Abstract
Background:
Conventional hemodynamic parameters may not accurately predict symptomatic improvement after percutaneous mitral valvuloplasty (PMV). Changes in left heart chamber compliance following adequate relief o0066 mitral stenosis (MS) may be useful in determining functional capacity after PMV. This study aims to determine the acute effects of PMV on compliance of the left heart and whether its changes relate to the patient’s functional capacity.
Methods:
One-hundred thirty-seven patients with severe MS undergoing PMV were enrolled. Left atrial (Ca) and left ventricular (Cv) compliance were invasively estimated and net atrioventricular compliance (Cav) was calculated before and immediately after the procedure. B-type natriuretic peptide (BNP) levels were obtained before and 24 hr after the procedure. The primary endpoint was functional status at 6-month follow-up, and the secondary endpoint was a composite of death, mitral valve (MV) replacement, repeat PMV, new onset of atrial fibrillation, or stroke in patients in whom PMV was successful.
Results:
The mean age was 43 ± 12 years, and 119 patients were female (87%). After PMV, Ca and Cav improved significantly from 5.3 [IQR 3.2–8.2] mL/mmHg to 8.7 [5.3–19.2] mL/mmHg (P < 0.001) and 2.2 [1.6–3.4] to 2.8 [2.1–4.1] mL/mmHg (P < 0.001), respectively, whereas Cv did not change (4.6 [3.2–6.8] to 4.4 [3.1–5.6]; P = 0.637). Plasma BNP levels significantly decreased after PMV, with no correlation between its variation and changes in left chamber compliance. At 6-month follow-up, NYHA functional class remained unchanged in 32 patients (23%). By multivariable analyses, changes in Ca immediately after PMV (adjusted OR 1.42; 95% CI 95% 1.02 to 1.97; P = 0.037) and younger age (adjusted OR 0.95; CI 95% 0.92–0.98; P = 0.004), predicted improvement in functional capacity at 6-month follow-up, independent of postprocedural data. The secondary endpoint were predicted by post-PMV mean gradient (adjusted HR 1.363; 95% CI 95% 1.027–1.809; P = 0.032), and lack of functional improvement at 6-month follow-up (adjusted HR 4.959; 95% 1.708–14.403; P = 0.003).
Conclusions:
Ca and Cav increase significantly after PMV with no change in Cv. The improvement of Ca is an important predictor of functional status at 6-month follow up, independently of other hemodynamic data. Postprocedural mean gradient and lack of short-term symptomatic improvement were predictors of adverse outcome.
Keywords: atrial compliance, functional capacity, mitral stenosis, net atrioventricular compliance, percutaneous mitral valvuloplasy, ventricular compliance
1 ∣. INTRODUCTION
Rheumatic heart disease (RHD) is an important cause of cardiovascular death and disability, particularly in low- and middle-income countries.1,2 Mitral stenosis (MS) is a frequent manifestation of RHD, which affects about a quarter of the patients.3,4 Several factors have been associated with clinical presentation and outcome in MS.3 The most important are transmitral pressure gradients, pulmonary artery pressure, and mitral valve (MV) area, which are used as indication criteria for valve intervention.5 Percutaneous mitral valvuloplasty (PMV) has become the preferred treatment in selected MS patients with appropriate MV morphology.6-8 The procedure results in a significant increase in MV area (MVA) and reduction of transmitral gradient, with improvement in symptoms.5 However, previous studies showed lack of correlation between the MVA or pressure gradient after PMV and exercise tolerance improvement9,10 This indicates that other hemodynamic parameters may play a potential role in determining functional capacity after the procedure.
Left atrial (LA) compliance (Ca) modulates the overall hemodynamic burden of MS, contributing to deterioration of functional capacity, pulmonary hypertension, and death.11-17 In this context, changes in Ca after PMV may have the potential role in predicting clinical outcomes. Thomas et al.18 originally evaluated the effect of PMV on net LA and ventricular compliance in 18 patients demonstrating that Ca increased, whereas ventricular compliance (Cv) decreased with increased of LV filling. As the rise of Ca compensated the fall in Cv, mean net chamber compliance increased. This pioneering study was performed before cumulative experience with PMV, which highlights the need of further studies, especially in the current era where alleviation of the valve obstruction can be reliably achieved. Additionally, the impact of acute changes in net chamber compliance on clinical outcome needs to be established.
Therefore, in this study, we sought to (1) determine the acute effects of PMV on the left heart chamber compliance; (2) assess the relationship between compliance changes and decline of BNP levels following the procedure; (3) examine the impact of compliance changes on short-term symptomatic status improvement; and (4) investigate whether NYHA functional class at 6-month follow up predicts long-term outcome.
2 ∣. METHODS
2.1 ∣. Study population
One hundred thirty-seven consecutive patients who underwent PMV between January 2012 and January 2015 for severe symptomatic rheumatic MS at Hospital das Clínicas of the Federal University of Minas Gerais (HC-UFMG) were enrolled. Patients were referred for PMV based on MS severity and symptoms. We included patients with mitral area ≤1.5 cm2 and NYHA functional class II or worse with favorable anatomy for the procedure.7,19 Asymptomatic patients with severe MS and pulmonary hypertension at rest or during exercise, who were suitable for the procedure, were also included.
Exclusion criteria included presence of other hemodynamically significant valve disease or systemic disease that could independently affect the compliance of the heart chambers.
This study was approved by the institutional ethics committee and written informed consent was obtained from all patients.
2.2 ∣. Echocardiographic evaluation
A standard transthoracic 2D echocardiogram was performed prior to and within 24–48 hr after PMV using commercially available equipment (iE33, Philips Medical Systems, Andover, MA) according to recommendations of the American Society of Echocardiography.20 A single investigator, blinded to clinical data, performed all measurements.
MV morphology was evaluated using the scoring system outlined by Wilkins and coworkers. Valve leaflet thickening, mobility, calcification, and subvalvular thickening were graded on a scale of 1–4 based on the severity of the lesion.19 MVA was measured using direct planimetry.21 Peak and mean transmitral diastolic pressure gradients were measured from Doppler profiles recorded in the apical four-chamber view. The presence and severity of mitral regurgitation was evaluated according to current guideline.22 Tricuspid regurgitant velocity was recorded with continuous-wave Doppler imaging and used to determine the systolic pulmonary artery pressure using the modified Bernoulli equation. LA volume was assessed by the biplane area-length method from apical 2 and 4-chamber views. All results were based on the average of three measurements for patients in sinus rhythm and five measurements for patients in atrial fibrillation. Transesophageal echocardiography was performed in all patients with atrial fibrillation or with previous embolic events to exclude LA and LA appendage thrombus.
2.3 ∣. Percutaneous mitral valvuloplasty
PMVs were conducted in the Interventional Cardiology Department of the HC-UFMG according to the technique described by Inoue et al.23 Sedation and oxygen supplementation were used only when strictly necessary. Standard hemodynamic measurements of the aorta, the left ventricular, LA, right ventricular, right atrial, and pulmonary artery pressures were recorded before and immediately after the procedure. Arterial and venous blood samples at baseline were collected. All procedures were performed at least 15 min after the vascular accesses were obtained and anesthetic procedures were concluded, to minimize variability. Cardiac output was determined by the Fick method.
After each balloon dilatation, transthoracic echocardiographic imaging was performed in order to evaluate the MVA and the degree of mitral regurgitation. If MVA and the degree of MR did not increase, dilatation was repeated with an additional 1 mL of volume in the balloon. This procedure was repeated until a significant increase in the MVA or in the degree of regurgitation was observed. Procedural success was defined by an increase in MVA to ≥1.5 cm2 without an increase in the degree of regurgitation to ≥3+. PMV was aborted and deemed unsuccessful if the degree of mitral regurgitation worsened to ≥3+, regardless of MVA.8
Continuous recordings of the pressure in aorta, pulmonary artery, right ventricle, right atrium and simultaneous records in the left atrium (LA) and left ventricle (LV) were obtained throughout the procedure, as well as subsequent blood gas sampling. Based on these data we made the following calculations:
CO = 135 × BSA/13.6 × Hb (Sat. O2 aorta – Sat. O2 pulmonary trunk) (L/min)
SV = CO × 1000/heart rate (mL)
Ca = SV/LA pressure rise during ventricular systole (peak of the V wave – nadir of the X descent) (mL/mmHg).
Cv = SV/left ventricular pressure rise during ventricular diastole (end diastolic LV pressure – initial diastolic LV pressure) (mL/mmHg)
Cav = (1/Ca + 1/Cv)−1 (mL/mmHg)*
*CO, cardiac output; BSA, body surface area; Hb, hemoglobin; Sat. O2, oxygen saturation; PT, pulmonary trunk; SV, systolic volume; HR, heart rate; Ca, LA compliance; Cv, left ventricular compliance; Cav, net atrioventricular compliance.
The LA and left ventricular pressure variations were measured in continuous and simultaneous pressure recordings of these chambers—as an average of three sequential beats if patient was in sinus rhythm and five beats if patient was in atrial fibrillation—obtained in a scale of 0.5 mmHg/mm (Figure 1).19
FIGURE 1.
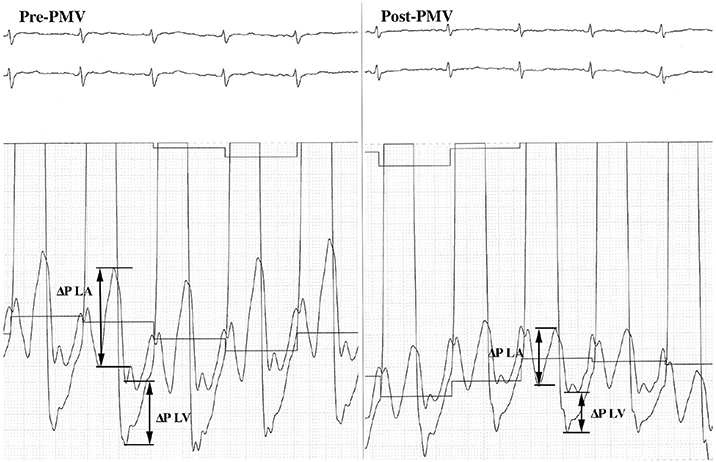
Simultaneous LA and LV pressure recordings of a patient preprocedure and postprocedure procedure used for measurement of left chamber compliance. The changes in LA and LV pressures were used to calculate mean LA and LV compliance before and after valvuloplasty
2.4 ∣. Measurement of B-type natriuretic peptide (BNP)
Direct samples of blood from the femoral vein were obtained in all patients during the procedure and repeated 24 hr after by peripheral venous puncture. The samples for BNP analysis were placed in chilled tubes containing protease inhibitors and BNP levels were measured using standard radioimmunoassay.
2.5 ∣. Endpoint definitions
The primary endpoint of this study was functional status at 6-month follow-up, assessed by NYHA functional class. The attending cardiologists who were independent of the study and unaware of the results of the left heart chambers compliance measurements assessed the patients’ functional status at follow-up. The secondary endpoint after PMV was a composite endpoint, defined as death, MV replacement, repeat PMV, new onset of atrial fibrillation, or stroke. Outcome data were obtained from clinic follow-up appointments.
2.6 ∣. Statistical analysis
All data was prospectively collected with standardized protocols in the outpatient clinics and interventional cardiology facility. All researchers were trained in the study protocol, and the clinical team was blinded to left heart chamber compliance measurements. Hemodynamic parameters were double-checked by two investigators.
All analyses were performed on the software SPSS 20.0 IBM Statistics for Mac OSX (SPSS Inc., Chicago, IL). Categorical variables, expressed as numbers and percentages, were compared using chi-square test. Shapiro–Wilk test was performed to evaluate the distribution of the continuous variables, expressed as mean ± standard deviation or median and interquartile range (Q1–Q3) as appropriate. To evaluate the effects of PMV on LA compliance we compared the values obtained before and after the procedure using the paired Student’s t-test (normal distribution) or the Wilcoxon paired test (non-normal distribution), as appropriate. Changes were calculated using this formula: Post-Pre/Pre. Ordinal logistic regression analysis was used to identify the factors associated with NYHA functional class at follow-up. Cox proportional hazards regression analyses were performed to identify independent predictors of long-term composite endpoint.
Reproducibility of Ca and Cv were assessed by the intra-class correlation coefficients and Bland–Altman method in a random sample of 10 patients.
3 ∣. RESULTS
3.1 ∣. Baseline characteristics of the study population
A total of 137 patients were included, with mean age of 43 ± 12 years and 119 (87%) were female. The majority of the patients were in NYHA class II and III (79%) while 13 patients (9%) were in class IV at the time of the procedure. Baseline clinical characteristics of the study population are presented in Table 1. Atrial fibrillation was present in 32 patients (23%) with MVA of 0.94 ± 0.3 (range, 0.5–1.5 cm2) and systolic pulmonary artery pressure of 53.2 ± 22.8 mmHg.
TABLE 1.
Baseline characteristics of the study population
| Variables | Value (n = 137) | |
|---|---|---|
| Clinical data | ||
| Age (years) | 43 ± 12 | |
| Body surface area (m2) | 1.64 ± 0.2 | |
| Female gender (n/%) | 119 (87) | |
| NYHA functional class | I | 16 (12) |
| II | 56 (41) | |
| III or IV | 65 (47) | |
| Atrial fibrillation (n/%) | 32 (23) | |
| Heart rate (bpm) | 73.9 ± 15.0 | |
| Systolic blood pressures (mmHg) | 112.3 ± 17.6 | |
| Diastolic blood pressures (mmHg) | 72.2 ± 10.0 | |
| Medication | ||
| Diuretics | 96 (70) | |
| Beta-blockers | 101 (74) | |
| Anticoagulants | 38 (28) | |
| Aspirin | 19 (14) |
Abbreviations: LV, left ventricular; NYHA, New York Heart Association. Data are expressed as the mean value ± SD or number (percentage) of patients.
PMV was successful in 112 patients (82%), whereas 7 patients developed severe mitral regurgitation (5%) and were excluded from the analysis of changes in compliance. Insufficient valve opening or increase in mitral regurgitation grade was observed in the remaining patients (18 patients). The echocardiographic and hemodynamic measurements pre and post PMV are summarized in Table 2. There was a significant increase in the cardiac output as well as a significant decrease in the mean pulmonary artery pressure (mPAP) and LA mean pressure after PMV.
TABLE 2.
Echocardiographic and hemodynamic data of the overall population pre- and post-PMV
| Variablesa | Pre | Post | P value |
|---|---|---|---|
| Echocardiographic data | |||
| LA diameter (mm) | 50.1 ± 6.2 | 48.1 ± 6.7 | <0.001 |
| Mitral peak gradient (mmHg) | 19.9 ± 7.3 | 12.3 ± 4.8 | <0.001 |
| Mitral mean gradient (mmHg) | 11.7 ± 5.1 | 5.7 ± 2.7 | <0.001 |
| Mitral valve area (cm2) | 0.94 ± 0.26 | 1.65 ± 0.23 | <0.001 |
| Hemodynamic data | |||
| LA pressure (mmHg) | 26.2 ± 8.2 | 17.1 ± 5.6 | <0.001 |
| SPAP (mmHg) | 53.2 ± 22.8 | 44.2 ± 14.6 | <0.001 |
| MPAP (mmHg) | 34.1 ± 13.7 | 28.3 ± 9.8 | <0.001 |
| LV end-diastolic pressure (mmHg) | 11.5 ± 3.9 | 13.4 ± 4.2 | <0.001 |
| Cardiac output (L/min) | 3.9 ± 1.2 | 4.1 ± 1.3 | 0.002 |
| Cardiac index (L/min/m2) | 2.4 ± 0.7 | 2.6 ± 0.8 | 0.003 |
| Compliancesb | |||
| Ca (mL/mmHg) | 5.3 [3.2–8.2] | 8.7 [5.3–19.2] | <0.001 |
| Cv (mL/mmHg) | 4.6 [3.2–6.8] | 4.4 [3.1–5.6] | 0.637 |
| Cav (mL/mmHg) | 2.2 [1.6–3.4] | 2.8 [2.1–4.1] | <0.001 |
| Natriuretic peptide | |||
| BNP (pg/mL) | 185 [121–327] | 107 [65–218] | <0.001 |
Data are expressed as the mean value ± SD when the data has a normal distribution or as the median [interquartile range] when the data has a non-normal distribution.
Including the patients who developed severe mitral regurgitation post PMV.
Abbreviations: Ca, LA compliance; Cav, net left atrioventricular compliance; Cv, left ventricular compliance; LV, left ventricle; MPAP, mean pulmonary artery pressure; SPAP, systolic pulmonary artery pressure.
PMV had marked effects on Ca, which improved from 5.3 [IQR 3.2–8.2] mL/mmHg preprocedure to 8.7 [5.3–19.2] mL/mmHg immediately post-PMV. Cav also increased significantly (2.2 [1.6–3.4] to 2.8 [2.1–4.1], P < 0.001), whereas Cv did not change (4.6 [3.2–6.8] to 4.4 [3.1–5.6]; P = 0.637). Table 2 summarizes the results of PMV on left chamber compliance. We found no difference in Ca values between patients with atrial fibrillation and sinus rhythm.
The effects of PMV on left chamber compliance varied according to the results of the procedure. In patients in whom the procedure was successful, there was a significant improvement of Ca and Cav. However, even in those who had suboptimal results with moderate mitral regurgitation or valve area <1.5 cm2, the Ca increased from 3.5 [2.7–7.9] to 5.5 [4.3–8.7]; P = 0.003. Cv and Cav did not change in this subgroup of patients. However, the patients who developed severe mitral regurgitation, both Ca and Cv decreased (from 8.8 to 8.2 and 3.8 to 3.4 mL/mmHg, respectively), but not statistically significant likely due to the small number of patients (n = 7).
To test the impact of the iatrogenic left-to-right shunt on the LA pressure and consequently on Ca after the procedure, the magnitude of the left-to-right atrial shunt was calculated in a subset of patients by oximetry and expressed as the ratio of pulmonary to systemic blood flow (Qp/Qs). This ratio was 1.01 [0.99/1.02] pre and 1.00 [0.98/1.03] after PMV, indicating the absence of significant shunt that would influence LA pressure reduction after the procedure.
Plasma BNP levels significantly decreased after PMV (185 [121–327] pg/mL pre vs. 107 [65–218] pg/mL post; P < 0.001). There were no correlations between preprocedural BNP and left chamber compliance, whereas post procedural BNP was negatively correlated with post Cv (r = −0.41; P < 0.001) and Cav (r = −0.33; P < 0.001). BNP concentrations were not correlated with Ca either before or after the procedure. We also tested the correlation between the delta of BNP levels and the delta of Ca, Cv, and Cav measurements, but found no significant correlations among these variables.
3.2 ∣. Clinical improvement following PMV
In symptomatic patients (classes II–IV) who did not develop postprocedural severe mitral regurgitation, NYHA functional class improved in 86 patients (66%), whereas it remained unchanged in 32 patients (23%). Twenty-one patients (16%) who were in class II before the procedure remained in class II afterwards, whereas 11 patients (8%) with class III symptoms remained in class III after. All patients in class IV experienced improvement in functional class, and 12 patients (9%) in class I preprocedure remained in class I at 6-month follow-up. The changes of Ca immediately after PMV were associated with NYHA functional class at 6-month follow up (Figure 2). Patients in functional class I at follow up had an increased in Ca with a median of 91% increase (ranging from −45% to 372%) compared with pre procedure, whereas those patients in class III had lower increase in Ca (median of increase of 43%, ranging from −43% to 268%). Cav and Cv were not associated with functional status at follow up.
FIGURE 2.
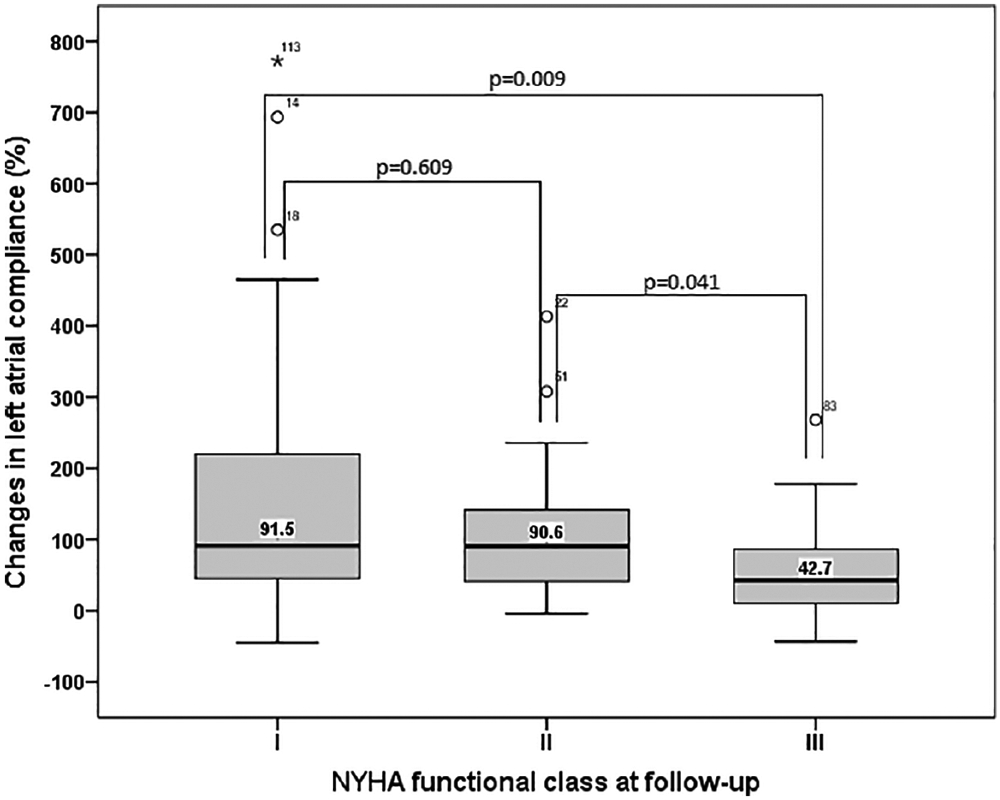
Changes in LA compliance immediately after PMV and NYHA functional class at 6 month follow-up. Erros bars represent error standard error
We further assessed the value of hemodynamic parameters changes in predicting functional status at follow up. The variables that reflect the results of the procedure were analyzed, including changes in valve area, pressure gradients, pulmonary artery pressure, and BNP concentrations (Table 3). By the multivariable ordinal logistic regression analysis, excluding those patients who developed severe mitral regurgitation after the procedure (n = 130), independent predictors of functional class at 6-month follow-up were changes in Ca immediately after PMV (adjusted odds ratio [OR] 1.42; 95% confidence interval [CI 95%] 1.02–1.97; P = 0.037), and age (OR 0.95; CI 95% 0.92–0.98; P = 0.004), after adjusting for immediate procedural results, including postprocedural MVA, pulmonary artery pressure, and transvalvular gradient.
TABLE 3.
Factors associated with NYHA functional class at follow-upa
| Covariates | Odds ratio | (95% CI) | P value | |
|---|---|---|---|---|
| Univariable analysis | ||||
| Baseline data | Age (years) | 0.96 | 0.93–0.99 | 0.010 |
| Postprocedural data | LA pressure (mmHg) | 1.03 | 0.96–1.10 | 0.387 |
| Transvalvular gradient (mmHg) | 1.06 | 0.94–1.19 | 0.368 | |
| MPAP (mmHg) | 1.01 | 0.97–1.05 | 0.664 | |
| Mitral valve area (cm2) | 4.88 | 0.95–25.07 | 0.058 | |
| Moderate mitral regurgitation | 0.75 | 0.29–1.91 | 0.544 | |
| Changes in the variablesb | Changes in LA pressure (mmHg) | 0.97 | 0.91–1.02 | 0.219 |
| Changes in LA compliance (mL/mmHg) | 1.36 | 1.00–1.85 | 0.045 | |
| Changes in BNP (pg/mL) | 0.48 | 0.22–1.07 | 0.075 | |
| Changes in mitral valve gradient (mmHg) | 0.45 | 0.17–1.20 | 0.110 | |
| Multivariable analysisc | ||||
| Age (years) | 0.95 | 0.92–0.98 | 0.004 | |
| Changes in LA compliance (mL/mmHg) | 1.42 | 1.02–1.97 | 0.037 |
Total of 130 patients were included in the analysis, 7 patients were excluded due to severe mitral regurgitation after the procedure
Percentage change was calculated using this formula: Post-Pre/Pre × 100%.
Multivariable analysis after adjustment for immediate procedural results: postprocedural mitral valve area, MPAP, and mean transvalvular gradient.
Abbreviation: LA, left atrial.
The performance of the model including conventional final postprocedural parameters was compared with the performance of the model adding Ca. The model performance after including Ca changes showed a substantial improvement compared with the model without Ca. The Nagelkerke pseudo R-squared of the model without Ca was 0.118 whereas the addition of Ca increased the pseudo R-squared value to 0.232. Therefore, the final model with Ca is more accurate to predict functional class at follow-up compared with the model with conventional measurements.
3.3 ∣. Adverse events during the follow-up
After exclusion of patients who developed severe mitral regurgitation, 20 adverse clinical events were observed during a median follow-up period of 27 months (range, 6–53), including 2 deaths, 6 MV replacements, 5 repeat PMV, 6 onset of AF, and 1 stroke. After adjustment for postprocedural LA pressure, MVA, and pulmonary artery pressure, the predictors of outcomes were post-PMV transmitral mean gradient (adjusted HR 1.363; 95% CI 95% 1.027–1.809; P = 0.032), and lack of functional improvement at 6-month follow-up (adjusted HR 4.959; 95% 1.708–14.403; P = 0.003) (Figure 3).
FIGURE 3.
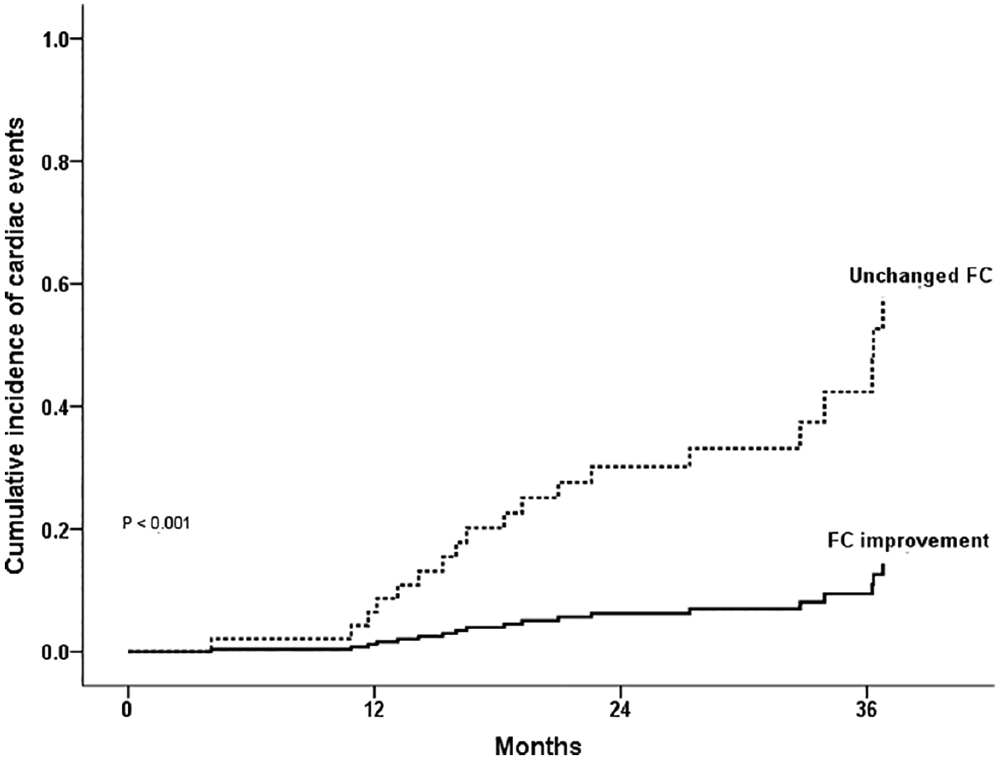
Cumulative incidence of adverse events in patients with functional capacity improvement compared to unchanged functional capacity. Adverse events were defined as death, MV replacement, repeat PMV, new onset of atrial fibrillation, and stroke. Abbreviation: FC, functional capacity
3.4 ∣. Reproducibility
For Ca and Cv measurements the two independent observers achieved a high level of agreement. The intraclass correlation coefficients for Ca were 0.992 for interobserver and 0.998 for intraobserver variability, and for Cv were 0.982 for interobserver and 0.983 for intraobserver variability. We also evaluated the interobserver and intraobserver agreement using the Bland–Altman statistic (Supporting Information Figure S1), which showed a good reproducibility.
4 ∣. DISCUSSION
PMV is an effective therapy for rheumatic MS, which results in an increase in MVA and reduction of LA pressure with improvement in clinical symptomatology.6,24,25 Successful valve splitting may also have effects on other hemodynamic parameters that can contribute to exercise tolerance. This study addresses the impact of PMV on left chamber compliance, and evaluates changes in compliance as potential predictors of functional capacity following valve intervention. The results show that PMV significantly improves Ca and Cav, without changing Cv. Moreover, the change in Ca appears to be an independent predictor of NYHA functional class improvement following the procedure. Although plasma BNP levels significantly decreased after PMV, there was no correlation between variation of BNP levels and changes in left chamber compliance. Long-term post PMV event-free survival was predicted by postprocedural mean gradient and functional status at 6-month follow-up.
4.1 ∣. Effects of PMV on left chamber compliance
PMV may have other beneficial effects besides simply increase in MVA and reduce pressure gradients. A substantial reduction in LA pressure was accompanied by the restoration of the LA function and reduction in LA stiffness. Stefanadis et al.26 including 15 patients showed that LA stiffness returns to normal after relieving the stenosis by valvuloplasty. As the atrial stiffness rapidly returns to normal, it suggests that the changes in LA chamber stiffness are unlikely to be related to structural derangements in the atrium. Similarly, Thomas et al.18 demonstrated that LA compliance rises after PMV, supporting the hypothesis that atrial compliance changes reflect shift along a pressure volume curve, rather than a change in the intrinsic properties of the atrial wall. In contrast, ventricular compliance falls with the acute increase in diastolic filling, which shifts the pressure volume curve to the right, in steeper portion of the curve. In this study, we found that Ca improves significantly after PMV, even in those patients who had suboptimal results. In agreement with Thomas et al. study,18 if we consider mean values of Ca, it rose by 117% after successful PMV. Ca decreased in those patients with postprocedural severe mitral regurgitation, but it was not statistically significant. This finding is also similar to the study of Thomas et al., that the pressure-compliance relations for patients with 2 or 3 + mitral regurgitation (n = 5) were analyzed as a separate subgroup, and these were statistically indistinguishable from the patients with 0 or 1+ mitral regurgitation.
In our study, the improvement in Cav is explained by increase in Ca given the lack of change in Cv. However, chronic adaptation of the LV to an increased stroke volume after PMV may improve Cv over time in a more gradual manner. It is therefore likely that following successful PMV, Cav may further increase by an improvement of Cv, and after several months the improvement of Cav might actually reflect a combination of an improvement of both Ca (acutely) and Cv (chronically).
Different from our findings, Liu et al.27 showed a marked increase in Cv acutely after valvuloplasty.
Reduced Cv has been attributed to a functional restriction related to the thickened immobile valve apparatus tethered to the ventricular chamber rather than a permanent myocardial abnormality.27,28 Therefore, PMV sufficiently improves valve apparatus mobility to release this constraining effect on the ventricle, thereby revealing relatively normal underlying myocardial chamber properties.27
Previous studies addressing the impact of atrial compliance on pulmonary artery pressure have used net atrioventricular compliance (Cn), derived by Doppler echocardiography,29 which is comparable to invasively measured Cav.11,13,17 We have previously demonstrated reasonable correlation (r = 0.60) between Cav and Doppler-derived Cn.17 As low atrial compliance is the main determinant of pathologic physiology in MS, Cn is used as a surrogate for Ca.12,17,26,27
4.2 ∣. Compliance and functional status
Previous studies have demonstrated that Cn is an important determinant of functional capacity in MS.11-14,16,17 Patients with similar MVA have different levels of symptoms according to Cn values. Those patients with the lowest levels of Cn are the most symptomatic14 and also seems to be those with the smallest VO2 max.13 Thus, the association between low Cn and worst functional capacity is well established. It would be expected that the patients who had improvement of this parameter and especially of its main determinant, Ca, would have the greatest benefits in functional improvement. Our study found compelling evidence for this hypothesis, since the changes in Ca flowing PMV were associated with improvement in NYHA functional class at follow up.
Additionally, in accordance to previous investigation, NYHA functional class following the procedure was an important predictor of adverse outcome.30 Yates et al. evaluating 132 patients showed that assessment of short-term symptomatic status after PMV, reflected in the NYHA classification at an initial 3-month follow-up, was a strong predictor of mid- and long-term outcome, independent of other prognostic variables.30 Furthermore, conventional hemodynamic and echocardiographic parameters may not accurately predict functional capacity improvement after PMV. Our study highlights that elevation of Ca predicts improvement in functional status, independently of changes in other procedural variables. Of note, this finding was not simply related to immediate results of the procedure, as the patients who had complications were excluded. Therefore, improvement in Ca has a potential to predict short-term patients’ functional status, which ultimately is an independent predictor of long-term adverse outcome. However, we are not able to derive this conclusion based on our data.
4.3 ∣. Changes in net chamber compliance and BNP levels following the procedure
Plasma BNP concentrations are elevated in MS in response to LA pressure overload and pulmonary hypertension, which decline with successful PMV.31 We previously showed that reduction of LA pressure after relief of obstruction was associated with a decrease in BNP levels.32 Although we expected that increase in Ca would result in a decrease in atrial pressure as well as in wall stretch with consequent reduction in BNP levels, in this study changes in Ca were not correlated to reduction of BNP. However, the negative correlation between post PMV BNP concentration and Cv indicates that post procedural ventricular compliance may influence BNP release. Indeed, acute changes in loading conditions after PMV may affect left ventricular systolic function.33 Relief valve obstruction improves diastolic filling with an increase in both end-diastolic volume and pressure, which may contribute to reduction in Cv, wall stretch and increase in BNP levels. Nonetheless, BNP concentrations are influenced by other factors, especially atrial fibrillation, which in turn has also an effect on left chamber compliance.
4.4 ∣. Study limitations
The outcome assessed in this study was NYHA functional class, which is a subjective estimate of a patient’s functional ability that do not always correlates with the objective measures of functional capacity and peak oxygen consumption. Other objective functional evaluations, such as 6-min walking test or even stress test with blood gas analysis could provide more precise insights. However, symptoms of dyspnea express severity of MS and indication for the valve intervention. Additionally, no correlation has previously been found between valve area or pressure gradient after PMV and exercise performance, even assessed by objective parameters including achieved workload or peak systemic oxygen consumption9,10 Furthermore, our data does not support the extrapolation of the changes in Ca as a predictor of long-term outcomes, limiting the conclusions to the NYHA functional class at 6 months.
Hemodynamically, the analysis of CO can be influenced by the shunt derived from the atrial septal defect created during PMV, once it was not occluded by a compliant balloon during blood sampling. Although the discontinuity of the septum is very small, the slight shunt may lead to inaccuracies. Nevertheless, we found minimal left-to-right atrial shunt after PMV. Finally, any degree of increasing mitral regurgitation could potentially affect left heart chamber compliance. Although patients with significant worsening of mitral regurgitation were excluded, this factor may have accounted for some degree of Ca variability.
4.5 ∣. Clinical implications
Although PMV has an immediate benefit on hemodynamics, improvements in subjective patient symptoms, and exercise tolerance rely on other factors. Currently, the results of PMV are evaluated by MVA and transmitral gradient, which may be not correlated with symptomatic improvement.9,10,30 Pulmonary artery pressure immediately after PMV may not return to normal and its change does not correlate with functional capacity improvement.13,14 Inappropriate increase of pulmonary arterial pressure beyond changes in the LA pressure is known to be a major exercise-limiting factor in patients with MS.34 In this context, this study shows that adequate relief of obstruction results in an increase in Ca and consequent improvement in patient symptomatic status. Importantly, our study assessed the magnitude of increased Ca on functional capacity at follow-up.
5 ∣. CONCLUSIONS
In a large series of patients with rheumatic MS, PMV increases significantly Ca and Cav with no changes in Cv. The improvement of Ca seems to be an important predictor of functional status at 6-month follow up, independently of other hemodynamic data. Postprocedural mean gradient and lack of short-term symptomatic improvement are predictors of long-term outcome. Assessing changes in Ca following the procedure may be a helpful tool for clinical risk stratification in patients with MS.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly supported by grants from CNPq (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Brasília, Brazil) and FAPEMIG (Fundação de Apoio à Pesquisa do Estado de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil). Dr. Nascimento was supported in part by Edwards Lifesciences Foundation (Every Heartbeat Matters Program).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Zuhlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: The global rheumatic heart disease registry (the remedy study). Eur Heart J. 2015;36:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377:713–722. [DOI] [PubMed] [Google Scholar]
- 3.Wood P An appreciation of mitral stenosis. I. Clinical features. Br Med J 1954;1:1051–1063; contd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009; 374:1271–1283. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Creager MA, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Yancy CW; American College of Cardiology; American College of Cardiology/American Heart Association; American Heart Association. 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/American heart association task force on practice guidelines. J Thorac Cardiovasc Surg 2014;148:e1–e132. [DOI] [PubMed] [Google Scholar]
- 6.Palacios I, Block PC, Brandi S, Blanco P, Casal H, et al. Percutaneous balloon valvotomy for patients with severe mitral stenosis. Circulation. 1987;75:778–784. [DOI] [PubMed] [Google Scholar]
- 7.Nunes MC, Tan TC, Elmariah S, et al. The echo score revisited: Impact of incorporating commissural morphology and leaflet displacement to the prediction of outcome for patients undergoing percutaneous mitral valvuloplasty. Circulation. 2014;129:886–895. [DOI] [PubMed] [Google Scholar]
- 8.Nunes MC, Nascimento BR, Lodi-Junqueira L, Tan TC, Athayde GR, Hung J. Update on percutaneous mitral commissurotomy. Heart. 2016;102:500–507. [DOI] [PubMed] [Google Scholar]
- 9.Barlow CW, Long JE, Brown G, Manga P, Meyer TE, Robbins PA. Exercise capacity and skeletal muscle structure and function before and after balloon mitral valvuloplasty. Am J Cardiol. 1995;76:684–688. [DOI] [PubMed] [Google Scholar]
- 10.Douard H, Gilles YM, Choussat A, Broustet JP. Lack of correlation between haemodynamic and cardiopulmonary exercise capacity improvement after catheter-balloon mitral valvuloplasty. Eur Heart J. 1995;16:1375–1379. [DOI] [PubMed] [Google Scholar]
- 11.Schwammenthal E, Vered Z, Agranat O, Kaplinsky E, Rabinowitz B, Feinberg MS. Impact of atrioventricular compliance on pulmonary artery pressure in mitral stenosis: An exercise echocardiographic study. Circulation. 2000;102:2378–2384. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Dery JP, Dumesnil JG, Boudreault JR, Jobin J, Pibarot P. Usefulness of measuring net atrioventricular compliance by doppler echocardiography in patients with mitral stenosis. Am J Cardiol. 2005;96:432–435. [DOI] [PubMed] [Google Scholar]
- 13.Choi EY, Shim J, Kim SA, et al. Value of echo-doppler derived pulmonary vascular resistance, net-atrioventricular compliance and tricuspid annular velocity in determining exercise capacity in patients with mitral stenosis. Circ J. 2007;71:1721–1727. [DOI] [PubMed] [Google Scholar]
- 14.Guray Y, Demirkan B, Karan A, Guray U, Boyaci A, Korkmaz S. Left atrial compliance and pulmonary venous flow velocities are related to functional status in patients with moderate-to-severe mitral stenosis. Echocardiography. 2009;26:1173–1178. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Kim YJ, Hwang SJ, et al. Hemodynamic and prognostic implications of net atrioventricular compliance in patients with mitral stenosis. J Am Soc Echocardiogr. 2008;21:482–486. [DOI] [PubMed] [Google Scholar]
- 16.Mahfouz RA, Elawady W, Hossein E, Yosri A. Impact of atrioventricular compliance on clinical outcome of patients undergoing successful percutaneous balloon mitral valvuloplasty. Echocardiography. 2013;30:1187–1193. [DOI] [PubMed] [Google Scholar]
- 17.Nunes MC, Hung J, Barbosa MM, et al. Impact of net atrioventricular compliance on clinical outcome in mitral stenosis. Circ Cardiovasc Imaging. 2013;6:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JD, Wilkins GT, Choong CY, et al. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation. 1988;78:980–993. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: An analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. quiz 101-102. [DOI] [PubMed] [Google Scholar]
- 22.Lancellotti P, Tribouilloy C, Hagendorff A, et al. Scientific document Committee of the European Association of cardiovascular I. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 24.Cruz-Gonzalez I, Sanchez-Ledesma M, Sanchez PL, et al. Predicting success and long-term outcomes of percutaneous mitral valvuloplasty: A multifactorial score. Am J Med. 2009;122:581. e511–581. e589. [DOI] [PubMed] [Google Scholar]
- 25.Bouleti C, Iung B, Himbert D, et al. Relationship between valve calcification and long-term results of percutaneous mitral commissurotomy for rheumatic mitral stenosis. Circ Cardiovasc Interv. 2014;7:381–389. [DOI] [PubMed] [Google Scholar]
- 26.Stefanadis C, Dernellis J, Stratos C, et al. Effects of balloon mitral valvuloplasty on left atrial function in mitral stenosis as assessed by pressure-area relation. J Am Coll Cardiol. 1998;32:159–168. [DOI] [PubMed] [Google Scholar]
- 27.Liu CP, Ting CT, Yang TM, et al. Reduced left ventricular compliance in human mitral stenosis. Role of reversible internal constraint. Circulation. 1992;85:1447–1456. [DOI] [PubMed] [Google Scholar]
- 28.Venkateshvaran A, Sola S, Govind SC, et al. The impact of arterial load on left ventricular performance: An invasive haemodynamic study in severe mitral stenosis. J Physiol. 2015;593:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flachskampf FA, Weyman AE, Guerrero JL, Thomas JD. Calculation of atrioventricular compliance from the mitral flow profile: Analytic and in vitro study. J Am Coll Cardiol. 1992;19:998–1004. [DOI] [PubMed] [Google Scholar]
- 30.Yates LA, Peverill RE, Harper RW, Smolich JJ. Usefulness of short-term symptomatic status as a predictor of mid- and long-term outcome after balloon mitral valvuloplasty. Am J Cardiol. 2001;87:912–916. [DOI] [PubMed] [Google Scholar]
- 31.Sharma V, Stewart RA, Lee M, et al. Plasma brain natriuretic peptide concentrations in patients with valvular heart disease. Open Heart. 2016;3:e000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esteves WA, Lodi-Junqueira L, Neto CP, et al. The impact of right ventricular stroke work on b-type natriuretic peptide levels in patients with mitral stenosis undergoing percutaneous mitral valvuloplasty. J Interv Cardiol. 2013;26:501–508. [DOI] [PubMed] [Google Scholar]
- 33.Klein AJ, Carroll JD. Left ventricular dysfunction and mitral stenosis. Heart Fail Clin. 2006;2:443–452. [DOI] [PubMed] [Google Scholar]
- 34.Song JK, Kang DH, Lee CW, et al. Factors determining the exercise capacity in mitral stenosis. Am J Cardiol. 1996;78:1060–1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


