Abstract
Ovarian cancer is the leading cause of death among gynecologic malignancies. Combining cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) can benefit patients with advanced ovarian cancer. We evaluate the role of small bowel peritoneal cancer index (sb-PCI) score as a prognostic factor. We retrospectively analyzed characteristics and clinical outcomes of patients that underwent intermediate cytoreductive surgery combined with HIPEC after neoadjuvant chemotherapy and patient’s characteristics underwent debulking surgery plus HIPEC for recurrence disease. One hundred thirty patients were included. Eighty-five of them (65.4%) were treated for recurrent ovarian cancer, while 45 (34.6%) underwent intermediate cytoreductive surgery after neoadjuvant chemotherapy with a mean age of 52 years. Mean intraoperative peritoneal cancer index (PCI) was 11.84 with a mean sb-PCI score of 5.57. Univariate analysis revealed that PCI, sb-PCI, and completeness of cytoreduction (CC) were parameters that correlated significantly with overall survival, while after multivariate analysis sb-PCI and CC were identified as independent prognostic factors of survival. A statistically significant correlation between sb-PCI score and overall survival of patients with advanced ovarian cancer was revealed. Further larger future studies are required to confirm our conclusion in order to change the treatment of advanced ovarian cancer patients.
Keywords: Ovarian cancer, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy (HIPEC), Peritoneal cancer index (PCI), Small bowel peritoneal cancer index (sb-PCI), Overall survival
Introduction
Ovarian cancer is the leading cause of death among gynecologic malignancies [1]. The GLOBOCAN 2018 estimates that 295,414 new epithelial ovarian cancer cases were diagnosed worldwide in 2018, while 184,799 deaths occurred by this cause [2]. The 5-year survival rate in stages I–II epithelial ovarian cancer patients is reported to be 40–90%, while in stages III–IV, this rate descends at 3–19% [3]. One of the main reasons for this outcome is the advanced stage of the disease at the time of diagnosis, after dissemination to the peritoneal cavity. Treatment options for advanced stage patients include neoadjuvant chemotherapy followed by interval debulking surgery, primary cytoreductive surgery combined with adjuvant chemotherapy with or **without the addition of hyperthermic intraperitoneal chemotherapy (HIPEC). In all options, the ultimate goal is the complete resection of the intraperitoneal tumor growth.
In 2010, a published study mentioned that neoadjuvant chemotherapy plus interval debulking surgery had no inferior outcome when compared to primary cytoreductive surgery combined with adjuvant chemotherapy [4]. Since then, this option is broadly used aiming complete cytoreduction, less complications, and no residual disease. Furthermore, the publication of a breakthrough study in 2018 showed the utility of HIPEC in the management of advanced ovarian cancer patients. This study reported both a statistically significant higher progression free survival and overall survival when compared to cytoreductive surgery without HIPEC [5]. However, several subsequent articles noticed criticism about the results of this study [6] with some of them to reveal no benefit from HIPEC addition in either primary or interval debulking surgery [7].
Several approaches have been attempted in order to predict the completeness of cytoreductive surgery which affects the result of surgery and patient survival. One of the most useful tools is the peritoneal cancer index (PCI), a score measuring the extend of intraperitoneal disease through quantitatively combining the cancer implant size with the tumor distribution throughout 13 abdominopelvic regions, reaching a maximum score of 39 [8, 9]. Such a score alongside with other scores is proposed to be used as the standard ovarian cancer operative report in all ESGO recognized centers of excellence for the treatment of ovarian cancer [10].
We report the results of a retrospective study in which we present the progression free and the overall survival of advanced ovarian cancer patients who underwent either intermediate cytoreductive surgery plus HIPEC after neoadjuvant chemotherapy or debulking surgery combined with HIPEC for recurrent disease bearing in mind the importance of small bowel PCI score.
Material and Methods
Study Design
The present study is a retrospective analysis of supported data of patients that underwent cytoreductive surgery plus HIPEC for ovarian cancer with peritoneal metastasis in three different centers between 2005 and 2019. Our study was approved by Institutional Ethics and Research Boards of all three centers.
Patient Population
The analysis included patients that were treated either for recurrent ovarian cancer or newly diagnosed advanced-stage disease (FIGO stage III or more) after 3 or 4 chemotherapy cycles. All cases went through complete preoperative assessment in order to decide the maximum cytoreduction extend that could be achieved. The assessment included thoracic, abdominal, and pelvic computed tomography and/or abdominal and pelvic magnetic resonance imaging. Also, all patients had been discussed in the multi-disciplinary team (MDT) meeting of each institution, and they all signed an informed consent before surgery.
For each patient, we evaluated preoperative, intraoperative, and postoperative parameters, which included age, eastern cooperative oncology group (ECOG) status, previous chemotherapy, PCI score, small bowel PCI score as a separate parameter, cytoreduction score, and length of hospital stay. Overall survival (OS) was defined as the time between the surgical procedure and the date of reported death, while progression-free survival (PFS) was defined as the time between surgical procedure and disease recurrence or progression.
Procedure Details
All patients underwent laparotomy, and PCI score was calculated intraoperatively, as described by Sugarbaker [11]. We performed cytoreduction carrying out tumor removal, organ resection when needed, as well as peritonectomy. Completeness of cytoreduction was calculated by the measurement of residual disease in the peritoneal cavity: A CC-0 indicated no visible tumor, a CC-1 residual tumor < 2.5 mm, a CC-2 residual tumor between 2.5 mm and 2.5 cm, and CC-3 residual tumor > 2.5 cm [11]. After this procedure, we performed the closed abdomen technique HIPEC with a dose of 100 mg/m2 cisplatin and 175 mg/m2 paclitaxel in the abdominal cavity heated to 40 °C for 90 min. These regimens were selected by our multi-disciplinary team (MDT) based on our experience, and results from our team’s already published the article that mentioned the use of cisplatin and paclitaxel for platinum-sensitive disease [12]. Same positive results about the use of cisplatin and paclitaxel for intraperitoneal use were reported by other studies such as Coccolini et al. [13].
Statistical Analysis
The primary endpoint of this study was the evaluation of overall survival. Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA). Overall survival and disease-free survival analyses were performed using the Kaplan-Meier method and compared using the log-rank test. In order to define independent prognostic factors of survival, multivariate analysis was performed using COX-regression models. A p value less than 0.05 was considered as stastistically significant.
Results
In total, 130 ovarian cancer patients with a mean age of 52 years (range 28–73) were treated between 2005 and 2019 and were included in this analysis. Eighty-five of them (65.4%) were treated for recurrent ovarian cancer, while 45 (34.6%) underwent intermediate cytoreductive surgery after neoadjuvant chemotherapy. Regarding the intermediate group, 4 patients (9%) were FIGO stage IIIA, 5 (11%) stage IIIB, 25 (55.6%) stage IIIC, 7 stage IVA (15.6%), and finally 4 (9%) FIGO stage IVB. Regarding the histologic type, most of cases were serous ovarian cancer with 33/45 (73.3%) and 65/85 (76.5%) in intermediate and recurrent group, respectively. Mean operation time for cytoreduction plus HIPEC administration in both groups was 5.64 ± 1.7 h. Mean intraoperative PCI score was calculated to be close to 12 with a range between 4 and 30, while mean small bowel PCI (sb-PCI) score was 5.6 (range 0–12). Complete cytoreduction (CC-0) was achieved in 74 patients (56.9%), CC-1 in 37 patients (28.5%), CC-2 in 15 patients (11.5%), while in 4 patients was achieved CC-3 (3.1%). The patients’ characteristics are summarized in Table 1.
Table 1.
Patients’ characteristics
| N (%) | |
|---|---|
| Number of patients | 130 |
| Intermediate | 45 (34.6%) |
| Recurrent | 85 (65.4%) |
| Mean age (years) | 52.2 years (range 28 − 73) |
| Mean PCI score | 11.84 (range 4 − 30) |
| Mean sb-PCI score | 5.57 (range 0 − 12) |
| Completeness of cytoreduction | |
| CC-0 | 74 (56.9%) |
| CC-1 | 37 (28.5%) |
| CC-2 | 15 (11.5%) |
| CC-3 | 4 (3.1%) |
Fifty-one patients (39.2%) were needed to dissect a part of bowel, and anastomoses were made in order to succeed cytoreduction, while in 31 patients (23.8%), stomas were made. Regarding the intra- or postoperative complications that treated patients faced, these are mainly due to surgical procedures and not the HIPEC use. More specifically, no intraoperative death was occurred in both recurrent and intermediate group. In the intermediate group, 5 patients suffered from complications classified as grade III or IV Clavien-Dindo Classification, while in the recurrent group, 11 patients suffered from such grade complications. Complications due to toxicity of HIPEC were rare and were limited to acute renal failure to 2 patients, one for each treatment group (Table 2). Mean period for all the included patients between surgical treatment and adjuvant chemotherapy with carboplatin, paclitaxel, and bevacizumab was calculated at approximately 35 days (range 26–49 days).
Table 2.
Observed complications
| Intermediate(N = 45) | Recurrent(N = 85) | |
|---|---|---|
| Mortality (N) | 0/45 | 0/85 |
| Morbidity (N %) | 17/45 (37.8%) | 34/85 (40%) |
| Clavien-Dindo I–II | 11 | 23 |
| Clavien-Dindo III–IV* | 6 | 11 |
| Enterocutaneous fistula | 4 | 5 |
| Bleeding—reoperation | 1 | 3 |
| Rectovaginal fistula | 0 | 1 |
| ARDS—pulmonary embolism | 0 | 1 |
| Acute renal failure | 1 | 1 |
*specifically
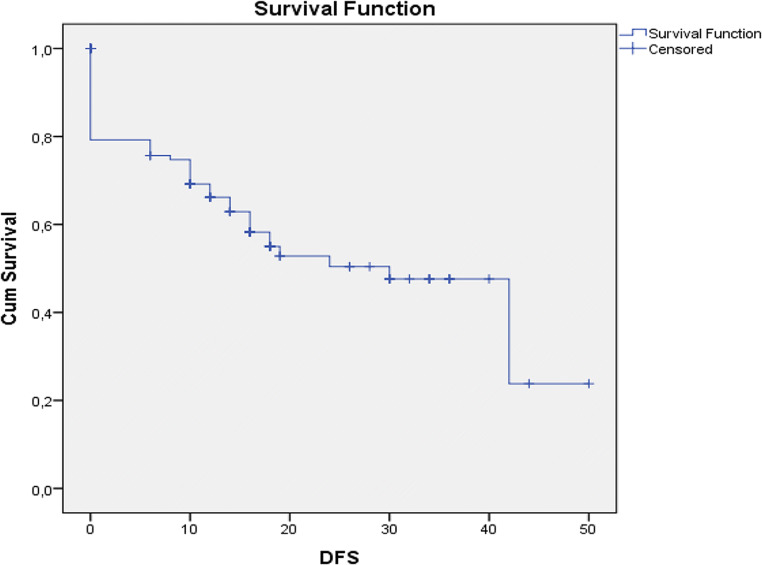
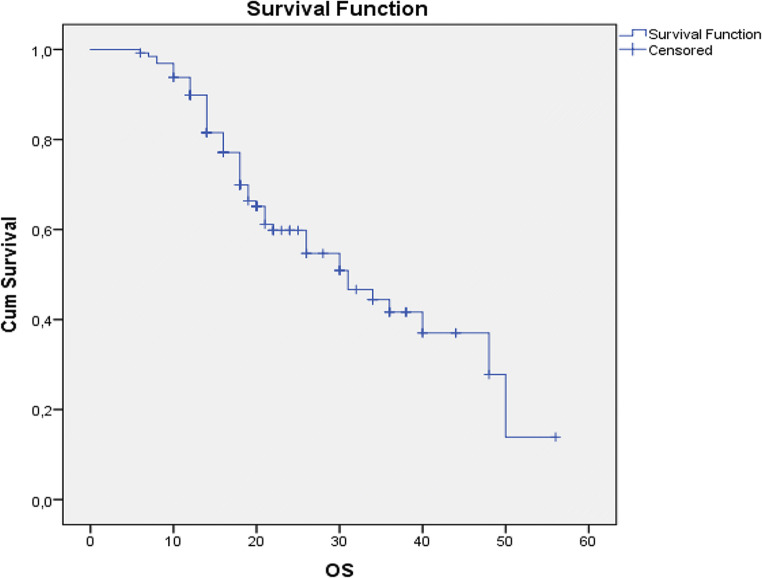
At the time of manuscript writing, 75 patients were alive (57.7%), while 45 have passed away (42.3%). Mean progression-free survival was 12.7 months with a range between 0 and 50 months (Fig. 1), whereas mean overall survival was calculated to be 21.24 months (range 6–56 months) (Fig. 2). Univariate regression analysis (age, timing of HIPEC, sb-PCI score, and completeness of cytoreduction) showed that the small bowel PCI score and the completeness of cytoreduction were parameters that correlated significantly with overall survival (p < 0.001).
Fig. 1.
Kaplan-Meier curve for progression-free survival
Fig. 2.
Kaplan-Meier curve for overall survival
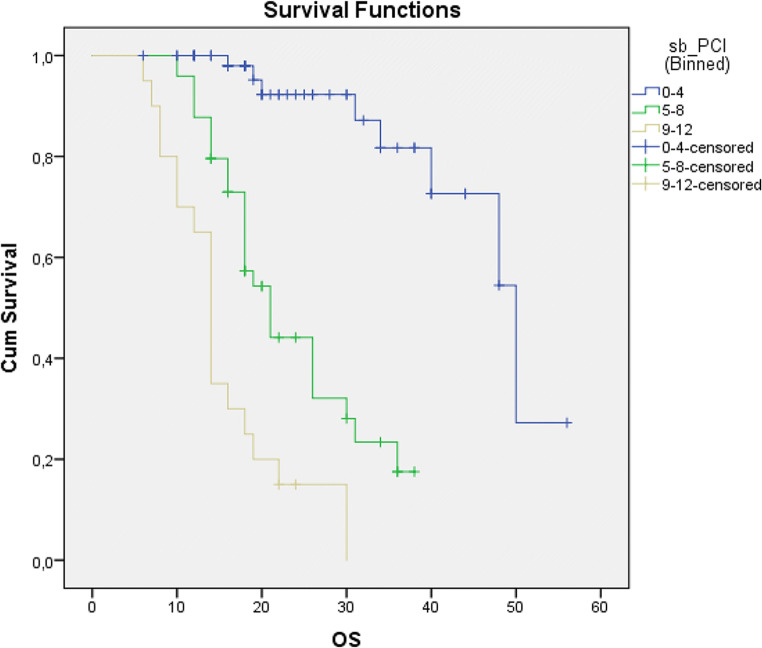
The aim of this study was to evaluate the effect of sb-PCI score in PFS and OS in ovarian cancer patients. So we divided the patients regarding the sb-PCI score in 3 groups: 0–4, 4–8, and 9–12 (sb-PCI score). Kaplan-Meier curve analysis showed a statistically significant difference in overall survival between these three groups (Table 3) (Fig. 3).
Table 3.
Overall survival analysis according to sb-PCI score category
| Sb-PCI score category | Number of patients (%) | OS (mean) | OS (median) | p |
|---|---|---|---|---|
| 0–4 | 60 (46.15%) | 46.09 months (95 % CI 41.2 − 50.98) | 50 months (95% CI 40.84–59.16) | |
| 5–8 | 50 (38.46%) | 23.67 months (95 % CI 20.69 − 26.66) | 21 months (95% CI 17.33–24.67) | p < 0.001 |
| 9–12 | 20 (15.38%) | 15.5 months (95 % CI 12.29 − 18.77) | 14 months (95% CI 12.6–15.39) |
Fig. 3.
Kaplan-Meier curves according to sb-PCI score category
Multivariate regression model analysis showed that both sb-PCI score and completeness of cytoreduction (CC) were independent prognostic factors of survival, while age and time of HIPEC use (recurrent or intermediate) did not manage to identify as independent prognostic factors (Table 4).
Table 4.
Multivariate analysis of overall survival
| p | |
|---|---|
| Age | p = 0.904 |
| Time of HIPEC | p = 0.84 |
| Sb-PCI score | p < 0.001 |
| Completeness of cytoreduction | p < 0.001 |
Discussion
Ovarian cancer constitutes the 3rd most common gynecologic cancer worldwide, being though the one with the highest mortality rate and worst prognosis, those being attributed to the lack of symptoms and screening methods resulting in late diagnosis of the disease [14]. It has been estimated that in 2012, 65,900 and 86,000 women in economically developed and developing countries respectively died due to the disease, whereas the median overall survival barely reaches 30% [15].
Primary surgical cytoreduction with maximal debulking surgery followed by platinum-based chemotherapy is considered the standard therapy and guarantees an accurate staging, aiming at improved survival of the patients [16]. Already back in 1934, Meigs was the first to propose cytoreductive surgery as a condition to enhance the results of postoperative therapy for advanced ovarian carcinoma [17]. More specifically, Griffith in 1975 described the correlation of the patient’s survival with the residual tumor diameter [18], a demonstration followed later by various large studies with climax the 2004 Consensus Statements on the Management of Ovarian Cancer of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) suggesting “up-front maximal surgical effort at cytoreduction with the goal of no residual disease” [19] Carter et al. also in 2004 described an increase in the median survival as high as double, following successful cytoreduction surgeries which in a meta-analyses by Bristow et al. is calculated as a percent of 5.5–6% with each 10% increase in cytoreduction [20].
While the importance of a maximal debulking surgery is recognized by the majority of gynecologic oncologists, the definition of optimal cytoreduction is less clear. The term has been widely varying between a maximal residual tumor diameter between 0 and 3 cm with the gynecologic oncology group (GOG) specifically describing residual tumor nodules with a maximum diameter of 1 cm as “optimal” cytoreduction [21]. On the other hand, Vergote et al. estimate the residual disease by the number of grams and correlates this score to differences in survival among the patients [22]. Another significant point advantage in the survival is demonstrated when the cytoreduction is performed by trained gynecologic oncologists in comparison to other physicians [23].
Whichever the definition, standard parameters have been suggested as negative predictors of incomplete tumor resection referring to preoperative imaging techniques, scores based on laparoscopy, and measurement of specific serum biomarkers [24]. Some of the parameters have been suggested to be more predictive than others though, and interestingly mesenteric disease/involvement is one of them. Ramachandran et al. in their study that correlate the pattern of spread of advanced epithelial ovarian cancer and the surgical outcome describe the small bowel serosa and mesentery disease as less amenable to optimal cytoreduction [25]. Additionally, Braicu et al. in a study that compared primary versus secondary cytoreduction for epithelial ovarian cancer found that secondary cytoreduction is associated with worse surgical outcomes regarding the tumor residuals, mainly because of the patterns of the recurrence of the disease at that case, which tend to include the upper abdomen, mesentery, and gastrointestinal serosa, practically confirming that those patterns have lower optimal debulking rates [26]. Similarly, in a large study by Fotopoulou et al., diseases at the same regions were found to have less favorable surgical outcomes and the highest risk of incomplete debulking [27].
In this present retrospective study, we report the importance of small bowel involvement in overall survival of advanced ovarian cancer patients. More specifically, our analysis showed that both completeness of cytoreduction and small bowel peritoneal cancer index score (sb-PCI) are independent prognostic factors and affect the overall survival of these patients. Moreover, when we divided our patients regarding their sb-PCI score in three groups (0–4, 5–8, 9–12), a statistically significant difference in their overall survivals was noticed.
Small bowel disease itself has been found to be negative predictive for complete cytoreduction in advanced ovarian cancer. Suidan et al. in a multicenter prospective trial using preoperative serum Ca-125 level and computed tomography to predict incomplete cytoreduction at primary debulking surgery attribute to diffuse small bowel disease in the form of adhesions or thickening with an OR of 1.87 for suboptimal surgical outcome [28]. More particularly, Pomel et al. classify the involvement of the small bowel as one of the three independent factors among infiltration of the porta hepatis and the right hemi diaphragm that oppose to complete cytoreduction [29]. Additionally, in a systematic analysis of 240 cases by Sehouli et al., residual tumors after primary and secondary surgery were compared, and it was demonstrated that recurrent cases were usually involving the serosa of the small bowel as well as the mesentery and the gastric serosa [30]. Deffieux et al. investigating the role of laparoscopy in the evaluation of complete cytoreduction candidates with an unsatisfactory preoperative evaluation described that the disease at the walls of the small intestine made the complete resection of the tumor residuals unlikely [31].
Besides ovarian cancer, small bowel involvement has been described as an adverse prognostic factor against optimal debulking in multiple other malignancies. Chandramohan et al. have found that the disease either in the small bowel serosa or at large volume in the upper abdomen is unlikely accompanied by optimal debulking in patients with malignant peritoneal mesothelioma who were treated with cytoreductive surgery plus HIPEC [32]. Regarding pseudomyxoma peritonei as well, Mittal et al. consider small bowel serosa and mesentery involvement to raise the difficulty of surgical excision which is also suggested in the CT and MRI investigations [33].
Moreover, concerning colorectal cancer, in a consensus statement by Esquivel et al. about the cytoreductive surgery in cases of colonic cancer with peritoneal involvement, the likelihood of total removal of the tumor is considered increased when no gross disease in the mesentery of the small bowel is observed [34]. In a retrospective analysis of 139 such patients, Elias et al. classified small bowel disease as an independent negative prognostic factor, since the complications of multiple bowel anastomoses preclude complete peritoneal metastasis resection. They specifically described the extent of the involvement with the small bowel peritoneal cancer index (SB-PCI), a score that ranges from 0 to 12 and measures all its four segments (upper jejunum, lower jejunum, upper ileum and lower ileum) [35]. These findings agree with those of a perspective study by Rosendahl et al., indicating that the PCI scores of specific regions and more specifically hepatoduodenal ligament and the small intestine are more predictive of a R0 debulking surgery than the overall PCI score, since penetrating carcinosis is a severely limiting factor [36]. Interestingly, Benizri et al. in another prospective study of 49 patients with peritoneal carcinomatosis from colorectal cancer regard the small intestine involvement as an independent prognostic factor of the survival, even when complete cytoreduction is achieved [37]. Similarly, regarding gastric cancer and peritoneal metastases, a retrospective study by Königsrainer et al. describes that irresectability was evidenced in all cases of extent disease on the surface of the small bowel [38], while Glehen et al. in their multiinstitutional study of patients with peritoneal carcinomatosis of gastric origin encompass the small bowel involvement into the exclusion criteria during the selection of candidates for laparotomy [39].
The present study was designed in order to evaluate the small bowel PCI score as a prognostic factor of survival in advanced ovarian cancer patients undergoing intermediate cytoreductive surgery plus HIPEC or debulking surgery in combination with HIPEC for recurrence of disease. Our study avails of a homogenous patient sample, a strictly follow-up schedule, and a high standardization of surgical technique. Still, there are some limitations. Our study is retrospective with all the disadvantages this kind of study can have. A prospective trial should be proceeding in order to confirm our results. Also, the included number of patients in our study is relatively small, and our results ought to be confirmed in larger future studies.
In conclusion, our results showed a significant correlation between small bowel PCI score and overall survival of patients with advanced ovarian cancer.
Authors’ Contributions
Iavazzo C: Protocol/project development, data management, consultation to the manuscript
Fotiou A: Data collection, data management and analysis, manuscript writing
Psomiadou V: Data collection and management, manuscript writing
Lekka S: Data collection and management, manuscript writing
Katsanos D: Data management and analysis
Spiliotis J: Protocol/project development, consultation to the manuscript
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed SA, Abou-Taleb H, Yehia A, el Malek NAA, Siefeldein GS, Badary DM, Jabir MA. The accuracy of multi-detector computed tomography and laparoscopy in the prediction of peritoneal carcinomatosis index score in primary ovarian cancer. Acad Radiol. 2019;26(12):1650–1658. doi: 10.1016/j.acra.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Whitwell HJ, Worthington J, Blyuss O, Gentry-Maharaj A, Ryan A, Gunu R, Kalsi J, Menon U, Jacobs I, Zaikin A, Timms JF. Improved early detection of ovarian cancer using longitudinal multimarker models. Br J Cancer. 2020;122(6):847–856. doi: 10.1038/s41416-019-0718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RHM, van der Burg MEL, Lacave AJ, Panici PB, Kenter GG, Casado A, Mendiola C, Coens C, Verleye L, Stuart GCE, Pecorelli S, Reed NS. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 5.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal chemotherapy in ovarian Cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 6.Fotopoulou C, Sehouli J, Mahner S, Harter P, van Nieuwenhuysen E, Gonzalez-Martin A, Vergote I, Chiva L, du Bois A. HIPEC: HOPE or HYPE in the fight against advanced ovarian cancer? Ann Oncol. 2018;29(8):1610–1613. doi: 10.1093/annonc/mdy198. [DOI] [PubMed] [Google Scholar]
- 7.Lim MC, et al. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J Clin Oncol. 2017;35(15_suppl):5520. doi: 10.1200/JCO.2017.35.15_suppl.5520. [DOI] [Google Scholar]
- 8.Muallem MZ, Sehouli J, Richter R, Babayeva A, Gasimli K, Parashkevova A. Pre-operative serum CA125, peritoneal cancer index and intra-operative mapping score as predictors of surgical results in primary epithelial ovarian cancer. Int J Gynecol Cancer. 2020;30(1):62–66. doi: 10.1136/ijgc-2019-000778. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH (2015) Management of peritoneal metastases - basic concepts. J Buon 20(Suppl 1):S2–S11 [PubMed]
- 10.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I, Vergote I, Baert T, Belaroussi I, Dashora A, Olbrecht S, Planchamp F, Querleu D, Baert T, Banerjee S, Belaroussi I, Blecharz P, Bruchim I, Cibula D, Colombo N, Concin N, Davidson B, Dashora A, Devouassoux-Shisheboran M, du Bois A, Ferrero A, Glasspool R, González-Martin A, Heinzelmann-Schwarz V, Joly F, Kim JW, Kridelka F, Ledermann J, Lorusso D, Mahner S, McCluggage WG, McNeish I, Mikami M, Mirza MR, Morice P, Nicum S, Olbrecht S, O’Donnell DM, Pautier P, Planchamp F, Pignata S, Querleu D, Ray-Coquard I, Rodolakis A, Sehouli J, Selcukbiricik F, Sessa C, Singh N, Tan DSP, Timmerman D, Tognon G, van der Velden J, Vergote I, Witteveen PO, Zeimet AG (2019) ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease dagger. Ann Oncol 30(5):672–705 [DOI] [PubMed]
- 11.Mehta SS, Bhatt A, Glehen O. Cytoreductive surgery and peritonectomy procedures. Indian J Surg Oncol. 2016;7(2):139–151. doi: 10.1007/s13193-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S (2015) Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22(5):1570–1575 [DOI] [PubMed]
- 13.Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, Lotti M, Magnone S, Napoli J, Rossetti D, de Iaco P, Frigerio L, Pinna A, Runnebaum I, Ansaloni L. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61. doi: 10.3802/jgo.2015.26.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Women's Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WL, Lu Z, Bast RC., Jr The role of biomarkers in the management of epithelial ovarian cancer. Expert Rev Mol Diagn. 2017;17(6):577–591. doi: 10.1080/14737159.2017.1326820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junor EJ, Hole DJ, McNulty L, Mason M, Young J. Specialist gynaecologists and survival outcome in ovarian cancer: a Scottish national study of 1866 patients. Br J Obstet Gynaecol. 1999;106(11):1130–1136. doi: 10.1111/j.1471-0528.1999.tb08137.x. [DOI] [PubMed] [Google Scholar]
- 17.JV M. Tumors of the female pelvic organs. New York: Macmillan; 1934. [Google Scholar]
- 18.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 19.du Bois A, et al. Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2004;16(Suppl 8):viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 20.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 21.Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25(20):2873–2883. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- 22.Vergote I, de Wever I, Tjalma W, van Gramberen M, Decloedt J, van Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998;71(3):431–436. doi: 10.1006/gyno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen T, Kjærheim K, KÆRN J, Tretli S, Tropé C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16(Suppl 1):11–17. doi: 10.1136/ijgc-00009577-200602001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Martin-Camean M, et al. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience. 2016;10:666. doi: 10.3332/ecancer.2016.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran A, Rajanbabu A, Bagul KG, Pavithran K, Vijaykumar DK. Correlation of pattern of spread and outcomes in advanced epithelial ovarian cancers. Indian J Surg Oncol. 2018;9(2):126–132. doi: 10.1007/s13193-017-0618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braicu EI, Sehouli J, Richter R, Pietzner K, Lichtenegger W, Fotopoulou C. Primary versus secondary cytoreduction for epithelial ovarian cancer: a paired analysis of tumour pattern and surgical outcome. Eur J Cancer. 2012;48(5):687–694. doi: 10.1016/j.ejca.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Fotopoulou C, Richter R, Braicu EI, Schmidt SC, Lichtenegger W, Sehouli J. Can complete tumor resection be predicted in advanced primary epithelial ovarian cancer? A systematic evaluation of 360 consecutive patients. Eur J Surg Oncol. 2010;36(12):1202–1210. doi: 10.1016/j.ejso.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, Zhou Q, Iasonos A, Paul H, Hosaka M, Aghajanian CA, Leitao MM, Jr, Gardner GJ, Abu-Rustum NR, Sonoda Y, Levine DA, Hricak H, Chi DS. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134(3):455–461. doi: 10.1016/j.ygyno.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomel C, Jeyarajah A, Oram D, Shepherd J, Milliken D, Dauplat J, Reynolds K. Cytoreductive surgery in ovarian cancer. Cancer Imaging. 2007;7:210–215. doi: 10.1102/1470-7330.2007.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sehouli J, Richter R, Braicu EI, Bühling KJ, Bahra M, Neuhaus P, Lichtenegger W, Fotopoulou C. Role of secondary cytoreductive surgery in ovarian cancer relapse: who will benefit? A systematic analysis of 240 consecutive patients. J Surg Oncol. 2010;102(6):656–662. doi: 10.1002/jso.21652. [DOI] [PubMed] [Google Scholar]
- 31.Deffieux X, Castaigne D, Pomel C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. Int J Gynecol Cancer. 2006;16(Suppl 1):35–40. doi: 10.1136/ijgc-00009577-200602001-00006. [DOI] [PubMed] [Google Scholar]
- 32.Chandramohan A, Thrower A, Shah N, Mohamed F. Radiological predictors of complete cytoreduction in 59 patients with peritoneal mesothelioma treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a UK referral centre. Br J Radiol. 2017;90(1079):20170361. doi: 10.1259/bjr.20170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperth. 2017;33(5):511–519. doi: 10.1080/02656736.2017.1310938. [DOI] [PubMed] [Google Scholar]
- 34.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P, Bieligk S, Bretcha-Boix P, Chang CK, Chu F, Chu Q, Daniel S, de Bree E, Deraco M, Dominguez-Parra L, Elias D, Flynn R, Foster J, Garofalo A, Gilly FN, Glehen O, Gomez-Portilla A, Gonzalez-Bayon L, Gonzalez-Moreno S, Goodman M, Gushchin V, Hanna N, Hartmann J, Harrison L, Hoefer R, Kane J, Kecmanovic D, Kelley S, Kuhn J, Lamont J, Lange J, Li B, Loggie B, Mahteme H, Mann G, Martin R, Misih RA, Moran B, Morris D, Onate-Ocana L, Petrelli N, Philippe G, Pingpank J, Pitroff A, Piso P, Quinones M, Riley L, Rutstein L, Saha S, Alrawi S, Sardi A, Schneebaum S, Shen P, Shibata D, Spellman J, Stojadinovic A, Stewart J, Torres-Melero J, Tuttle T, Verwaal V, Villar J, Wilkinson N, Younan R, Zeh H, Zoetmulder F, Sebbag G, Society of Surgical Oncology Annual Meeting Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 35.Elias D, Mariani A, Cloutier AS, Blot F, Goéré D, Dumont F, Honoré C, Billard V, Dartigues P, Ducreux M. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol. 2014;40(11):1467–1473. doi: 10.1016/j.ejso.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Rosendahl M, Harter P, Bjørn SF, Høgdall C. Specific regions, rather than the entire peritoneal carcinosis index, are predictive of complete resection and survival in advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2018;28(2):316–322. doi: 10.1097/IGC.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 37.Benizri EI, Bernard JL, Rahili A, Benchimol D, Bereder JM. Small bowel involvement is a prognostic factor in colorectal carcinomatosis treated with complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2012;10:56. doi: 10.1186/1477-7819-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konigsrainer I, et al. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer. 2014;14(2):117–122. doi: 10.5230/jgc.2014.14.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glehen O, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]