 of 10 patients), and arthroscopy (93%; 14 of 15 patients) in predicting lesion stability using an ultrasound classification system (Table II)115. Ultrasound is heavily operator-dependent and therefore is not the diagnostic modality of choice in most cases22,91.
TABLE II.
Classification Systems for OCD of the Elbow*
| Radiography | MRI | Ultrasound | Lesion Stability | Arthroscopy |
|---|---|---|---|---|
| Minami et al.260 (1979) Grade 1: Translucent cystic shadow in the lateral or middle aspect of the capitellum Grade 2: Clear zone or split line between the lesion and the adjacent subchondral bone Grade 3: Loose bodies |
Dipaola et al.253 (1991) Stage I: Thickening of articular cartilage and low-signal changes Stage II: Articular cartilage breached and low-signal rim behind fragment indicating fibrous attachment Stage III: Articular cartilage breached and high-signal changes behind fragment indicating synovial fluid between fragment and underlying subchondral bone Stage IV: Loose body |
Takahara et al.115 (2000) Type I: Lesion with localized subchondral bone flattening and cartilaginous thickening Type II: Lesion with nondisplaced fragments and an intact articular surface Type III: Lesion with displaced fragment Type IV: Lesion with osteochondral defect |
Takahara et al.131 (2007) Stable (ICRS I): open capitellar physis, normal elbow motion, and Minami grade I Unstable (ICRS II-IV): Closed capitellar physis, restricted elbow motion >20°, and Minami grade II or III Kolmodin and Saluan modification261 (2014) Type I (stable): open capitellar physis, Minami grade I, and normal elbow motion Type II (unstable): Closed capitellar physis, Minami grade II or III, and motion restricted >20°, medial to the radial head center line Type IIIa (unstable): Closed capitellar physis, Minami grade II or III, and motion restricted >20°, extending lateral to the radial head center line Type IIIb (unstable): Closed capitellar physis, Minami grade II or III, and motion restricted >20°, extending lateral to the radial head center line including the lateral margin |
Brittberg and Winalski256 (2003) ICRS OCD I: Stable lesions with a continuous but softened area covered by intact cartilage ICRS OCD II: Lesions with partial discontinuity that are stable when probed ICRS OCD III: Lesions with a complete discontinuity that are not yet dislocated (“dead in situ”) ICRS OCD IV: Empty defects as well as defects with a dislocated fragment or a loose fragment within the bed |
OCD = osteochondritis dissecans, MRI = magnetic resonance imaging, and ICRS = International Cartilage Repair Society.
Bone Scintigraphy
Scintigraphy was historically used to detect OCD and assess healing on the basis of perfusion94,121,122. Despite excellent sensitivity, scintigraphy became obsolete because of the lack of specificity, MRI availability, and radioisotope exposure6,18,36,104,123–126. Recently, a nonradioactive, non-contrast-enhanced 3-T MRI method, called arterial spin labeling, was demonstrated to visualize distal femoral perfusion similarly to scintigraphy in children with knee OCD127.
Classification Systems
There are numerous OCD classifications systems based on the joint involved and the diagnostic modality (Tables I, II, and III). Arthroscopy is the gold standard for determining lesion stability128. The ROCK arthroscopic classification system for the knee is the most comprehensive, including 6 mutually exclusive categories divided into immobile and mobile lesions but omitting salvageability, which requires considering skeletal maturity, prior treatments, and imaging (Table I)129,130. Contributing to MRI-based systems, Ellermann et al. devised a classification system for juvenile OCD that stages natural history from necrotic epiphyseal cartilage to lesions that are healed or not healed51. While no stand-alone preoperative method can definitively determine stability, the Takahara classification system uses radiography and clinical parameters for capitellar OCD, demonstrating the utility of a multifactorial system (Table II)131.
TABLE III.
Classification Systems for OCD of the Talus*
| Radiography | CT | MRI | Arthroscopy |
|---|---|---|---|
| Berndt and Harty251 (1959) Stage 1: Small subchondral compression Stage 2: Partially detached osteochondral fragment Stage 3: Completely detached but nondisplaced fragment Stage 4: Completely detached and displaced fragment (loose body) Scranton and McDermott modification252 (2001) Stage 5: Subchondral cyst |
Ferkel et al.216 (2008) Stage I: Cystic lesion within dome of talus, and intact roof on all views Stage IIA: Cystic lesion with communication to talar dome surface Stage IIB: Open articular surface lesion with overlying nondisplaced fragment Stage III: Nondisplaced lesion with radiolucency Stage IV: Displaced fragment Other classification systems: Loomer et al.124 (1993) |
Hepple et al.262 (1999) Stage 1: Articular cartilage damage only Stage 2a: Cartilage injury with underlying fracture and surrounding osseous edema Stage 2b: Stage 2a without surrounding osseous edema Stage 3: Detached but nondisplaced fragment Stage 4: Detached and displaced fragment Stage 5: Subchondral cyst formation |
Ferkel et al.216 (2008) Grade A: Smooth, intact, but soft or “ballottable” (springy) Grade B: Rough surface Grade C: Fibrillations and/or fissures Grade D: Flap present or bone exposed Grade E: Loose, nondisplaced fragment Grade F: Displaced fragment Other classification systems: Pritsch et al.263 (1986) |
CT = computed tomography, and MRI = magnetic resonance imaging.
Treatment Alternatives
The 2010 American Academy of Orthopaedic Surgeons clinical practice guidelines revealed limited existing high-level evidence for guiding OCD management132. Skeletal maturity and lesion stability are generally considered the most important information for clinical decision-making7,18,22,133. General treatment algorithms for knee, elbow, and talus are shown in Figures 4, 5, and 6.
Fig. 4.
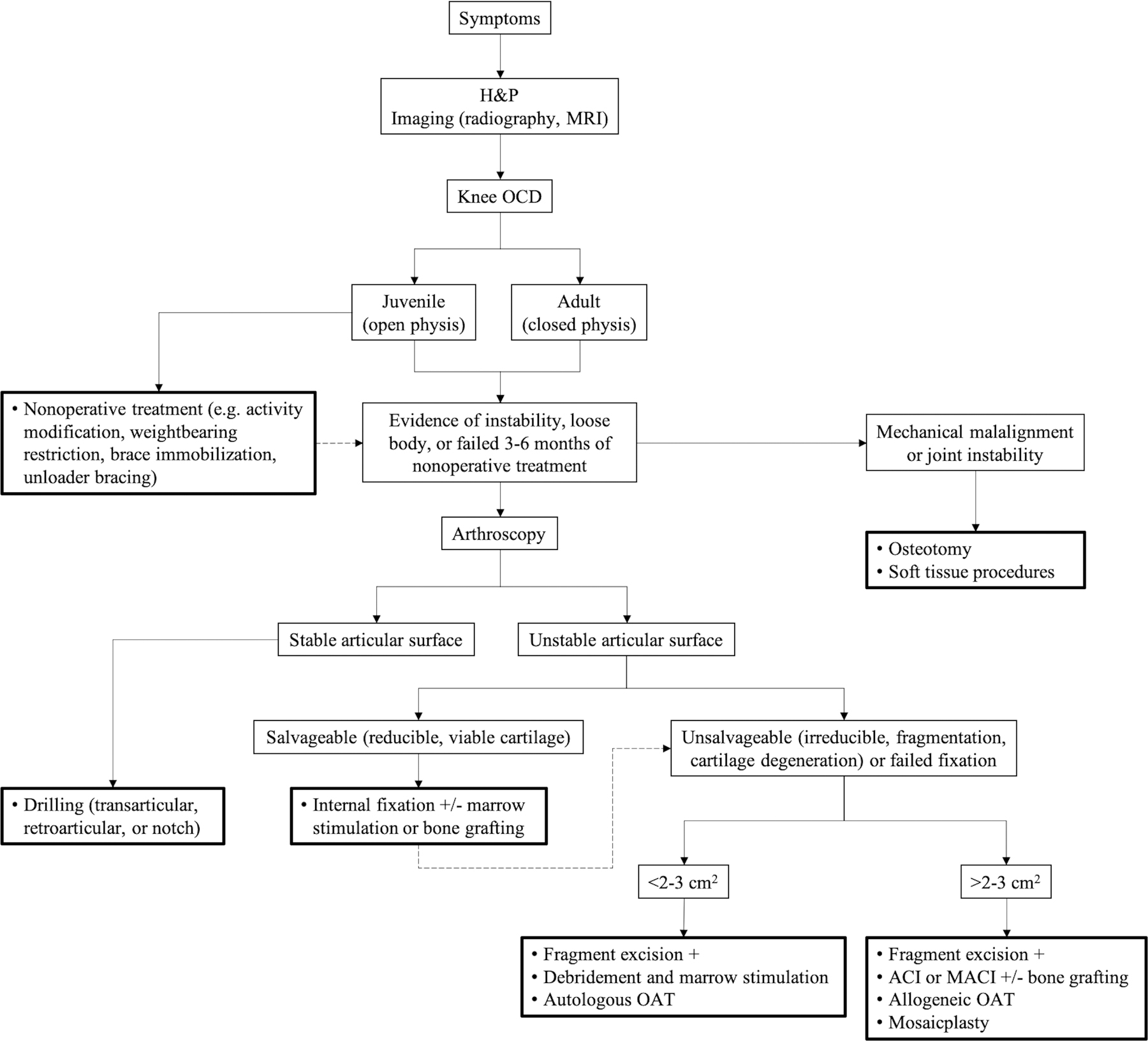
General treatment algorithm for OCD of the knee. Dotted arrow indicates alternative treatment after initial failure. ACI = autologous chondrocyte implantation, H&P = history and physical examination, MACI = matrix-assisted autologous chondrocyte implantation, MRI = magnetic resonance imaging, and OAT = osteochondral autograft or allograft transplantation.
Fig. 5.
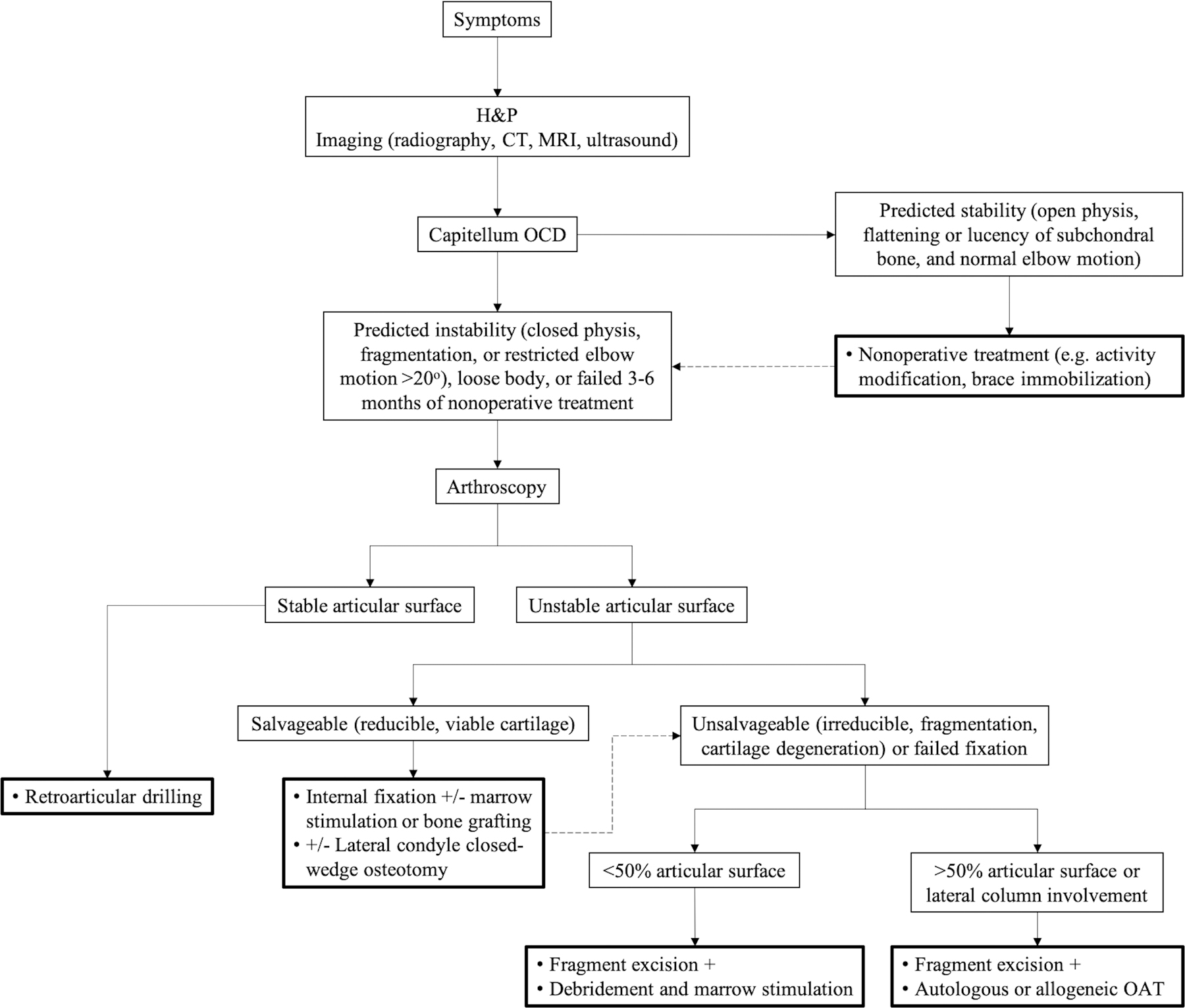
General treatment algorithm for OCD of the capitellum. Dotted arrow indicates alternative treatment after initial failure. CT = computed tomography, H&P = history and physical examination, MRI = magnetic resonance imaging, and OAT = osteochondral autograft or allograft transplantation.
Fig. 6.
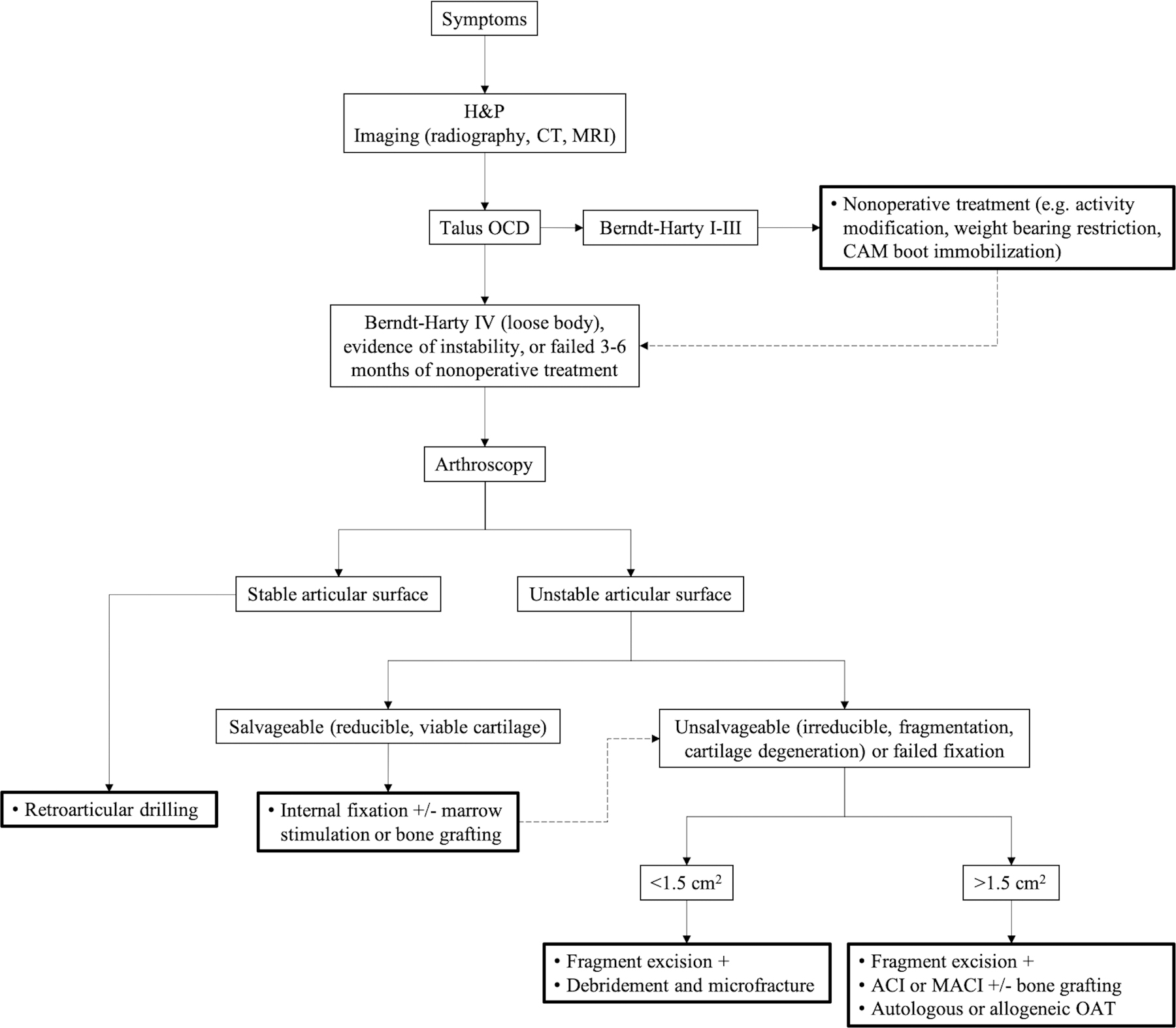
General treatment algorithm for OCD of the talus. Dotted arrow indicates alternative treatment after initial failure. ACI = autologous chondrocyte implantation, CAM = controlled ankle motion, CT = computed tomography, H&P = history and physical examination, MACI = matrix-assisted autologous chondrocyte implantation, MRI = magnetic resonance imaging, and OAT = osteochondral autograft or allograft transplantation.
Nonoperative Treatment
Nonoperative treatment is well accepted for patients with open physes, stable lesions, or minimal symptoms. Duration is typically a minimum of 3 to 6 months followed by gradual return to activity. Protocols on activity modification, weight-bearing restriction, and immobilization vary19,96. Immediate cessation of athletics and impact activities is usually recommended. Some authors prefer protected weight-bearing for minimizing compression across the lesion, while others have reported success with unloader bracing protecting the lesion18,96. Controversy over immobilization stems from incomplete understanding of the etiology. Those who believe OCD is localized to articular cartilage advocate maintaining motion for chondral health, whereas those who believe OCD originates from subchondral bone advocate immobilization to promote osseous healing17,125. Nomograms have been developed to predict the likelihood of healing with nonoperative treatment based on patient age, mechanical symptoms, lesion size, lesion location, and cyst-like lesions95,96,134.
Operative Treatment
Surgery is primarily indicated for patients with unstable lesions, failed conservative treatment, or poor nomogram predictions. The technique depends on the lesion characteristics. Concomitant pathology, including limb malalignment and joint instability, may require surgical correction (Fig. 7).
Fig. 7.
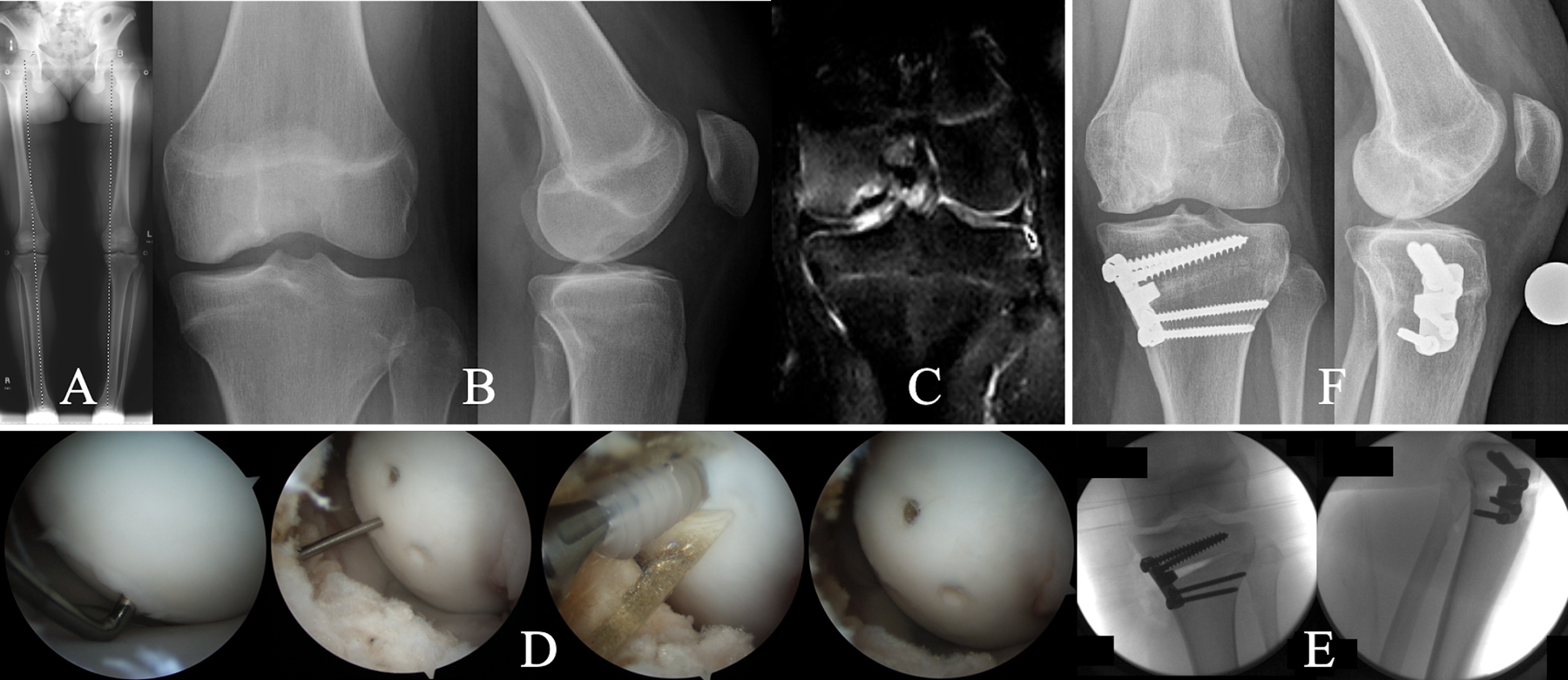
Figs. 7-A through 7-F A 14-year-old girl who underwent internal fixation with bioabsorbable headless compression screws and proximal tibial medial opening-wedge osteotomy for treatment of an OCD lesion on the medial femoral condyle with associated varus deformity at the knee. Fig. 7-A Preoperative full-length radiograph of the lower limbs, made with the patient standing, demonstrating left genu varum. Fig. 7-B Preoperative anteroposterior and lateral radiographs of the left knee reveal concave parent bone, suggesting a largely cartilaginous OCD lesion. Fig. 7-C Preoperative T2-weighted MRI demonstrating marrow edema and mild fluid at the parent-progeny interface. Fig. 7-D Arthroscopic photographs showing an OCD lesion delineated by unstable articular cartilage but not displaceable with gentle probing, implantation of 3-mm bioabsorbable headless compression screws, and final appearance of the articular surface. Fig. 7-E Fluoroscopy demonstrating a medial opening-wedge high tibial osteotomy. Fig. 7-F Radiographs, made 2 years postoperatively, showing interval healing of the lesion and consolidation of the osteotomy. The patient returned to all activities.
Stable Lesions
Subchondral drilling is well-established for stable lesions to stimulate influx of mesenchymal cells and growth factors135,136. Techniques include transarticular (Fig. 8) and retroarticular (transepiphyseal) drilling135,137. Retroarticular drilling avoids cartilage penetration but requires fluoroscopy. A systematic review concluded that there were comparable patient-oriented and radiographic outcomes138. Adjunctive bone-grafting has also been described139.
Fig. 8.
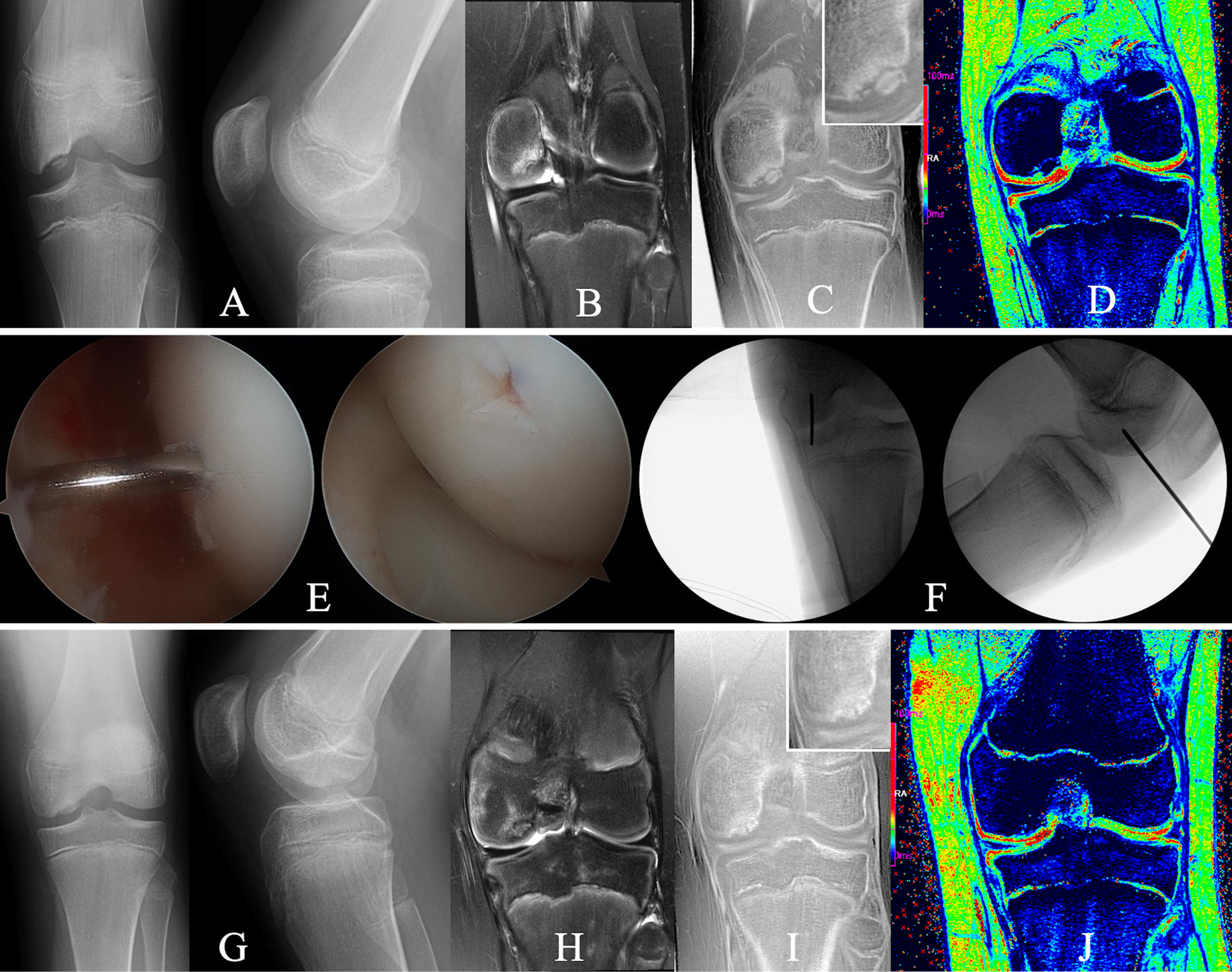
Figs. 8-A through 8-J An 11-year-old boy who underwent transarticular drilling for treatment of an OCD lesion in the medial femoral condyle of the left knee. Fig. 8-A Preoperative tunnel and lateral radiographs reveal an osseus OCD lesion. Figs. 8-B, 8-C, and 8-D Preoperative images made with advanced clinical 3-T MRI protocol, obtained with same imaging parameters as in Figure 3, including a T2-weighted coronal image demonstrating marrow edema (Fig. 8-B), a CT-like image contrast bone window (Fig. 8-C) depicting a partially ossified progeny lesion without evidence of osseous bridging with the parent bone (insert shows magnification of the lesion), and T2* map (Fig. 8-D), which provides additional information, specifically on cartilage integrity showing intact residual epiphyseal cartilage (green) and articular cartilage (red). Fig. 8-E Arthroscopic photographs made during transarticular drilling with a 0.045-in (0.114-cm) Kirschner wire to penetrate the lesion in a perpendicular fashion through the articular cartilage. Fig. 8-F Fluoroscopy confirming wire placement. Fig. 8-G Radiographs made 3 months postoperatively demonstrating interval healing. Figs. 8-H, 8-I, and 8-J Comprehensive MRI assessment demonstrating mild residual edema in parent bone and lesion in T2-weighted coronal MRI images (Fig. 8-H); solid osseous bridging and further ossification throughout the entire lesion in the CT-like contrast bone window images (insert shows magnification of the lesion) (Fig. 8-I), which was demonstrated on all slices (not shown); and preserved articular cartilage (red) after surgical intervention (see T2* map), which is mostly for further prognostication (Fig. 8-J). The patient returned to all activities.
Unstable Salvageable Lesions
Principles for treating unstable salvageable lesions include articular surface restoration, fracture fixation, and vascular enhancement121. Fixation devices include headless, partially threaded, variable-pitch compression screws, and bioabsorbable implants (Video 2). Metal screws can provide excellent purchase to help in healing but are dependent on lesion quality and tissue composition (e.g., cartilaginous versus osseous), may need to be removed before full weight-bearing, and interfere with MRI assessment140–143. Bioabsorbable implants are equally efficacious but can be associated with loosening, synovitis, and cyst formation144–147. Fixation using bone pegs or osteochondral plugs and hybrid techniques have been described with positive short-term outcomes148–151. Adjunctive strategies to address nonosseous tissues at the parent-progeny interface include debridement, marrow stimulation, and bone-grafting.
Unstable Unsalvageable Lesions
Lesions may be unsalvageable because of substantial osteochondral fragmentation, subchondral necrosis, or cartilage degeneration. Cartilage salvage and resurfacing techniques include marrow stimulation, autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OAT), and osteochondral allograft transplantation (OCA). Loose body excision alone yields poor outcomes152,153. Biomechanically inferior fibrocartilage formation is a shortcoming of marrow stimulation and ACI. Newer-generation ACI can treat deeper defects using bilayered collagen membranes interposed with chondrocytes154. Matrix-induced autologous chondrocyte implantation (MACI) addresses potential problems of uneven cell distribution or leakage by incorporating chondrocytes into collagen scaffolds155. OAT is biomechanically superior but limited by a maximum lesion size of 2 to 3 cm2 and donor site morbidity156,157. OCA is an alternative for larger lesions158–161.
Outcomes
Knee
Nonoperative success rates between 49% and 100% have been reported for skeletally immature patients with stable knee lesions8,12,16,95,96,101,123,162–165. Weiss et al. found that 33.5% (69) of 206 patients with juvenile OCD in the knee required surgery, and patients between the ages 12 and 19 years had a 7.4-times greater risk than 6 to 11-year-old patients166. Conversely, patients with adult OCD often require surgery17. Sanders et al. calculated that the cumulative rates of osteoarthritis and arthroplasty were 30% and 8%, respectively, at 35 years in 86 patients who had been treated nonoperatively167. A body mass index (BMI) of >25 kg/m2, patellar lesions, and adult onset were risk factors167. With multifocal lesions, Backes et al. found that only 25.5% (12) of 47 lesions healed conservatively after 12 months21. For stable lesions that had failure of nonoperative treatment, subchondral drilling has provided good outcomes136,138,168–175. Transarticular drilling in 23 children yielded radiographic healing at 4.4 months and improved functional outcomes176. Edmonds et al. performed retroarticular drilling in 59 children who had had failure after at least 6 months of nonoperative treatment of OCD lesions; they were able to return to activities at a mean of 2.8 months and 98.2% had radiographic healing177. For unstable salvageable lesions, Kocher et al. that found 84.6% (22) of 26 juvenile OCD lesions healed at 6 months after treatment with fixation, independent of the method or lesion location178. Similarly, Wu et al. reported that lesions in 76% (66) of 87 patients had healed with fixation at ≥2 years postoperatively despite skeletal maturity179. For unstable unsalvageable lesions, surgical outcomes have been encouraging180–184. Carey et al. reported that treatment of 55 patients (age range, 14 to 52 years) with ACI yielded 87% and 82% survivorship at 10 and 20 years, respectively, with 2 arthroplasties in the fifth and sixth decades185. Newer-generation techniques have also shown promise182,186,187. Roffi et al. described 19 children who were treated with MACI and bone-grafting, which provided functional improvement, but 16% of them had failure at 10 years155. A randomized clinical trial in which OAT was compared with microfracture in 50 children who had OCD (International Cartilage Repair Society [ICRS] grade III or IV) found superiority with OAT at 4.2 years188. Similarly, OCA achieved good short-term outcomes in a small cohort of children158. Emmerson et al. performed OCA in 64 adults (age range, 15 to 54 years), which resulted in good-to-excellent outcomes in 72% after 7.7 years; however, 15% required reoperation, including 3 arthroplasties (in patients who were 30, 36, and 54 years old), at 3 to 5.5 years159.
Elbow
Nonoperative treatment provides reliable healing for most early and stable capitellar lesions189–192. Risk factors include advanced skeletal age, larger lesions, cyst-like lesions, and radial head enlargement190,191,193. Matsuura et al. showed that compliance with discontinuing heavy elbow use for at least 6 months influenced outcomes, with healing in only 22.7% (17) of 75 noncompliant patients versus 78.6% (66) of 84 and 52.9% (9) of 17 compliant patients with Minami stage-I and II lesions, respectively189. For stable lesions that have failed nonoperative treatment, marrow stimulation has provided satisfactory outcomes194–198. Bexkens et al. reported good clinical results with microfracture at 1 year, with 62% (44) of 71 patients returning to sports199. For unstable salvageable lesions, reliable outcomes have been achieved with fragment fixation46,91,131,200. Hennrikus et al. reported that 77% (20) of 26 elbows healed with fixation, and 67% (12) of 18 questionnaire respondents returned to sports at 36 months201. For unstable unsalvageable lesions, osteochondral transfer techniques have demonstrated acceptable outcomes202–205. Bae et al., in a study of 28 patients treated with knee-to-elbow OAT, reported that 73% (19) of 26 patients had pain resolution and 100% (13) of 13 patients with >6 months of follow-up returned to sports at an average of 9 months206. Mirzayan and Lim performed OCA in 9 patients with juvenile OCD, and all had functional improvement and returned to sports after 2 years207. Lateral humeral condyle closing-wedge osteotomy, which is a less common technique, has shown promise208–210. Koda et al. performed 77 osteotomies for advanced lesions, with concomitant bone-grafting and fixation, yielding good remodeling in 69% (53) of 77 elbows; however, 53% (41) of the 77 elbows had osteoarthritic changes at 9 years209.
Talus
Skeletally immature patients and early talar lesions generally respond to nonoperative treatment15,54,211–213. Nonetheless, Perumal et al. found that 39% (12) of 31 children with OCD lesions (Berndt and Harty stages II, III, or IV) treated nonoperatively for 6 months eventually required surgery with drilling52. Similarly, Letts et al. reported that 54% (13) of 24 children with juvenile OCD (Berndt and Harty stages I through IV) had failure of conservative management and required surgery with a combination of drilling, pinning, and excision53. For osteochondral lesions of <150 mm2, microfracture has demonstrated positive outcomes214–220. Choi et al. reported that 165 osteochondral lesions (mean size, 73 mm2) treated with microfracture provided significant improvements in functional scores at 6.7 years221. Subchondral drilling has shown comparable results222–225. Masquijo et al. performed retroarticular drilling in 6 children with OCD lesions (Berndt and Harty stage I or II), and they showed improvements at 37 months226. For osteochondral lesions of >150 mm2, advanced cartilage restoration techniques are available and feasible227–232. Kwak et al. described ACI for 29 osteochondral lesions (mean size, 198 mm2) and reported that 79% (23) of 29 patients had good-to-excellent outcomes at 70 months233. A systematic review comprising 343 patients with lesions of <250 mm2 demonstrated equivalence between MACI and ACI234. Additionally, a study of 9 patients with osteochondral lesions treated with MACI after failing microfracture found substantial functional improvements at 7 years235. OAT has also demonstrated favorable outcomes236–242. Lee et al. reported on 17 patients with OCD (Berndt and Harty stage III or IV) who were managed with knee-to-ankle OAT and had good-to-excellent outcomes at 36 months243. OCA has provided comparable success244–248. VanTienderen et al. performed a systematic review comprising 91 osteochondral lesions treated with OCA and demonstrated improvements at 45 months249.
Overview
OCD is a relatively common cause of joint pain and dysfunction that may lead to premature osteoarthritis. The etiology is being elucidated by clinical and cadaveric studies, animal models, advanced imaging, and genetic analyses. Improved understanding will guide treatment and identify risk factors that can be addressed to reduce the prevalence of the disorder. Similar management principles are applicable to OCD of the knee, elbow, and talus. Skeletally immature patients with stable lesions usually improve with nonoperative treatment, whereas those who have failure of conservative management likely respond to arthroscopic surgery. Older patients and those with unstable lesions often require surgical intervention, and various techniques exist to stimulate subchondral revascularization, promote parent-progeny healing, restore articular congruity, and offload the lesion. Newer-generation strategies, including tissue-engineered scaffolds and biologics, are promising. Outcomes have room for improvement, and prospective randomized trials are needed to determine optimal management protocols. The current best evidence on highlighted topics related to OCD is summarized in Table IV.
TABLE IV.
Grades of Recommendation for OCD*
| Recommendation | Grade† |
|---|---|
| OCD occurs most frequently in the active pediatric and young adult populations, commonly affecting the knee, elbow, or ankle and may lead to premature osteoarthritis. | A |
| While generally considered an idiopathic phenomenon, various etiopathogenetic theories are being investigated, including local ischemia, aberrant endochondral ossification of the secondary subarticular physis, repetitive microtrauma, and genetic predisposition. | B |
| Diagnosis is based on the history, physical examination, radiography, and advanced imaging, with elbow ultrasonography and novel MRI protocols potentially enabling early detection and staging. | A |
| Treatment largely depends on skeletal maturity and lesion stability, defined by the presence or absence of articular cartilage fracture and subchondral bone separation, as determined by imaging and arthroscopy, and is typically nonoperative for stable lesions in skeletally immature patients and operative for those who have failed conservative management or have unstable lesions. | B |
| Clinical practice guidelines have been limited by a paucity of high-level evidence, but a multicenter effort is ongoing to develop accurate and reliable classification systems and multimodal decision-making algorithms with prognostic value. | A |
| There is currently no association between gadolinium deposition in the brain and poorer health outcomes in children; however, gadolinium-based contrast agents should be used judiciously and avoided when possible. | I |
OCD = osteochondritis dissecans, and MRI = magnetic resonance imaging.
According to Wright264, grade A indicates good evidence (Level-I studies with consistent findings) for or against recommending intervention; grade B, fair evidence (Level-II or III studies with consistent findings) for or against recommending intervention; grade C, poor-quality evidence (Level-IV or V studies with consistent findings) for or against recommending intervention; and grade I, insufficient or conflicting evidence not allowing a recommendation for or against intervention.
Supplementary Material
Video 1
Three-dimensional reconstruction of susceptibility-weighted MRI data obtained from a distal femoral specimen from a 3-month-old human cadaveric donor demonstrating central and peripheral vascular beds with a watershed region in the midsagittal plane.
Video 2
A 14-year-old female patient undergoing internal fixation with bioabsorbable headless compression screws for treatment of an osteochondritis dissecans lesion on the medial femoral condyle.
Osteochondritis dissecans occurs most frequently in the active pediatric and young adult populations, commonly affecting the knee, elbow, or ankle, and may lead to premature osteoarthritis.
While generally considered an idiopathic phenomenon, various etiopathogenetic theories are being investigated, including local ischemia, aberrant endochondral ossification of the secondary subarticular physis, repetitive microtrauma, and genetic predisposition.
Diagnosis is based on the history, physical examination, radiography, and advanced imaging, with elbow ultrasonography and novel magnetic resonance imaging protocols potentially enabling early detection and in-depth staging.
Treatment largely depends on skeletal maturity and lesion stability, defined by the presence or absence of articular cartilage fracture and subchondral bone separation, as determined by imaging and arthroscopy, and is typically nonoperative for stable lesions in skeletally immature patients and operative for those who have had failure of conservative management or have unstable lesions.
Clinical practice guidelines have been limited by a paucity of high-level evidence, but a multicenter effort is ongoing to develop accurate and reliable classification systems and multimodal decision-making algorithms with prognostic value.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (R01 AR070020 and K01 OD021293).
Footnotes
Investigation performed at the Department of Orthopedic Surgery, University of Minnesota, Minneapolis, Minnesota
On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/XXXXXXX).
References
- 1.König F The classic: On loose bodies in the joint. 1887. Clin Orthop Relat Res 2013. April;471(4):1107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federico DJ, Lynch JK, Jokl P. Osteochondritis dissecans of the knee: a historical review of etiology and treatment. Arthroscopy 1990;6(3):190–7. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds EW, Shea KG. Osteochondritis dissecans: editorial comment. Clin Orthop Relat Res 2013. April;471(4):1105–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall E, Von Stein D. Juvenile osteochondritis dissecans. Orthop Clin North Am 2003. July;34(3):341–53. [DOI] [PubMed] [Google Scholar]
- 5.Schenck RC Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am 1996. March;78(3):439–56. [PubMed] [Google Scholar]
- 6.Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res 2013. April;471(4):1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg 2006. February;14(2):90–100. [DOI] [PubMed] [Google Scholar]
- 8.Cahill BR. Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg 1995. July;3(4):237–47. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JM, Shea KG, Jacobs JC Jr, Cannamela PC, Becker I, Portman M, Kessler JI. Incidence of osteochondritis dissecans in adults. Am J Sports Med 2018. June;46(7):1592–5. Epub 2018 Apr 3. [DOI] [PubMed] [Google Scholar]
- 10.Lindén B The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand 1976. December;47(6):664–7. [DOI] [PubMed] [Google Scholar]
- 11.Kessler JI, Nikizad H, Shea KG, Jacobs JC Jr, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med 2014. February;42(2):320–6. Epub 2013 Nov 22. [DOI] [PubMed] [Google Scholar]
- 12.Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am 1984. December;66(9):1340–8. [PubMed] [Google Scholar]
- 13.Kessler JI, Weiss JM, Nikizad H, Gyurdzhyan S, Jacobs JC Jr, Bebchuk JD, Shea KG. Osteochondritis dissecans of the ankle in children and adolescents: demographics and epidemiology. Am J Sports Med 2014. September;42(9):2165–71. Epub 2014 Jul 2. [DOI] [PubMed] [Google Scholar]
- 14.Kessler JI, Jacobs JC Jr, Cannamela PC, Weiss JM, Shea KG. Demographics and epidemiology of osteochondritis dissecans of the elbow among children and adolescents. Orthop J Sports Med 2018. December 19;6(12):2325967118815846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer M, Jonsson K, Lindén B. Osteochondritis dissecans of the ankle. A 20-year follow-up study. J Bone Joint Surg Br 1987. January;69(1):93–6. [DOI] [PubMed] [Google Scholar]
- 16.Hefti F, Beguiristain J, Krauspe R, Möller-Madsen B, Riccio V, Tschauner C, Wetzel R, Zeller R. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B 1999. October;8(4):231–45. [PubMed] [Google Scholar]
- 17.Cruz AI Jr, Shea KG, Ganley TJ. Pediatric knee osteochondritis dissecans lesions. Orthop Clin North Am 2016. October;47(4):763–75. Epub 2016 Aug 6. [DOI] [PubMed] [Google Scholar]
- 18.Heyworth BE, Kocher MS. Osteochondritis dissecans of the knee. JBJS Rev 2015. July 7;3(7):01874474-201503070-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med 2006. July;34(7):1181–91. [DOI] [PubMed] [Google Scholar]
- 20.Aichroth P Osteochondritis dissecans of the knee. A clinical survey. J Bone Joint Surg Br 1971. August;53(3):440–7. [PubMed] [Google Scholar]
- 21.Backes JR, Durbin TC, Bentley JC, Klingele KE. Multifocal juvenile osteochondritis dissecans of the knee: a case series. J Pediatr Orthop 2014. June;34(4):453–8. [DOI] [PubMed] [Google Scholar]
- 22.Ruchelsman DE, Hall MP, Youm T. Osteochondritis dissecans of the capitellum: current concepts. J Am Acad Orthop Surg 2010. September;18(9):557–67. [DOI] [PubMed] [Google Scholar]
- 23.Cooper T, Boyles A, Samora WP, Klingele KE. Prevalence of bilateral JOCD of the knee and associated risk factors. J Pediatr Orthop 2015. July-Aug;35(5):507–10. [DOI] [PubMed] [Google Scholar]
- 24.Wall EJ, Heyworth BE, Shea KG, Edmonds EW, Wright RW, Anderson AF, Eismann EA, Myer GD. Trochlear groove osteochondritis dissecans of the knee patellofemoral joint. J Pediatr Orthop 2014. September;34(6):625–30. [DOI] [PubMed] [Google Scholar]
- 25.Kida Y, Morihara T, Kotoura Y, Hojo T, Tachiiri H, Sukenari T, Iwata Y, Furukawa R, Oda R, Arai Y, Fujiwara H, Kubo T. Prevalence and clinical characteristics of osteochondritis dissecans of the humeral capitellum among adolescent baseball players. Am J Sports Med 2014. August;42(8):1963–71. Epub 2014 Jun 18. [DOI] [PubMed] [Google Scholar]
- 26.Kessler JI, Jacobs JC Jr, Cannamela PC, Shea KG, Weiss JM. Childhood obesity is associated with osteochondritis dissecans of the knee, ankle, and elbow in children and adolescents. J Pediatr Orthop 2018. May/Jun;38(5):e296–9. [DOI] [PubMed] [Google Scholar]
- 27.Pareek A, Sanders TL, Wu IT, Larson DR, Saris DBF, Krych AJ. Incidence of symptomatic osteochondritis dissecans lesions of the knee: a population-based study in Olmsted County. Osteoarthritis Cartilage 2017. October;25(10):1663–71. Epub 2017 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nissen CW. Osteochondritis dissecans of the elbow. Clin Sports Med 2014. April;33(2):251–65. Epub 2014 Jan 10. [DOI] [PubMed] [Google Scholar]
- 29.Rogers DL, Klyce W, Kajstura T, Lee RJ. Adolescent obesity is associated with more severe presentations of osteochondritis dissecans of the knee. Orthop J Sports Med 2019;7(3 Suppl): 2325967119S00033. [Google Scholar]
- 30.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Epiphyseal cartilage canal blood supply to the tarsus of foals and relationship to osteochondrosis. Equine Vet J 2008. January;40(1):30–9. [DOI] [PubMed] [Google Scholar]
- 31.Carlson CS, Meuten DJ, Richardson DC. Ischemic necrosis of cartilage in spontaneous and experimental lesions of osteochondrosis. J Orthop Res 1991. May;9(3):317–29. [DOI] [PubMed] [Google Scholar]
- 32.Olstad K, Ekman S, Carlson CS. An update on the pathogenesis of osteochondrosis. Vet Pathol 2015. September;52(5):785–802. Epub 2015 Jun 16. [DOI] [PubMed] [Google Scholar]
- 33.Shea KG, Jacobs JC Jr, Carey JL, Anderson AF, Oxford JT. Osteochondritis dissecans knee histology studies have variable findings and theories of etiology. Clin Orthop Relat Res 2013. April;471(4):1127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusumi T, Ishibashi Y, Tsuda E, Kusumi A, Tanaka M, Sato F, Toh S, Kijima H. Osteochondritis dissecans of the elbow: histopathological assessment of the articular cartilage and subchondral bone with emphasis on their damage and repair. Pathol Int 2006. October;56(10):604–12. [DOI] [PubMed] [Google Scholar]
- 35.Kamhi E, MacEwen GD. Osteochondritis dissecans in Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1975. June;57(4):506–9. [PubMed] [Google Scholar]
- 36.Kobayashi K, Burton KJ, Rodner C, Smith B, Caputo AE. Lateral compression injuries in the pediatric elbow: Panner’s disease and osteochondritis dissecans of the capitellum. J Am Acad Orthop Surg 2004. July-Aug;12(4):246–54. [DOI] [PubMed] [Google Scholar]
- 37.Andriolo L, Crawford DC, Reale D, Zaffagnini S, Candrian C, Cavicchioli A, Filardo G. Osteochondritis dissecans of the knee: etiology and pathogenetic mechanisms. A systematic review. Cartilage 2020. July;11(3):273–90. Epub 2018 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tóth F, Nissi MJ, Ellermann JM, Wang L, Shea KG, Polousky J, Carlson CS. Novel application of magnetic resonance imaging demonstrates characteristic differences in vasculature at predilection sites of osteochondritis dissecans. Am J Sports Med 2015. October;43(10):2522–7. Epub 2015 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellermann JM, Ludwig KD, Nissi MJ, Johnson CP, Strupp JP, Wang L, Zbýň Š, Tóth F, Arendt E, Tompkins M, Shea K, Carlson CS. Three-dimensional quantitative magnetic resonance imaging of epiphyseal cartilage vascularity using vessel image features: new insights into juvenile osteochondritis dissecans. JB JS Open Access 2019. December 5;4(4):e0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tóth F, Nissi MJ, Wang L, Ellermann JM, Carlson CS. Surgical induction, histological evaluation, and MRI identification of cartilage necrosis in the distal femur in goats to model early lesions of osteochondrosis. Osteoarthritis Cartilage 2015. February;23(2):300–7. Epub 2014 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of osteochondrosis in the distal tibia of foals. J Orthop Res 2007. August;25(8):1094–105. [DOI] [PubMed] [Google Scholar]
- 42.Tóth F, Tompkins MA, Shea KG, Ellermann JM, Carlson CS. Identification of areas of epiphyseal cartilage necrosis at predilection sites of juvenile osteochondritis dissecans in pediatric cadavers. J Bone Joint Surg Am 2018. December 19;100(24):2132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCoy AM, Toth F, Dolvik NI, Ekman S, Ellermann J, Olstad K, Ytrehus B, Carlson CS. Articular osteochondrosis: a comparison of naturally-occurring human and animal disease. Osteoarthritis Cartilage 2013. November;21(11):1638–47. Epub 2013 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lomax A, Miller RJ, Fogg QA, Jane Madeley N, Senthil Kumar C. Quantitative assessment of the subchondral vascularity of the talar dome: a cadaveric study. Foot Ankle Surg 2014. March;20(1):57–60. Epub 2013 Oct 31. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi K, Sweet FA, Bindra R, Morrey BF, Gelberman RH. The extraosseous and intraosseous arterial anatomy of the adult elbow. J Bone Joint Surg Am 1997. November;79(11):1653–62. [DOI] [PubMed] [Google Scholar]
- 46.Baker CL 3rd, Romeo AA, Baker CL Jr. Osteochondritis dissecans of the capitellum. Am J Sports Med 2010. September;38(9):1917–28. Epub 2010 Jan 23. [DOI] [PubMed] [Google Scholar]
- 47.Ribbing S The hereditary multiple epiphyseal disturbance and its consequences for the aetiogenesis of local malacias—particularly the osteochondrosis dissecans. Acta Orthop Scand 1955;24(4):286–99. [DOI] [PubMed] [Google Scholar]
- 48.Barrie HJ. Hypertrophy and laminar calcification of cartilage in loose bodies as probable evidence of an ossification abnormality. J Pathol 1980. October;132(2):161–8. [DOI] [PubMed] [Google Scholar]
- 49.Barrie HJ. Hypothesis—a diagram of the form and origin of loose bodies in osteochondritis dissecans. J Rheumatol 1984. August;11(4):512–3. [PubMed] [Google Scholar]
- 50.Laor T, Zbojniewicz AM, Eismann EA, Wall EJ. Juvenile osteochondritis dissecans: is it a growth disturbance of the secondary physis of the epiphysis? AJR Am J Roentgenol 2012. November;199(5):1121–8. [DOI] [PubMed] [Google Scholar]
- 51.Ellermann J, Johnson CP, Wang L, Macalena JA, Nelson BJ, LaPrade RF. Insights into the epiphyseal cartilage origin and subsequent osseous manifestation of juvenile osteochondritis dissecans with a modified clinical MR imaging protocol: a pilot study. Radiology 2017. March;282(3):798–806. Epub 2016 Sep 15. [DOI] [PubMed] [Google Scholar]
- 52.Perumal V, Wall E, Babekir N. Juvenile osteochondritis dissecans of the talus. J Pediatr Orthop 2007. October-Nov;27(7):821–5. [DOI] [PubMed] [Google Scholar]
- 53.Letts M, Davidson D, Ahmer A. Osteochondritis dissecans of the talus in children. J Pediatr Orthop 2003. September-Oct;23(5):617–25. [DOI] [PubMed] [Google Scholar]
- 54.Higuera J, Laguna R, Peral M, Aranda E, Soleto J. Osteochondritis dissecans of the talus during childhood and adolescence. J Pediatr Orthop 1998. May-Jun;18(3):328–32. [PubMed] [Google Scholar]
- 55.McElroy MJ, Riley PM, Tepolt FA, Nasreddine AY, Kocher MS. Catcher’s knee: posterior femoral condyle juvenile osteochondritis dissecans in children and adolescents. J Pediatr Orthop 2018. September;38(8):410–7. [DOI] [PubMed] [Google Scholar]
- 56.Price MJ, Tuca M, Nguyen J, Silberman J, Luderowski E, Uppstrom TJ, Green DW. Juvenile osteochondritis dissecans of the trochlea: a cohort study of 34 trochlear lesions associated with sporting activities that load the patellofemoral joint. J Pediatr Orthop 2020. March;40(3):103–9. [DOI] [PubMed] [Google Scholar]
- 57.Chow RM, Guzman MS, Dao Q. Intercondylar notch width as a risk factor for medial femoral condyle osteochondritis dissecans in skeletally immature patients. J Pediatr Orthop 2016. September;36(6):640–4. [DOI] [PubMed] [Google Scholar]
- 58.Wechter JF, Sikka RS, Alwan M, Nelson BJ, Tompkins M. Proximal tibial morphology and its correlation with osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc 2015. December;23(12):3717–22. Epub 2014 Sep 25. [DOI] [PubMed] [Google Scholar]
- 59.Nambu T, Gasser B, Schneider E, Bandi W, Perren SM. Deformation of the distal femur: a contribution towards the pathogenesis of osteochondrosis dissecans in the knee joint. J Biomech 1991;24(6):421–33. [DOI] [PubMed] [Google Scholar]
- 60.Cavaignac E, Perroncel G, Thépaut M, Vial J, Accadbled F, De Gauzy JS. Relationship between tibial spine size and the occurrence of osteochondritis dissecans: an argument in favour of the impingement theory. Knee Surg Sports Traumatol Arthrosc 2017. August;25(8):2442–6. Epub 2015 Dec 12. [DOI] [PubMed] [Google Scholar]
- 61.Ishikawa M, Adachi N, Yoshikawa M, Nakamae A, Nakasa T, Ikuta Y, Hayashi S, Deie M, Ochi M. Unique anatomic feature of the posterior cruciate ligament in knees associated with osteochondritis dissecans. Orthop J Sports Med 2016. May 27;4(5):2325967116648138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fairbanks H Osteo-chondritis dissecans. Br J Surg 1933;21(81):67–82. [Google Scholar]
- 63.Smillie IS. Treatment of osteochondritis dissecans. J Bone Joint Surg Br 1957. May;39-B(2):248–60. [DOI] [PubMed] [Google Scholar]
- 64.Mitsuoka T, Shino K, Hamada M, Horibe S. Osteochondritis dissecans of the lateral femoral condyle of the knee joint. Arthroscopy 1999. January-Feb;15(1):20–6. [DOI] [PubMed] [Google Scholar]
- 65.Brown ML, McCauley JC, Gracitelli GC, Bugbee WD. Osteochondritis dissecans lesion location is highly concordant with mechanical axis deviation. Am J Sports Med 2020. March;48(4):871–5. [DOI] [PubMed] [Google Scholar]
- 66.Jacobi M, Wahl P, Bouaicha S, Jakob RP, Gautier E. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: radiographic study on 103 knees. Am J Sports Med 2010. July;38(7):1425–8. Epub 2010 Mar 29. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Herranz P, Rodriguez ML, de la Fuente C. Femoral osteochondritis of the knee: prognostic value of the mechanical axis. J Child Orthop 2017;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res 1979. May;(140):131–6. [PubMed] [Google Scholar]
- 69.Petrie PW. Aetiology of osteochondritis dissecans. Failure to establish a familial background. J Bone Joint Surg Br 1977. August;59(3):366–7. [DOI] [PubMed] [Google Scholar]
- 70.Matsuura T, Wada K, Suzue N, Iwame T, Fukuta S, Sairyo K. Bilateral osteochondritis dissecans of the capitellum in fraternal twins: a case report. JBJS Case Connect 2017. July-Sep;7(3):e44. [DOI] [PubMed] [Google Scholar]
- 71.Mei-Dan O, Mann G, Steinbacher G, Cugat RB, Alvarez PD. Bilateral osteochondritis dissecans of the knees in monozygotic twins: the genetic factor and review of the etiology. Am J Orthop (Belle Mead NJ) 2009. September;38(9):E152–5. [PubMed] [Google Scholar]
- 72.Richie LB, Sytsma MJ. Matching osteochondritis dissecans lesions in identical twin brothers. Orthopedics 2013. September;36(9):e1213–6. [DOI] [PubMed] [Google Scholar]
- 73.Gans I, Sarkissian EJ, Grant SFA, Ganley TJ. Identical osteochondritis dissecans lesions of the knee in sets of monozygotic twins. Orthopedics 2013. December;36(12):e1559–62. [DOI] [PubMed] [Google Scholar]
- 74.Mackie T, Wilkins RM. Case report: Osteochondritis dissecans in twins: treatment with fresh osteochondral grafts. Clin Orthop Relat Res 2010. March;468(3):893–7. Epub 2009 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kenniston JA, Beredjiklian PK, Bozentka DJ. Osteochondritis dissecans of the capitellum in fraternal twins: case report. J Hand Surg Am 2008. October;33(8):1380–3. Epub 2008. [DOI] [PubMed] [Google Scholar]
- 76.Woods K, Harris I. Osteochondritis dissecans of the talus in identical twins. J Bone Joint Surg Br 1995. March;77(2):331. [PubMed] [Google Scholar]
- 77.Hammett RB, Saxby TS. Osteochondral lesion of the talus in homozygous twins—the question of heredity. Foot Ankle Surg 2010. September;16(3):e55–6. Epub 2010 Apr 13. [DOI] [PubMed] [Google Scholar]
- 78.Gornitzky AL, Mistovich RJ, Atuahuene B, Storey EP, Ganley TJ. Osteochondritis dissecans lesions in family members: does a positive family history impact phenotypic potency? Clin Orthop Relat Res 2017. June;475(6):1573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tobin WJ. Familial osteochondritis dissecans with associated tibia vara. J Bone Joint Surg Am 1957. October;39-A(5):1091–105. [PubMed] [Google Scholar]
- 80.White J Osteochondritis dissecans in association with dwarfism. J Bone Joint Surg Br 1957. May;39-B(2):261–7. [DOI] [PubMed] [Google Scholar]
- 81.Al Kaissi A, Klaushofer K, Grill F. Osteochondritis dissecans and Osgood Schlatter disease in a family with Stickler syndrome. Pediatr Rheumatol Online J 2009. February 4;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jackson GC, Marcus-Soekarman D, Stolte-Dijkstra I, Verrips A, Taylor JA, Briggs MD. Type IX collagen gene mutations can result in multiple epiphyseal dysplasia that is associated with osteochondritis dissecans and a mild myopathy. Am J Med Genet A 2010. April;152A(4):863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg A. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet 2010. February 12;86(2):126–37. Epub 2010 Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gkourogianni A, Andrew M, Tyzinski L, Crocker M, Douglas J, Dunbar N, Fairchild J, Funari MF, Heath KE, Jorge AA, Kurtzman T, LaFranchi S, Lalani S, Lebl J, Lin Y, Los E, Newbern D, Nowak C, Olson M, Popovic J, Pruhová Š, Elblova L, Quintos JB, Segerlund E, Sentchordi L, Shinawi M, Stattin EL, Swartz J, Angel AG, Cuéllar SD, Hosono H, Sanchez-Lara PA, Hwa V, Baron J, Nilsson O, Dauber A. Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. J Clin Endocrinol Metab 2017. February 1;102(2):460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stattin EL, Tegner Y, Domellöf M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage 2008. August;16(8):890–6. Epub 2008 Jan 15. [DOI] [PubMed] [Google Scholar]
- 86.Yellin JL, Trocle A, Grant SFA, Hakonarson H, Shea KG, Ganley TJ. Candidate loci are revealed by an initial genome-wide association study of juvenile osteochondritis dissecans. J Pediatr Orthop 2017. January;37(1):e32–6. [DOI] [PubMed] [Google Scholar]
- 87.Bates JT, Jacobs JC Jr, Shea KG, Oxford JT. Emerging genetic basis of osteochondritis dissecans. Clin Sports Med 2014. April;33(2):199–220. Epub 2014 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conrad JM, Stanitski CL. Osteochondritis dissecans: Wilson’s sign revisited. Am J Sports Med 2003. September-Oct;31(5):777–8. [DOI] [PubMed] [Google Scholar]
- 89.Bauer KL, Polousky JD. Management of osteochondritis dissecans lesions of the knee, elbow and ankle. Clin Sports Med 2017. July;36(3):469–87. Epub 2017 Mar 11. [DOI] [PubMed] [Google Scholar]
- 90.Steele JR, Dekker TJ, Federer AE, Liles JL, Adams SB, Easley ME. Osteochondral lesions of the talus: current concepts in diagnosis and treatment. Foot Ankle Orthop 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Churchill RW, Munoz J, Ahmad CS. Osteochondritis dissecans of the elbow. Curr Rev Musculoskelet Med 2016. June;9(2):232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Looze CA, Capo J, Ryan MK, Begly JP, Chapman C, Swanson D, Singh BC, Strauss EJ. Evaluation and management of osteochondral lesions of the talus. Cartilage 2017. January;8(1):19–30. Epub 2016 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maruyama M, Takahara M, Satake H. Diagnosis and treatment of osteochondritis dissecans of the humeral capitellum. J Orthop Sci 2018. March;23(2):213–9. Epub 2017 Dec 22. [DOI] [PubMed] [Google Scholar]
- 94.Cahill BR, Phillips MR, Navarro R. The results of conservative management of juvenile osteochondritis dissecans using joint scintigraphy. A prospective study. Am J Sports Med 1989. September-Oct;17(5):601–5, discussion :605–6. [DOI] [PubMed] [Google Scholar]
- 95.Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med 2013. October;41(10):2384–91. Epub 2013 Jul 22. [DOI] [PubMed] [Google Scholar]
- 96.Wall EJ, Vourazeris J, Myer GD, Emery KH, Divine JG, Nick TG, Hewett TE. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am 2008. December;90(12):2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Smet AA, Ilahi OA, Graf BK. Untreated osteochondritis dissecans of the femoral condyles: prediction of patient outcome using radiographic and MR findings. Skeletal Radiol 1997. August;26(8):463–7. [DOI] [PubMed] [Google Scholar]
- 98.Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop 2003. January-Feb;23(1):102–8. [PubMed] [Google Scholar]
- 99.Wall EJ, Polousky JD, Shea KG, Carey JL, Ganley TJ, Grimm NL, Jacobs JC Jr, Edmonds EW, Eismann EA, Anderson AF, Heyworth BE, Lyon R; Research on OsteoChondritis Dissecans of the Knee (ROCK) Study Group. Novel radiographic feature classification of knee osteochondritis dissecans: a multicenter reliability study. Am J Sports Med 2015. February;43(2):303–9. Epub 2015 Jan 12. [DOI] [PubMed] [Google Scholar]
- 100.Bruns J, Werner M, Habermann C. Osteochondritis dissecans: etiology, pathology, and imaging with a special focus on the knee joint. Cartilage 2018. October;9(4):346–62. Epub 2017 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshida S, Ikata T, Takai H, Kashiwaguchi S, Katoh S, Takeda Y. Osteochondritis dissecans of the femoral condyle in the growth stage. Clin Orthop Relat Res 1998. January;(346):162–70. [PubMed] [Google Scholar]
- 102.O’Connor MA, Palaniappan M, Khan N, Bruce CE. Osteochondritis dissecans of the knee in children. A comparison of MRI and arthroscopic findings. J Bone Joint Surg Br 2002. March;84(2):258–62. [DOI] [PubMed] [Google Scholar]
- 103.Accadbled F, Vial J, Sales de Gauzy J. Osteochondritis dissecans of the knee. Orthop Traumatol Surg Res 2018. February;104(1S)(Supplement):S97–105. Epub 2017 Nov 29. [DOI] [PubMed] [Google Scholar]
- 104.Moktassi A, Popkin CA, White LM, Murnaghan ML. Imaging of osteochondritis dissecans. Orthop Clin North Am 2012. April;43(2):201–11: v-vi. Epub 2012 Feb 21. [DOI] [PubMed] [Google Scholar]
- 105.Kramer J, Stiglbauer R, Engel A, Prayer L, Imhof H. MR contrast arthrography (MRA) in osteochondrosis dissecans. J Comput Assist Tomogr 1992. March-Apr;16(2):254–60. [DOI] [PubMed] [Google Scholar]
- 106.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017. July;16(7):564–70. Epub 2017 Jun 13. [DOI] [PubMed] [Google Scholar]
- 107.Blumfield E, Moore MM, Drake MK, Goodman TR, Lewis KN, Meyer LT, Ngo TD, Sammet C, Stanescu AL, Swenson DW, Slovis TL, Iyer RS. Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a Quality and Safety Committee report. Pediatr Radiol 2017. May;47(6):665–73. Epub 2017 Mar 10. [DOI] [PubMed] [Google Scholar]
- 108.Hu H, Zhang C, Chen J, Li P, Zhang XE, Deng Z, Du Y. Clinical value of MRI in assessing the stability of osteochondritis dissecans lesions: a systematic review and meta-analysis. AJR Am J Roentgenol 2019. July;213(1):147–54. Epub 2019 Apr 17. [DOI] [PubMed] [Google Scholar]
- 109.Ellermann JM, Donald B, Rohr S, Takahashi T, Tompkins M, Nelson B, Crawford A, Rud C, Macalena J. Magnetic resonance imaging of osteochondritis dissecans: validation study for the ICRS classification system. Acad Radiol 2016. June;23(6):724–9. Epub 2016 Mar 11. [DOI] [PubMed] [Google Scholar]
- 110.Samora WP, Chevillet J, Adler B, Young GS, Klingele KE. Juvenile osteochondritis dissecans of the knee: predictors of lesion stability. J Pediatr Orthop 2012. January-Feb;32(1):1–4. [DOI] [PubMed] [Google Scholar]
- 111.De Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for stability of osteochondritis dissecans in the knee and ankle. Skeletal Radiol 1996. February;25(2):159–63. [DOI] [PubMed] [Google Scholar]
- 112.Kijowski R, Blankenbaker DG, Shinki K, Fine JP, Graf BK, De Smet AA. Juvenile versus adult osteochondritis dissecans of the knee: appropriate MR imaging criteria for instability. Radiology 2008. August;248(2):571–8. Epub 2008 Jun 13. [DOI] [PubMed] [Google Scholar]
- 113.Verhagen RA, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br 2005. January;87(1):41–6. [PubMed] [Google Scholar]
- 114.Holland P, Davies AM, Cassar-Pullicino VN. Computed tomographic arthrography in the assessment of osteochondritis dissecans of the elbow. Clin Radiol 1994. April;49(4):231–5. [DOI] [PubMed] [Google Scholar]
- 115.Takahara M, Ogino T, Tsuchida H, Takagi M, Kashiwa H, Nambu T. Sonographic assessment of osteochondritis dissecans of the humeral capitellum. AJR Am J Roentgenol 2000. February;174(2):411–5. [DOI] [PubMed] [Google Scholar]
- 116.Harada M, Takahara M, Sasaki J, Mura N, Ito T, Ogino T. Using sonography for the early detection of elbow injuries among young baseball players. AJR Am J Roentgenol 2006. December;187(6):1436–41. [DOI] [PubMed] [Google Scholar]
- 117.Takenaga T, Goto H, Nozaki M, Yoshida M, Nishiyama T, Otsuka T. Ultrasound imaging of the humeral capitellum: a cadaveric study. J Orthop Sci 2014. November;19(6):907–12. Epub 2014 Sep 8. [DOI] [PubMed] [Google Scholar]
- 118.Yoshizuka M, Sunagawa T, Nakashima Y, Shinomiya R, Masuda T, Makitsubo M, Adachi N. Comparison of sonography and MRI in the evaluation of stability of capitellar osteochondritis dissecans. J Clin Ultrasound 2018. May;46(4):247–52. Epub 2017 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahara M, Shundo M, Kondo M, Suzuki K, Nambu T, Ogino T. Early detection of osteochondritis dissecans of the capitellum in young baseball players. Report of three cases. J Bone Joint Surg Am 1998. June;80(6):892–7. [DOI] [PubMed] [Google Scholar]
- 120.Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology 2000. July;216(1):207–12. [DOI] [PubMed] [Google Scholar]
- 121.Cahill B Treatment of juvenile osteochondritis dissecans and osteochondritis dissecans of the knee. Clin Sports Med 1985. April;4(2):367–84. [PubMed] [Google Scholar]
- 122.Litchman HM, McCullough RW, Gandsman EJ, Schatz SL. Computerized blood flow analysis for decision making in the treatment of osteochondritis dissecans. J Pediatr Orthop 1988. March-Apr;8(2):208–12. [PubMed] [Google Scholar]
- 123.Paletta GA Jr, Bednarz PA, Stanitski CL, Sandman GA, Stanitski DF, Kottamasu S. The prognostic value of quantitative bone scan in knee osteochondritis dissecans. A preliminary experience. Am J Sports Med 1998. January-Feb;26(1):7–14. [DOI] [PubMed] [Google Scholar]
- 124.Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med 1993. January-Feb;21(1):13–9. [DOI] [PubMed] [Google Scholar]
- 125.Chan C, Richmond C, Shea KG, Frick SL. Management of osteochondritis dissecans of the femoral condyle: a critical analysis review. JBJS Rev 2018. March;6(3):e5. [DOI] [PubMed] [Google Scholar]
- 126.Schulz JF, Chambers HG. Juvenile osteochondritis dissecans of the knee: current concepts in diagnosis and management. Instr Course Lect 2013;62:455–67. [PubMed] [Google Scholar]
- 127.Li X, Johnson CP, Ellermann J. Measuring knee bone marrow perfusion using arterial spin labeling at 3 T. Sci Rep 2020. March 24;10(1):5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacobs JC Jr, Archibald-Seiffer N, Grimm NL, Carey JL, Shea KG. A review of arthroscopic classification systems for osteochondritis dissecans of the knee. Orthop Clin North Am 2015. January;46(1):133–9. [DOI] [PubMed] [Google Scholar]
- 129.Carey JL, Wall EJ, Grimm NL, Ganley TJ, Edmonds EW, Anderson AF, Polousky J, Murnaghan ML, Nissen CW, Weiss J, Lyon RM, Chambers HG; Research in OsteoChondritis of the Knee (ROCK) Group. Novel arthroscopic classification of osteochondritis dissecans of the knee: a multicenter reliability study. Am J Sports Med 2016. July;44(7):1694–8. Epub 2016 Apr 6. [DOI] [PubMed] [Google Scholar]
- 130.Carey JL, Wall EJ, Kevin G, Shea MD, Grimm NL, Anderson AF, Edmonds EW, Chambers HG, Heyworth BE, Kocher MS, Lyon RM, Murnaghan ML, Nissen CW, Polousky J, Weiss J, Wright RW. Reliability of the ROCK osteochondritis dissecans knee arthroscopy classification system - multi-center validation study. Orthop J Sports Med 2013;1(4 Suppl):2325967113S00074. [Google Scholar]
- 131.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am 2007. June;89(6):1205–14. [DOI] [PubMed] [Google Scholar]
- 132.Chambers HG, Shea KG, Anderson AF, Brunelle TJ, Carey JL, Ganley TJ, Paterno MV, Weiss JM, Sanders JO, Watters WC 3rd, Goldberg MJ, Keith MW, Turkelson CM, Wies JL, Raymond L, Boyer KM, Hitchcock K, Anderson S, Sluka P, Boone C, Patel N; American Academy of Orthopedic Surgeons. Diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg 2011. May;19(5):297–306. [DOI] [PubMed] [Google Scholar]
- 133.Talusan PG, Milewski MD, Toy JO, Wall EJ. Osteochondritis dissecans of the talus: diagnosis and treatment in athletes. Clin Sports Med 2014. April;33(2):267–84. [DOI] [PubMed] [Google Scholar]
- 134.Uppstrom TJ, Haskel JD, Gausden EB, Meyer R, Shin YW, Nguyen JT, Green DW. Reliability of predictive models for non-operative healing potential of stable juvenile osteochondritis dissecans knee lesions. Knee 2016. August;23(4):698–701. Epub 2016 Apr 23. [DOI] [PubMed] [Google Scholar]
- 135.Heyworth BE, Edmonds EW, Murnaghan ML, Kocher MS. Drilling techniques for osteochondritis dissecans. Clin Sports Med 2014. April;33(2):305–12. Epub 2014 Feb 18. [DOI] [PubMed] [Google Scholar]
- 136.Donaldson LD, Wojtys EM. Extraarticular drilling for stable osteochondritis dissecans in the skeletally immature knee. J Pediatr Orthop 2008. December;28(8):831–5. [DOI] [PubMed] [Google Scholar]
- 137.Lee CS, Larsen CG, Marchwiany DA, Chudik SC. Extra-articular, intraepiphyseal drilling for osteochondritis dissecans of the knee: characterization of a safe and reproducible surgical approach. Orthop J Sports Med 2019. February 28;7(2):2325967119830397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gunton MJ, Carey JL, Shaw CR, Murnaghan ML. Drilling juvenile osteochondritis dissecans: retro- or transarticular? Clin Orthop Relat Res 2013. April;471(4):1144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lykissas MG, Wall EJ, Nathan S. Retro-articular drilling and bone grafting of juvenile knee osteochondritis dissecans: a technical description. Knee Surg Sports Traumatol Arthrosc 2014. February;22(2):274–8. Epub 2013 Jan 18. [DOI] [PubMed] [Google Scholar]
- 140.Makino A, Muscolo DL, Puigdevall M, Costa-Paz M, Ayerza M. Arthroscopic fixation of osteochondritis dissecans of the knee: clinical, magnetic resonance imaging, and arthroscopic follow-up. Am J Sports Med 2005. October;33(10):1499–504. Epub 2005 Jul 11. [DOI] [PubMed] [Google Scholar]
- 141.Kouzelis A, Plessas S, Papadopoulos AX, Gliatis I, Lambiris E. Herbert screw fixation and reverse guided drillings, for treatment of types III and IV osteochondritis dissecans. Knee Surg Sports Traumatol Arthrosc 2006. January;14(1):70–5. Epub 2005 Jun 21. [DOI] [PubMed] [Google Scholar]
- 142.Magnussen RA, Carey JL, Spindler KP. Does operative fixation of an osteochondritis dissecans loose body result in healing and long-term maintenance of knee function? Am J Sports Med 2009. April;37(4):754–9. Epub 2009 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Webb JE, Lewallen LW, Christophersen C, Krych AJ, McIntosh AL. Clinical outcome of internal fixation of unstable juvenile osteochondritis dissecans lesions of the knee. Orthopedics 2013. November;36(11):e1444–9. [DOI] [PubMed] [Google Scholar]
- 144.Adachi N, Deie M, Nakamae A, Okuhara A, Kamei G, Ochi M. Functional and radiographic outcomes of unstable juvenile osteochondritis dissecans of the knee treated with lesion fixation using bioabsorbable pins. J Pediatr Orthop 2015. January;35(1):82–8. [DOI] [PubMed] [Google Scholar]
- 145.Camathias C, Festring JD, Gaston MS. Bioabsorbable lag screw fixation of knee osteochondritis dissecans in the skeletally immature. J Pediatr Orthop B 2011. March;20(2):74–80. [DOI] [PubMed] [Google Scholar]
- 146.Camathias C, Gögüs U, Hirschmann MT, Rutz E, Brunner R, Haeni D, Vavken P. Implant failure after biodegradable screw fixation in osteochondritis dissecans of the knee in skeletally immature patients. Arthroscopy 2015. March;31(3):410–5. Epub 2014 Nov 6. [DOI] [PubMed] [Google Scholar]
- 147.Millington KL, Shah JP, Dahm DL, Levy BA, Stuart MJ. Bioabsorbable fixation of unstable osteochondritis dissecans lesions. Am J Sports Med 2010. October;38(10):2065–70. Epub 2010 Jul 1. [DOI] [PubMed] [Google Scholar]
- 148.Lintz F, Pujol N, Pandeirada C, Boisrenoult P, Beaufils P. Hybrid fixation: evaluation of a novel technique in adult osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc 2011. April;19(4):568–71. Epub 2010 Sep 18. [DOI] [PubMed] [Google Scholar]
- 149.Slough JA, Noto AM, Schmidt TL. Tibial cortical bone peg fixation in osteochondritis dissecans of the knee. Clin Orthop Relat Res 1991. June;(267):122–7. [PubMed] [Google Scholar]
- 150.Navarro R, Cohen M, Filho MC, da Silva RT. The arthroscopic treatment of osteochondritis dissecans of the knee with autologous bone sticks. Arthroscopy 2002. October;18(8):840–4. [DOI] [PubMed] [Google Scholar]
- 151.Melugin HP, Desai VS, Levy BA, Tanaka Y, Horibe S, Nakamura N, Krych AJ. Osteochondritis dissecans of the knee: short-term outcomes of a hybrid technique to restore a partially salvageable progeny fragment. Cartilage 2020. July;11(3):300–8. Epub 2018 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanders TL, Pareek A, Obey MR, Johnson NR, Carey JL, Stuart MJ, Krych AJ. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am J Sports Med 2017. July;45(8):1799–805. Epub 2017 Apr 18. [DOI] [PubMed] [Google Scholar]
- 153.Wright RW, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res 2004. July;(424):239–43. [PubMed] [Google Scholar]
- 154.Minas T, Ogura T, Headrick J, Bryant T. Autologous chondrocyte implantation “sandwich” technique compared with autologous bone grafting for deep osteochondral lesions in the knee. Am J Sports Med 2018. February;46(2):322–32. Epub 2017 Nov 10. [DOI] [PubMed] [Google Scholar]
- 155.Roffi A, Andriolo L, Di Martino A, Balboni F, Papio T, Zaffagnini S, Filardo G. Long-term results of matrix-assisted autologous chondrocyte transplantation combined with autologous bone grafting for the treatment of juvenile osteochondritis dissecans. J Pediatr Orthop 2020. February;40(2):e115–21. [DOI] [PubMed] [Google Scholar]
- 156.Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, Smailys A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 2005. September;21(9):1066–75. [DOI] [PubMed] [Google Scholar]
- 157.Chadli L, Cottalorda J, Delpont M, Mazeau P, Thouvenin Y, Louahem D. Autologous osteochondral mosaicplasty in osteochondritis dissecans of the patella in adolescents. Int Orthop 2017. January;41(1):197–202. Epub 2016 Apr 27. [DOI] [PubMed] [Google Scholar]
- 158.Lyon R, Nissen C, Liu XC, Curtin B. Can fresh osteochondral allografts restore function in juveniles with osteochondritis dissecans of the knee? Clin Orthop Relat Res 2013. April;471(4):1166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med 2007. June;35(6):907–14. Epub 2007 Mar 16. [DOI] [PubMed] [Google Scholar]
- 160.Sadr KN, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation in patients with osteochondritis dissecans of the knee. Am J Sports Med 2016. November;44(11):2870–5. Epub 2016 Aug 5. [DOI] [PubMed] [Google Scholar]
- 161.Cotter EJ, Frank RM, Wang KC, Totlis T, Poland S, Meyer MA, Cole BJ. Clinical outcomes of osteochondral allograft transplantation for secondary treatment of osteochondritis dissecans of the knee in skeletally mature patients. Arthroscopy 2018. April;34(4):1105–12. Epub 2018 Jan 2. [DOI] [PubMed] [Google Scholar]
- 162.Sales de Gauzy J, Mansat C, Darodes PH, Cahuzac JP. Natural course of osteochondritis dissecans in children. J Pediatr Orthop B 1999. January;8(1):26–8. [PubMed] [Google Scholar]
- 163.Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee. A long-term study. J Bone Joint Surg Br 1991. May;73(3):461–4. [DOI] [PubMed] [Google Scholar]
- 164.Linden B Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am 1977. September;59(6):769–76. [PubMed] [Google Scholar]
- 165.Green WT, Banks HH. Osteochondritis dissecans in children. J Bone Joint Surg Am 1953. January;35-A(1):26–47, passim. [PubMed] [Google Scholar]
- 166.Weiss JM, Nikizad H, Shea KG, Gyurdzhyan S, Jacobs JC, Cannamela PC, Kessler JI. The incidence of surgery in osteochondritis dissecans in children and adolescents. Orthop J Sports Med 2016. March 16;4(3):2325967116635515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sanders TL, Pareek A, Johnson NR, Carey JL, Maak TG, Stuart MJ, Krych AJ. Nonoperative management of osteochondritis dissecans of the knee: progression to osteoarthritis and arthroplasty at mean 13-year follow-up. Orthop J Sports Med 2017. July 24;5(7):2325967117704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bradley J, Dandy DJ. Results of drilling osteochondritis dissecans before skeletal maturity. J Bone Joint Surg Br 1989. August;71(4):642–4. [DOI] [PubMed] [Google Scholar]
- 169.Aglietti P, Buzzi R, Bassi PB, Fioriti M. Arthroscopic drilling in juvenile osteochondritis dissecans of the medial femoral condyle. Arthroscopy 1994. June;10(3):286–91. [DOI] [PubMed] [Google Scholar]
- 170.Anderson AF, Richards DB, Pagnani MJ, Hovis WD. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy 1997. June;13(3):319–24. [DOI] [PubMed] [Google Scholar]
- 171.Louisia S, Beaufils P, Katabi M, Robert H; French Society of Arthroscopy. Transchondral drilling for osteochondritis dissecans of the medial condyle of the knee. Knee Surg Sports Traumatol Arthrosc 2003. January;11(1):33–9. Epub 2002 Dec 17. [DOI] [PubMed] [Google Scholar]
- 172.Lee CK, Mercurio C. Operative treatment of osteochondritis dissecans in situ by retrograde drilling and cancellous bone graft: a preliminary report. Clin Orthop Relat Res 1981. July-Aug;(158):129–6. [PubMed] [Google Scholar]
- 173.Adachi N, Deie M, Nakamae A, Ishikawa M, Motoyama M, Ochi M. Functional and radiographic outcome of stable juvenile osteochondritis dissecans of the knee treated with retroarticular drilling without bone grafting. Arthroscopy 2009. February;25(2):145–52. Epub 2008 Nov 1. [DOI] [PubMed] [Google Scholar]
- 174.Ojala R, Kerimaa P, Lakovaara M, Hyvönen P, Lehenkari P, Tervonen O, Blanco-Sequeiros R. MRI-guided percutaneous retrograde drilling of osteochondritis dissecans of the knee. Skeletal Radiol 2011. June;40(6):765–70. Epub 2011 Feb 15. [DOI] [PubMed] [Google Scholar]
- 175.Boughanem J, Riaz R, Patel RM, Sarwark JF. Functional and radiographic outcomes of juvenile osteochondritis dissecans of the knee treated with extra-articular retrograde drilling. Am J Sports Med 2011. October;39(10):2212–7. Epub 2011 Aug 9. [DOI] [PubMed] [Google Scholar]
- 176.Kocher MS, Micheli LJ, Yaniv M, Zurakowski D, Ames A, Adrignolo AA. Functional and radiographic outcome of juvenile osteochondritis dissecans of the knee treated with transarticular arthroscopic drilling. Am J Sports Med 2001. September-Oct;29(5):562–6. [DOI] [PubMed] [Google Scholar]
- 177.Edmonds EW, Albright J, Bastrom T, Chambers HG. Outcomes of extra-articular, intra-epiphyseal drilling for osteochondritis dissecans of the knee. J Pediatr Orthop 2010. December;30(8):870–8. [DOI] [PubMed] [Google Scholar]
- 178.Kocher MS, Czarnecki JJ, Andersen JS, Micheli LJ. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med 2007. May;35(5):712–8. Epub 2007 Mar 2. [DOI] [PubMed] [Google Scholar]
- 179.Wu IT, Custers RJH, Desai VS, Pareek A, Stuart MJ, Saris DBF, Krych AJ. Internal fixation of unstable osteochondritis dissecans: do open growth plates improve healing rate? Am J Sports Med 2018. August;46(10):2394–401. Epub 2018 Jul 11. [DOI] [PubMed] [Google Scholar]
- 180.Macmull S, Parratt MTR, Bentley G, Skinner JA, Carrington RW, Morris T, Briggs TW. Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med 2011. August;39(8):1723–30. Epub 2011 Apr 29. [DOI] [PubMed] [Google Scholar]
- 181.Micheli LJ, Moseley JB, Anderson AF, Browne JE, Erggelet C, Arciero R, Fu FH, Mandelbaum BR. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop 2006. July-Aug;26(4):455–60. [DOI] [PubMed] [Google Scholar]
- 182.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2003;85-A(Suppl 2):17–24. [DOI] [PubMed] [Google Scholar]
- 183.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 2000. May;(374):212–34. [DOI] [PubMed] [Google Scholar]
- 184.Moradi B, Schönit E, Nierhoff C, Hagmann S, Oberle D, Gotterbarm T, Schmitt H, Zeifang F. First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years’ clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy 2012. December;28(12):1851–61. Epub 2012 Oct 2. [DOI] [PubMed] [Google Scholar]
- 185.Carey JL, Shea KG, Lindahl A, Vasiliadis HS, Lindahl C, Peterson L. Autologous chondrocyte implantation as treatment for unsalvageable osteochondritis dissecans: 10- to 25-year follow-up. Am J Sports Med 2020. April;48(5):1134–40. Epub 2020 Mar 17. [DOI] [PubMed] [Google Scholar]
- 186.Steinhagen J, Bruns J, Deuretzbacher G, Ruether W, Fuerst M, Niggemeyer O. Treatment of osteochondritis dissecans of the femoral condyle with autologous bone grafts and matrix-supported autologous chondrocytes. Int Orthop 2010. August;34(6):819–25. Epub 2009 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med 2013. August;41(8):1786–93. Epub 2013 Jun 12. [DOI] [PubMed] [Google Scholar]
- 188.Gudas R, Simonaitytė R, Čekanauskas E, Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop 2009. October-Nov;29(7):741–8. [DOI] [PubMed] [Google Scholar]
- 189.Matsuura T, Kashiwaguchi S, Iwase T, Takeda Y, Yasui N. Conservative treatment for osteochondrosis of the humeral capitellum. Am J Sports Med 2008. May;36(5):868–72. Epub 2008 Jan 24. [DOI] [PubMed] [Google Scholar]
- 190.Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med 2009. February;37(2):298–304. Epub 2008 Dec 4. [DOI] [PubMed] [Google Scholar]
- 191.Niu EL, Tepolt FA, Bae DS, Lebrun DG, Kocher MS. Nonoperative management of stable pediatric osteochondritis dissecans of the capitellum: predictors of treatment success. J Shoulder Elbow Surg 2018. November;27(11):2030–7. [DOI] [PubMed] [Google Scholar]
- 192.Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med 1999. November-Dec;27(6):728–32. [DOI] [PubMed] [Google Scholar]
- 193.Funakoshi T, Furushima K, Miyamoto A, Kusano H, Horiuchi Y, Itoh Y. Predictors of unsuccessful nonoperative management of capitellar osteochondritis dissecans. Am J Sports Med 2019. September;47(11):2691–8. Epub 2019 Jul 26. [DOI] [PubMed] [Google Scholar]
- 194.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am 2008. March;90(Suppl 2 Pt 1):47–62. [DOI] [PubMed] [Google Scholar]
- 195.Ueda Y, Sugaya H, Takahashi N, Matsuki K, Tokai M, Onishi K, Hoshika S, Hamada H. Arthroscopic fragment resection for capitellar osteochondritis dissecans in adolescent athletes: 5- to 12-year follow-up. Orthop J Sports Med 2017. December 15;5(12):2325967117744537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bexkens R, van Bergen CJA, van den Bekerom MPJ, Kerkhoffs GMMJ, Eygendaal D. Decreased defect size and partial restoration of subchondral bone on computed tomography after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med 2018. October;46(12):2954–9. Epub 2018 Aug 24. [DOI] [PubMed] [Google Scholar]
- 197.Tis JE, Edmonds EW, Bastrom T, Chambers HG. Short-term results of arthroscopic treatment of osteochondritis dissecans in skeletally immature patients. J Pediatr Orthop 2012. April-May;32(3):226–31. [DOI] [PubMed] [Google Scholar]
- 198.Lewine EB, Miller PE, Micheli LJ, Waters PM, Bae DS. Early results of drilling and/or microfracture for grade IV osteochondritis dissecans of the capitellum. J Pediatr Orthop 2016. December;36(8):803–9. [DOI] [PubMed] [Google Scholar]
- 199.Bexkens R, van den Ende KIM, Ogink PT, van Bergen CJA, van den Bekerom MPJ, Eygendaal D. Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. Am J Sports Med 2017. August;45(10):2312–8. Epub 2017 May 18. [DOI] [PubMed] [Google Scholar]
- 200.Uchida S, Utsunomiya H, Taketa T, Sakoda S, Hatakeyama A, Nakamura T, Sakai A. Arthroscopic fragment fixation using hydroxyapatite/poly-L-lactate Acid thread pins for treating elbow osteochondritis dissecans. Am J Sports Med 2015. May;43(5):1057–65. Epub 2015 Mar 3. [DOI] [PubMed] [Google Scholar]
- 201.Hennrikus WP, Miller PE, Micheli LJ, Waters PM, Bae DS. Internal fixation of unstable in situ osteochondritis dissecans lesions of the capitellum. J Pediatr Orthop 2015. July-Aug;35(5):467–73. [DOI] [PubMed] [Google Scholar]
- 202.Maruyama M, Takahara M, Harada M, Satake H, Takagi M. Outcomes of an open autologous osteochondral plug graft for capitellar osteochondritis dissecans: time to return to sports. Am J Sports Med 2014. September;42(9):2122–7. Epub 2014 Jun 20. [DOI] [PubMed] [Google Scholar]
- 203.Lyons ML, Werner BC, Gluck JS, Freilich AM, Dacus AR, Diduch DR, Chhabra AB. Osteochondral autograft plug transfer for treatment of osteochondritis dissecans of the capitellum in adolescent athletes. J Shoulder Elbow Surg 2015. July;24(7):1098–105. Epub 2015 May 7. [DOI] [PubMed] [Google Scholar]
- 204.Funakoshi T, Momma D, Matsui Y, Kamishima T, Matsui Y, Kawamura D, Nagano Y, Iwasaki N. Autologous osteochondral mosaicplasty for centrally and laterally located, advanced capitellar osteochondritis dissecans in teenage athletes: clinical outcomes, radiography, and magnetic resonance imaging findings. Am J Sports Med 2018. July;46(8):1943–51. Epub 2018 May 8. [DOI] [PubMed] [Google Scholar]
- 205.Westermann RW, Hancock KJ, Buckwalter JA, Kopp B, Glass N, Wolf BR. Return to sport after operative management of osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Orthop J Sports Med 2016. June 28;4(6):2325967116654651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bae DS, Ingall EM, Miller PE, Eisenberg K. Early results of single-plug autologous osteochondral grafting for osteochondritis dissecans of the capitellum in adolescents. J Pediatr Orthop 2020. February;40(2):78–85. [DOI] [PubMed] [Google Scholar]
- 207.Mirzayan R, Lim MJ. Fresh osteochondral allograft transplantation for osteochondritis dissecans of the capitellum in baseball players. J Shoulder Elbow Surg 2016. November;25(11):1839–47. [DOI] [PubMed] [Google Scholar]
- 208.Kiyoshige Y, Takagi M, Yuasa K, Hamasaki M. Closed-Wedge osteotomy for osteochondritis dissecans of the capitellum. A 7- to 12-year follow-up. Am J Sports Med 2000. July-Aug;28(4):534–7. [DOI] [PubMed] [Google Scholar]
- 209.Koda H, Moriya K, Ueki M, Endo N, Yoshizu T. Long-term results of closed-wedge osteotomy of the lateral humeral condyle for osteochondritis dissecans of the capitellum. J Shoulder Elbow Surg 2019. September;28(9):e313–20. Epub 2019 Jul 17. [DOI] [PubMed] [Google Scholar]
- 210.Ueki M, Moriya K, Yoshizu T, Tsubokawa N, Kouda H, Endo N. Closed-wedge osteotomy of the distal humerus for treating osteochondritis dissecans of the capitellum in young patients. Orthop J Sports Med 2019. October 24;7(10):2325967119876247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lam KY, Siow HM. Conservative treatment for juvenile osteochondritis dissecans of the talus. J Orthop Surg (Hong Kong) 2012. August;20(2):176–80. Epub 2012 Aug 1. [DOI] [PubMed] [Google Scholar]
- 212.McCullough CJ, Venugopal V. Osteochondritis dissecans of the talus: the natural history. Clin Orthop Relat Res 1979. October;(144):264–8. [PubMed] [Google Scholar]
- 213.Bruns J, Rosenbach B. Osteochondrosis dissecans of the talus. Comparison of results of surgical treatment in adolescents and adults. Arch Orthop Trauma Surg 1992;112(1):23–7. [DOI] [PubMed] [Google Scholar]
- 214.Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med 2009. October;37(10):1974–80. Epub 2009 Aug 4. [DOI] [PubMed] [Google Scholar]
- 215.Polat G, Erşen A, Erdil ME, Kızılkurt T, Kılıçoğlu Ö, Aşık M. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc 2016. April;24(4):1299–303. Epub 2016 Feb 1. [DOI] [PubMed] [Google Scholar]
- 216.Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, Dopirak RM. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med 2008. September;36(9):1750–62. [DOI] [PubMed] [Google Scholar]
- 217.Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc 2010. May;18(5):656–63. Epub 2010 Feb 4. [DOI] [PubMed] [Google Scholar]
- 218.van Bergen CJA, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GMMJ, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am 2013. March 20;95(6):519–25. [DOI] [PubMed] [Google Scholar]
- 219.Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy 2008. January;24(1):106–12. Epub 2007 Nov 19. [DOI] [PubMed] [Google Scholar]
- 220.Donnenwerth MP, Roukis TS. Outcome of arthroscopic debridement and microfracture as the primary treatment for osteochondral lesions of the talar dome. Arthroscopy 2012. December;28(12):1902–7. Epub 2012 Aug 11. [DOI] [PubMed] [Google Scholar]
- 221.Choi SW, Lee GW, Lee KB. Arthroscopic microfracture for osteochondral lesions of the talus: functional outcomes at a mean of 6.7 years in 165 consecutive ankles. Am J Sports Med 2020. January;48(1):153–8. [DOI] [PubMed] [Google Scholar]
- 222.Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am 1999. September;81(9):1229–35. [DOI] [PubMed] [Google Scholar]
- 223.Kono M, Takao M, Naito K, Uchio Y, Ochi M. Retrograde drilling for osteochondral lesions of the talar dome. Am J Sports Med 2006. September;34(9):1450–6. Epub 2006 Apr 24. [DOI] [PubMed] [Google Scholar]
- 224.Anders S, Lechler P, Rackl W, Grifka J, Schaumburger J. Fluoroscopy-guided retrograde core drilling and cancellous bone grafting in osteochondral defects of the talus. Int Orthop 2012. August;36(8):1635–40. Epub 2012 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Choi JI, Lee KB. Comparison of clinical outcomes between arthroscopic subchondral drilling and microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 2016. July;24(7):2140–7. Epub 2015 Feb 4. [DOI] [PubMed] [Google Scholar]
- 226.Masquijo JJ, Ferreyra A, Baroni E. Arthroscopic retrograde drilling in juvenile osteochondritis dissecans of the talus. J Pediatr Orthop 2016. September;36(6):589–93. [DOI] [PubMed] [Google Scholar]
- 227.Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am 2006. February;88(2):303–8. [DOI] [PubMed] [Google Scholar]
- 228.Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med 2009. February;37(2):274–84. Epub 2008 Dec 22. [DOI] [PubMed] [Google Scholar]
- 229.Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br 2005. February;87(2):179–83. [DOI] [PubMed] [Google Scholar]
- 230.Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc 2012. September;20(9):1696–703. Epub 2011 Oct 30. [DOI] [PubMed] [Google Scholar]
- 231.Schneider TE, Karaikudi S. Matrix-Induced Autologous Chondrocyte Implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int 2009. September;30(9):810–4. [DOI] [PubMed] [Google Scholar]
- 232.Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, Walton J. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int 2010. September;31(9):747–53. [DOI] [PubMed] [Google Scholar]
- 233.Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med 2014. September;42(9):2156–64. Epub 2014 Jul 23. [DOI] [PubMed] [Google Scholar]
- 234.Erickson B, Fillingham Y, Hellman M, Parekh SG, Gross CE. Surgical management of large talar osteochondral defects using autologous chondrocyte implantation. Foot Ankle Surg 2018. April;24(2):131–6. Epub 2017 Jan 17. [DOI] [PubMed] [Google Scholar]
- 235.Kreulen C, Giza E, Walton J, Sullivan M. Seven-year follow-up of matrix-induced autologous implantation in talus articular defects. Foot Ankle Spec 2018. April;11(2):133–7. Epub 2017 Jun 6. [DOI] [PubMed] [Google Scholar]
- 236.Emre TY, Ege T, Cift HT, Demircioğlu DT, Seyhan B, Uzun M. Open mosaicplasty in osteochondral lesions of the talus: a prospective study. J Foot Ankle Surg 2012. September-Oct;51(5):556–60. Epub 2012 Jul 11. [DOI] [PubMed] [Google Scholar]
- 237.Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 2006. October;22(10):1085–92. [DOI] [PubMed] [Google Scholar]
- 238.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 2003;85-A(Suppl 2):25–32. [DOI] [PubMed] [Google Scholar]
- 239.Scranton PE Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br 2006. May;88(5):614–9. [DOI] [PubMed] [Google Scholar]
- 240.Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2013. March;41(3):519–27. Epub 2013 Feb 7. [DOI] [PubMed] [Google Scholar]
- 241.McGahan PJ, Pinney SJ. Current concept review: osteochondral lesions of the talus. Foot Ankle Int 2010. January;31(1):90–101. [DOI] [PubMed] [Google Scholar]
- 242.Sammarco GJ, Makwana NK. Treatment of talar osteochondral lesions using local osteochondral graft. Foot Ankle Int 2002. August;23(8):693–8. [DOI] [PubMed] [Google Scholar]
- 243.Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int 2003. November;24(11):815–22. [DOI] [PubMed] [Google Scholar]
- 244.Adams SB Jr, Viens NA, Easley ME, Stinnett SS, Nunley JA 2nd. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am 2011. April 6;93(7):648–54. [DOI] [PubMed] [Google Scholar]
- 245.El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am 2011. September 7;93(17):1634–40. [DOI] [PubMed] [Google Scholar]
- 246.Bugbee WD, Khanna G, Cavallo M, McCauley JC, Görtz S, Brage ME. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am 2013. March 6;95(5):426–32. [DOI] [PubMed] [Google Scholar]
- 247.Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int 2001. May;22(5):385–91. [DOI] [PubMed] [Google Scholar]
- 248.Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am 2009. December;91(12):2818–26. [DOI] [PubMed] [Google Scholar]
- 249.VanTienderen RJ, Dunn JC, Kusnezov N, Orr JD. Osteochondral allograft transfer for treatment of osteochondral lesions of the talus: a systematic review. Arthroscopy 2017. January;33(1):217–22. Epub 2016 Aug 18. [DOI] [PubMed] [Google Scholar]
- 250.Cahill BR, Berg BC. 99m-Technetium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med 1983. September-Oct;11(5):329–35. [DOI] [PubMed] [Google Scholar]
- 251.Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am 1959. September;41-A:988–1020. [PubMed] [Google Scholar]
- 252.Scranton PE Jr, McDermott JE. Treatment of type V osteochondral lesions of the talus with ipsilateral knee osteochondral autografts. Foot Ankle Int 2001. May;22(5):380–4. [DOI] [PubMed] [Google Scholar]
- 253.Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy 1991;7(1):101–4. [DOI] [PubMed] [Google Scholar]
- 254.Guhl JF. Arthroscopic treatment of osteochondritis dissecans. Clin Orthop Relat Res 1982. July;(167):65–74. [PubMed] [Google Scholar]
- 255.Guhl JF. Arthroscopic treatment of osteochondritis dissecans: preliminary report. Orthop Clin North Am 1979. July;10(3):671–83. [PubMed] [Google Scholar]
- 256.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 2003;85(Suppl 2):58–69. [DOI] [PubMed] [Google Scholar]
- 257.Ewing JW, Voto SJ. Arthroscopic surgical management of osteochondritis dissecans of the knee. Arthroscopy 1988;4(1):37–40. [DOI] [PubMed] [Google Scholar]
- 258.Johnson LL, Uitvlugt G, Austin MD, Detrisac DA, Johnson C. Osteochondritis dissecans of the knee: arthroscopic compression screw fixation. Arthroscopy 1990;6(3):179–89. [DOI] [PubMed] [Google Scholar]
- 259.Chen CH, Liu YS, Chou PH, Hsieh CC, Wang CK. MR grading system of osteochondritis dissecans lesions: comparison with arthroscopy. Eur J Radiol 2013. March;82(3):518–25. Epub 2012 Oct 25. [DOI] [PubMed] [Google Scholar]
- 260.Minami M, Nakashita K, Ishii S, Usui M, Muramatsu I, Ogino T, Fukuda K, Sugawara M. Twenty-five cases of osteochondritis dissecans of the elbow. Rinsho Seikei Geka 1979;14(8):805–10. [Google Scholar]
- 261.Kolmodin J, Saluan P. Osteochondritis dissecans of the humeral capitellum: the significance of lesion location. Orthop J Sports Med 2014. April 22;2(4):2325967114530840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int 1999. December;20(12):789–93. [DOI] [PubMed] [Google Scholar]
- 263.Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am 1986. July;68(6):862–5. [PubMed] [Google Scholar]
- 264.Wright JG. Revised grades of recommendation for summaries or reviews of orthopaedic surgical studies. J Bone Joint Surg Am 2006. May;88(5):1161–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1
Three-dimensional reconstruction of susceptibility-weighted MRI data obtained from a distal femoral specimen from a 3-month-old human cadaveric donor demonstrating central and peripheral vascular beds with a watershed region in the midsagittal plane.
Video 2
A 14-year-old female patient undergoing internal fixation with bioabsorbable headless compression screws for treatment of an osteochondritis dissecans lesion on the medial femoral condyle.


