Abstract
Breast cancer is emerging as the most common malignancy in Indian women. Mammography is one of the few screening modalities available to the modern world that has proved itself of much use by aiding early detection and treatment of non-palpable, node-negative breast cancers. However, due to its two-dimensional nature, many cases of malignancies are still missed, to be detected at a later date or by an alternate modality. In 2011, FDA approved the supplemental use of digital breast tomosynthesis (DBT) in screening and diagnostic set ups. The acquisition of multiple low-dose projection images of the compressed parenchyma provided a ‘third’ dimension to the mammogram whereby the breast tissue could be seen layer by layer on the workstation. It improves cancer detection rate, and reduces recall rate and false-positive findings by improving lesion characterization. The current review discusses the principle of DBT with a comprehensive study of the literature.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13193-021-01310-y.
Keywords: Mammography, Digital breast tomosynthesis, Breast carcinoma, Synthesized mammogram
Introduction
Breast cancer is the most common malignancy and leading cause of cancer-related mortality among women across the world. It has an age-adjusted incidence rate as high as 25.8 per 100,000 women and mortality upto 12.7 per 100,000 women [1]. In India, approximately 1,62, 468 new cases were detected in the year 2018 [2]. In spite of having lower incidence rates than in the western world, breast carcinoma is soon to become the most common cancer killer in urban Indian women surpassing cervical carcinoma [3]. Mortality and morbidity has reduced significantly with initiation of screening programs due to early detection of non-palpable and node-negative cancers [4]. The onus of diagnosis of breast malignancy rests on triple assessment which consists of clinical breast examination, imaging and histopathology. Digital mammography, ultrasonography (US) and MRI are the modalities suitable for breast imaging. Among these three, mammography has established itself as a screening tool causing reduction in mortality rate by 30% or more with early detection of cancer [5–7]. The standard single breast mammogram (MMG) consists of two views: craniocaudal and mediolateral oblique views, according to the position of breast with respect to the X-ray tube [8]. The role of MMG has undergone revolution with emergence of full-field digital mammography (FFDM) which is considered as the imaging modality of choice for females above 40–45 years of age [9, 10]. However, its sensitivity drops down to 47.8–64.4% in younger population due to dense breasts [11]. On the other hand, it is shown that increased breast density is associated with two to sixfold increased risk of breast cancer [12], which mandates further evaluation with other adjunct imaging modalities like US or MRI.
With continuous advancements in field of imaging, Digital breast tomosynthesis (DBT) has emerged as adjunct tool in breast imaging. It basically adds another dimension to standard two-dimensional (2D) mammogram, which is depth of the tissue, by obtaining multiple slices of the breast at fixed intervals. This has led to increase in detection rate, reduction in recall rates and increase in confidence of reporting radiologists [13]. Thus, FDA approved DBT as a supplementary technique to FFDM in 2011 for breast cancer screening and diagnosis (https://www.fda.gov/radiation-emitting-products/facility-certification-and-inspection-mqsa/digital-accreditation). In this article, we will discuss the principle of DBT, its utility and limitations followed by its current status worldwide in field of breast imaging.
Principle and Technique of Digital Breast Tomosynthesis
Like any radiograph, the standard views of mammogram are also two dimensional. This leads to anatomical noise due to superimposition of normal glandular parenchyma and the pathological changes. These can be seen as either pseudo-masses or obscuration of true mass [14]. The technique of DBT involves acquisition of multiple projection radiographs of breast tissue at fixed intervals which are seen on the high-resolution workstations, as reading MMG layer by layer.
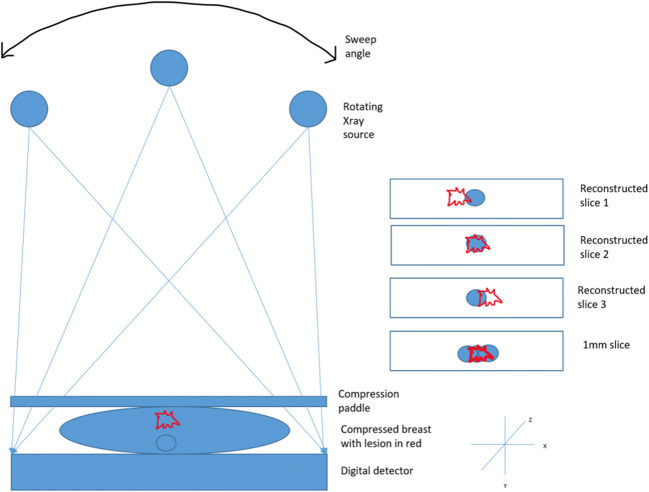
Acquisition of DBT is performed in similar breast position as 2D FFDM. However, for the former, the X-ray tube rotates in an arc (varying from 15 to 60 degrees depending on the vendor—referred to as the sweep angle) acquiring multiple low-dose projections in a plane aligned to the chest wall [15] (Fig. 1). This motion of the X-ray tube varies with the manufacturer and can be described as continuous or step-and-shoot depending on whether it emits X-rays continuously or comes to a complete stop in between image exposures. Continuous motion of the tube, although reduces the acquisition time, decreases resolution by focal spot blur whereas the step-and-shoot method takes a longer time for acquisition and hence is prone to motion artefact [16]. Multiple 1-mm thickness sections are then reconstructed from the projection images by using either filtered back projection or iterative reconstruction algorithms [17]. The number of reconstructions depends on the thickness of the compressed breast tissue and they can be grouped together as slabs of various thickness for assessment on the workstation [18].
Fig. 1.
Diagrammatic representation of the technique of digital breast tomosynthesis which separates the breast tissue layer by layer by projection radiography and helps in better visualization of lesions by ‘unmasking’ it from overlying breast parenchyma
For the same radiation dose and number of projections, the wider the arc or sweep angle, the better is the tomographic separation and z axis resolution which increases the conspicuity of masses or architectural distortions. However, this reduces the in-plane resolution compromising the visualization of microcalcifications [15, 19, 20]. While increasing the number of projections increases the in-plane resolution, it also increases the radiation dose. Parameters such as sweep angle, number of projections and acquisition parameters are fixed for vendors (Table 1).
Table 1.
FDA-approved DBT systems (https://www.fda.gov/radiation-emitting-products/facility-certification-and-inspection-mqsa/digital-accreditation) [17]
| Hologic Selenia dimensions | GE SenoClaire | Siemens Mammomat | Fujifilm Aspire | GE Senographe | |
|---|---|---|---|---|---|
| Sweep angles (degrees) | 15 | 25 | 50 |
15 (standard mode) 40 (high resolution) |
25 |
| Tube motion | Continuous | Step and shoot | Continuous | Continuous | Step and shoot |
| Number of projections | 15 | 9 | 25 | 15 | 9 |
By virtue of providing third-dimensional information, that is, depth of the tissue, it unfolds the breast parenchyma layer by layer or like a drill (Fig. 2). DBT is not in the exact sense a 3D mammogram as the third dimension is derived from the planar data [21]. Thus, the lesions are seen in focus only in the specific plane of their respective section, and some other lesion not in that plane is out of focus. The amount of blurring is proportional to its distance from the currently displayed plane and the lesion’s size.
Fig. 2.
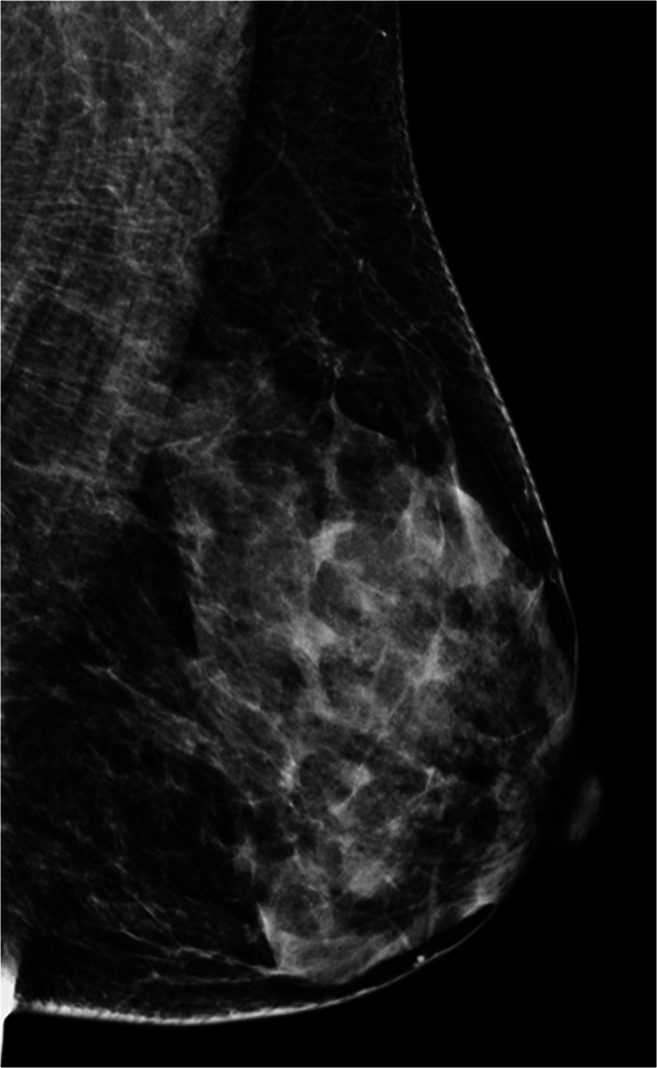
2D FFDM image in MLO view and corresponding DBT cine stack-vide (Supplementary Material) of an ACR category c (heterogeneously dense which may obscure small masses) left breast. Scrolling through the tomosynthesis stack shows normal fibroglandular parenchyma slice by slice (1 mm) without overlap, thus increasing the confidence of the reporting radiologist
In addition to craniocaudal and mediolateral oblique views, DBT can also be used for mediolateral view, spot compression and implant displaced views however not for spot magnification [18].
Advantages of DBT
The added technology of DBT provides an edge over 2D mammography, spanning scopes of both diagnostic and screening breast imaging.
Improves Cancer Detection Rate (CDR)
Supplementing DBT with 2D mammography showed significant improved CDR in Oslo Tomosynthesis Screening Trial (OTST) from 6.1 to 8.0 [22, 23] and STORM (Screening with Tomosynthesis or Mammography) trial from 5.3 to 8.1 [24–26]. Another observational study conducted by Friedewald et al. had shown increase in CDR by 29% in both fatty and dense breasts by improving lesion conspicuity, more so in dense breasts [27]. Subsequently in 2011, DBT was approved by FDA for supplemental use with screening and diagnostic FFDM. In the diagnostic population too, addition of one or two view DBT to FFDM increases the sensitivity for detection of malignancy as compared to FFDM alone [28–30]. The improvement was seen more so in the detection of invasive cancers with relatively good prognosis like tubular, papillary and mucinous subtypes [31, 32]. Determination and delineation of multicentricity and multifocality of malignancy are better seen on tomosynthesis images [33] (Fig. 3). Benefits of using DBT extend beyond the first round of screening with further increase in detection rates every year [34].
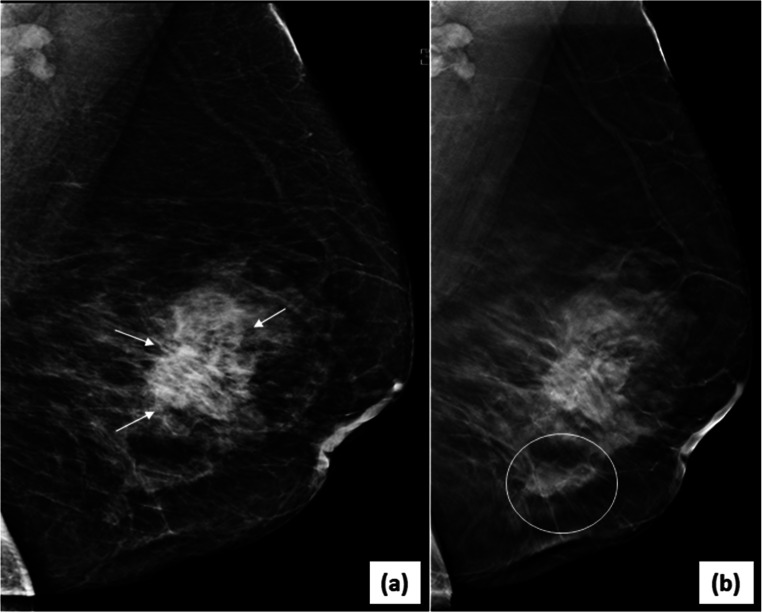
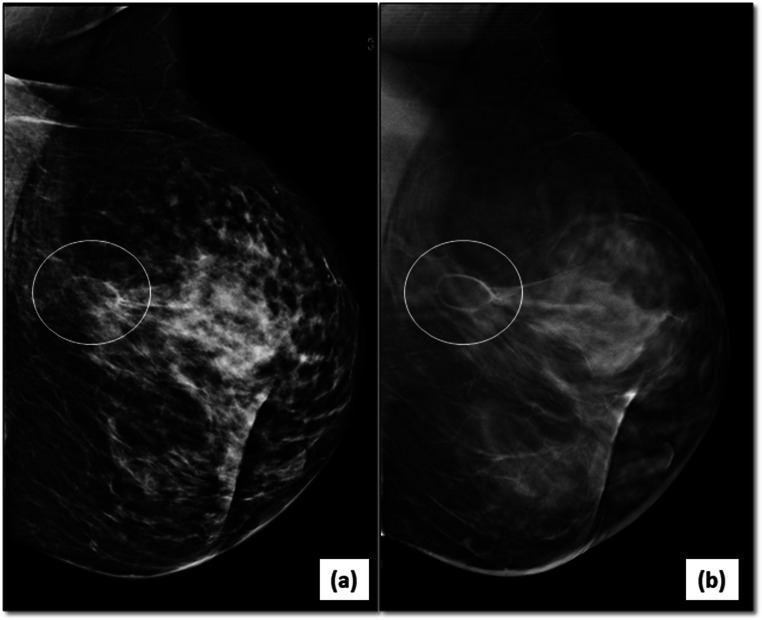
Fig. 3.
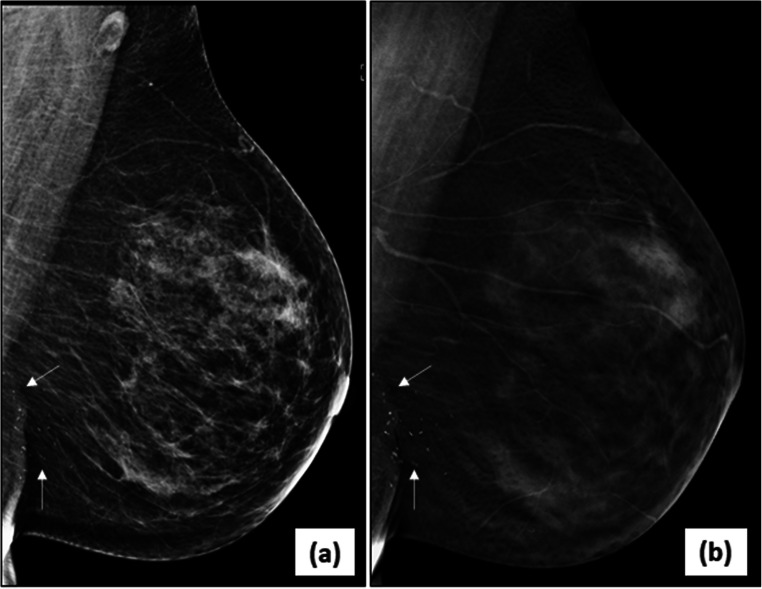
Multifocal/multicentric malignancy on digital breast tomosynthesis (DBT)-2D-FFDM image in MLO view (a) of the left breast reveals an irregular, high density mass with spiculated margins (arrows) in the central breast with associated nipple retraction and skin thickening. DBT image (b) of same patient shows another smaller mass with spiculations (circle) inferior to the index mass representing multicentric disease
Reduces False-Positive Rates
Due to superimposition of glandular parenchyma in 2D mammography, false positives occur due to obscuration of margins or appearance of pseudo-masses (Fig. 4). DBT makes malignant masses appear more malignant and benign masses to be more benign and hence reduce the false positives for malignancy. The OTST trial reported reduced false-positive rates from 6.1 to 5.3% comparing FFDM vs. DBT-FFDM [22, 23]. The estimated reduction in false-positive rate in the STORM trial was by 17% when using DBT-FFDM [24–26]. These benefits extended to the diagnostic evaluation of breast findings as well [29].
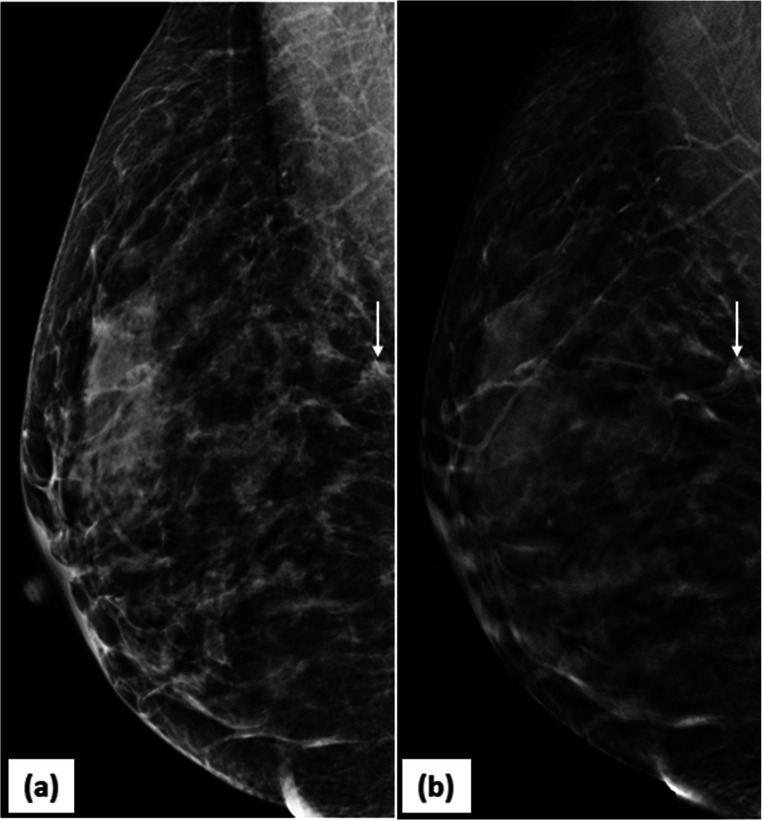
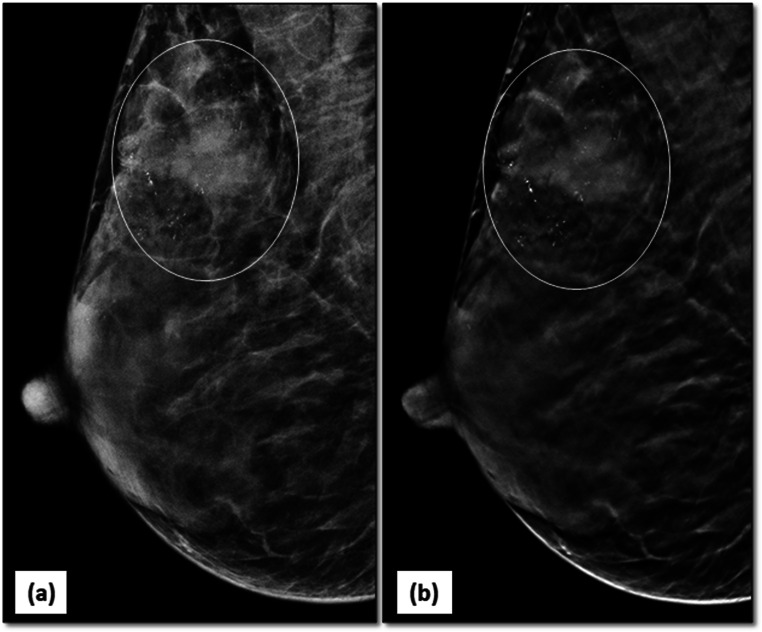
Fig. 4.
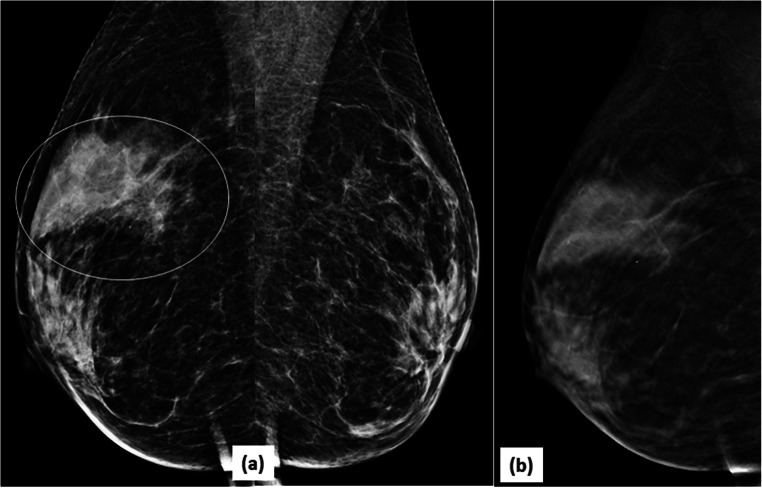
DBT reduces false positives: 2D-MLO (a) mammogram of right breast raised suspicion of an irregular mass in posterior third of breast tissue (arrow). DBT slice at that level (b) clearly showed a looping blood vessel, obviating the need of any further investigation. BI-RADS category 1 was assigned to the mammogram
Reduction in Recall Rates (RR)
A multitude of suspicious abnormalities seen on 2D mammography need further characterization with USG or MRI for appropriate BI-RADS assignment. Patients have to be called back for review in such circumstances. Various studies have shown that these RR for further evaluation were substantially reduced when DBT was used in addition to FFDM rather than FFDM alone due to better mass characterization [27, 35]. Reduction upto 5.5% in RR along with increase in positive predictive value (PPV) of the recalls by approximately 5% are reported [36]. There are however other conflicting studies which have shown use of DBT to increase RR possibly due to higher number of masses detected on DBT than FFDM [26, 37–39].
Reduces un-necessary investigations like more views, USG or MRI
The three-dimensional information given by DBT has shown to improve the workflow by reducing the need of supplemental views such as spot compressions and tangential views [40, 41]. Philpotts et al. reported a 32% reduction in need of supplementary mammographic views, with no requirement of additional views in 72% patients 1 year after introduction of DBT in their setup [42].
-
2.
Enables depth determination or lesion localization
DBT is of particular importance when the mass is visible on only one view. With the use of the scroll bar (a tool in the workstation used to navigate through the contiguous sections of the specific CC or MLO- DBT stack), the reader can determine the exact clockface of the lesion which can aid in a targeted USG and further guided biopsy (Fig. 5). When specifying the location of a lesion on DBT, the exact slice numbers where it is best visualized should be identified in the report.
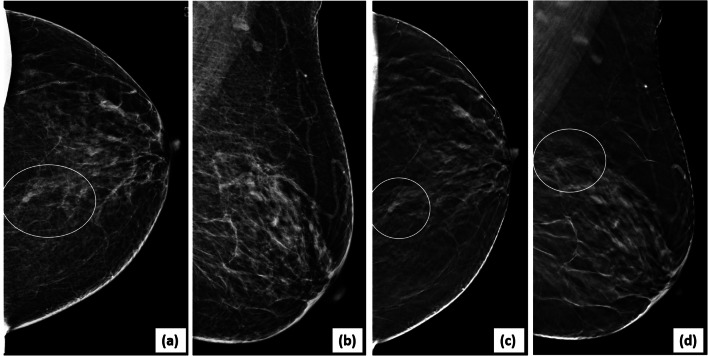
Fig. 5.
Lesion localization and characterization on DBT: a screening mammogram showed a small equal density mass (circle) with indistinct margins in the posterior third depth of left breast on 2D CC view (a). 2D MLO view (b) appeared normal. On scrolling through the CC DBT stack, the mass was best seen in the mid-slices (26/60). A targeted search in the central breast in the MLO DBT stack, revealed the lesion, as seen in selected MLO DBT slice (d). DBT (c, d) showed associated spiculations allowing accurate assessment as BI-RADS category 5 (stereotactic biopsy-invasive carcinoma)
Findings can be readily localized to the skin and thus avoid any unnecessary further workup. Lesions/calcifications localized to the skin are often seen in the peripheral stacks of the DBT within the same sections showing the skin surface [43] (Fig. 6).
-
3.
Evaluation of architectural distortion and asymmetries
Fig. 6.
Localization of dermal calcifications on DBT: MLO view (a) of a 2D mammogram of left breast showed fine pleomorphic calcification (arrow) in regional distribution in the lower part of the breast. However, these were seen in the peripheral sections of DBT slice (b) indicating that these were dermal calcifications (arrow). Hence, it avoided further workup and additional views in this patient
Architectural distortion is one of the most reliable and often missed signs of a malignant lesion on mammogram and should be suspected whenever straight lines are seen converging to a point. DBT can demonstrate architectural distortions better than 2D mammography and can guide the site for focussed ultrasonography [44–46]. The increase in cancer detection is primarily due to the ‘decamouflaging’ effect of DBT, rendering architectural distortions more conspicuous to the reader.
This also leads to increase in detection rate of benign differentials like radial scars or complex sclerosing lesions. The PPV of biopsies of architectural distortions seen on DBT (10.2%) was lower than those detected by FFDM alone (43.4%) [45]. Different authors have addressed this issue and attempted to have an algorithm for approaching such architectural distortions picked on DBT. In brief, the abnormality which is seen on DBT as well as FFDM having an USG correlate bears higher chances of being malignant and should definitely be sampled as compared to the ones which do not have correlate on USG [47]. The role of USG has also been emphasized by Bahl et al. stating that finding of ultrasound correlate for a mammographically detected architectural distortion had a higher chance of harbouring a malignancy (82.9%) compared to one without an ultrasound correlate(27.9%) [48].
An asymmetric density seen on a 2D mammogram may be due to overlapping of normal fibroglandular parenchyma, a true mass obscured by overlapping tissue, or a true asymmetry. Additional supplemental views likely spot compression may be required with FFDM to solve this query; however, the thin slices of DBT can demonstrate the cause of the ‘asymmetry’ and reduce recall rates (Fig. 7). Studies have shown higher probability-for-malignancy based area under curve with DBT than with FFDM [49].
-
4.
Mass detection and characterization
Fig. 7.
Accurate characterization of focal asymmetry on DBT: focal asymmetry in upper outer quadrant of right breast on MLO view (a) was confirmed to be due to non-involuted fibroglandular parenchyma on DBT (b)
The present modality of choice to detect the exact extent of breast disease is MRI. Studies have demonstrated that DBT is comparable to MRI in determining the exact size of the mass, with increased sensitivity than FFDM [50–52]. Margin discrimination can be done better with DBT due to thin slice reconstruction. Suspicious margin characteristics like spiculations and microlobulation can be better discerned with DBT (Figs. 8 and 9).
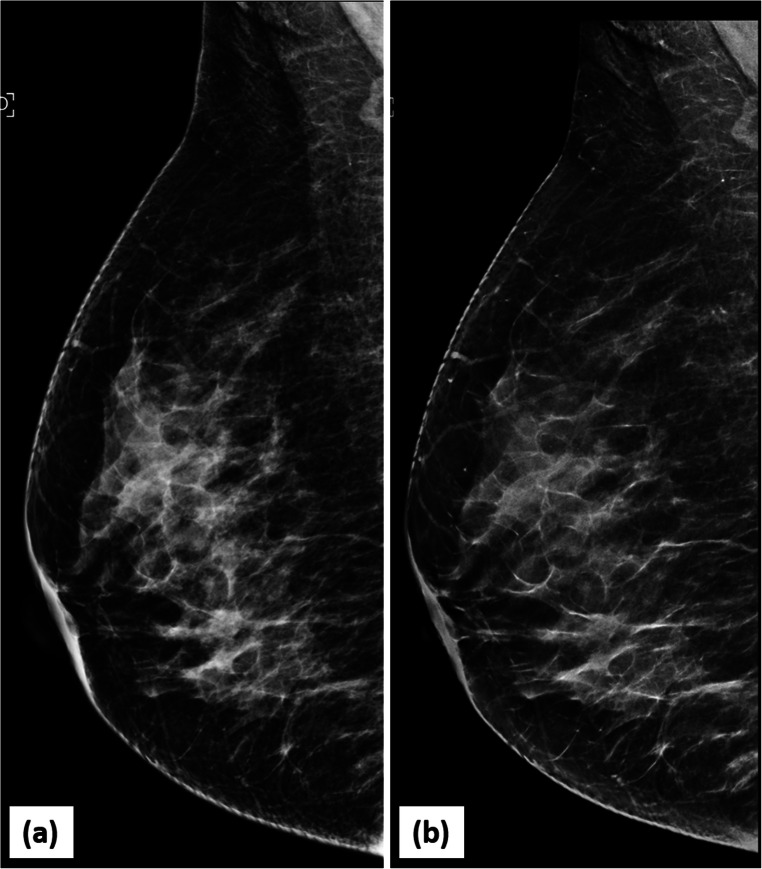
Fig. 8.
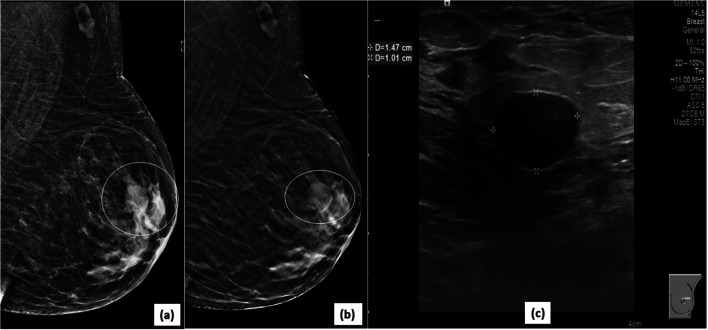
Better characterization of mass margins on DBT: 2D MLO view (a) of left breast demonstrates an oval, equal density mass (circle) in the upper quadrant of left breast with partly circumscribed and partly obscured margins (superior and inferior). The corresponding DBT image (b) delineates the previously obscured margins very well, showing them to be circumscribed (circle). The mass was designated as a BIRADS 3 lesion (likely fibroadenoma). The 6-month follow up USG image (c) of the mass confirms the circumscribed nature and stability of the mass
Fig. 9.
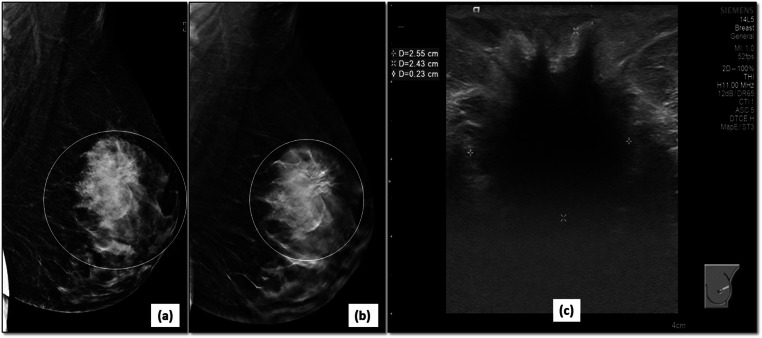
Better margin characterization in a dense breast: 2D-MLO view (a) of the left breast shows a large irregular mass (circle) in the upper quadrant of left breast. The spiculated margins (circle) are better demonstrated on DBT image (b). Corroborative ultrasound (c) confirmed the malignant features of mass as irregular, hypoechoic mass with spiculated margins and posterior shadowing. It was correctly assigned BI-RADS category 5 and biopsy confirmed an invasive ductal carcinoma
DBT can show areas of fat within masses like fat necrosis, lipomas, galactoceles and hamartomas due thin stacks (Fig. 10). However, presence of fat within a mass does not rule out malignancy due to frequent engulfment of surrounding fat within a cancer; hence, the margins, shape and other factors should also be kept in mind to assess the mass. Also, density of the masses may appear less on individual sections of tomosynthesis than on FFDM [18]. Tomosynthesis used over time improves the specificity of the final BIRADS category assigned. The number of BIRADS 3 has shown to be reduced with more findings being assigned to BIRADS 1 or 2 categories with gradual shift of patients to annual screening and higher PPV for biopsies [53].
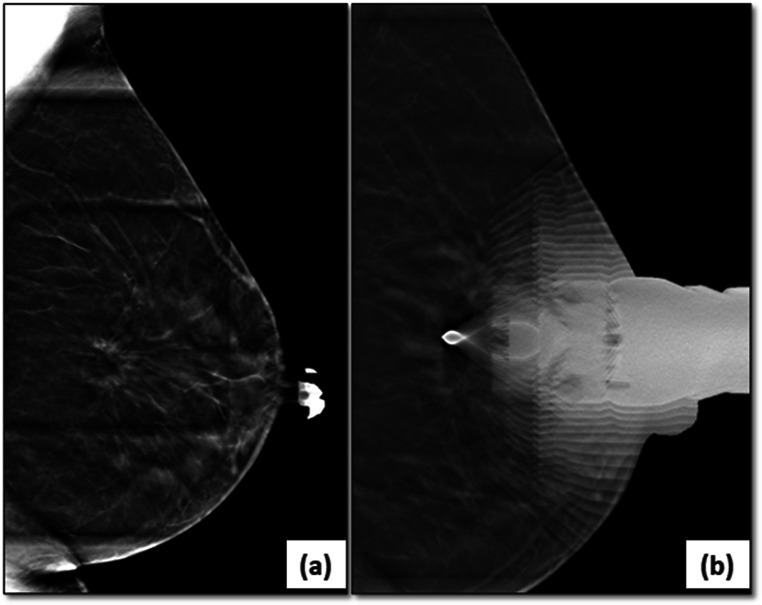
Fig. 10.
Fat necrosis on DBT: suspicious architectural distortion (circle) was seen on a follow-up left mammogram (MLO view) (a) in a patient who had undergone breast conservation surgery. The DBT slice (b) showed a circumscribed lesion with central fat lucency (circle) within this suspicious area representing post-operative fat necrosis
Limitations and Disadvantages
Radiation dose and DBT
The radiation dose to the breast is assessed as mean glandular dose (MGD) which depends on the measurement of air kerma (or exposure) incident on the breast and normalized glandular dose coefficient that is specific to the X-ray beam quality. The variation of this coefficient in different projections relative to the central projection depends not so much on the amount on fibroglandular parenchyma and X-ray spectrum, more so on the size and thickness of breast tissue on MLO view than on CC view [54]. Generally, the MGD to the breast is higher in DBT than in FFDM. Addition of 2 tomosynthesis view to the 2D mammogram increases the radiation dose to ~2 times; however, it is still below the 3 mGy/view limit set by the FDA. This difference in dose to the breast between DBT and FFDM reduces as the density of breast increases [55]. Further reduction is possible with the technique of synthesized mammogram by ~45% [56], which will be discussed in further sections.
Apart from the debate about the radiation dose, the use of an antiscatter grid poses some problems due to projection geometry of images and already increased dose of the study. This requires the use of post-processing scatter-reducing softwares to preserve the image resolution [57].
-
2.
Complex sclerosing lesions:
Increase in detection of complex sclerosing lesions due to better ‘unmasking’ of pathology, and the detection of both benign and malignant findings are at a rise with DBT. However, PPV of biopsies of architectural distortions detected by DBT (10.2%) is lower than those detected by FFDM alone (43.4%) [45]. In spite of this, persisting distortions with no known prior surgery or trauma detected by DBT should be sampled under USG or tomosynthesis guidance as they have a high risk of malignancy [35].
-
3.
Artefacts due to surgical staples
Any high-density object such as surgical clips or markers placed on skin produces blurring-ripple artefacts. The margins of these clips appear ill-defined and wider than their true self in sections out of plane (blur) and the skin appears artefactually thickened. As the distance between the true object and reconstructed slice increases, it appears as ripples. This occurs due to a phenomenon similar to volume averaging in CT when the number of acquired projections is much less than the reconstructed slices leading to noise [58, 59].
-
4.
Calcification
DBT alone is not reliable to characterize or detect microcalcifications. On individual DBT sections, only a limited number of calcifications may be detected (Fig. 11). Currently, it is advocated that for detection of calcifications, FFDM with spot magnification views are to be obtained for their characterization [60, 61]. The emerging role of SM+ DBT vs. FFDM+DBT is being evaluated.
-
5.
Storage
Fig. 11.
Limitation of DBT in evaluating microcalcifications: 2D MLO view (a) of right breast reveals an irregular mass with obscured margins in the posterior third of breast parenchyma in upper quadrant. Associated fine pleomorphic calcifications (circle) are seen in segmental distribution. Similar findings can be seen in the DBT image (b); however, some calcifications are accentuated however others are not well seen as the sections shows only the in-plane calcifications (circle)
DBT files are much larger than 2D FFDM or even CT or MRI files, about 200–450 MB compared to 8–24 MB size of a FFDM file. Storage of such large files can pose a problem as the number of DBT examinations increases. New workstations are needed for reading DBT studies having rapid scrolling and cine facilities in addition to the requirements of mammographic workstation [4].
-
6.
Increased reading time
As the reading radiologist must interpret a separate set of data, it increases the reading time for each study and may affect the productivity in screening programs. Studies have shown that the reading time may be increased to double [22, 62].
Current Status
Digital breast tomosynthesis is deemed as an appropriate modality for breast cancer screening in all women by the American College of Radiology [63]. Studies have also shown cost benefits of addition of DBT to screening programs [64]. No definite screening programs exist in India, and the screening which is being done is mostly opportunistic with protocols varying between institutes. Being a middle-income group nation and the inequitable distribution of healthcare and oncology services, devising such a program is a mammoth task. Studying the incidence of breast carcinoma among the Indian population, it contrasted significantly with western population. The peak incidence in India is mostly in the premenopausal age groups around the 40s [65], in comparison to peak in the 50s to 60s in the western world. Even though mammography has established itself as one of the few screening imaging modalities to substantially reduce the cancer mortality, it is not very effective in screening dense breasts encountered in these younger women. Ultrasonography is an important inexpensive adjunct to breast imaging and yields better results in dense breasts than mammography [66, 67]. However, unavailability of adequately trained breast radiologists limits its widespread use.
As the major benefit of DBT is seen in dense breasts, a call for additional acquiring of tomosynthesis’ images could be taken at the same time by the radiologist (even possible remotely) after assessing the digital mammograms. Depending on individual case, only single view DBT may be acquired.
The Debate of the Dense Breast: DBT or USG
In spite of mammography being the primary screening modality for breast carcinoma detection, a large number of cancers are missed in dense breasts due to decreased sensitivity of mammography in the same [11]. Nonetheless, the risk of breast cancer is also 4–6-fold increased with increasing density of the tissue [12]. Adjunct modalities like breast tomosynthesis and ultrasonography come handy in such scenarios and should be offered to the patients on case-by-case basis. A large number of false positives are detected by USG and the estimates for incremental cancer detection also vary widely from 2.4 to 4.2/1000 screens [68, 69]. A large multicenter prospective trial in Italy, from 2015 to 2017 recruiting 5300 screen negative women with dense breasts on FFDM, detected additional 2.83 cancers per 1000 screens with DBT, with ultrasound having an incremental CDR of 4.9/1000 screens (P = 0.015). However, the significant increase in false positives (1%) with USG underscored its utility compared to DBT having a false-positive recall rate of 0.3% [70]. These findings were reiterated by Starikov et al. [71].
Future Trends
With better sensitivity, specificity and reader confidence offered by breast tomosynthesis, there is increased incorporation of this technology in most of the institutes or centers. Also, there is increased patient acceptability due to reduced recall rates and need of supplemental views. Computer-aided diagnosis (CAD) use with DBT has been investigated by some authors.
Synthesized or composite mammogram:
In the abovementioned technique, a 2D mammogram image is ‘condensed’ from the tomosynthesis image eliminating the need for a separate acquisition of 2D mammogram to circumvent the issue of an additional exposure [4] (Fig. 12). These images are comparable to the FFDM image for diagnostic and screening purpose and were approved by FDA for use in 2013 [72–74]. However, as these images are derived from the tomosynthesis acquisitions, they may have less resolution if motion had occurred during the taking of the image. Also, as the tube moves in an arc to acquire the image, the voxels are shifted, only slightly in a direction perpendicular to the movement, which may blur the microcalcifications [75]. The calcifications may appear enhanced in the SM image due to the intrinsic reconstruction algorithm which is designed to preserve high attenuation voxels [76]. ‘Pseudocalcification’ on SM images may be seen due to overlapping structures such as cooper’s ligaments; however, these will not be visible on any of the stacks, in contrast to true calcifications. Reports have shown inferiority of SM for detection of microcalcifications and still recommend a spot compression FFDM for characterization [77].
Tomosynthesis guided procedures
Fig. 12.
Synthesized mammogram-2D FFDM image (a) and synthesized mammography image (b) of the same patient (MLO view of right breast) showing comparable image quality
The ultrasound correlate of a suspicious finding on DBT should be looked for and ultrasound-guided biopsy of the same be planned. However, there will be subtle findings particularly architectural distortions and asymmetries which would only be visible on DBT. Tomosynthesis-guided core needle biopsy scores over the traditional prone stereotactic biopsy. It has better yields than stereotactic biopsy nearing 100% [78, 79]. Guidance is better, as 3D information is obtained without the need of stereotactic image pairs which is prone to more error (Fig. 13). It also permits the use of the entire detector, compared to stereotactic biopsy, where only a part of breast is seen. Hence, tomosynthesis guidance requires less than half of the time. In spite of increased mean glandular dose with DBT, the reduced need of exposures and shorter procedure time, the actual dose may be lesser [80].
Fig. 13.
Tomosynthesis guided breast biopsy: architectural distortion only seen on tomosynthesis (a) is targeted for biopsy (b), from the peripheral aspect of the lesion using tomosynthesis image as the scout image
In the author’s institute, DBT in single or both views is being done with each mammogram: screening or diagnostic. Synthesized mammogram is being evaluated in comparison to FFDM and tomosynthesis-/DBT-guided biopsies are being conducted for architectural distortions seen only in the latter.
Conclusion
DBT is a promising tool with wide array of advantages and utility in academic and non-academic institutions with notable increase in cancer detection with reduced recall rates and better lesion characterization. Introduction and increasing research on synthesized mammograms (SM) may overcome the drawbacks of the radiation dose and detection of microcalcifications.
Supplementary Information
(MPEG 2.74 mb)
Availability of Data and Material
NA
Code Availability
NA
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women: breast cancer epidemiology. Asia Pac J Clin Oncol. 2017;13:289–295. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Mathew A, George PS, Jagathnath Krishna KM, et al. Transition of cancer in populations in India. Cancer Epidemiol. 2019;58:111–120. doi: 10.1016/j.canep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hooley RJ, Durand MA, Philpotts LE. Advances in digital breast tomosynthesis. Am J Roentgenol. 2017;208:256–266. doi: 10.2214/AJR.16.17127. [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AMF, Chen THH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin N Am. 2004;42:793–806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Tabár L, Yen AM-F, Wu WY-Y, Chen SLS, Chiu SYH, Fann JCY, Ku MMS, Smith RA, Duffy SW, Chen THH. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13–20. doi: 10.1111/tbj.12354. [DOI] [PubMed] [Google Scholar]
- 7.Webb ML, Cady B, Michaelson JS, Bush DM, Calvillo KZ, Kopans DB, Smith BL. A failure analysis of invasive breast cancer: most deaths from disease occur in women not regularly screened: most breast cancer deaths not screened. Cancer. 2014;120:2839–2846. doi: 10.1002/cncr.28199. [DOI] [PubMed] [Google Scholar]
- 8.Popli MB, Teotia R, Narang M, Krishna H (2014) Breast positioning during mammography: mistakes to be avoided. Breast Cancer(Auckl) 8:BCBCR.S17617. 10.4137/BCBCR.S17617 [DOI] [PMC free article] [PubMed]
- 9.Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, Brenner RJ, Bassett L, Berg W, Feig S, Hendrick E, Mendelson E, D'Orsi C, Sickles E, Burhenne LW. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Lerda D, Quinn C, Follmann M, Alonso-Coello P, Rossi PG, Lebeau A, Nyström L, Broeders M, Ioannidou-Mouzaka L, Duffy SW, Borisch B, Fitzpatrick P, Hofvind S, Castells X, Giordano L, Canelo-Aybar C, Warman S, Mansel R, Sardanelli F, Parmelli E, Gräwingholt A, Saz-Parkinson Z, for the European Commission Initiative on Breast Cancer (ECIBC) Contributor Group Breast cancer screening and diagnosis: a synopsis of the European Breast Guidelines. Ann Intern Med. 2020;172:46–56. doi: 10.7326/M19-2125. [DOI] [PubMed] [Google Scholar]
- 11.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 12.Porter GJR, Evans AJ, Cornford EJ, Burrell HC, James JJ, Lee AHS, Chakrabarti J. Influence of mammographic parenchymal pattern in screening-detected and interval invasive breast cancers on pathologic features, mammographic features, and patient survival. Am J Roentgenol. 2007;188:676–683. doi: 10.2214/AJR.05.1950. [DOI] [PubMed] [Google Scholar]
- 13.Rangarajan K, Hari S, Thulkar S, et al. Characterization of lesions in dense breasts: does tomosynthesis help? Indian J Radiol Imaging. 2016;26:210. doi: 10.4103/0971-3026.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vedantham S, Karellas A, Vijayaraghavan GR, Kopans DB. Digital breast tomosynthesis: state of the art. Radiology. 2015;277:663–684. doi: 10.1148/radiol.2015141303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong A, Weinstein SP, McDonald ES, Conant EF. Digital breast tomosynthesis: concepts and clinical practice. Radiology. 2019;292:1–14. doi: 10.1148/radiol.2019180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jousi MO, Erkkilä J, Varjonen M, Soiva M, Hukkinen K, Blanco Sequeiros R. A new breast tomosynthesis imaging method: continuous sync-and-shoot – technical feasibility and initial experience. Acta Radiol Open. 2019;8:205846011983625. doi: 10.1177/2058460119836255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirada N, Li G, Dreizin D, Robinson L, Khorjekar G, Dromi S, Ernst T. Digital breast tomosynthesis: physics, artifacts, and quality control considerations. RadioGraphics. 2019;39:413–426. doi: 10.1148/rg.2019180046. [DOI] [PubMed] [Google Scholar]
- 18.Peppard HR, Nicholson BE, Rochman CM, Merchant JK, Mayo RC, III, Harvey JA. Digital breast tomosynthesis in the diagnostic setting: indications and clinical applications. RadioGraphics. 2015;35:975–990. doi: 10.1148/rg.2015140204. [DOI] [PubMed] [Google Scholar]
- 19.Sechopoulos I, Ghetti C. Optimization of the acquisition geometry in digital tomosynthesis of the breast: acquisition geometry optimization of breast tomosynthesis. Med Phys. 2009;36:1199–1207. doi: 10.1118/1.3090889. [DOI] [PubMed] [Google Scholar]
- 20.Sechopoulos I. A review of breast tomosynthesis. Part I. The image acquisition process: breast tomosynthesis review. I. Image acquisition. Med Phys. 2013;40:014301. doi: 10.1118/1.4770279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CH, Destounis SV, Friedewald SM, Newell MS. Digital breast tomosynthesis (DBT) guidance (a supplement to ACR BI-RADS mammography 2013) Reston: American College of Radiology; 2013. [Google Scholar]
- 22.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, Izadi M, Jebsen IN, Jahr G, Krager M, Niklason LT, Hofvind S, Gur D. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 23.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, Izadi M, Jebsen IN, Jahr G, Krager M, Hofvind S. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol. 2013;23:2061–2071. doi: 10.1007/s00330-013-2820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caumo F, Bernardi D, Ciatto S, Macaskill P, Pellegrini M, Brunelli S, Tuttobene P, Bricolo P, Fantò C, Valentini M, Montemezzi S, Houssami N. Incremental effect from integrating 3D-mammography (tomosynthesis) with 2D-mammography: increased breast cancer detection evident for screening centres in a population-based trial. Breast. 2014;23:76–80. doi: 10.1016/j.breast.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Bernardi D, Caumo F, Macaskill P, Ciatto S, Pellegrini M, Brunelli S, Tuttobene P, Bricolo P, Fantò C, Valentini M, Montemezzi S, Houssami N. Effect of integrating 3D-mammography (digital breast tomosynthesis) with 2D-mammography on radiologists’ true-positive and false-positive detection in a population breast screening trial. Eur J Cancer. 2014;50:1232–1238. doi: 10.1016/j.ejca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, Tuttobene P, Bricolo P, Fantò C, Valentini M, Montemezzi S, Macaskill P. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14:583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, Hayes MK, Copit DS, Carlson KL, Cink TM, Barke LD, Greer LN, Miller DP, Conant EF. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499–2507. doi: 10.1001/jama.2014.6095. [DOI] [PubMed] [Google Scholar]
- 28.Svahn TM, Chakraborty DP, Ikeda D, Zackrisson S, Do Y, Mattsson S, Andersson I. Breast tomosynthesis and digital mammography: a comparison of diagnostic accuracy. BJR. 2012;85:e1074–e1082. doi: 10.1259/bjr/53282892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, Niklason LT. Assessing radiologist performance using combined digital mammography and breast tomosynthesis compared with digital mammography alone: results of a multicenter, multireader trial. Radiology. 2013;266:104–113. doi: 10.1148/radiol.12120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-Ray imaging observer study. Radiology. 2012;262:788–796. doi: 10.1148/radiol.11103514. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Kang HJ, Shin JK, Lee NK, Song YS, Nam KJ, Choo KS. Biologic profiles of invasive breast cancers detected only with digital breast tomosynthesis. Am J Roentgenol. 2017;209:1411–1418. doi: 10.2214/AJR.17.18195. [DOI] [PubMed] [Google Scholar]
- 32.Skaane P, Sebuødegård S, Bandos AI, Gur D, Østerås BH, Gullien R, Hofvind S. Performance of breast cancer screening using digital breast tomosynthesis: results from the prospective population-based Oslo Tomosynthesis Screening Trial. Breast Cancer Res Treat. 2018;169:489–496. doi: 10.1007/s10549-018-4705-2. [DOI] [PubMed] [Google Scholar]
- 33.Fontaine M, Tourasse C, Pages E, Laurent N, Laffargue G, Millet I, Molinari N, Taourel P. Local tumor staging of breast cancer: digital mammography versus digital mammography plus tomosynthesis. Radiology. 2019;291:594–603. doi: 10.1148/radiol.2019182457. [DOI] [PubMed] [Google Scholar]
- 34.McDonald ES, Oustimov A, Weinstein SP, et al. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol. 2016;2:737–743. doi: 10.1001/jamaoncol.2015.5536. [DOI] [PubMed] [Google Scholar]
- 35.Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269:694–700. doi: 10.1148/radiol.13130307. [DOI] [PubMed] [Google Scholar]
- 36.Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R., Jr Implementation of breast tomosynthesis in a routine screening practice: an observational study. Am J Roentgenol. 2013;200:1401–1408. doi: 10.2214/AJR.12.9672. [DOI] [PubMed] [Google Scholar]
- 37.Zackrisson S, Lång K, Rosso A, Johnson K, Dustler M, Förnvik D, Förnvik H, Sartor H, Timberg P, Tingberg A, Andersson I. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol. 2018;19:1493–1503. doi: 10.1016/S1470-2045(18)30521-7. [DOI] [PubMed] [Google Scholar]
- 38.Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings at digital breast tomosynthesis occult to conventional digital mammography: imaging features and pathology findings. Breast J. 2015;21:538–542. doi: 10.1111/tbj.12446. [DOI] [PubMed] [Google Scholar]
- 39.Lourenco AP, Barry-Brooks M, Baird GL, Tuttle A, Mainiero MB. Changes in recall type and patient treatment following implementation of screening digital breast tomosynthesis. Radiology. 2015;274:337–342. doi: 10.1148/radiol.14140317. [DOI] [PubMed] [Google Scholar]
- 40.Park JM, Franken EA, Garg M, et al. Breast tomosynthesis: present considerations and future applications. RadioGraphics. 2007;27:S231–S240. doi: 10.1148/rg.27si075511. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg JS, Javitt MC, Katzen J, Michael S, Holland AE. Clinical performance metrics of 3D digital breast tomosynthesis compared with 2D digital mammography for breast cancer screening in community practice. Am J Roentgenol. 2014;203:687–693. doi: 10.2214/AJR.14.12642. [DOI] [PubMed] [Google Scholar]
- 42.Philpotts LE, Kalra VB, Crenshaw J, Butler RS (2013) How tomosynthesis optimizes patient work-up, throughput, and resource utilization [abstr]. In: Radiological Society of North America Scientific Assembly and Annual Meeting Program. Oak Brook, Ill: Radiological Society of North America, 191
- 43.Roth RG, Maidment ADA, Weinstein SP, et al Digital breast tomosynthesis: lessons learned from early clinical implementation. 34:15 [DOI] [PMC free article] [PubMed]
- 44.Patel BK, Covington M, Pizzitola VJ, Lorans R, Giurescu M, Eversman W, Lewin J. Initial experience of tomosynthesis-guided vacuum-assisted biopsies of tomosynthesis-detected (2D mammography and ultrasound occult) architectural distortions. Am J Roentgenol. 2018;210:1395–1400. doi: 10.2214/AJR.17.18802. [DOI] [PubMed] [Google Scholar]
- 45.Alshafeiy TI, Nguyen JV, Rochman CM, Nicholson BT, Patrie JT, Harvey JA. Outcome of architectural distortion detected only at breast tomosynthesis versus 2D mammography. Radiology. 2018;288:38–46. doi: 10.1148/radiol.2018171159. [DOI] [PubMed] [Google Scholar]
- 46.Partyka L, Lourenco AP, Mainiero MB. Detection of Mammographically occult architectural distortion on digital breast tomosynthesis screening: initial clinical experience. Am J Roentgenol. 2014;203:216–222. doi: 10.2214/AJR.13.11047. [DOI] [PubMed] [Google Scholar]
- 47.Pujara AC, Hui J, Wang LC. Architectural distortion in the era of digital breast tomosynthesis: outcomes and implications for management. Clin Imaging. 2019;54:133–137. doi: 10.1016/j.clinimag.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Bahl M, Baker JA, Kinsey EN, Ghate SV. Architectural distortion on mammography: correlation with pathologic outcomes and predictors of malignancy. Am J Roentgenol. 2015;205:1339–1345. doi: 10.2214/AJR.15.14628. [DOI] [PubMed] [Google Scholar]
- 49.Zuley ML, Bandos AI, Ganott MA, Sumkin JH, Kelly AE, Catullo VJ, Rathfon GY, Lu AH, Gur D. Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology. 2013;266:89–95. doi: 10.1148/radiol.12120552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luparia A, Mariscotti G, Durando M, Ciatto S, Bosco D, Campanino PP, Castellano I, Sapino A, Gandini G. Accuracy of tumour size assessment in the preoperative staging of breast cancer: comparison of digital mammography, tomosynthesis, ultrasound and MRI. Radiol Med. 2013;118:1119–1136. doi: 10.1007/s11547-013-0941-z. [DOI] [PubMed] [Google Scholar]
- 51.Mun HS, Kim HH, Shin HJ, Cha JH, Ruppel PL, Oh HY, Chae EY. Assessment of extent of breast cancer: comparison between digital breast tomosynthesis and full-field digital mammography. Clin Radiol. 2013;68:1254–1259. doi: 10.1016/j.crad.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Mann RM, Veltman J, Barentsz JO, Wobbes T, Blickman JG, Boetes C. The value of MRI compared to mammography in the assessment of tumour extent in invasive lobular carcinoma of the breast. Eur J Surg Oncol. 2008;34:135–142. doi: 10.1016/j.ejso.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 53.Raghu M, Durand MA, Andrejeva L, Goehler A, Michalski MH, Geisel JL, Hooley RJ, Horvath LJ, Butler R, Forman HP, Philpotts LE. Tomosynthesis in the diagnostic setting: changing rates of BI-RADS final assessment over time. Radiology. 2016;281:54–61. doi: 10.1148/radiol.2016151999. [DOI] [PubMed] [Google Scholar]
- 54.Sechopoulos I, Suryanarayanan S, Vedantham S, D'Orsi C, Karellas A. Computation of the glandular radiation dose in digital tomosynthesis of the breast: computation of dose in digital breast tomosynthesis. Med Phys. 2006;34:221–232. doi: 10.1118/1.2400836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng SSJ, Sechopoulos I. Clinical digital breast tomosynthesis system: dosimetric characterization. Radiology. 2012;263:35–42. doi: 10.1148/radiol.11111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svahn TM, Houssami N, Sechopoulos I, Mattsson S. Review of radiation dose estimates in digital breast tomosynthesis relative to those in two-view full-field digital mammography. Breast. 2015;24:93–99. doi: 10.1016/j.breast.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sechopoulos I, Suryanarayanan S, Vedantham S, D'Orsi CJ, Karellas A. Scatter radiation in digital tomosynthesis of the breast: scatter radiation in breast tomosynthesis. Med Phys. 2007;34:564–576. doi: 10.1118/1.2428404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu Y-H, Zhao B, Zhao W. Image artifacts in digital breast tomosynthesis: investigation of the effects of system geometry and reconstruction parameters using a linear system approach: image artifacts in digital breast tomosynthesis. Med Phys. 2008;35:5242–5252. doi: 10.1118/1.2996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machida H, Yuhara T, Mori T, Ueno E, Moribe Y, Sabol JM. Optimizing parameters for flat-panel detector digital tomosynthesis. RadioGraphics. 2010;30:549–562. doi: 10.1148/rg.302095097. [DOI] [PubMed] [Google Scholar]
- 60.Tagliafico A, Mariscotti G, Durando M, Stevanin C, Tagliafico G, Martino L, Bignotti B, Calabrese M, Houssami N. Characterisation of microcalcification clusters on 2D digital mammography (FFDM) and digital breast tomosynthesis (DBT): does DBT underestimate microcalcification clusters? Results of a multicentre study. Eur Radiol. 2015;25:9–14. doi: 10.1007/s00330-014-3402-8. [DOI] [PubMed] [Google Scholar]
- 61.Spangler ML, Zuley ML, Sumkin JH, Abrams G, Ganott MA, Hakim C, Perrin R, Chough DM, Shah R, Gur D. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. Am J Roentgenol. 2011;196:320–324. doi: 10.2214/AJR.10.4656. [DOI] [PubMed] [Google Scholar]
- 62.Dang PA, Freer PE, Humphrey KL, Halpern EF, Rafferty EA. Addition of Tomosynthesis to conventional digital mammography: effect on image interpretation time of screening examinations. Radiology. 2014;270:49–56. doi: 10.1148/radiol.13130765. [DOI] [PubMed] [Google Scholar]
- 63.Mainiero MB, Moy L, Baron P, Didwania AD, Green ED, Heller SL, et al. ACR appropriateness criteria® breast cancer screening. J Am Coll Radiol. 2017;14:S383–S390. doi: 10.1016/j.jacr.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 64.Lee CI, Cevik M, Alagoz O, Sprague BL, Tosteson ANA, Miglioretti DL, Kerlikowske K, Stout NK, Jarvik JG, Ramsey SD, Lehman CD. Comparative effectiveness of combined digital mammography and tomosynthesis screening for women with dense breasts. Radiology. 2015;274:772–780. doi: 10.1148/radiol.14141237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Indian Council of Medical Research. Three year report of Population Based Cancer Registries, 2009–2011. Bangalore: National Centre for Disease Informatics and Research/National Cancer Registry Programme, 2013
- 66.Buchberger W, DeKoekkoek-Doll P, Springer P, Obrist P, Dünser M. Incidental findings on sonography of the breast: clinical significance and diagnostic workup. Am J Roentgenol. 1999;173:921–927. doi: 10.2214/ajr.173.4.10511149. [DOI] [PubMed] [Google Scholar]
- 67.Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US--diagnostic yield and tumor characteristics. Radiology. 1998;207:191–199. doi: 10.1148/radiology.207.1.9530316. [DOI] [PubMed] [Google Scholar]
- 68.Corsetti V, Houssami N, Ghirardi M, Ferrari A, Speziani M, Bellarosa S, Remida G, Gasparotti C, Galligioni E, Ciatto S. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1year follow-up. Eur J Cancer. 2011;47:1021–1026. doi: 10.1016/j.ejca.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Venturini E, Losio C, Panizza P, Rodighiero MG, Fedele I, Tacchini S, Schiani E, Ravelli S, Cristel G, Panzeri MM, de Cobelli F, del Maschio A. Tailored breast cancer screening program with microdose mammography, US, and MR imaging: short-term results of a pilot study in 40–49-year-old women. Radiology. 2013;268:347–355. doi: 10.1148/radiol.13122278. [DOI] [PubMed] [Google Scholar]
- 70.Tagliafico AS, Mariscotti G, Valdora F, Durando M, Nori J, la Forgia D, Rosenberg I, Caumo F, Gandolfo N, Sormani MP, Signori A, Calabrese M, Houssami N. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2) Eur J Cancer. 2018;104:39–46. doi: 10.1016/j.ejca.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 71.Starikov A, Drotman M, Hentel K, Katzen J, Min RJ, Arleo EK. 2D mammography, digital breast tomosynthesis, and ultrasound: which should be used for the different breast densities in breast cancer screening? Clin Imaging. 2016;40:68–71. doi: 10.1016/j.clinimag.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Zuley ML, Guo B, Catullo VJ, Chough DM, Kelly AE, Lu AH, Rathfon GY, Lee Spangler M, Sumkin JH, Wallace LP, Bandos AI. Comparison of two-dimensional synthesized mammograms versus original digital mammograms alone and in combination with tomosynthesis images. Radiology. 2014;271:664–671. doi: 10.1148/radiol.13131530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skaane P, Bandos AI, Eben EB, Jebsen IN, Krager M, Haakenaasen U, Ekseth U, Izadi M, Hofvind S, Gullien R. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology. 2014;271:655–663. doi: 10.1148/radiol.13131391. [DOI] [PubMed] [Google Scholar]
- 74.Zuckerman SP, Conant EF, Keller BM, Maidment ADA, Barufaldi B, Weinstein SP, Synnestvedt M, McDonald ES. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology. 2016;281:730–736. doi: 10.1148/radiol.2016160366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson JS, Wells JR, Baker JA, Samei E. How does c - view image quality compare with conventional 2D FFDM?: comparing c - view and FFDM image quality. Med Phys. 2016;43:2538–2547. doi: 10.1118/1.4947293. [DOI] [PubMed] [Google Scholar]
- 76.Zuckerman SP, Maidment ADA, Weinstein SP, McDonald ES, Conant EF. Imaging with synthesized 2D mammography: differences, advantages, and pitfalls compared with digital mammography. Am J Roentgenol. 2017;209:222–229. doi: 10.2214/AJR.16.17476. [DOI] [PubMed] [Google Scholar]
- 77.Gur D, Zuley ML, Anello MI, Rathfon GY, Chough DM, Ganott MA, Hakim CM, Wallace L, Lu A, Bandos AI. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images. Acad Radiol. 2012;19:166–171. doi: 10.1016/j.acra.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bahl M, Maunglay M, D’Alessandro HA, Lehman CD. Comparison of upright digital breast tomosynthesis–guided versus prone stereotactic vacuum-assisted breast biopsy. Radiology. 2019;290:298–304. doi: 10.1148/radiol.2018181788. [DOI] [PubMed] [Google Scholar]
- 79.Schrading S, Distelmaier M, Dirrichs T, Detering S, Brolund L, Strobel K, Kuhl CK. Digital breast tomosynthesis–guided vacuum-assisted breast biopsy: initial experiences and comparison with prone stereotactic vacuum-assisted biopsy. Radiology. 2015;274:654–662. doi: 10.1148/radiol.14141397. [DOI] [PubMed] [Google Scholar]
- 80.Amir T, Barafaldi B, Zuckerman SP, Maidment ADA, Conant EF. Comparison between radiation dose of 2D digital versus digital tomosynthesis guided stereotactic breast biopsies: tomosynthesis wins! [abstr]. In: Radiological Society of North America scientific assembly and annual meeting program. Oak Brook: Radiological Society of North America; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MPEG 2.74 mb)
Data Availability Statement
NA
NA