Abstract
Objectives.
Diagnosis-to-treatment interval is an important quality measure that is recognized by the National Accreditation Program for Breast Centers, and the American Society of Breast Surgeons and the National Quality Measures for Breast Care. The aim of this study was to assess factors related to delays in receiving breast cancer treatment.
Methods:
This retrospective cohort study (2002 to 2010) used data from the South Carolina Central Cancer Registry (SCCCR) and Office of Revenue and Fiscal Affairs (RFA) to examine racial differences in diagnosis-to-treatment time (in days), with adjuvant hormone receipt, surgery, chemotherapy, and radiotherapy assessed separately. Chi-square tests, logistic regression and generalized linear models were used to compare diagnosis-to-treatment days.
Results:
Black women on average received adjuvant hormone therapy, surgery, chemotherapy, and radiotherapy 25, 8, 7, and 3 days later than their White counterparts, respectively. Black women with local stage cancer had later time to surgery (OR: 1.6; CI: 1.2-2.2) compared with White women with local stage cancer. Black women living in rural areas had higher odds (OR: 2.0; CI: 1.1-3.7) of receiving late chemotherapy compared with White women living in rural areas. Unmarried Black women also had greater risk (OR: 2.0; CI: 1.0-4.0) of receiving late radiotherapy compared to married White women.
Conclusions:
To improve timely receipt of effective BrCA treatments, programs aimed at reducing racial disparities may need to target subgroups of Black breast cancer patients based on their social determinants of health and geographic residence.
Keywords: Race, Breast Cancer, Health Disparities, Treatment Delay
Introduction
Survival studies have associated delay in receipt of treatment with less favorable survival among breast cancer (BrCA) cases but have not clearly identified specific disparities that contribute to mortality; [1, 2] Potential factors in minority women delays related to diagnosis, surgery, chemotherapy, adjuvant hormonal therapy, and all forms of treatment combined. [3-6] Factors documented to affect delay in treatment among Black BrCA patients include age, hospital type, trust in oncologists, and communication with physicians, likely rooted in systemic racism that persists in the healthcare system. [4, 6-9] Delays in adjuvant hormone therapy (AHT) among BrCA patients decrease survival and increase patient anxiety.[10] McGee et al. showed that younger Black women experienced greater delay than White women of the same age. [4]
Although studies in other states consistently show that Black women with BrCA experience delays in receipt of surgery, chemotherapy, AHT, and radiation, this has not been examined in SC, which has a high representation of minority and rural populations, which tend to have generally worse outcomes. [1, 3, 4, 11, 12] SC also has a centralized data warehouse that allows for unique linkages between diagnostic data from its central cancer registry and complete treatment data from administrative sources from Medicaid or private insurance plans. Only a few other states have the capacity to complete these types of investigations (e.g., with the national data source, Surveillance, Epidemiology, and End Results Program cancer registry, only linked with Medicare enrollees aged 65 years and older) and of these, SC has the highest proportion of economically disadvantaged, rural, and Black residents. By combining these two resources, we have the unique ability to examine BrCA disparities in younger, racially, socio-economically, and geographically diverse populations. The aim of this study was to assess factors related to delays in receiving BrCA treatment.
Materials and Methods
Data Sources
This retrospective cohort study (2002 to 2010) included data on all BrCA patients derived from linked files from the SC Central Cancer Registry (SCCCR) and Office of Revenue and Fiscal Affairs (RFA) (which maintains the administrative medical claims data for the South Carolina Public Employee Benefits plan and Medicaid). The dataset was deidentified; hence, the study was exempt from review by the University of South Carolina’s Institutional Review Board. However, the protocol was reviewed by the SC Department of Health and Environmental Control (DHEC, which houses the SCCCR) prior to data release. Data in the SCCCR include information on demographics, diagnosis date, cancer location and histology, first course of treatment, and overall survival. [13] All BrCA cases between 2002 and 2010 who met eligibility criteria (that could be ascertained from their files) were given to RFA. Then RFA matched the data to determine which cases linked and further met our eligibility criteria (that required claims data to ascertain). This resulted in 2155 cases with evaluable data that constituted the combined dataset that was used to conduct all analyses.
To create an analytic dataset, we utilized datasets from the RFA (Medicaid and State Health Plan), Best Chance Network (BCN), the CDC-sponsored National Breast and Cervical Cancer Early Detection Program in SC [14, 15], and SCCCR to create an extensive look at BrCA treatment in SC for Black and White women. Women who are eligible for the BCN program are those that are aged 47-64 years, residents of SC, underserved/underinsured or do not have insurance, and are of low income (less than 200% of the Federal Poverty Level). The cancer registry has an indicator variable for BCN enrollment (yes or no) and this was provided as part of the data from SCCCR. BCN enrollment was neither an inclusion nor an exclusion criteria.
Inclusion and Exclusion Criteria
To qualify for inclusion in the analytic sample, BrCA cases had to be diagnosed with a first primary BrCA between 2002 and 2010, have either Medicaid or State Health Plan insurance at the time of diagnosis and have had 36 months of continuous eligibility with the insurance carrier. This was to ensure that complete treatment data were available for at least 36 months post diagnosis for each case, thus reducing the risk of bias from misclassification.
Variables
The main predictor variable was race, dichotomized as Black or White. Race was self-reported and extracted from the SCCCR records. The main outcome variable was time from diagnosis to receipt of first treatment for various forms of treatment, i.e., surgery, AHT, chemotherapy and radiotherapy. The analysis on the receipt of AHT was performed on all patients who were estrogen receptor positive and/or progesterone receptor positive utilizing the cancer registry data.
The first treatment received was determined and the number of days from diagnosis to the treatment modality also was determined. The outcome variable for AHT was treated as numeric because records met the assumptions for a linear model using skewness. Also, it met the assumption for a linear model because the kurtosis was −2 to +2 when assessed by race.
The outcome variables for chemotherapy, radiotherapy and surgery were dichotomized (early and late) because they did not meet the assumptions for linear models. The cut-off for dichotomization was based on the median (median split) for the distribution, which was 17, 39, and 135 days for surgery, post-surgery chemotherapy and post-surgery radiotherapy respectively. [16]
Variables that were considered as covariates or effect modifiers were age, marital status, urban vs. rural designation (based on Rural Urban Commuting Codes/RUCA 2003), year of diagnosis (2002-2004, 2005-2007, 2008-2010), hormone receptor status (positive or negative), enrollment in BCN (yes or no), stage of BrCA at diagnosis (in situ, local, regional, or distant), grade of BrCA at diagnosis (well differentiated, moderately differentiated, poorly differentiated and undifferentiated) network distance to health care provider for each of the four treatment modalities (in miles), early/late surgery (early or late) for non-surgery outcomes, and insurance provider (provider 1 or provider 2). One of the health insurance provider categories was Medicaid, while the other one was private (e.g., State Employee Health Plan); the specific payer source was not identified in the analytic dataset as per the data use agreements.
Network distance to provider refers to distance from the location (house) of the patient to provider of service (hospital/clinical site) which performed each type of treatment. This was calculated using ArcGIS by utilizing the latitude and longitude of the patient’s residential address and latitude and longitude of the provider’s location. This was calculated for us by the RFA because of confidentiality issues.
Analysis
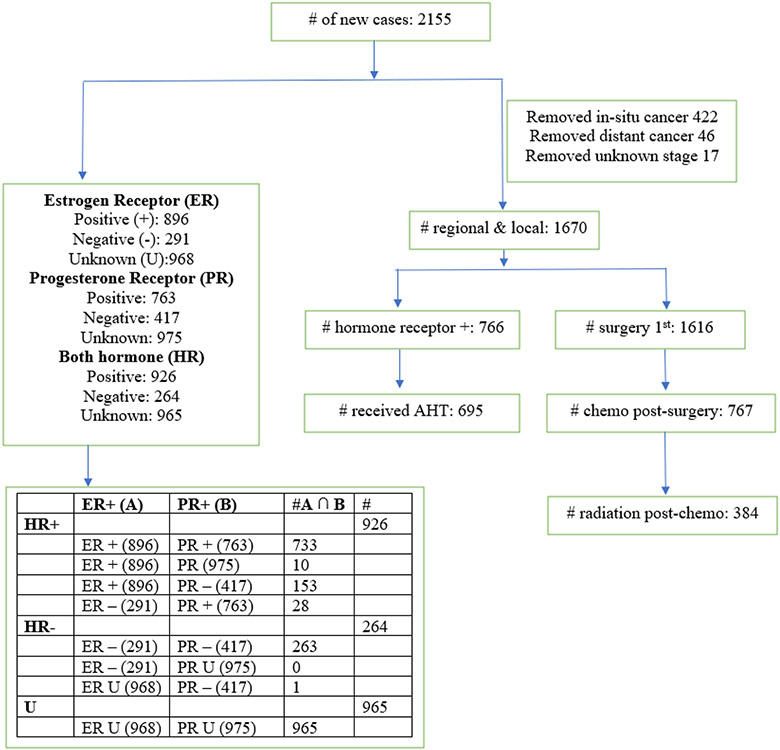
In all analysis, we assessed racial disparity in time to treatment. Race was utilized as the main exposure; additional variables were then added into the model and backward selection was utilized to select the best fitting model. Time to treatment considered all women who received AHT (hormone receptor positive breast cancer); all women who received surgery; women who received chemotherapy (post-surgery) and women who received radiation (post-chemotherapy). This analysis was performed in a manner consistent with recommended guidelines by the National Comprehensive Cancer Network’s (NCCN) Clinical Guidelines in Oncology for locoregional breast cancer where women received surgery, chemotherapy and radiation in that order. Bleicher et al and Recht et al also found that when chemotherapy is given before radiotherapy, local recurrence is higher; however, when radiotherapy is given before chemotherapy, metastasis increases thereby suggesting the order: surgery, chemotherapy and radiotherapy.[17, 18] This analysis focused on local and regional breast cancer only. In situ and metastatic BrCA were removed from the analysis (Figure 1).
Figure 1.
Data Flow Diagram
A generalized linear model was used to compute adjusted means and SD for AHT (dependent variable that met linear assumptions) overall and by race after adjustment for age, cancer grade, cancer stage and age of patients. This analysis was performed on patients who were hormone receptor positive and also received AHT (see Figure 1). A model-building procedure was used to fit the best model. Generally, a two-way interaction was first assessed between race*(diagnosis year, urban-rural status, distance, age, marital status, cancer stage, cancer grade, BCN enrollment, hormone receptor status, and insurance type) as part of the statistical analyses and stratified analyses were conducted to further clarify statistically significant interactions. In the assessment of racial disparity and receipt of post-surgery chemotherapy, additional interaction, race*time to surgery was assessed; this interaction was significant, so we stratified by time to surgery. In the assessment of racial disparity and receipt of radiation, race*time to surgery and race*time to chemotherapy were assessed; race*time to chemotherapy showed significant interaction but race*time to surgery did not show significant interaction so we did not stratify by time to chemotherapy. Based on statistically significant interactions, the tables were stratified. After stratification, variable selection began as a series of bivariate analyses (i.e., exposure + potential covariate) where potential covariates were added to a “full” model. Backward elimination procedures in SAS were used to develop “final” models that included all covariates that were statistically significant.
Results
Descriptive statistics for this study sample are shown in Table 1. Overall, there were 2155 patients with BrCA, of which the majority were White women (1557, 72%). In bivariate analyses, there were significant differences between Black or White women in age, rural/urban status, year of diagnosis, hormone receptor status, cancer grade, cancer stage and insurance provider. Black women were younger (45-54 years of age; 46%) than Whites (42%, p<0.01). Black women were more likely to be unmarried (47%) compared to White women (23%, p:<0.01). The proportion of White women who lived in urban areas (79% vs 67 %) and had hormone receptor positive cancer (46% vs 36%) were higher compared to Black women (both p<0.01). More Black women were participants in BCN compared to their White counterparts (10% vs 4%, p<0.01).
Table 1.
Summary of patients’ characteristics by race, Retrospective Cohort of South Carolina Cancer Registry, 2002 to 2010.
| N (%) | N (%) | N (%) | |||
|---|---|---|---|---|---|
| Characteristic | Total (N=2155) |
White N=1557 (72.25) |
Black N=598 (27.75) |
P- value |
|
| Age (mean±SD) | 51.2(7.2) | 51.6(7.0) | 50.2(7.6) | <0.01 | |
| Age categories | Under 45 years old | 386(17.9) | 256(16.4) | 130(21.7) | <0.01 |
| 45-54 years old | 924(42.9) | 649(41.7) | 275(46.0) | ||
| 55-64 years old | 845(39.2) | 652(41.9) | 193(32.3) | ||
| Marital status | Not married | 639(29.7) | 359(23.1) | 280(46.8) | <0.01 |
| Married | 1256(58.3) | 1020(65.5) | 236(39.5) | ||
| Unknown/Missing | 260 (12.0) | 178 (11.4) | 82 (13.7) | ||
| Rural/Urban status | Urban | 1634(75.8) | 1232(79.1) | 402(67.2) | <0.01 |
| Rural | 521(24.2) | 325(20.9) | 196(32.8) | ||
| Year of diagnosis | 2002-2004 | 611(28.3) | 452(29.0) | 159(26.6) | 0.02 |
| 2005-2007 | 693(32.2) | 518(33.3) | 175(29.3) | ||
| 2008-2010 | 851(39.5) | 587(37.7) | 264(44.2) | ||
| Hormone receptor status | Positive | 926(43.0) | 709(45.5) | 217(36.3) | <0.01 |
| Negative | 264(12.2) | 166(10.7) | 98(16.4) | ||
| Unknown/Missing | 965(44.8) | 682(43.8) | 283(47.3) | ||
| Stage at Diagnosis | In-situ | 422(19.6) | 299(19.2) | 123(20.6) | 0.68 |
| Local | 1013(47.0) | 741(47.6) | 272(45.5) | ||
| Regional | 657(30.5) | 470(30.2) | 187(31.3) | ||
| Distant | 46(2.1) | 36(2.3) | 10(1.7) | ||
| Unknown | 17 (0.8) | 11(0.7) | 6(1.0) | ||
| Cancer grade | I | 392(18.2) | 300(19.3) | 92(15.4) | <0.01 |
| II | 785(36.4) | 608(39.0) | 177(29.6) | ||
| III | 749(34.8) | 479(30.8) | 270(45.1) | ||
| IV | 32(1.5) | 22(1.4) | 10(1.7) | ||
| Unknown | 197(9.1) | 148(9.5) | 49(8.2) | ||
| Best Chance Network | Yes | 119(5.5) | 60(3.8) | 59(9.9) | <0.01 |
| No | 2036(94.5) | 1497(96.2) | 539(90.1) | ||
| Insurance provider | 1 | 1640(76.1) | 1285(82.3) | 358(59.9) | <0.01 |
| 2 | 515(23.9) | 275(17.7) | 240(40.1) | ||
| Average distance to AHT provider (miles) | 7.3±17.0 | 7.2±18.1 | 7.4±13.4 | 0.79 | |
| Average distance to Surgery provider (miles) | 19.9±23.5 | 20.0±25.0 | 19.6±19.1 | 0.72 | |
| Average distance to Radiation provider (miles) | 18.6±18.5 | 18.2±18.3 | 19.8±19.1 | 0.17 | |
| Average distance to Chemotherapy provider (miles) | 20.6±20.7 | 19.7±20.3 | 22.6±21.5 | 0.03 |
P-values were obtained comparing Black women with White women using Student’s t-test for numeric variables and Chi-square for categorical variables.
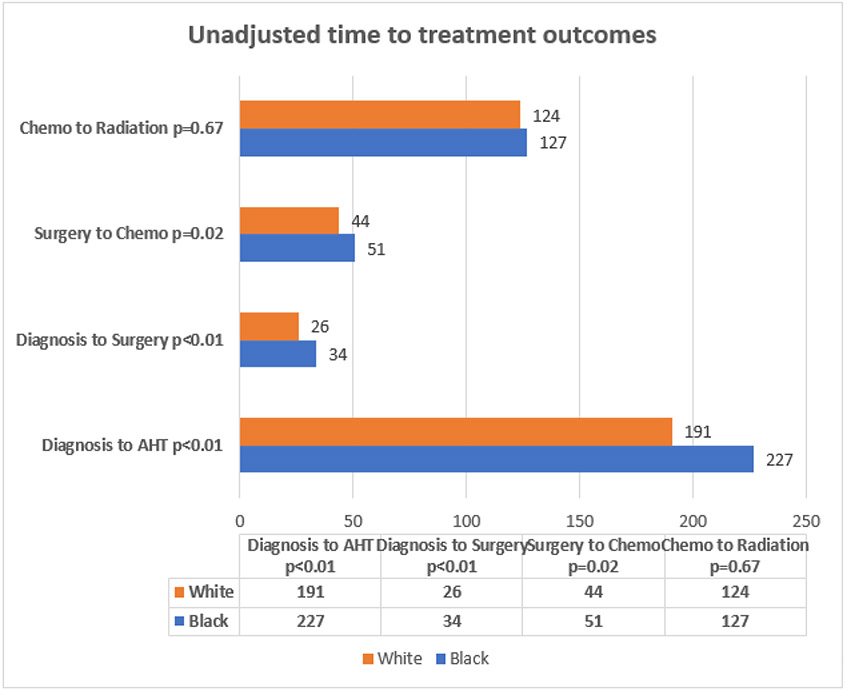
Figure 1 showed the number of BrCA patients with local and regional stage BrCA were 1670 (78%). Of the 1670 that had local and regional stage BrCA, 766 (46%) had hormone receptor positive BrCA and 695 (91%) received AHT. Also, of the 1670 that had local and regional stage BrCA, 1616 (97%) had surgery. Of the 1616 that had surgery, 767 (47%) had chemotherapy post-surgery. Of the 767 that had chemotherapy post-surgery, 384 (50%) had radiation post-chemotherapy. Black women, on average, received adjuvant hormone therapy, surgery, chemotherapy, and radiotherapy 25, 8, 7, and 3 days later than their White counterparts. (not presented in tables).
Table 2 presents diagnosis to AHT, time-adjusted and unadjusted. The unadjusted White-Black difference in time to AHT was 36 days while the adjusted time to AHT was 25 days. In the full model, the White-Black difference in time to AHT was 29 days; however, in the reduced final model the White-Black difference was 25 days. The reduced final model adjusted for cancer stage, cancer grade and age of patients.
Table 2.
Mean diagnosis-to-hormone treatment times, Retrospective Cohort of South Carolina Cancer Registry, 2002 to 2010.
| Unadjusted Mean Days Model | |
|---|---|
| Mean Days (+ SD) | |
| White | 191.1 ± 107.1 |
| Black | 227.4 ± 109.2 |
| Black-White | 36.3 |
| % Increase | 19.0 |
| p-value | <0.01 |
| Adjusted Model (Full model) * | |
| Mean Days(+ SD) | |
| White | 185.9 ± 392.4 |
| Black | 214.5 ± 211.1 |
| Black-White | 28.6 |
| % Increase | 15.4 |
| p-value | 0.01 |
| (+ SD) Adjusted Model (Final model) ** Mean Days(+ SD) |
|
| White | 200.5 ± 201.5 |
| Black | 225.1 ± 138.6 |
| Black-White | 24.6 |
| % Increase | 12.3 |
| p-value | <0.01 |
Model adjusted for age, cancer stage, cancer grade, diagnosis year, BCN enrollment, urban status, distance, marital status, county and insurance type.
Model adjusted for age, cancer stage and cancer grade.
Table 3 showed that the odds of late receipt of surgery was 1.3 times (95% CI: 1.0-1.7) more likely among Black women compared with White women. Black women with local cancer stage were 1.6 (CI: 1.2-2.2) times more likely as White women with local cancer stage to receive late surgery. Both Black and White women diagnosed at a more recent date (2008-2010) were 2.0 (CI: 1.3-3.0) and 1.7 (CI: 1.2-2.3), respectively, times more likely to receive late surgery compared to White women who were diagnosed in 2002-2004. A similar pattern was seen among Black women who were diagnosed in the years 2005-2007 (OR: 2.1; CI: 1.4-3.3).
Table 3.
Racial disparities among breast cancer patients and the odds of late receipt of surgery stratified by marital status, urban status and distance, Retrospective Cohort of South Carolina Cancer Registry, 2002 to 2010.
| Stratum | Race | Total | Early receipt (<17 days) 794 (49.1%) |
Late receipt (≥17 days) 822 (50.1%) |
Crude OR* | Adjusted OR Full model** |
Adjusted OR Final model*** |
|---|---|---|---|---|---|---|---|
| Overall | No (%) | No (%) | OR (CI) | OR (CI) | OR (CI) | ||
| White | 1180(73.0) | 606(51.4) | 574(48.6) | Reference | Reference | Reference | |
| Black | 436(27.0) | 188(43.1) | 248(56.9) | 1.4(1.1-1.7) | 1.4(1.1-1.8) | 1.3 (1.0-1.7) | |
| Stage | |||||||
| Local | White | 721(44.6) | 372(51.6) | 349(48.4) | Reference | Reference | Reference |
| Local | Black | 261(16.2) | 105(40.2) | 156(59.8) | 1.6(1.2-2.1) | 1.6(1.2-2.2) | 1.6(1.2-2.2) |
| Regional | White | 459(28.4) | 234(51.0) | 225(49.0) | 1.0(0.8-1.3) | 1.0(0.8-1.3) | 1.0(0.8-1.3) |
| Regional | Black | 175(10.8) | 83(47.4) | 92(52.6) | 1.2(0.8-1.6) | 1.0(0.7-1.5) | 1.0(0.7-1.5) |
| Diagnosis year | |||||||
| 2002-2004 | White | 354(21.9) | 208(58.8) | 146(41.2) | Reference | Reference | Reference |
| 2002-2004 | Black | 116(7.2) | 63(54.3) | 53(45.7) | 1.2(0.8-1.8) | 1.1(0.7-1.8) | 1.1(0.7-1.8) |
| 2005-2007 | White | 399(24.7) | 215(53.9) | 184(46.1) | 1.2(0.9-1.6) | 1.2(0.9-1.6) | 1.2(0.9-1.6) |
| 2005-2007 | Black | 135(8.3) | 52(38.5) | 83(61.5) | 2.3(1.5-3.4) | 2.1(1.3-3.3) | 2.1(1.4-3.3) |
| 2008-2010 | White | 427(26.4) | 183(42.9) | 244(57.1) | 1.9(1.4-2.5) | 1.7(1.2-2.3) | 1.7(1.2-2.3) |
| 2008-2010 | Black | 185(11.5) | 73(38.5) | 112(60.5) | 2.2(1.5-3.4) | 2.0(1.3-3.0) | 2.0(1.3-3.0) |
| Insurance | **** | ||||||
| 1 | White | 965(59.7) | 517(53.6) | 448(46.4) | Reference | Reference | Reference |
| 1 | Black | 262(16.2) | 123(47.0) | 139(53.0) | 1.3(1.0-1.7) | 1.3(1.0-1.8) | 1.3(1.0-1.8) |
| 2 | White | 215(13.3) | 89(41.4) | 126(58.6) | 1.6(1.2-2.2) | 1.4(1.0-2.1) | 1.4(1.0-2.0) |
| 2 | Black | 174(10.8) | 65(37.4) | 109(62.6) | 1.9(1.4-2.7) | 2.1(1.4-3.2) | 2.0(1.3-3.0) |
Crude model with race as only predictor for time to surgery
Full model with race as main predictor for time to surgery with confounders: age, marital status, urban status, cancer grade, distance, BCN enrollment, hormone receptor status, county and insurance provider.
Final model with race as main predictor for time to surgery with select confounders based on backward selection in SAS: marital status, distance, BCN enrollment and insurance provider.
For stratification by insurance status, full model had race as main predictor for time to surgery with confounders: age, marital status, urban status, cancer grade, distance, BCN enrollment, hormone receptor status and county; while final model had race as main predictor for time to surgery with select confounders based on backward selection in SAS: marital status, distance and BCN enrollment.
For Table 4 an additional interaction, race*time to surgery was assessed; this interaction was significant, so we stratified by time to surgery in Table 4. For Table 5, race*time to surgery and time to chemotherapy were assessed; race*time to chemotherapy showed significant interaction but race*time to surgery did not show significant interaction so we not stratify by time to chemotherapy in Table 5. Based on statistically significant interactions, we stratified the tables based on the significant interactions that were found. Among women who received surgery as their first treatment modality showed that Black women who lived in rural areas were twice (2.0; CI: 1.1-3.7) more likely than White women who lived in rural areas (Table 4). Black women who were not married were twice (2.0; CI: 1.0-4.0) as likely to receive late radiotherapy compared to White married women. Black women who lived in a home with distance ≤10 miles from the treatment facility were 2.6 times (CI: 1.1-6-1) more likely as White women who lived ≤10 miles from their care (Table 5).
Table 4.
Racial disparities among breast cancer patients and the odds of late receipt of post-surgery chemotherapy, Retrospective Cohort of South Carolina Cancer Registry, 2002 to 2010.
| Stratum | Race | Total n(%) |
Early receipt (<39 days) 373(48.6%) |
Late receipt (≥39 days) 394(51.4%) |
Crude OR* | Adjusted OR Full model** |
Adjusted OR Final model*** |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| White | 547(71.3) | 275(50.3) | 272(49.7) | Reference | Reference | Reference | |
| Black | 220(28.7) | 98(44.6) | 122(55.4) | 1.3(0.9-1.7) | 1.1(0.8-1.5) | 1.0(0.7-1.4) | |
| Stage | |||||||
| Local | White | 234(30.5) | 112(47.9) | 122(52.1) | Reference | Reference | Reference |
| Local | Black | 105(13.7) | 50(47.6) | 55(52.4) | 1.0(0.6-1.6) | 0.9(0.6-1.5) | 0.9(0.6-1.5) |
| Regional | White | 313(40.8) | 163(52.1) | 150(47.9) | 0.8(0.6-1.2) | 0.8(0.6-1.2) | 0.9(0.6-1.2) |
| Regional | Black | 115(15.0) | 48(41.7) | 67(58.3) | 1.3(0.8-2.0) | 1.1(0.7-1.8) | 1.1(0.7-1.8) |
| Urban | |||||||
| Rural | White | 119(15.5) | 64(53.8) | 55(46.2) | Reference | Reference | Reference |
| Rural | Black | 72(9.4) | 26(36.1) | 46(63.9) | 2.1(1.1-3.8) | 2.0(1.0-3.7) | 2.0(1.1-3.7) |
| Urban | White | 428(55.8) | 211(49.3) | 217(50.7) | 1.2(0.8-1.8) | 1.2(0.8-1.8) | 1.2(0.8-1.8) |
| Urban | Black | 148(19.3) | 72(48.6) | 76(51.4) | 1.2(0.8-2.0) | 1.1(0.6-1.8) | 1.0(0.6-1.7) |
| Surgery date | |||||||
| Early | White | 294(38.3) | 148(50.3) | 146(49.7) | Reference | Reference | Reference |
| Early | Black | 112(14.6) | 44(39.3) | 68(60.7) | 1.6(1.0-2.4) | 1.4(0.9-2.2) | 1.4(0.9-2.1) |
| Late | White | 253(33.0) | 127(50.2) | 126(49.8) | 1.0(0.7-1.4) | 0.9(0.6-1.3) | 0.9(0.7-1.3) |
| Late | Black | 108(14.1) | 54(50.0) | 54(50.0) | 1.0(0.7-1.6) | 0.8(0.6-1.3) | 0.8(0.5-1.3) |
Crude model with race as only predictor for time to chemotherapy (post-surgery)
Full model with race as main predictor for time to chemotherapy with confounders: age, marital status, diagnosis year, cancer grade, BCN enrollment, hormone receptor status, county, distance and insurance provider.
Final model with race as main predictor for time to surgery with select confounders based on backward selection in SAS: marital status, diagnosis year and BCN enrollment.
Table 5.
Racial disparities among breast cancer patients and the odds of late receipt of radiation (post-chemotherapy), Retrospective Cohort of South Carolina Cancer Registry, 2002 to 2010.
| Stratum | Race | Total n(%) |
Early receipt (<135 days) 188(49.0%) |
Late receipt (≥135 days) 196(51.0%) |
Crude OR* | Adjusted OR Full model** |
Adjusted OR Final model*** |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| White | 274(71.4) | 138(50.4) | 136(49.6) | Reference | Reference | Reference | |
| Black | 110(28.7) | 50(45.4) | 60(54.6) | 1.2(0.8-1.9) | 1.2(0.7-2.0) | 1.3(0.8-2.2) | |
| Married | |||||||
| Yes | White | 192(55.0) | 95(49.5) | 97(50.5) | Reference | Reference | Reference |
| Yes | Black | 50(14.3) | 28(56.0) | 22(44.0) | 0.8(0.4-1.4) | 0.7(0.4-1.5) | 0.9(0.4-1.7) |
| No | White | 57(16.3) | 27(47.4) | 30(52.6) | 1.1(0.6-2.0) | 1.2(0.6-2.5) | 1.2(0.7-2.3) |
| No | Black | 50(14.3) | 19(38.0) | 31(62.0) | 1.6(0.8-3.0) | 1.8(0.9-3.8) | 2.0(1.0-4.0) |
| Diagnosis year | |||||||
| 2008-2010 | White | 91(23.7) | 47(51.7) | 44(48.4) | Reference | Reference | Reference |
| 2008-2010 | Black | 40(10.4) | 14(35.0) | 26(65.0) | 1.9(0.9-4.3) | 1.7(0.7-3.9) | 1.9(0.8-4.2) |
| 2005-2007 | White | 90(23.4) | 43(47.8) | 47(52.2) | 1.2(0.7-2.1) | 0.9(0.5-1.7) | 0.9(0.5-1.6) |
| 2005-2007 | Black | 34(8.9) | 20(58.8) | 14(41.2) | 0.7(0.3-1.7) | 0.6(0.3-1.5) | 0.7(0.3-1.7) |
| 2002-2004 | White | 93(24.2) | 48(51.6) | 45(48.4) | 1.0(0.6-1.8) | 0.9(0.5-1.8) | 0.9(0.5-1.6) |
| 2002-2004 | Black | 36(9.4) | 16(44.4) | 20(55.6) | 1.3(0.6-2.9) | 1.3(0.6-3.0) | 1.4(0.6-3.1) |
| Distance | |||||||
| <=10 miles | White | 90(27.3) | 45(50.0) | 45(50.0) | Reference | Reference | Reference |
| <=10 miles | Black | 39(11.8) | 12(30.8) | 27(69.2) | 2.2(1.0-5.0) | 2.4(1.0-5.8) | 2.6(1.1-6.1) |
| >10 miles | White | 143(43.3) | 71(49.7) | 72(50.4) | 1.0(0.6-1.7) | 1.1(0.6-1.9) | 1.1(0.6-1.9) |
| >10 miles | Black | 58(17.6) | 32(55.2) | 26(44.8) | 0.8(0.4-1.6) | 0.8(0.4-1.7) | 0.8(0.4-1.8) |
| Chemotherapy date | |||||||
| Early | White | 158(41.2) | 73(46.2) | 85(53.8 | Reference | Reference | Reference |
| Early | Black | 56(14.6) | 19(33.3) | 37(66.1) | 1.7(0.9-3.2) | 1.8(0.8-3.6) | 1.8(0.9-3.6) |
| Late | White | 116(30.2) | 65(56.0) | 51(44.0) | 0.7(0.4-1.1) | 0.8(0.4-1.1) | 0.7(0.4-1.0) |
| Late | Black | 54(14.1) | 31(57.4) | 23(42.6) | 0.6(0.3-1.2) | 0.5(0.3-1.0) | 0.6(0.3-1.2) |
Crude model with race as only predictor for time to radiation (post-chemotherapy)
Full model with race as main predictor for time to chemotherapy with confounders: age, urban status, cancer stage, cancer grade, BCN enrollment, insurance provider, time to surgery and time to chemotherapy.
Final model with race as main predictor for time to surgery with select confounders based on backward selection in SAS: cancer stage, time to surgery, time to chemotherapy and BCN enrollment.
Discussion
This study demonstrated that late receipt of AHT was higher among Black women by 25 days, on average, compared with White women. We also found that the odds of late receipt of surgery was higher among Black women who had local-stage cancer (OR: 1.6; CI: 1.2-2.2) compared with White women with local-stage cancer. Black women who had a more recent date of diagnosis i.e., 2008-2010 (OR: 2.0; CI: 1.3-3.0) had a higher odds of receiving late surgery compared with White women who were diagnosed at an earlier date (2002-2004). Black women who lived in rural areas had a greater likelihood (OR: 2.0; CI: 1.1-3.7) of receiving late chemotherapy compared with White women who lived in rural areas. Black women who were not married and who lived in homes ≤10 miles from their providers had higher odds (OR: 2.0 and 2.6 respectively) of receiving late radiotherapy compared to their White counterparts.
In addition to showing that there were longer diagnosis-to-treatment times, which has been previously demonstrated, [10, 14] we were able to show the impact of factors such as being unmarried, living in rural areas, distance to providers, local stage cancer and time of diagnosis on late receipt of treatment. Our data that is uniquely linked between diagnostic data from central cancer registry and complete treatment data from administrative sources from Medicaid or private insurance plans in a state that has the highest proportion of economically disadvantaged, rural, and Black residents gave us the unique ability to examine BrCA disparities in younger, racially, socio-economically, and geographically diverse populations. Our study, therefore, helped to identify subgroups of BrCA patients that may benefit from more intense navigation to care in order to shorten the diagnosis-to-treatment times for various BrCA patients.
The diagnosis-to-treatment interval is an important quality measure that is recognized by the National Accreditation Program for Breast Centers (NAPBC), the American Society of Breast Surgeons (ASBS) and the National Quality Measures for Breast Care (NQMBC). [19] The NAPBC, ASBC and NQMBC all serve to validate BrCA care rendered by hospitals. Although, there has been no formal agreement on what constitutes an acceptable delay in terms of quality of BrCA care measures, our study demonstrated racial disparities in diagnosis to treatment in the receipt of surgery and AHT. [19]
In North Carolina (NC), statistically significant racial disparities in commencement of AHT was demonstrated: Black women were less likely to commence AHT, and this racial disparity was more pronounced among the subpopulation of patients that did not receive chemotherapy. [5] However, in a previous study in SC, there was no racial difference found between Black and White women in the commencement and early use of AHT, but further analysis showed that receipt of chemotherapy/radiation was independently associated with commencement/early use of AHT. (6) In the study described above in NC, the BrCA cases studied were privately insured [5]; however, in the previous study in SC [20], the BrCA cases only include women with Medicaid insurance. Because our study had both groups of insurance providers (Medicaid and a private payor health plan), it provided a deeper understanding; showing that racial disparities exist in receipt of AHT, with Black women entering care at least 25 days later after adjustments were made for age, cancer stage and cancer grade.
A study that used three cutoff points (30, 60, 90 days) to define a diagnosis-to-surgery delay showed that Black women were more likely to experience delays compared to White women, and this association was independent of health insurance status, age at diagnosis and cancer stage at diagnosis [21]. Additionally, studies have shown that after controlling for socio-economic status and stratifying by stage at diagnosis, there is still residual disparity in the cancer burden that is unfavorable to Black women when compared to White women [22, 23]. Our study provided an additional insight by demonstrating that local cancer stage and more recent date of diagnosis were factors that increased the odds of receiving late surgery among Black BrCA patients compared with their White counterparts.
One of our findings is that Black BrCA patients who lived in rural areas had higher odds of receiving late chemotherapy. This is similar to another study where our team identified lower treatment adherence rates among Black BrCA patients living in rural versus urban areas.[24] The rural-urban differences may be due to the fact that a higher proportion of the Black BrCA patients in our study lived in rural areas (33% compared to 21% White BrCA patients). Also, in rural areas, there may be fewer pharmacies; [25] and all counties in SC have some medically underserved areas while 65% are completely medically underserved. [26]
We also found that Black BrCA patients who were not married were at greater risk of receiving late radiotherapy. In a previous study that our team conducted, we also found that Black BrCA patients who were not married had a higher risk of death from BrCA compared with White women who were married.[27]. This information helps to identify sub-populations among Black BrCA patients who may be targets of more intense navigation efforts to preventive and curative services that are directed towards reducing mortality and morbidity from BrCA and reduction of racial disparities among BrCA patients. Similarly, another previous study showed that unmarried women with BrCA have higher rates of mortality compared to married women. [28] Also, in one of our prior research studies, we found that Black participants who had a partner enrolled in the study had a higher odds of retention in the behavioral trial study compared with participants that do not have a partner. [29] In a sense, having a partner (or being married) may be a proxy measure of social support that enhances healthful behavior. [29]
We also showed that Black women who lived ≤ 10 miles to providers of radiation therapy had a higher likelihood of receiving later treatment compared with White women in same category. This may be because breast cancer patients able to travel greater distances for treatment have increased access to tertiary National Cancer Institute-designated cancer centers. Previous studies have also shown that Black populations usually live close to inner-city academic medical centers, while white higher-socioeconomic populations usually lived in areas that are more suburban. [30, 31]
A potential explanation for the results showing consistent racial differences across various treatment outcomes and, in some cases, intersections with rurality and marital status reflects structural and institutional racism. [8, 9] The lack of interventions and educational materials contributes to systemic racism as Black women may not identify or find useful materials that does not feature or portray and individuals like them. In addition to navigation, our study suggested a patient literacy and cultural sensitivity intervention to assure that patient understand the purpose and value of the various treatments. Patients may not understand the reason for AHT and its value in decreasing recurrence and improving mortality. This is an issue because minimal educational materials or interventions are available that are specific to black women. [32]
Overall, our findings have implications for designing interventions that can help reduce racial disparities and help enhance the effectiveness of existing navigation programs. An example of a previously successful navigation program among breast cancer patient is the Breast Oncology Navigation Program. [33] The success of that program was based on the utility of fundamental knowledge base by a trained navigator to address 4 areas of navigation namely, diagnostic, treatment, financial and support. [33] Navigation programs or other intervention programs may benefit from our findings by making specific plans to reach and retain Black women who live in rural areas, or live close to tertiary oncology centers, or are unmarried. Our findings may be useful for other NBCCEDP programs as it has the potential to identify subsets of women that are at high risk of having delays in times to treatment. Specifically, Black women residing in a rural area and who are unmarried may be at high risk and could benefit from targeted intervention and support. [14, 15].
One strength of this study is the availability of a wide range of effect modifiers and covariates that were utilized in the analysis. To our knowledge, this is the first study that assessed all 4 treatment modalities among both privately and publicly insured patients using both multivariable regression models to understand disparities in time-to-treatment among BrCA patients. Our study also was able to analyze the role of distance, marital status, rurality, cancer stage, diagnosis time and race in late receipt of treatment. Additionally, our data consisted of both Medicaid and privately insured women that have socioeconomic differences. However, we could not access the interaction between race and socioeconomic status which could have played a role in driving these disparities because we did not have enough information patients’ socioeconomic status.
In conclusion, late receipt of AHT was higher among Black women by 25 days on average compared with White women; late receipt of surgery was higher among Black women who had local stage cancer and a more recent date of diagnosis i.e., 2008-2010. Black women who lived in rural areas had a higher odd of receiving late chemotherapy and Black women who were not married and who lived in homes <=10 miles from their providers had a higher odd of receiving late radiotherapy compared to their White counterparts. To improve overall timely receipt of BrCA treatments, efforts need to be directed at Black BrCA patients that are not married, have localized cancer stage, live in rural areas and live ≤10 miles from health providers. This may be achieved through interventions such as navigation programs and other programs by NBCCEDP.
Supplementary Material
Figure 2.
Unadjusted mean time to treatment by race.
Acknowledgments
Financial support.
Oluwole A. Babatunde was supported by a National Cancer Institute’s K00 Fellowship grant (F99 CA 222722 and K00 CA 222722).
Swann A. Adams was supported by a National Cancer Institute’s R15 grant (R15CA179355) as principal investigator.
Samantha Truman is being supported by Interdisciplinary Graduate Training Program in Cancer Disparities (IGniTE-CD) program (GTDR17500160 ) sponsored by Susan G. Komen.
Tisha M. Felder was supported by a National Cancer Institute Mentored Research Scientist Development Award to Promote Diversity (K01CA193667).
Chanita Hughes-Halbert was supported by a National Cancer Institute’s R21 grant (R21 CA 235852).
Footnotes
Conflicts.
No Author declares a conflict of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.George P, et al. , Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt), 2015. 24(3): p. 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EC, Ziogas A, and Anton-Culver H, Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg, 2013. 148(6): p. 516–23. [DOI] [PubMed] [Google Scholar]
- 3.Freedman RA, He Y, and Keating NL, Racial disparities in chemotherapy for breast cancer: Are delays explained by hospital quality and volume? J Clin Oncol, 2011. 29(15_suppl): p. 6034. [Google Scholar]
- 4.McGee SA, et al. , Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev, 2013. 22(7): p. 1227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeder-Hayes KE, et al. , Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat, 2014. 145(3): p. 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard VB, et al. , Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient-provider relationship. Breast Cancer Res Treat, 2013. 139(1): p. 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman HP and Rodriguez RL, History and principles of patient navigation. Cancer, 2011. 117(15 Suppl): p. 3539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey ZD, et al. , Structural racism and health inequities in the USA: evidence and interventions. Lancet, 2017. 389(10077): p. 1453–1463. [DOI] [PubMed] [Google Scholar]
- 9.Pallok K, De Maio F, and Ansell DA, Structural Racism - A 60-Year-Old Black Woman with Breast Cancer. N Engl J Med, 2019. 380(16): p. 1489–1493. [DOI] [PubMed] [Google Scholar]
- 10.Liederbach E, et al. , Wait times for breast surgical operations, 2003-2011: a report from the National Cancer Data Base. Ann Surg Oncol, 2015. 22(3): p. 899–907. [DOI] [PubMed] [Google Scholar]
- 11.Sheppard VB, et al. , Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol, 2015. 22(9): p. 2902–11. [DOI] [PubMed] [Google Scholar]
- 12.Eberth JM, et al. , Mortality-to-incidence ratios by US Congressional District: Implications for epidemiologic, dissemination and implementation research, and public health policy. Prev Med, 2019. 129s: p. 105849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babatunde OA, et al. , Racial disparities in endometrial cancer mortality-to-incidence ratios among Blacks and Whites in South Carolina. Cancer Causes Control, 2016. 27(4): p. 503–11. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Precision Medicine Initiative. 2020. Available at https://ghr.nlm.nih.gov/primer/precisionmedicine/initiative. Downloaded on 04/February/2020.
- 15.Smith ER, et al. , Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev, 2008. 17(10): p. 2882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brand EC, et al. , Development and validation of a prediction model for adenoma detection during screening and surveillance colonoscopy with comparison to actual adenoma detection rates. PLoS One, 2017. 12(9): p. e0185560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleicher RJ, Timing and Delays in Breast Cancer Evaluation and Treatment. Ann Surg Oncol, 2018. 25(10): p. 2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recht A, et al. , The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med, 1996. 334(21): p. 1356–61. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman CS, et al. , National Quality Measures for Breast Centers (NQMBC): a robust quality tool: breast center quality measures. Ann Surg Oncol, 2010. 17(2): p. 377–85. [DOI] [PubMed] [Google Scholar]
- 20.Felder TM, et al. , Racial differences in receipt of adjuvant hormonal therapy among Medicaid enrollees in South Carolina diagnosed with breast cancer. Breast Cancer Res Treat, 2016. 157(1): p. 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedewa SA, et al. , Race and ethnicity are associated with delays in breast cancer treatment (2003-2006). J Health Care Poor Underserved, 2011. 22(1): p. 128–41. [DOI] [PubMed] [Google Scholar]
- 22.American Cancer Society (2015) Cancer Facts and Figures 2015. Atlanta: American Cancer Society; 2015 [cited October 22nd 2017]. Available from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. [Google Scholar]
- 23.Newman LA, et al. , Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol, 2006. 24(9): p. 1342–9. [DOI] [PubMed] [Google Scholar]
- 24.Heiney SP, et al. , Racial and Geographic Disparities in Endocrine Therapy Adherence Among Younger Breast Cancer Survivors. Am J Clin Oncol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey MM, Klingner J, and Moscovice I, Pharmacy services in rural areas: is the problem geographic access or financial access? The Journal of Rural Health, 2002. 18(3): p. 467–477. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health and Environmetal Control. Medically Underserved Areas in South Carolina. Data Source: Federal Health Resources and Service Administration (HRSA). Downloaded on 06/December/2020. Available at https://scdhec.gov/sites/default/files/docs/Health/docs/SC%20Medically%20Underserved%20Areas%20-%20Map.pdf.
- 27.Babatunde OA, et al. , Social Determinants of Racial Disparities in Breast Cancer Mortality Among Black and White Women. J Racial Ethn Health Disparities, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez ME, et al. , Prognostic significance of marital status in breast cancer survival: A population-based study. PLoS One, 2017. 12(5): p. e0175515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babatunde OA, et al. , Predictors of Retention among African Americans in a Randomized Controlled Trial to Test the Healthy Eating and Active Living in the Spirit (HEALS) Intervention. Ethn Dis, 2017. 27(3): p. 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamont EB, et al. , Do socially deprived urban areas have lesser supplies of cancer care services? J Clin Oncol, 2012. 30(26): p. 3250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markossian TW, Hines RB, and Bayakly R, Geographic and racial disparities in breast cancer-related outcomes in Georgia. Health Serv Res, 2014. 49(2): p. 481–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiney SP, et al. , A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat, 2019. 173(3): p. 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castaldi M, et al. , A Multidisciplinary Patient Navigation Program Improves Compliance With Adjuvant Breast Cancer Therapy in a Public Hospital. Am J Med Qual, 2017. 32(4): p. 406–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.