Abstract
Objective:
To determine whether clinically normal older adults with remote, mild traumatic brain injury (mTBI) show evidence of higher cortical Aβ burden.
Participants and Measurements:
We studied 134 clinically normal older adults (age 74.1±6.8 years, 59.7% female, 85.8% white) who underwent Aβ positron emission tomography (Aβ-PET) and who completed the Ohio State University Traumatic Brain Injury Identification questionnaire. We limited participants to those reporting injuries classified as mTBI. A subset (N=30) underwent a second Aβ-PET scan (mean 2.7 years later). We examined the effect of remote mTBI on Aβ-PET burden, interactions between remote mTBI and age, sex, and APOE status, longitudinal Aβ accumulation, and the interaction between remote mTBI and Aβ burden on memory and executive functioning.
Results:
Of 134 participants, 48 (36%) reported remote mTBI (0, N=86; 1, N=31, 2+, N=17; mean 37±23 years since last mTBI). Effect size estimates were small to negligible for the association of remote mTBI with Aβ burden (p=.94, η2<0.01), and for all interaction analyses. Longitudinally, we found a non-statistically significant association of those with remote mTBI (N=11) having a faster rate of Aβ accumulation (B=.01, p=.08) than those without (N=19). There was no significant interaction between remote mTBI and Aβ burden on cognition.
Conclusion:
In clinically normal older adults, history of mTBI is not associated with greater cortical Aβ burden and does not interact with Aβ burden to impact cognition. Longitudinal analyses suggest remote mTBI may be associated with more rapid cortical Aβ accumulation. This finding warrants further study in larger and more diverse samples with well-characterized lifelong head trauma exposure.
Keywords: traumatic brain injury, concussion, amyloid, PET, aging, neurodegenerative, dementia
INTRODUCTION
There is conflicting evidence linking remote head trauma exposure with altered cognitive aging trajectories or increased dementia risk. Mild traumatic brain injury (mTBI), especially when coupled with repetitive, asymptomatic (i.e., “subconcussive”) exposure, might predispose to diverse neuropathology (Mackay et al., 2019; Mez et al., 2017). One focus is beta-amyloid (Aβ) production and deposition, which has been attributed to a range of severities and timing of prior head trauma (DeKosky & Asken, 2017; Johnson, Stewart, & Smith, 2010). Direct evidence of increased cortical Aβ burden and the relationship to cognition in clinically normal older adults with detailed characterization of remote, mild head trauma exposure is lacking.
Several investigations of remote mTBI within aging studies showed no significant effect on the likelihood of developing dementia (Dams-O’Connor et al., 2013; Grasset et al., 2020) or cortical Aβ burden (Crane et al., 2016; Sugarman et al., 2019; Wang, Wei, Yu, Li, & Li, 2017; Weiner et al., 2017) among cognitively normal adults. Others suggested an association of remote mTBI with higher Aβ burden in older adults with mild cognitive impairment or significant medical comorbidities, but not cognitively normal older adults (Mielke et al., 2014; Schneider et al., 2019; Yang et al., 2015). Medical comorbidities can make accurately attributing later-life brain changes to remote mTBI exceedingly difficult. A targeted study of Aβ burden in clinically normal older adults without significant medical comorbidities, but with head trauma exposure representative of older adult populations, would mitigate these challenges. Doing so requires validated, comprehensive ascertainment of lifelong brain injury exposure.
Characterization of head trauma history in aging studies typically is limited by unknown or binarized TBI frequency (Mielke et al., 2014; Sugarman et al., 2019; Tripodis et al., 2017; Wang et al., 2017; Weiner et al., 2017), unknown TBI severities (Schneider et al., 2019), unknown exposure to repetitive, asymptomatic impacts (e.g., collision sports) (Mielke et al., 2014; Schneider et al., 2019; Sugarman et al., 2019; Tripodis et al., 2017; Wang et al., 2017; Weiner et al., 2017), and exposure ascertainment methods with low sensitivity to mTBI history (Gardner et al., 2020). The Ohio State University Traumatic Brain Injury Identification method (OSU TBI-ID) is a National Institute of Neurological Disorders and Stroke (NINDS) Common Data Element (Corrigan & Bogner, 2007) for TBI research with presumed higher sensitivity to lifelong head trauma exposure (Gardner et al., 2020). Inaccurate ascertainment of brain injury history inherently limits precise quantification of exposure-related risk estimates for poor neurologic outcomes. Incorporating the OSU TBI-ID into aging studies would refine our understanding of associations between lifelong head trauma exposure and cortical Aβ burden.
In our cohort of clinically normal older adults, we investigated the association between history and frequency of mTBI (Gardner et al., 2020) with cortical Aβ burden. We additionally examined several interactions to determine whether the association between remote mTBI and Aβ burden was altered by age, sex, or APOE status. In a subset of participants, we investigated the effect of remote mTBI on longitudinal cortical Aβ accumulation. Lastly, we tested the hypothesis that remote mTBI synergistically interacts with Aβ burden to negatively impact cognition.
METHODS
Data Source and Ethics
Data are from clinically normal, functionally independent, community-dwelling older adults participating in the UCSF Memory and Aging Center Hillblom Aging Network (see Supplementary Methods enrollment criteria). Briefly, participants lack cognitive concerns and neurologic and other medical conditions (e.g., history of stroke, sleep apnea, psychiatric disorders), and are physically healthy at the time of study enrollment (see Supplementary Table 1 for frequency of comorbid medical diagnoses and data from common lab tests). Participants in our study completed the older adult modification of the OSU TBI-ID (Gardner et al., 2020) plus at least one Aβ-PET scan. All participants were classified as clinically normal during multidisciplinary case conference after undergoing a neurologic examination, neuropsychological testing, and informant interview including the Clinical Dementia Rating scale (CDR; all CDR=0).
Head Trauma Exposure Ascertainment
We captured remote head trauma exposure using the older adult version of the OSU TBI-ID (OA OSU TBI-ID) (Corrigan & Bogner, 2007; Gardner et al., 2020). Participants first were asked about past exposure to common TBI mechanisms (e.g., falls, motor vehicle accidents, assault). If exposure was reported, they then were asked if the exposure resulted in loss of consciousness (LOC) or a period of feeling dazed or forgetfulness (i.e., posttraumatic amnesia; PTA). We additionally asked participants whether the head trauma resulted in “other” symptoms that commonly are reported with a diagnosed mTBI despite absence of LOC or PTA (often diagnosed as a concussion), such as dizziness, nausea, or vomiting. The last portion of the questionnaire queries previous repetitive head impact exposure (RHIE) through activities like collision sports or military service.
For our primary analyses, we classified the number of remote mTBIs with LOC or PTA as 0, 1, or 2+. We then performed secondary analyses that either removed or re-coded remote head injuries with “other” symptoms only (“ambiguous mTBI”) to see if findings differed when considering broader head trauma symptoms.
Study-Specific Inclusion/Exclusion Criteria
Our study focused on remote mild TBI. Exclusion criteria therefore were prior TBI with LOC >30 minutes or mTBI within 1 year of completing Aβ-PET. We initially excluded participants reporting prior RHIE from collision sports or military service without symptomatic TBI since RHIE alone may increase risk for neurodegenerative disease. Older adults reporting RHIE and prior mTBI were included and were classified based on mTBI frequency (1 or 2+). We performed a sensitivity analysis that additionally included the “RHIE only” group and separated out the RHIE+mTBI groups.
Amyloid PET Imaging
All participants underwent one Aβ-PET scan and a subset completed a second Aβ-PET scan. PET was completed with either 18F-florbetapir (92.5% of sample) or 11C-Pittsburgh compound B (PiB; 7.5% of sample). For cross-sectional analyses, we calculated cortical composite (frontal, cingulate, temporal, and parietal areas) standardized uptake value ratios (SUVR) using cerebellum gray matter (PiB) or whole cerebellum (florbetapir) as the reference region. Cortical composite SUVR values were then converted to the Centiloid (CL) scale. A value of 100 CLs corresponds with the average Aβ deposition observed in patients diagnosed with Alzheimer’s dementia, while 0 CLs corresponds with average Aβ in young, healthy adults. Aβ-PET positivity was defined as: PiB SUVR > 1.21 (8.6 CLs); florbetapir SUVR > 1.11 (22.5 CLs). Longitudinal amyloid PET analysis (SUVR values derived from florbetapir only) was based on a longitudinal processing pipeline, applying eroded white matter as the reference region (see Supplementary Methods).
Neuropsychological Testing
Participants completed memory testing (California Verbal Learning Test, 2nd Edition [CVLT-2], and the Benson Figure) (Kramer et al., 2003) plus executive function tests from the National Institutes of Health Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research (NIH EXAMINER) (Kramer et al., 2014). The NIH EXAMINER is a test battery designed to assess executive function domains reliably and validly for clinical investigations that is adaptable to a wide range of ages and disorders and captures real-life social and executive deficits (Kramer et al., 2014). A composite memory score was used in this study and defined as the mean of z-scores for immediate (total trials 1–5) and delayed recall performance from CVLT-2 and delayed recall of the Benson figure. Z-scores reflect performance relative to the larger Hillblom Aging Network (N≈500). Executive functioning was based on the overall EXAMINER composite z-score (Kramer et al., 2014).
Statistical Analyses
All analyses were performed using IBM SPSS v.25 (Armonk, NY). Demographic and medical history variables were compared using chi square, Fisher’s Exact Test, or analysis of variance (ANOVA). We investigated the main effect of remote mTBI group (0 vs. 1 vs. 2+ mTBI with LOC or PTA) on cortical Aβ burden (Aβ-PET CLs) using analysis of covariance (ANCOVA) adjusting for age, sex, and APOE ε4 carrier status. Aβ-PET CLs was log transformed to better approximate a normal distribution. We compared the likelihood of remote mTBI groups being Aβ-PET positive using Fisher’s Exact Test. The association of remote mTBI (dichotomized, 0 or 1+) with rate of change in Aβ burden was assessed in 30 older adults using a linear mixed effects model (mTBI × time interaction) controlling for baseline age and APOE ε4 status. We also analyzed interactions between remote mTBI and age, sex, and APOE status on Aβ-PET CLs using sequential linear regression models each including one interaction term of interest.
ANCOVAs were also used to examine the effect of remote mTBI on memory and executive functioning composite (z) scores controlling for age, sex, education, and APOE ε4 carrier status. Lastly, we investigated interactions between mTBI and Aβ-PET on cognition to test the hypothesis that other chronic pathophysiologic effects of remote mTBI could exacerbate the effect of Alzheimer’s-related pathology on memory and executive functioning (i.e., decreased cognitive resilience).
To inform how injury definition might impact associations with Aβ burden, we performed two sets of secondary analyses considering remote head trauma defined by presence of LOC, PTA, or “other” symptoms. Specifically, we identified participants who reported injuries with “other” symptoms only (without LOC or PTA), which we term “ambiguous mTBI.” We first removed participants with only ambiguous mTBI who were classified as “0 mTBI” for primary analyses and repeated the analyses. This was done to further increase confidence that the “0 mTBI” group was free of all potentially relevant head trauma exposure. Second, we re-coded the ambiguous mTBI into either the “1 mTBI” or “2+ mTBI” groups to be treated as equivalent to injuries with LOC or PTA. Effect sizes were estimated using partial eta squared (η2; small=0.01, medium=0.06, large=0.14), Cramer’s V (small=0.1, medium=0.3, large=0.5), and R2 change (small=0.01, medium=0.09, large=0.25). Statistical significance was defined a priori as p<0.05 for all analyses.
A priori power analyses are provided in Supplementary Methods.
RESULTS
Sample Characteristics
A total of 146 older adults underwent at least one Aβ-PET scan and completed the OA OSU TBI-ID. Of these, 3 were excluded due to reporting prior TBI with LOC >30 minutes. There were 9 participants initially excluded due to prior RHIE without any TBI, leaving 134 study participants for primary analyses (age 74.1±6.8 years, 59.7% female, education 17.4±2.1 years, 85.8% white). Forty-eight older adults (36%) reported a total of 77 remote mTBI with LOC or PTA (0 mTBI, N=86; 1, N=31; 2+, N=17). The most common mTBI mechanism was motor vehicle or bike accident (N=23, 30%) followed by falls (N=20, 26%), sport/recreation/military (N=17, 22%), hit by an object (N=13, 17%), fight/assault (N=3, 4%), and other (N=1, 1%). Six participants with remote mTBI also reported RHIE, 3 of which reported multiple RHIE sources: American football (N=5), boxing, military/blast exposure, mixed martial arts, lacrosse (N=1 each). All reported mTBI occurred more than 2 years prior to Aβ-PET scan completion (mean 37±23 years, range 2–70 years). Table 1 further characterizes the study sample based on remote mTBI history.
Table 1:
Sample characteristics stratified by remote mTBI group (LOC or PTA required)
| Overall | Remote mTBI with LOC or Dazed/PTA | Sig. (p) | Effect Sizec | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2+ | ||||
| N | 134 | 86 | 31 | 17 | ||
| Age, M (SD) y | 74.1 (6.8) | 74.1 (6.4) | 73.5 (8.1) | 74.6 (6.2) | .88 | η2<0.01 |
| Sex, N (%) female | 80 (59.7) | 55 (64) | 14 (45) | 11 (65) | .17 | V=0.16 |
| Education, M (SD) y | 17.4 (2.1) | 17.4 (2.1) | 17.4 (2.1) | 17.6 (2.0) | .93 | η2<0.01 |
| Race, N (%) white | 115 (85.8) | 75 (87) | 27 (87) | 13 (76) | .32 | V=0.13 |
| Missing, N (%) | 6 (4.5) | 4 (5) | 0 (0) | 2 (12) | - | - |
| APOE, N (%) e4 carrier | 26 (19.4) | 12 (14) | 7 (23) | 7 (41) | .03 | V=0.23 |
| mTBI Characteristics | d | |||||
| Time since first mTBI, M (SD) y | 45.8 (21.5) | - | 41.5 (22.6) | 53.4 (17.7) | .07 | 0.57 |
| Time since last mTBI, M (SD) y | 37.1 (23.2) | - | 41.5 (22.6) | 29.5 (22.9) | .09 | 0.53 |
| Age at first mTBI, M (SD) y | 27.9 (22.2) | - | 31.8 (24.2) | 21.2 (16.9) | .11 | 0.48 |
| Age at last mTBI, M (SD) y | 36.6 (23.9) | - | 31.8 (24.2) | 45.2 (21.4) | .06 | 0.58 |
| ≥1 mTBI w/ LOC<30min, N (%) | 29 (21.6) | 0 (0) | 15 (48) | 14 (82) | - | - |
| PTA<24hrs only, N (%) | 19 (14.2) | 0 (0) | 16 (52) | 3 (18) | - | - |
| Aβ-PET | ||||||
| Positive, N (%) | 26 (19.4) | 16 (19) | 8 (26) | 2 (17) | .52 | V=0.11 |
| Centiloid, Mdn. (IQR) | 5.2 (−3.7, 16.9) | 4.6 (−4.3, 17.4) 7 | .5 (−4.3, 17.6) | 6.6 (0.5, 14.1) | .94a | η2<0.01 |
| Cognition (Z-scores), M (SD) | η2 | |||||
| Memory | 0.11 (0.80) | 0.08 (0.76) | 0.18 (0.79) | 0.13 (1.1) | .57b | 0.01 |
| Executive Function | 0.86 (058) | 0.91 (0.58) | 0.85 (0.52) | 0.64 (0.64) | .21b | 0.03 |
| Missing, Memory/Exec. N (%) | 15 (11.2)/7 (5.2) | 9 (10)/3 (3) | 5 (16)/3 (10) | 1 (6)/1 (6) | - | - |
ANCOVA with Centiloid as the dependent variable and age, sex, and APOE e4 carrier status covariates
ANCOVA with cognitive z-scores as the dependent variable and age, sex, education, and APOE e4 covariates
Effect size estimates presented either as eta squared (η2) or Cohen’s d for continuous variables and Cramer’s V for categorical variables
Abbrev: Aβ-PET – amyloid positron emission tomography, APOE – apolipoprotein E, M (SD) – mean (standard deviation) Mdn (IQR) – median (interquartile range), mTBI – mild traumatic brain injury, y – years
Remote mTBI groups did not differ significantly on most demographic or medical history factors except that older adults with 2+ remote mTBI were more likely to be APOE ε4 carriers (N=7, 41%) than those with 1 (N=7, 23%) or 0 (N=12, 14%) remote mTBI (p=.03, Cramer’s V=0.23).
Remote mTBI (LOC or PTA required) and Amyloid Burden
There was not a significant main effect of remote mTBI on Aβ-PET CLs (F[5, 133]=0.07, p=.94, η2<0.01; Figure 1). Post hoc pairwise comparisons are shown in Supplementary Table 2. Remote mTBI also was not significantly associated with the likelihood of being Aβ-PET positive (Fisher’s Exact Test=1.38, p=.52, V=0.11). We did not observe any significant interactions between remote mTBI group and age (R2 change=.003, p=.56), sex (R2 change=.001, p=.68), or APOE ε4 status (R2 change=.011, p=.24; Figure 2) on Aβ-PET CLs (Supplemental Table 3). We performed an additional analysis that compared the participants with prior RHIE but no mTBI (N=9; 7.3±11.0 CLs), participants with RHIE and mTBI (N=6; 8.9±16.5 CLs), prior mTBI only (N=55; 11.3±21.2 CLs), and no mTBI or RHIE (N=73; 10.8±27.5 CLs). Results similarly showed group differences of negligible magnitude, no significant main effect of remote head trauma group (F[6, 136]=0.06, p=.98, η2<0.01), and no significant pairwise post hoc differences.
Figure 1:
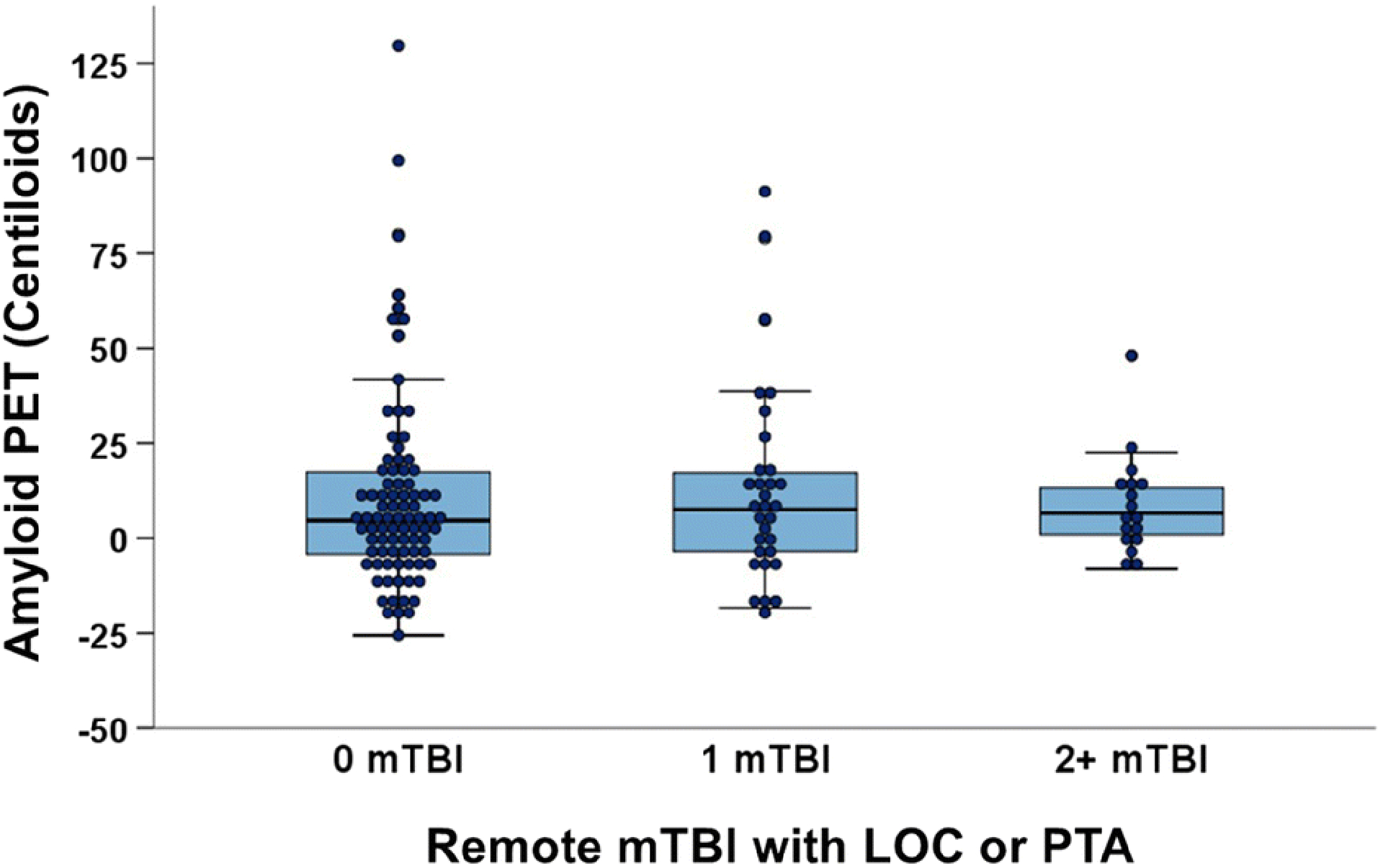
No main effect of remote mTBI on cortical Aβ burden in clinically normal older adults. Data show remote mTBI groups defined as head trauma followed by loss of consciousness <30mins or a period of posttraumatic daze/amnesia.
Figure 2:
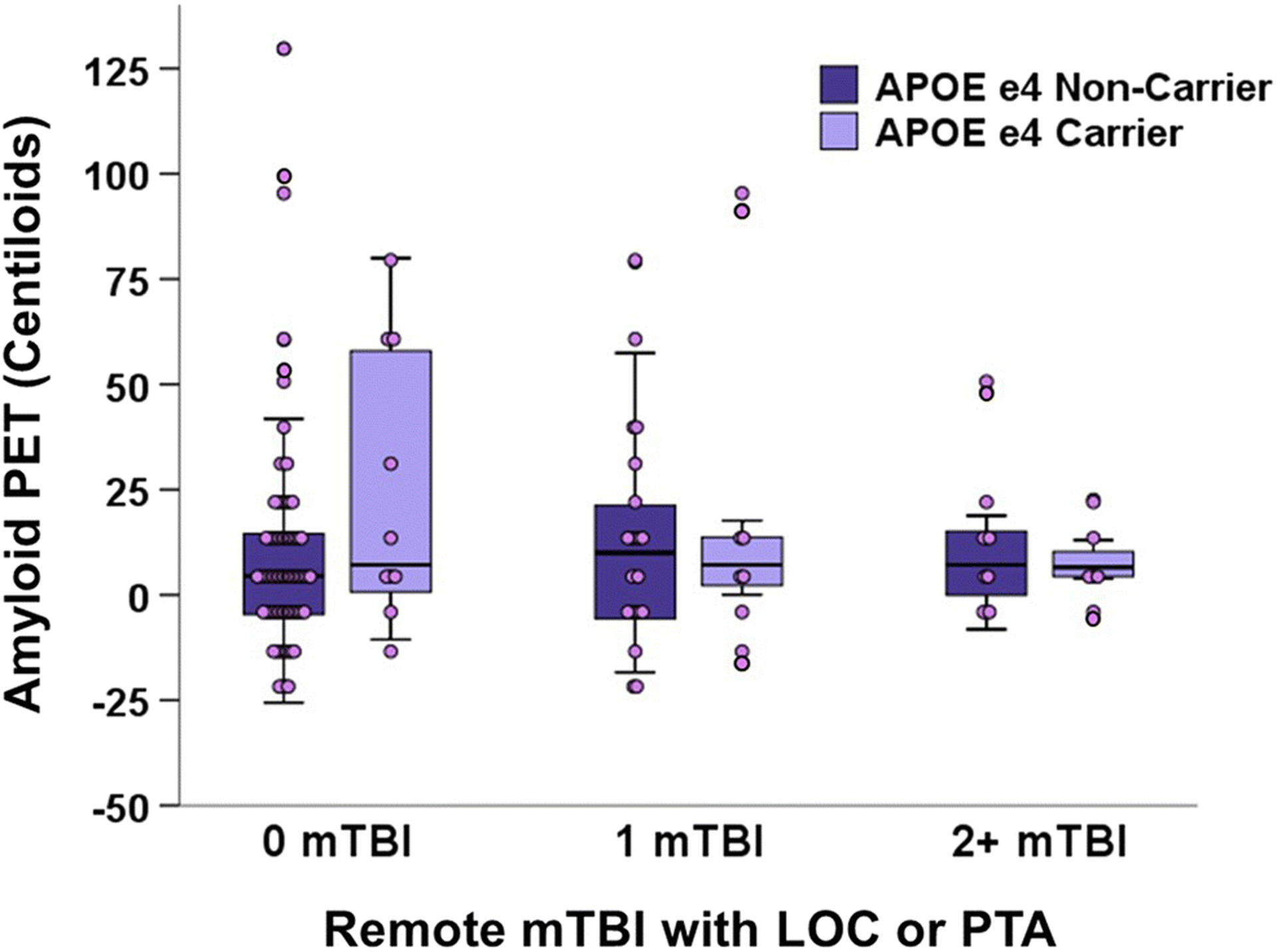
No remote mTBI × APOE ε4 interaction on cortical Aβ burden in clinically normal older adults. Data show remote mTBI groups defined as head trauma followed by loss of consciousness <30mins or a period of posttraumatic daze/amnesia.
Remote mTBI and Longitudinal Amyloid Changes
A subgroup of 30 older adults (N=19 with no remote mTBI and N=11 with mTBI composed of N=7 with 1 mTBI, N=3 with 2, N=1 with 3) underwent a second Aβ-PET scan (mean 2.7 years later, range 1.3–4.1 years). These 30 participants did not significantly differ from those without a second Aβ-PET scan in age (t=−1.06, p=.29) or sex (χ2=.001, p=.97). Of the 11 participants with remote mTBI, 4 were APOE ε4 carriers compared to 0 of the 19 participants with no mTBI or RHIE. Controlling for age and APOE carrier status, the association of remote mTBI with rate of Aβ accumulation was not statistically significant, though this analysis may have been underpowered (remote mTBI × time; B [unstandardized]=.012, SE=.007, p=.08; Figure 3). Models were re-run excluding the 4 APOE ε4 carriers from the remote mTBI group and results were similar (B=.012, SE=.003, p=.13).
Figure 3:
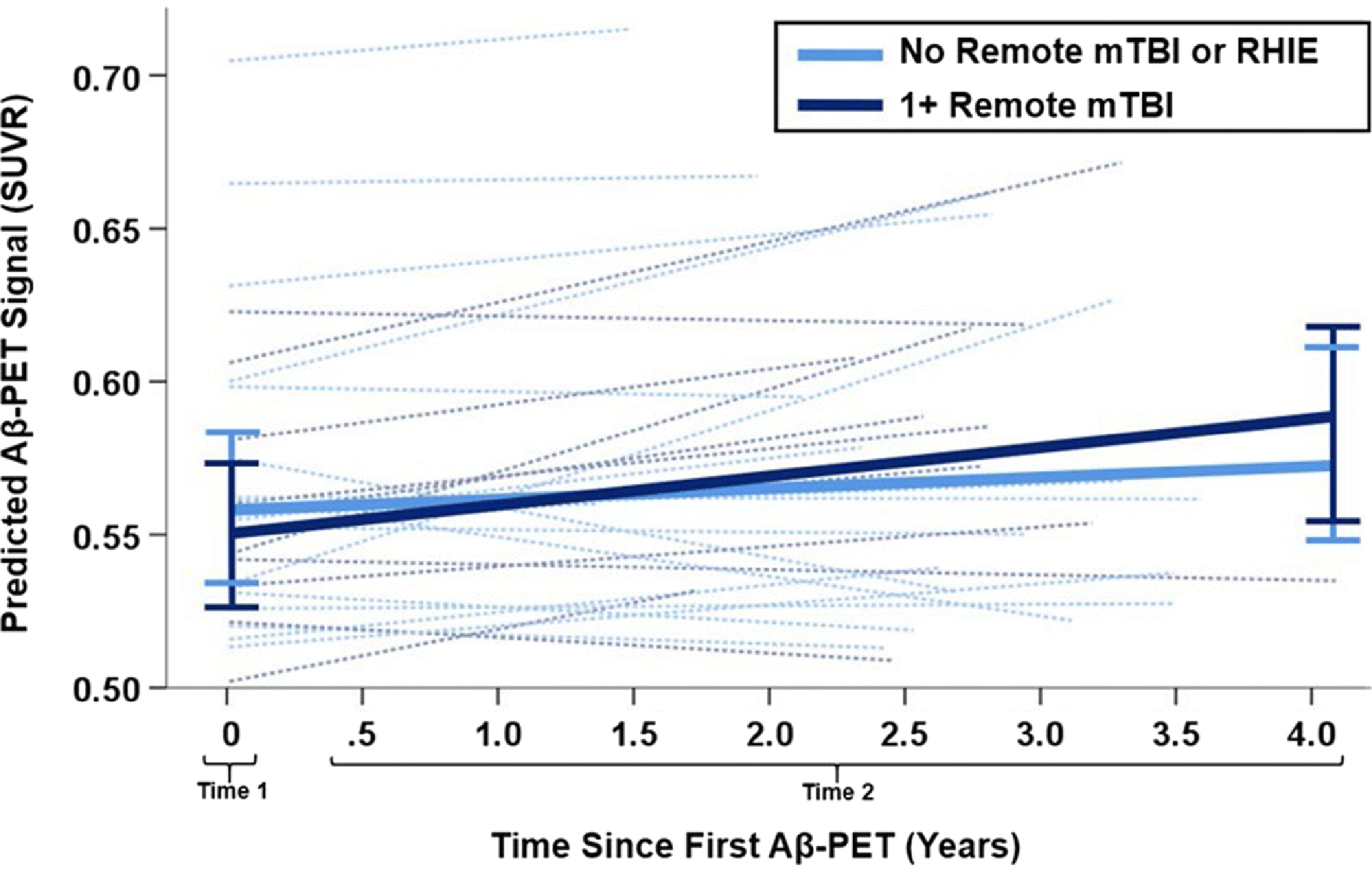
Change in cortical Aβ burden over time in subgroup of 30 older adults who underwent two PET scans (N=19 without remote mTBI or repetitive head impact exposure, N=11 with 1+ remote mTBI). Mixed effects models suggested a possible, but not statistically significant, association (p=.10) of typically aging older adults with remote mTBI (dark blue) having a higher rate of Aβ accumulation over time than those with no remote head trauma (light blue). Bars represent 95% confidence interval of model predicted cortical Aβ burden for each group. Dotted lines show individual participants.
Secondary Analyses of Ambiguous mTBI (“Other” Symptoms without LOC or PTA)
Analyses were first repeated with 13 participants who reported ambiguous mTBI (head trauma with no LOC or PTA, but “other” symptoms) removed from the “0 mTBI” group. Similar to primary analyses, neither the main effect of remote mTBI (F[5,120]=0.05, p=.95, η2=0.01]) nor the demographic and APOE ε4 carrier status interactions (R2 changes=.001–.014, p’s≥.20) were significantly associated with Aβ-PET CLs (Supplemental Table 3). We then treated all ambiguous injuries as equivalent to mTBI with LOC or PTA, which regrouped participants into 0 (N=73), 1 (N=36), and 2+ (N=25) remote “mTBI” groups reporting a total of 103 mTBIs. Again, the main effect of remote mTBI and interactions with other factors were not significantly associated with Aβ-PET CLs.
Interaction Effects of Remote mTBI and Amyloid on Cognition
There was no significant main effect of Aβ burden on either memory (β=0.05, 95%CI[−.13, .23], p=.59) or executive functioning (β=−0.04, 95%CI[−.22, .13], p=.62), and no significant main effect of remote mTBI (memory: β=0.03, 95%CI[−.15, .22], p=.71; executive functioning: β=−0.13, 95%CI[−.30, .05], p=.16). There also was no significant interaction between remote mTBI and Aβ burden on memory (R2 change=.002, p=.62) or executive function (R2 change=.001, p=.75). Results were similar when removing ambiguous mTBI or recoding as equivalent to mTBI with LOC or PTA.
DISCUSSION
We evaluated clinically normal older adults without significant health comorbidities and with well-characterized head trauma history, Aβ-PET scans, and cognitive testing. Unique facets of our study included 1) a standardized and validated collection of lifelong head trauma exposure using the OA OSU TBI-ID (Gardner et al., 2020), 2) a wider range of reported remote mTBI than similar studies (0, 1, or 2+), 3) investigating head trauma without classical mTBI symptoms like LOC or PTA (more akin to common diagnoses like “concussion”), 4) comprehensive and validated neuropsychological test measures to evaluate cognition, 5) thorough characterization of participants as clinically normal, and 6) having a subgroup with longitudinal Aβ-PET data.
We found no associations between remote mTBI and cortical Aβ burden. Interactions between remote mTBI and age, sex, or APOE status were similarly unremarkable. We also found no associations between remote mTBI and cortical Aβ burden on cognitive function. Data from a small subset of our sample with a second Aβ PET scan suggested that remote mTBI may be associated with a faster rate of cortical Aβ burden increase over time, though this analysis likely was underpowered and the finding was not statistically significant.
Consolidating findings across this literature requires careful consideration of TBI severity and timing. Researchers investigating brain autopsies performed acutely after severe TBI have reported accumulation of Alzheimer’s-like Aβ plaques (Gentleman et al., 1997; Ikonomovic et al., 2004; Johnson et al., 2010), similar to in vivo Aβ-PET studies of moderate-to-severe TBI cases within 1 year of injury (Hong et al., 2014). Longer-term severe TBI survivors interestingly showed an absence of Aβ plaques at autopsy despite marked accumulation of intra-axonal amyloid precursor protein (Chen, Johnson, Uryu, Trojanowski, & Smith, 2009). This finding aligned with a report of low frequency Aβ-PET positivity (1 of 9 cases) several years after severe TBI (Kawai et al., 2013). The TBI severity continuum is extremely heterogeneous, even within the relatively arbitrary delineations of “mild,” “moderate,” or “severe” TBI. Our study lends further support to the strikingly consistent findings across aging studies of no association of remote mild TBI and cross-sectional Aβ burden, particularly within clinically normal older adults (Crane et al., 2016; Mielke et al., 2014; Sugarman et al., 2019; Wang et al., 2017; Weiner et al., 2017) and including veteran populations (Peltz et al., 2020; Weiner et al., 2017).
Cognitively, recent work has shown remote mTBI negatively affects aspects of executive functioning in non-demented older adults (Alosco et al., 2020), particularly among military veterans often harboring multiple medical comorbidities (Kaup et al., 2017; Peltz et al., 2017). Complicated health histories may partly explain why veterans in general score below the normative average on cognitive tests (Kaup et al., 2017). The lack of association of remote mTBI with cortical Aβ burden in both veteran (Weiner et al., 2017) and non-veteran studies (Mielke et al., 2014; Sugarman et al., 2019) suggests that these cognitive differences are not driven by underlying TBI-related Alzheimer’s pathologic changes. However, genetic susceptibility and other medical history interactions warrant further study (Hayes et al., 2017). It is also important to note that presence of neurodegenerative disease, with or without overt symptoms, is itself a risk factor for common mTBI mechanisms like falls (Stark et al, 2013; Welmer et al., 2016). This suggests potential bidirectional influences in at-risk older adults. We failed to identify associations of remote mTBI and Aβ burden on memory or executive functioning in our exceptionally healthy sample cross-sectionally, but longitudinal studies including cognitive tests more sensitive to subtle variability in clinically normal older adults (e.g., processing speed) may be necessary to elucidate such findings. Regardless, epidemiologic data show consistently that remote mTBI increases dementia risk, while studies capable of more deeply phenotyping participants paint a murkier picture.
Epidemiologic studies (Nordström & Nordström, 2018) often rely on medical record and insurance-based diagnostic coding. This potentially leads to inaccurate identification of both head trauma history (Bazarian, Veazie, Mookerjee, & Lerner, 2006) and dementia diagnosis (Zhu et al., 2019). Aging studies like ours directly measure long-term outcomes through comprehensive collection of cognitive and neurodegenerative (e.g., Aβ-PET) biomarker data, but often are less representative of the general population than epidemiologic studies due to recruitment and survival biases. Accurate characterization of head trauma history remains tenuous in many study protocols (Gardner et al., 2020).
Ascertaining head trauma exposure using the OA OSU TBI-ID is a clear study strength. However, we directly measured cortical Aβ burden only. Cortical Aβ burden is relatively less correlated with cognition than tau or other neurodegenerative proteins. It is possible that another strength of epidemiologic studies is that they better reflect diverse neuropathologic outcomes of head trauma exposure (Crane et al., 2016). We targeted a medically uncomplicated sample of clinically normal older adults whom we suspect possess factors promoting both resistance to developing neuropathology and resilience to age-related brain changes (Arenaza-Urquijo & Vemuri, 2018). Simply put, the totality of evidence suggests that mild head trauma moderates the later-life neurologic health of some, but not all, older adults. Frequently unmeasured variables like lifestyle factors, social determinants of health, or impulsive behaviors linked to both risk for and poor outcomes after head trauma might reduce resilience to dementia even if head trauma does not directly facilitate neurodegenerative pathology per se (Asken et al., 2016; Visser-Kaizer et al., 2016). Our findings in highly educated, clinically normal older adults who have maintained good mental and physical health highlight the importance of further studying how such factors might mitigate links between lifelong head trauma, Alzheimer’s pathologic changes, and associated dementia.
The OSU TBI-ID represents a gold-standard assessment of lifelong traumatic brain injury. Recent iterations include a cursory indicator of repetitive, asymptomatic head impacts through activities like collision sports and military service. Thorough characterization of such impacts is not obtained on the OSU TBI-ID but may be important when studying long-term neurologic outcomes of head trauma exposure. For example, former elite American football athletes shown to be at increased risk for neurodegenerative disease often have many years of repetitive, asymptomatic head blows (i.e., “subconcussive” exposure) in addition to self-reporting hundreds to thousands of symptomatic injuries (often without LOC or PTA) (Alosco et al., 2017; Mez et al., 2017). In contrast, 92% of our sample had less than 3 remote mTBI (with or without LOC or PTA). Just 10% reported collision sport and/or military participation, the effects of which we could not thoroughly investigate due to relatively small N and unknown relevant details like the ages and total years of exposure. Deeper characterization of both mTBI and sources of repetitive asymptomatic exposure (e.g., collision sports, military blast, domestic violence) within broader aging populations is clearly needed for informing risks for later-life neurologic changes as well as whether the type of exposure confers differential risk. Targeting clinically normal older adults improves generalizability of related findings but also has its own limitations.
Medical comorbidities like cardiovascular disease may interact with mTBI to increase risk of neurodegenerative disease and cognitive impairment (“multi-hit hypothesis”). Eligibility for the Hillblom Aging Network requires absence of common medical comorbidities (e.g., sleep apnea) and significant neurologic events (e.g., stroke) prior to enrollment. Frequency of lifelong mTBI in our sample (36%) was commensurate with older adult estimates (Whiteneck, Cuthbert, Corrigan, & Bogner, 2016). However, frequency of relevant medical comorbidities may be lower than the general population. For example, 40% of our sample reported hypertension, which is lower than 2015–2016 national estimates of 63% for adults 60 and older (National Health and Nutrition Examination Survey). One prior study showed a dose-response risk of number of prior TBI (all severity) with Aβ-PET positivity rates, though the overall sample had high frequencies of hypertension (71%), diabetes (42%), and former smokers (50%) (Schneider et al., 2019). Similarly, veteran cohorts with high rates of cardiovascular risk factors and substance abuse more consistently show detrimental effects of remote mTBI on later-life brain health (Kaup et al., 2017). Studying synergistic effects of remote head trauma with other common medical risk factors would further clarify how head trauma impacts aging and help identify factors that promote resistance and resilience to long-term brain changes.
Limitations
In addition to having a rather healthy older adult sample, other limitations to generalizability included being predominantly white/Caucasian and highly educated. Cognitive testing was limited to memory and executive functioning. Including other domains with greater variability among older adults and associated with head trauma (e.g., processing speed) may improve sensitivity to subtle differences. Subgroups of older adults with 1 or 2+ remote mTBI were relatively small but were larger than similarly designed studies (Weiner et al., 2017). Post hoc estimates of the magnitude of pairwise group differences suggested negligible effect sizes. Of note, we quantified Aβ burden using a cortical SUVR composite, which may obscure region-specific differences in cortical Aβ associated with mTBI. PET tracers like florbetapir are thought to reflect primarily cortical neuritic plaque deposition with lower affinity to diffuse plaques, so our results cannot speak to other potential amyloid-related changes (e.g., intra-axonal/white matter plaque deposition). We likely were underpowered to detect the observed effect of remote mTBI on longitudinal Aβ-PET changes as statistically significant, and there was variability in the time between Aβ-PET scans. This finding warrants further investigation. Like almost all studies of remote head trauma exposure, self-report data are an inherent limitation.
Conclusions
In clinically normal older adults with minimal medical comorbidities, history of remote mTBI is not associated with greater cortical Aβ burden measured by PET. Remote mTBI also does not interact synergistically with Aβ burden to influence memory or executive function test scores. Remote mTBI may accelerate Aβ burden accumulation over time, but clarifying this relationship requires larger longitudinal samples with well-characterized lifelong head trauma exposure.
Supplementary Material
ACKNOWLEDGMENTS
We thank the research participants and their families whose time and effort made this work possible.
FUNDING SOURCES
We thank the following funding sources that have supported our work: UCSF ADRC (NIA P50AG023501 and P30AG062422; PI: Bruce Miller), NIH-NIA (R01 AG045611) to GDR; NIH-NIA (R01(s) AG032289 and AG048234) and Larry L. Hillblom Network Grant (2014-A-004-NET) to JHK; NIH-NINDS (K23 NS095755), American Federation for Aging Research, and Global Brain Health Institute to RCG; NIH-NIA (K23 AG058752) to KBC; NIH-NIA (K23 AG061253) and Larry L. Hillblom Fellowship (2018-A-025-FEL) to AMS; NIH-NIA (K99AG065501) and Alzheimer’s Association (AARF-16-443577) to RLJ; and the Robert W. Katzman Fellowship Training Grant through the American Academy of Neurology in conjunction with the American Brain Foundation and Alzheimer’s Association to WGM.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
COMPLIANCE WITH ETHICAL STANDARDS
The study was approved by the UCSF IRB on human research and all participants provided written, informed consent before enrolling in accordance with guidelines of the Helsinki declaration.
DATA AVAILABILITY STATEMENT
Data are available upon request from qualified, not-for-profit researchers (see https://memory.ucsf.edu/research-trials/professional/open-science).
DISCLOSURES/CONFLICTS OF INTEREST
The authors report no conflicts with any product mentioned or concept discussed in this article.
REFERENCES
- Alosco ML, Jarnagin J, Tripodis Y, Martin B, Chaisson C, Baugh CM, Torres A, Nowinski CJ, Cantu RC, & Stern RA (2017). Utility of providing a concussion definition in the assessment of concussion history in former NFL players. Brain injury, 31(8), 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Baucom ZH, Mez J, Stein TD, Martin B, … Stern RA (2020). The Late Contributions of Repetitive Head Impacts and TBI to Depression Symptoms and Cognition. Neurology. doi: 10.1212/wnl.0000000000010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, & Vemuri P (2018). Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology, 90(15), 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asken BM, Sullan MJ, Snyder AR, Houck ZM, Bryant VE, Hizel LP, McLaren ME, Dede DE, Jaffee MS, DeKosky ST, & Bauer RM (2016). Factors Influencing Clinical Correlates of Chronic Traumatic Encephalopathy (CTE): a Review. Neuropsychology review, 26(4), 340–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Veazie P, Mookerjee S, & Lerner EB (2006). Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Academic emergency medicine, 13(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Chen XH, Johnson VE, Uryu K, Trojanowski JQ, & Smith DH (2009). A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol, 19(2), 214–223. doi: 10.1111/j.1750-3639.2008.00176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, & Bogner J (2007). Initial reliability and validity of the Ohio State University TBI identification method. The Journal of head trauma rehabilitation, 22(6), 318–329. [DOI] [PubMed] [Google Scholar]
- Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, … Leurgans S (2016). Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA neurology, 73(9), 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, & Crane PK (2013). Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry, 84(2), 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, & Asken BM (2017). Injury cascades in TBI-related neurodegeneration. Brain injury, 31(9), 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Rivera E, O’Grady M, Doherty C, Yaffe K, Corrigan J, … Wilson F (2020). Screening for lifetime history of traumatic brain injury among older American and Irish adults at risk for dementia: development and validation of a web-based survey. Journal of Alzheimer’s Disease(Preprint), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Greenberg BD, Savage MJ, Noori M, Newman SJ, Roberts GW, … Graham DI (1997). A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport, 8(6), 1519–1522. doi: 10.1097/00001756-199704140-00039 [DOI] [PubMed] [Google Scholar]
- Grasset L, Glymour MM, Yaffe K, Swift SL, Gianattasio KZ, Power MC, & Zeki Al Hazzouri A (2020). Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimers Dement. doi: 10.1002/alz.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Logue MW, Sadeh N, Spielberg JM, Verfaellie M, Hayes SM, … Miller MW (2017). Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain, 140(3), 813–825. doi: 10.1093/brain/aww344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YT, Veenith T, Dewar D, Outtrim JG, Mani V, Williams C, … Canales R (2014). Amyloid imaging with carbon 11–labeled Pittsburgh compound B for traumatic brain injury. JAMA neurology, 71(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, … DeKosky ST (2004). Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol, 190(1), 192–203. doi: 10.1016/j.expneurol.2004.06.011 [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, & Smith DH (2010). Traumatic brain injury and amyloid-β pathology: a link to Alzheimer’s disease? Nature Reviews Neuroscience, 11(5), 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Peltz C, Kenney K, Kramer JH, Diaz-Arrastia R, & Yaffe K (2017). Neuropsychological Profile of Lifetime Traumatic Brain Injury in Older Veterans. J Int Neuropsychol Soc, 23(1), 56–64. doi: 10.1017/s1355617716000849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Kawanishi M, Kudomi N, Maeda Y, Yamamoto Y, Nishiyama Y, & Tamiya T (2013). Detection of brain amyloid β deposition in patients with neuropsychological impairment after traumatic brain injury: PET evaluation using Pittsburgh Compound-B. Brain Inj, 27(9), 1026–1031. doi: 10.3109/02699052.2013.794963 [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sharon JS, Rankin KP, Rosen HJ, Johnson JK, & Miller BL (2003). Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology, 16(4), 211–218. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, Rosen HJ, … Widmeyer M (2014). NIH EXAMINER: conceptualization and development of an executive function battery. Journal of the international neuropsychological society, 20(1), 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, & Stewart W (2019). Neurodegenerative disease mortality among former professional soccer players. N Engl J Med, 381, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, … McHale L (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. Jama, 318(4), 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Savica R, Wiste HJ, Weigand SD, Vemuri P, Knopman DS, … Jack CR Jr. (2014). Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology, 82(1), 70–76. doi: 10.1212/01.wnl.0000438229.56094.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A, & Nordström P (2018). Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS medicine, 15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz CB, Gardner RC, Kenney K, Diaz-Arrastia R, Kramer JH, & Yaffe K (2017). Neurobehavioral Characteristics of Older Veterans With Remote Traumatic Brain Injury. J Head Trauma Rehabil, 32(1), E8–e15. doi: 10.1097/htr.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz CB, Kenney K, Gill J, Diaz-Arrastia R, Gardner RC, & Yaffe K (2020). Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology. doi: 10.1212/wnl.0000000000010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ALC, Selvin E, Liang M, Latour L, Turtzo LC, Koton S, … Gottesman RF (2019). Association of Head Injury with Brain Amyloid Deposition: The ARIC-PET Study. J Neurotrauma, 36(17), 2549–2557. doi: 10.1089/neu.2018.6213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SL, Roe CM, Grant EA, Hollingsworth H, Benzinger TL, Fagan AM, Buckles VD, & Morris JC (2013). Preclinical Alzheimer disease and risk of falls. Neurology, 81(5), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman MA, McKee AC, Stein TD, Tripodis Y, Besser LM, Martin B, … Au R (2019). Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimer’s & Dementia, 15(5), 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman MA, McKee AC, Stein TD, Tripodis Y, Besser LM, Martin B, … Alosco ML (2019). Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement, 15(5), 686–698. doi: 10.1016/j.jalz.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodis Y, Alosco ML, Zirogiannis N, Gavett BE, Chaisson C, Martin B, … Stern RA (2017). The effect of traumatic brain injury history with loss of consciousness on rate of cognitive decline among older adults with normal cognition and Alzheimer’s disease dementia. Journal of Alzheimer’s Disease, 59(1), 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser-Keizer AC, Westerhof-Evers HJ, Gerritsen MJ, van der Naalt J, Spikman JM. To Fear Is to Gain? The Role of Fear Recognition in Risky Decision Making in TBI Patients and Healthy Controls. PLoS One. 2016. November 21;11(11):e0166995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Wei XE, Yu MM, Li PY, & Li WB (2017). Self-reported traumatic brain injury and in vivo measure of AD-vulnerable cortical thickness and AD-related biomarkers in the ADNI cohort. Neurosci Lett, 655, 115–120. doi: 10.1016/j.neulet.2017.06.055 [DOI] [PubMed] [Google Scholar]
- Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, … Decarli C (2017). Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer’s disease in Vietnam veterans using the Alzheimer’s Disease Neuroimaging Initiative: preliminary report. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 3(2), 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welmer AK, Rizzuto D, Laukka EJ, Johnell K, & Fratiglioni L (2017). Cognitive and Physical Function in Relation to the Risk of Injurious Falls in Older Adults: A Population-Based Study. The journals of gerontology. Series A, Biological sciences and medical sciences, 72(5), 669–675. [DOI] [PubMed] [Google Scholar]
- Whiteneck GG, Cuthbert JP, Corrigan JD, & Bogner JA (2016). Prevalence of Self-Reported Lifetime History of Traumatic Brain Injury and Associated Disability: A Statewide Population-Based Survey. J Head Trauma Rehabil, 31(1), E55–62. doi: 10.1097/htr.0000000000000140 [DOI] [PubMed] [Google Scholar]
- Yang S-T, Hsiao T, Hsieh C-J, Chiang Y-H, Yen T-C, Chiu W-T, … Hu C-J (2015). Accumulation of amyloid in cognitive impairment after mild traumatic brain injury. Journal of the neurological sciences, 349(1–2), 99–104. [DOI] [PubMed] [Google Scholar]
- Zhu CW, Ornstein KA, Cosentino S, Gu Y, Andrews H, & Stern Y (2019). Misidentification of dementia in Medicare claims and related costs. Journal of the American Geriatrics Society, 67(2), 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


