Abstract
Objective
Cinnamon is a cooking spice and a medicinal herb. It is increasingly used as a health supplement due to its perceived benefit to prevent and or manage type 2 diabetes and metabolic disorders. However, it is unclear if regular consumption of this medicinal plant will interfere with normal physiological functions. Therefore, this study investigated the impact of daily cinnamon supplements on glucose and lipid metabolic profiles in healthy rats.
Methods
Male rats (Sprague Dawley, 8 weeks) were supplied with cinnamon in their diet (equivalent to ∼1 g/day in humans) for two weeks. Blood glucose and lipid levels, as well as metabolic markers in both liver and abdominal white adipose tissue, were measured.
Results
Cinnamon significantly increased fat mass and blood cholesterol and low-density lipoprotein (LDL) levels, but reduced fasting blood glucose level by 12%. Liver functional enzymes were normal in rats consuming cinnamon. However, several lipid metabolic markers were impaired which may contribute to dyslipidemia, including two main switches for energy metabolism (sirtuin 1 and peroxisome proliferator-activated receptor-gamma coactivator-1α) and the LDL receptor. However, de novo lipid synthesis enzymes and inflammatory markers were also reduced in the liver by cinnamon treatment, which may potentially prevent the development of steatosis. Markers for lipid oxidation were downregulated in fat tissue in cinnamon-treated rats, contributing to increased fat accumulation.
Conclusion
Daily low-dose cinnamon supplementation seems to promote abdominal adipose tissue accumulation and disturb lipid homeostasis in healthy rats, raising the concerns regarding daily use in healthy people.
1. Introduction
Cinnamon is a commonly used food spice in many countries. As a medicinal herb, cinnamon has also been traditionally used to promote coronary and microcirculation in the extremities, as well as in treating diabetes in several countries [1–3]. Human trials have provided evidence supporting the blood glucose lowing effect of cinnamon in patients with type 2 diabetes [4, 5]. This effect seems largely attributed to cinnamaldehyde [6]. As such, commercial cinnamon supplements (1-2 g per day) also claim beneficial effects on blood glucose control and cardiovascular health. Due to its perceived health benefits, cinnamon is increasingly used as a supplement, especially among individuals with diabetes [7].
While the antidiabetic effect of cinnamon is supported by evidence from clinical trials, its impacts on blood lipids vary between studies. In humans and animals with diabetes, cinnamon supplements reduced blood low-density lipoprotein (LDL) and cholesterol levels, whereas a recent systematic review and meta-analysis on human trials of cinnamon showed no effect on blood LDL levels [5, 8–11]. The confirmed efficacy of cinnamon powder on blood lipid control has been shown mainly in patients with type 2 diabetes, which can be affected by confounding factors, such as strategies to promote healthy lifestyles that reduce both blood glucose and lipids and the introduction of other medications to manage complications (e.g., antihypertensive drugs). It is also possible that cinnamon alone may not be potent enough to counteract complex risk factors (e.g., drinking and social eating). However, in well-controlled animal studies, lipid-lowering effects of cinnamon powder and cinnamon extracts have been shown in rats fed a high-fat diet [12–14], although the chemical extracts have not been approved to be used by humans.
Cinnamon is also consumed by healthy people in the form of powder or dry bark. Due to the perceived benefit in people with metabolic disorders, there is increasing popularity for it to be used as a health supplement to prevent the development of such disorders. As a medicinal plant, it is likely that there will be both positive and negative effects of some of the active chemical species. While identifying individual chemicals is of interest to medicinal chemists, the raw unextracted plant is the likely form to be consumed by the general public. Blood glucose and lipids are commonly used biomarkers for the initial screening of metabolic disorders in the clinic. Therefore, it is useful to investigate the influence of unextracted cinnamon on blood glucose and lipid profiles in the absence of disease. This formed our first aim, which was to investigate how cinnamon bark affects the blood lipid and glucose profile in healthy rats fed a standard diet (i.e., rodent chow balanced with nutrients).
The liver plays a key role in lipid metabolism, especially in the synthesis of cholesterol and major lipoproteins (e.g., LDL and HDL). Suppressing the rate-controlling enzyme in cholesterol synthesis, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), in the liver, can induce the expression of LDL receptors (LDLR). This further increases the breakdown of LDL and reduces blood cholesterol levels. Squalene monooxygenase (SQLE) is another rate-limiting enzyme in sterol biosynthesis. Also, sirtuins (SIRTs) are a group of deacetylases and mono-ADP-ribosyl transferases that function as the main switch for energy metabolism [15]. SIRT1-peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) pathway plays a vital role in cellular substrate metabolism [16]. Therefore, all the abovementioned metabolic markers were measured in the liver and adipose tissue, which are vital mechanisms for systemic glucose and lipid metabolic homeostasis.
2. Methods
2.1. Animal Study
The study was approved by the Animal Ethics Committee of the Chengdu Dossy Experimental Animal Co., Ltd. (SCXK (Chuan) 2015-030). Sprague Dawley rats (male, 8 weeks, n = 10) were fed either standard rodent chow (14% fat) or rodent chow containing cinnamon powder (1 g/kg chow) for two weeks. Medical grade cinnamon (Sichuan New Lotus Chinese Medicine Pieces Co., Ltd.) for human use was added to the standard rodent chow by Chengdu Dossy Experimental Animal Co., Ltd. The average daily food consumption of the rats was 25.5 g/day/rat as measured by our laboratory in this strain, making the treatment dose equivalent to ∼1 g/day in humans of 75 kg based on the calculation using a published method [17]. At the endpoint, after overnight fasting and anesthesia (pentobarbital sodium, 40 mg/kg), blood was collected for serum via cardiac puncture, and blood glucose was measured using a glucose meter (Accu-Chek®, Roche Diagnostics). The organs were dissected and weighed. White adipose tissue and liver were snap-frozen and kept at −80°C.
2.2. Bioassays
Commercial ELISA kits were used to measure serum lipids LDL (Cat. F4562, Shanghai Westang Biotechnology) and cholesterols (Cat. ab65390, Abcam, Cambridge, United Kingdom), according to the instructions provided by the manufactures. The liver enzymes alanine aminotransferase (ALT) and aspartate transaminase (AST) in the serum were also measured using commercial kits (ALT kit Cat. C009-3-1 and AST kit Cat. C010-3-1, Nanjing Jiancheng Bioengineering Institute, China), following the manufacturer's instructions.
2.3. Real-Time PCR
mRNA expression of energy metabolic markers was measured in the liver and white adipose tissue using real-time PCR. The tissues were homogenized in RNAzol (Sigma-Aldrich, USA) and total RNA was isolated according to the manufacturer's instructions [18, 19]. Quantification was performed with a two-step reaction process: reverse transcription and qPCR. Reverse transcription was carried out with the M-MLV reverse transcriptase kit (Accurate Biology, China) according to the manufacturer's protocol in a GeneAmp® PCR System 9700 (Applied Biosystems 7500 Real-Time PCR System, USA). qPCR experiments were carried out using the SYBR Green qPCR kit from KAPA on an Applied Biosystems 7500 Real-Time PCR System. The primer sequences were designed in the laboratory and synthesized by TSINGKE Biological Technology (Guangzhou, China) based on the mRNA sequences obtained from the NCBI database (Table 1). At the end of the PCR cycles, the melting curve analysis was performed to validate the specific generation of the expected PCR product. mRNA expression was calculated using 2−∆∆Ct methods using the 18s as the housekeeping gene [18, 19]. The control group was assigned as the calibrator against which all other results were expressed as fold changes [18, 19].
Table 1.
Primers used for real-time PCR.
| Gene symbol | Forward primer (5 ≥ 3) | Reverse primer (5 ≥ 3) |
|---|---|---|
| ATGL | CGCAATCTCTACCGCCTCTC | GGGTTGGTTCAGTAGGCCATT |
| CPT-1α | CGGCAGACCTATTTTGCACG | TGGACTTGTCAAACCACCTGT |
| FASN | AGCCTGAGCTTGTCCCTAGA | CACTGGTACACTTTCCCGCT |
| F4/80 | CCACAACACCTACCTGCACC | ATGATAGCGCAAGCTGTCTGG |
| Glut4 | TTCCAGTATGTTGCGGATGCT | AATGTCCGGCCTCTGGTTTC |
| HMGCR | TGCAGAGCGATCAGTCTTGG | AATCTGCTCGTGCTGTCGAA |
| LPL | GGTCGCCTGGTCGAAGTATT | CAGCTGGTCCACATCTCCAA |
| LDLR | AGACCCAGAGCCATCGTAGT | GGCCACTGGGAAGATCTAGTG |
| PGC-1α | TGGAGTGACATAGAGTGTGCTG | TATGTTCGCGGGCTCATTGT |
| SIRT1 | TTTATGCTCGCCTTGCTGTG | GCTTCAATGCTGTTTCTTCTTTGC |
| SQLE | TCAGTGAACAAACGAGGCGT | GCCTGGAAAATAGCGGCATC |
| TNFα | ATGGGCTCCCTCTCATCAGT | GCTTGGTGGTTTGCTACGAC |
| 18S | ATTCCCAGTAAGTGCGGGTC | AAGTTCGACCGTCTTCTCAGG |
2.4. Statistical Methods
The results are expressed as mean ± SEM. The data were analyzed by unpaired student's t-test. P < 0.05 was considered statistically significant.
3. Results
Cinnamon supplementation did not affect body or organ weights (Table 2). However, it increased retroperitoneal white fat mass (P < 0.05, Table 2). It also reduced fasting blood glucose levels by 12%, consistent with the literature [20–22]. However, cinnamon supplementation increased blood LDL (P < 0.01 vs. control, Table 2) and cholesterol levels (P < 0.05 vs. control, Table 2).
Table 2.
The effect of the cinnamon supplement on anthropometry markers.
| Control | Cinnamon | |
|---|---|---|
| Body weight (g) | 460 ± 14 | 468 ± 9 |
| Liver (g) | 13.2 ± 0.7 | 13.2 ± 0.3 |
| Liver (%) | 2.88 ± 0.08 | 2.82 ± 0.03 |
| Kidney (g) | 2.59 ± 0.11 | 2.53 ± 0.05 |
| Kidney (%) | 0.56 ± 0.02 | 0.54 ± 0.01 |
| White adipose tissue (g) | 5.18 ± 0.41 | 7.10 ± 0.79∗ |
| White adipose tissue (%) | 1.13 ± 0.08 | 1.50 ± 0.15∗ |
| Skeletal muscle (g) | 0.87 ± 0.03 | 0.88 ± 0.04 |
| Skeletal muscle (%) | 0.19 ± 0.01 | 0.19 ± 0.01 |
| Fasting blood glucose (mM) | 9.38 ± 0.26 | 8.35 ± 0.50 |
| Cholesterol (mM) | 2.16 ± 0.08 | 2.36 ± 0.06∗ |
| LDL (mM) | 0.94 ± 0.10 | 1.36 ± 0.07∗∗ |
| ALT (U/L) | 24.1 ± 1.34 | 25.7 ± 2.51 |
| AST (U/L) | 53.8 ± 4.73 | 53.0 ± 5.66 |
Results are expressed as mean ± SE, n = 10. ∗P < 0.05; ∗∗P < 0.01. ALT, alanine aminotransferase; AST, aspartate transaminase; LDL, low-density lipoprotein.
In the liver, mRNA expression of several lipid metabolic markers including SIRT1 (P < 0.05, Figure 1(a)), PCG-1α (P=0.085, Figure 1(b)), fatty acid synthase (FASN, P=0.083, Figure 1(d)), and LDLR (P=0.069, Figure 1(i)) was reduced by cinnamon supplementation. Also, the markers for macrophage number F4/80 (Figure 1(j)) and the proinflammatory cytokine TNFα (Figure 1(k)) were downregulated by cinnamon supplementation (P < 0.05 for both).
Figure 1.
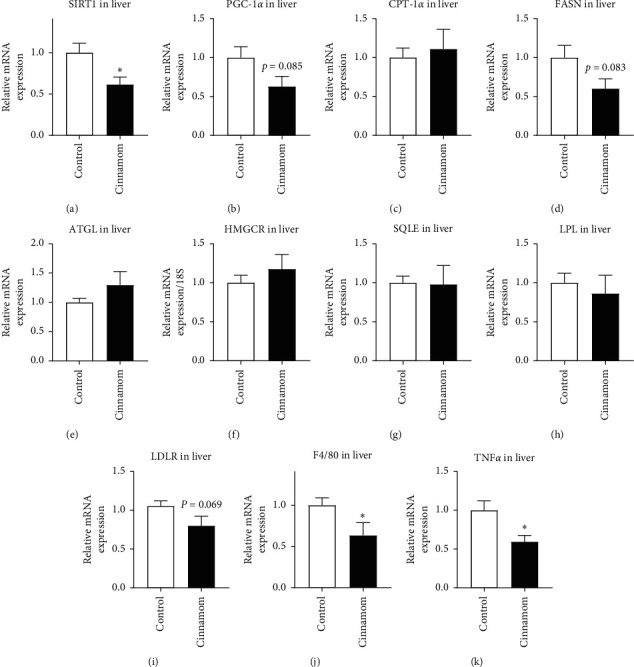
mRNA expression of metabolic markers, SIRT1 (a), PGC-1α (b), CPT-1α (c), FASN (d), ATGL (e), HMGCR (f), SQLE (g), LPL (h), LDLR (i), F4/80 (j), and TNFα (k), in the liver. Results are expressed as mean ± SE, n = 8–10. ∗P < 0.05.
In the fat tissue, markers for lipolysis, adipose triglyceride lipase (ATGL, P < 0.05, Figure 2(b)), and free fatty acid oxidation, CPT-1α (P < 0.05, Figure 2(c)), were significantly reduced, with halved levels of SQLE (Figure 2(d)) and glucose transporter 4 (Glut4, Figure 2(f)), albeit without statistical significance.
Figure 2.
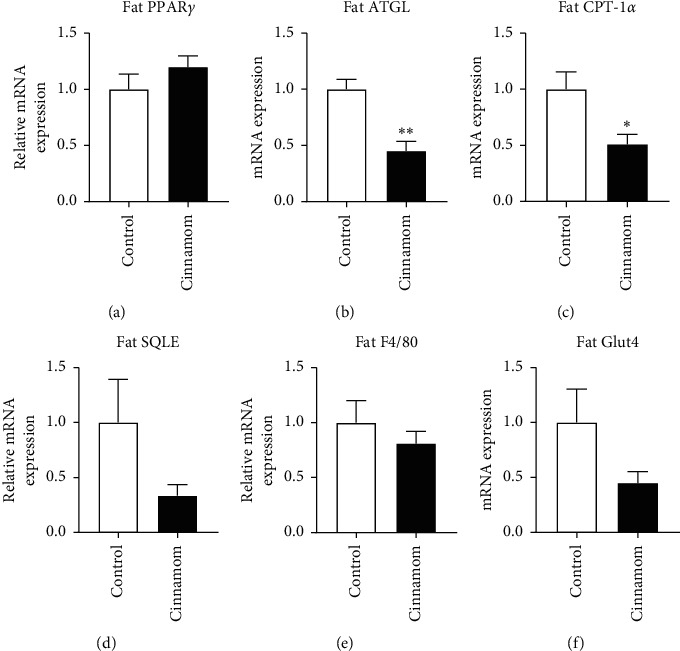
mRNA expression of metabolic markers, fat PPARγ (a), ATGL (b), CPT-1α (c), SQLE (d), F4/80 (e), and Glut4 (f), in the abdominal white adipose tissue. Results are expressed as mean ± SE, n = 6. ∗P < 0.05; ∗∗P < 0.01.
4. Discussion
In the literature, the impact of cinnamon supplements on metabolic disorders has only been examined in the context of diabetes or high-fat diet consumption. The outcomes promoted the use of cinnamon supplements even among healthy individuals, although without any evidence of the health benefit. This makes the major findings of this study particularly important to inform the use in healthy people, in that low-dose cinnamon dietary supplementation may increase blood cholesterol and LDL levels, as well as abdominal fat mass. We also found that such adverse impacts on lipid metabolic profiles may be related to impaired lipid metabolic markers in both the liver and fat tissue.
Liver function is critical for maintaining systemic glucose and lipid metabolic homeostasis [23]. Blood ALT and AST levels are used in the clinic as an indicator of hepatocyte integrity, which are highly concentrated in healthy hepatocytes with a low amount released into the circulation. The blood levels of both enzymes rise when hepatocytes are damaged during conditions such as drug-induced liver damage and liver steatosis. In this study, unchanged blood ALT and AST levels suggest that daily consumption of a low dose of cinnamon is safe for hepatocyte integrity. However, it is not so optimistic for the circulating lipid profile in rats consuming cinnamon daily, reflected by increased cholesterol and LDL levels in the blood. This effect is opposite to the beneficial effects observed in those with preexisting metabolic conditions. It is well accepted that high cholesterol levels in the blood are strongly associated with vascular disorders. Cholesterol in the circulation is mainly synthesized in the liver. LDL is rich in cholesterol, and when increased, it is associated with the development of atherosclerosis and cardiovascular and cerebrovascular diseases [24]. In the literature, cinnamon has been shown to reduce blood lipid levels in patients with type 2 diabetes [10]. Such effects may be secondary to the improvement in glycaemic control, where ketoacidosis, a common complication due to reduced cellular glucose uptake, can result in hyperlipidemia due to the mobilization of alternative fuels (i.e., lipids restored in the fat tissue). In the current study, in healthy rats, both LDL and cholesterol were increased by cinnamon supplement, albeit with normoglycemia. This may attribute to several downregulated lipid metabolic markers in the liver. SIRT1 is essential for cellular survival, especially during stress, which promotes nutrient metabolism to maximize energy availability [16]. SIRT1 activates its downstream signal PGC-1α, which is another essential metabolic switch to promote fatty acid oxidation [25]. Thus, the suppression of SIRT1 by obesity leads to metabolic dysfunction, such as hyperlipidemia and hyperglycemia [26]. In this study, liver SIRT1 and PCG-1α are downregulated in cinnamon-treated rats, which may indirectly lead to their dyslipidemia. Several enzymes and mediators involved in cholesterol and LDL production in the liver were not significantly affected by cinnamon, perhaps due to the short duration of the treatment. However, a trend of increased HMGCR and reduced LDLR can be observed, which may contribute to the increased blood total cholesterol and LDL levels.
However, it needs to be noted that liver FASN, the enzyme that regulates de novo lipid synthesis, was suppressed by cinnamon supplementation. Concurrently, there was also a reduction in macrophage (Kupfer cells) number and inflammation, reflected by F4/80 and TNFα expression, respectively. Both increased FASN and Kupfer cells have been shown to promote the development of liver steatosis, while the suppression of FASN and Kupfer cells can ameliorate alcohol-induced steatosis [27–29]. These changes in our study suggest that cinnamon may help to prevent liver steatosis. This effect may be promising in controlling the fatty liver disease. In a small randomized clinical trial on patients with nonalcoholic fatty liver disease (NAFLD), 1.5 g of cinnamon was administered daily for 12 weeks with the implementation of both a balanced diet and physical activity [30]. Although the study reported improved blood lipid profile and reduced blood ALT, AST, and inflammatory markers suggesting improved liver function, it did not directly examine the liver lipid accumulation using imagining analysis [30]. While the therapeutic effect of cinnamon is important to those with lipid metabolic disorders, the preventive effects of cinnamon against NAFLD may be of more interest to individuals either at risk or no risk (i.e., healthy population), which needs to be confirmed in models of hepatic steatosis in future studies.
The increase in abdominal fat mass by cinnamon is small, but yet is of concern. Abdominal fat is now considered a risk for early all-cause mortality, with the highest incidence in cardiovascular diseases and metabolic disorders [31]. In this study, fat mass was increased in the face of hyperlipidemia in cinnamon-treated rats. This fat accumulation does not seem to be due to fat cell differentiation reflected by unchanged PPARγ, which regulates the differentiation of new adipocytes. The reduced breakdown and oxidation of the lipids in the fat tissue may be the contributors, as suggested by the lipolysis marker ATGL and fatty acid oxidation markers CPT-1α and SQLE. When fat storage is increased, blood monocytes are attracted to the fat tissue forming resident macrophages, which in turn produce the proinflammatory cytokine TNFα leading to insulin resistance and glucose intolerance. However, in this study, the initial fat accumulation has not increased macrophage number or inflammation. This may be due to the short duration of treatment and the small increase in fat mass from 1.13% to 1.50% of total body weight. Long-term treatment (e.g., 12 weeks) is needed in future studies to confirm this observation further.
It also needs to be noted that Glut4 is downregulated in the fat. Glut4 is essential for insulin-stimulated glucose uptake [32]. Cinnamon has been suggested to improve insulin resistance caused by high-fat diet consumption [32, 33]. However, in the setting of a balanced healthy diet, the response of Glut4 observed in this study may suggest otherwise. Also, cinnamaldehyde in cinnamon has been identified to exert the hypoglycemia effect [34]. In this study, we used cinnamon in rats with normoglycemia, which may act differently. It is likely that the downregulation of Glu4 only occurs during normoglycemia to prevent hypoglycemia, and cinnamaldehyde increases Glu4 level when there is increased blood glucose or the presence of insulin resistance [6, 35].
There are limitations to this study. We only tested the metabolic effect of a single low dose of cinnamon in a short-term setting, which showed significant dyslipidemia and increased fat mass in healthy rats. A 16-week cinnamon treatment using a higher dose (3 g/day) has been shown to improve body composition and metabolic parameters in an Indian cohort with preexisting metabolic disorders [36]. Therefore, future studies need to evaluate the effects of high doses to determine whether such metabolic benefits of cinnamon only occur in the setting of metabolic disorder.
5. Conclusion
A low level of cinnamon supplementation in the daily diet seems to encourage fat accumulation and disturb lipid homeostasis in healthy rats, opposite to the beneficial effects reported in individuals with metabolic disorders. Thus, healthy individuals or those with liver dysfunction may need to be cautious with the frequent consumption of cinnamon.
Acknowledgments
This study was funded by an International Young Scientist Fellowship (81750110554) awarded to A/Prof Hui Chen from the National Natural Science Foundation of China and research grants awarded to A/Prof Chenju Yi from the National Natural Science Foundation of China (NSFC 81971309), Guangdong Basic and Applied Basic Research Foundation (2019A1515011333), the Fundamental Research Funds for the Central Universities (19ykzd04), and Shenzhen Fundamental Research Program (JCYJ20190809161405495 and RCYX20200714114644167).
Data Availability
All data are included in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Xiaomin Huang and Haiyang Cai contributed equally to this work.
References
- 1.Santos H. O., Da Silva G. A. R. To what extent does cinnamon administration improve the glycemic and lipid profiles? Clinical Nutrition ESPEN. 2018;27:1–9. doi: 10.1016/j.clnesp.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Deyno S., Eneyew K., Seyfe S., et al. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: a meta-analysis and meta-regression. Diabetes Research and Clinical Practice. 2019;156 doi: 10.1016/j.diabres.2019.107815.107815 [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe J. H., Gheewala N. M., O’Keefe J. O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. Journal of the American College of Cardiology. 2008;51(3):249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Xu J., Zhong L. Impact of cinnamon on glycogen in the liver and muscle in a rat model of type 2 diabetes. Chinese Journal of Traditional Medical Science and Technology. 2007;14(3):171–172. [Google Scholar]
- 5.Khan A., Safdar M., Ali Khan M. M., Khattak K. N., Anderson R. A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 6.Camacho S., Michlig S., De Senarclens-Bezençon C., et al. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Scientific Reports. 2015;5(1):p. 7919. doi: 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manya K., Champion B., Dunning T. The use of complementary and alternative medicine among people living with diabetes in Sydney. BMC Complementary and Alternative Medicine. 2012;12(1):p. 2. doi: 10.1186/1472-6882-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subash Babu P., Prabuseenivasan S., Ignacimuthu S. Cinnamaldehyde-A potential antidiabetic agent. Phytomedicine. 2006;14(1):15–22. doi: 10.1016/j.phymed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Maierean S. M., Serban M.-C., Sahebkar A., et al. The effects of cinnamon supplementation on blood lipid concentrations: a systematic review and meta-analysis. Journal of Clinical Lipidology. 2017;11(6):1393–1406. doi: 10.1016/j.jacl.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Allen R. W., Schwartzman E., Baker W. L., Coleman C. I., Phung O. J. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. The Annals of Family Medicine. 2013;11(5):452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan M. P., Proulx S. Nutritional supplements and serum lipids: does anything work? Current Atherosclerosis Reports. 2009;11(6):470–476. doi: 10.1007/s11883-009-0070-2. [DOI] [PubMed] [Google Scholar]
- 12.Qin B., Polansky M. M., Anderson R. A. Cinnamon extract regulates plasma levels of adipose-derived factors and expression of multiple genes related to carbohydrate metabolism and lipogenesis in adipose tissue of fructose-fed rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2010;42(3):187–193. doi: 10.1055/s-0029-1242746. [DOI] [PubMed] [Google Scholar]
- 13.Tuzcu Z., Orhan C., Sahin N., Juturu V., Sahin K. Cinnamon polyphenol extract inhibits hyperlipidemia and inflammation by modulation of transcription factors in high-fat diet-fed rats. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/1583098.1583098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsoodeeri F. N., Alqabbani H. M., Aldossari N. M. Effects of cinnamon (Cinnamomum cassia) consumption on serum lipid profiles in albino rats. Journal of Lipids. 2020;2020 doi: 10.1155/2020/8469830.8469830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang H.-C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends in Endocrinology & Metabolism. 2014;25(3):138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen L. T., Chen H., Pollock C. A., Saad S. Sirtuins-mediators of maternal obesity‐induced complications in offspring? The FASEB Journal. 2016;30(4):1383–1390. doi: 10.1096/fj.15-280743. [DOI] [PubMed] [Google Scholar]
- 17.Nair A., Jacob S. A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Chan Y. L., Sukjamnong S., et al. A mitochondrial specific antioxidant reverses metabolic dysfunction and fatty liver induced by maternal cigarette smoke in mice. Nutrients. 2019;11(7):p. 1669. doi: 10.3390/nu11071669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Chan Y. L., Wang B., et al. E‐cigarettes damage the liver and alter nutrient metabolism in pregnant mice and their offspring. Annals of the New York Academy of Sciences. 2020;1475(1):64–77. doi: 10.1111/nyas.14411. [DOI] [PubMed] [Google Scholar]
- 20.Sahib A. Antidiabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. Journal of Intercultural Ethnopharmacology. 2016;5(2):108–113. doi: 10.5455/jice.20160217044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasanzade F., Toliat M., Emami S. A., Emamimoghaadam Z. The effect of cinnamon on glucose of type II diabetes patients. Journal of Traditional and Complementary Medicine. 2013;3(3):171–174. doi: 10.4103/2225-4110.114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costello R. B., Dwyer J. T., Saldanha L., Bailey R. L., Merkel J., Wambogo E. Do cinnamon supplements have a role in glycemic control in type 2 diabetes? a narrative review. Journal of the Academy of Nutrition and Dietetics. 2016;116(11):1794–1802. doi: 10.1016/j.jand.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rui L. Energy metabolism in the liver. Comprehensive Physiology. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borén J., Chapman M. J., Krauss R. M., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal. 2020;41(24):2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugden M. C., Caton P. W., Holness M. J. PPAR control: it’s SIRTainly as easy as PGC. Journal of Endocrinology. 2010;204(2):93–104. doi: 10.1677/joe-09-0359. [DOI] [PubMed] [Google Scholar]
- 26.Costa C. D. S., Hammes T. O., Rohden F., et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obesity Surgery. 2010;20(5):633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 27.Järveläinen H. A., Fang C., Ingelman-Sundberg M., Lukkari T. A., Sippel H., Lindros K. O. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. Journal of Hepatology. 2000;32(6):900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 28.Dorn C., Riener M. O., Kirovski G., et al. Expression of fatty acid synthase in nonalcoholic fatty liver disease. International Journal of Clinical and Experimental Pathology. 2010;3(5):505–514. [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng G., Palanisamy A. P., Evans Z. P., et al. Cerulenin blockade of fatty acid synthase reverses hepatic steatosis in ob/ob mice. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075980.e75980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Askari F., Rashidkhani B., Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutrition Research. 2014;34(2):143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Jayedi A., Soltani S., Zargar M. S., Khan T. A., Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370 doi: 10.1136/bmj.m3324.m3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saadeldeen F. S. A., Niu Y., Wang H., et al. Natural products: regulating glucose metabolism and improving insulin resistance. Food Science and Human Wellness. 2020;9(3):214–228. doi: 10.1016/j.fshw.2020.04.005. [DOI] [Google Scholar]
- 33.Couturier K., Hininger I., Poulet L., et al. Cinnamon intake alleviates the combined effects of dietary-induced insulin resistance and acute stress on brain mitochondria. The Journal of Nutritional Biochemistry. 2016;28:183–190. doi: 10.1016/j.jnutbio.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhu R., Liu H., Liu C., et al. Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharmacological Research. 2017;122:78–89. doi: 10.1016/j.phrs.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Nikzamir A., Palangi A., Kheirollaha A., et al. Expression of glucose transporter 4 (GLUT4) is increased by cinnamaldehyde in C2C12 mouse muscle cells. Iranian Red Crescent Medical Journal. 2014;16(2) doi: 10.5812/ircmj.13426.e13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta Jain S., Puri S., Misra A., Gulati S., Mani K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: a randomized double -blind control trial. Lipids in Health and Disease. 2017;16(1):p. 113. doi: 10.1186/s12944-017-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in this manuscript.


