Abstract
Pharmacological tools for chronic visceral pain management are still limited and inadequate. A3 adenosine receptor (A3AR) agonists are effective in different models of persistent pain. Recently, their activity has been related to the block of N-type voltage-gated Ca2+ channels (Cav2.2) in dorsal root ganglia (DRG) neurons. The present work aimed to evaluate the efficacy of A3AR agonists in reducing postinflammatory visceral hypersensitivity in both male and female rats. Colitis was induced by the intracolonic instillation of 2,4-dinitrobenzenesulfonic acid (DNBS; 30 mg in 0.25 mL 50% EtOH). Visceral hypersensitivity was assessed by measuring the visceromotor response and the abdominal withdrawal reflex to colorectal distension. The effects of A3AR agonists (MRS5980 and Cl-IB-MECA) were evaluated over time after DNBS injection and compared to that of the selective Cav2.2 blocker PD173212, and the clinically used drug linaclotide. A3AR agonists significantly reduced DNBS-evoked visceral pain both in the postinflammatory (14 and 21 days after DNBS injection) and persistence (28 and 35 days after DNBS) phases. Efficacy was comparable to effects induced by linaclotide. PD173212 fully reduced abdominal hypersensitivity to control values, highlighting the role of Cav2.2. The effects of MRS5980 and Cl-IB-MECA were completely abolished by the selective A3AR antagonist MRS1523. Furthermore, patch-clamp recordings showed that A3AR agonists inhibited Cav2.2 in dorsal root ganglia neurons isolated from either control or DNBS-treated rats. The effect on Ca2+ current was PD173212-sensitive and prevented by MRS1523. A3AR agonists are effective in relieving visceral hypersensitivity induced by DNBS, suggesting a potential therapeutic role against abdominal pain.
Keywords: A3 adenosine receptor, DRG, Abdominal pain, IBS, IBD
1. Introduction
Visceral pain management is a major clinical problem, because of the lack of effective and safe drugs.11 Abdominal pain is the most common form of visceral hypersensitivity and is often the result of inflammatory bowel diseases (IBDs).14,51,84,85 The poor correlations between reported abdominal pain intensity and IBD activity raise close similarities with the irritable bowel syndrome (IBS), a symptom-based clinical condition defined by the presence of persistent abdominal pain and altered bowel habits, in the absence of any other disease able to account for these symptoms.24,35,51,58,84,85 Frequently, IBS results from a previous intestinal damage caused by severe infections or prolonged inflammatory processes.24,83 In the inflamed gut, there is breakdown of intestinal barrier function, abnormal secretion, changes in the patterns of motility, and altered visceral sensation, which altogether contribute to generation of symptoms (diarrhoea, cramping, and pain). Pain, in particular, persists beyond the relief of inflammation, thus revealing a peculiar kind of chronic hypersensitivity thought to be due to inflammatory, immune, and neuropathic mechanisms.9,26,40,53,86 Currently, the most efficacious therapies against visceral hypersensitivity are mainly directed toward treating bowel dysfunction, whereas drugs able to directly target the associated pain are still unsatisfactory.11
The neuromodulator adenosine exerts potent and long-lasting pain suppression in preclinical models as well as in human studies.105 Moreover, adenosinergic signalling is known to modulate intestinal functionality.2-4 For decades, it was thought that the analgesic effects of adenosine were mediated by A1 adenosine receptor (A1AR) activation,8,54,65,77,105 but research efforts over the past several years have also implicated a key role for the A3AR subtype.43,75 For example, A3AR agonists are able to block the development of trauma- and chemotherapeutic-induced neuropathic pain17,42 and are also effective in reducing inflammatory and cancer-related pain.50,93,100,101 Recently, we found that selective A3AR stimulation inhibits the opening of N-type voltage-gated Ca2+ channels (Cav2.2) and decreases the electrically evoked excitation of isolated rat dorsal root ganglia (DRG) neurons. This mechanism could explain the antihyperalgesic effect of A3AR agonists across different models.19 Therapeutic targeting of A1AR has been limited to severe cardiovascular side effects; by contrast, A3AR agonists are already in advanced clinical trials for different indications with a good safety profile.29,80
The aims of this study were to: (1) evaluate the efficacy of selective A3AR agonists in postinflammatory visceral hypersensitivity induced in male and female rats by the intrarectal administration of 2,4-dinitrobenzenesulfonic acid (DNBS); (2) investigate the electrophysiological effects of selective A3AR stimulation on DRG neurons isolated from control and DNBS-treated animals.
2. Materials and methods
An extensive description of materials and methods has been reported in the supplemental digital content (available at http://links.lww.com/PAIN/B10).
2.1. Animals
We used male and female Sprague-Dawley rats (Envigo, Varese, Italy) weighing approximately 220 to 250 g at the beginning of the experimental procedure. All animal manipulations were conducted according to the Directive 2010/63/EU of the European Parliament and of the European Union Council (September 22, 2010) on the protection of animals used for scientific purposes. The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85-23, revised 1996; University of Florence assurance number: A5278-01). Formal approval to conduct the described experiments was obtained from the Animal Subjects Review Board of the University of Florence. Experiments involving animals have been reported according to ARRIVE guidelines.57 Gastrointestinal motility assays (GI) were conducted using Sprague-Dawley male rats (Envigo, 250-350 g) in accordance with the University of Arizona Institutional Animal Care and Use Committee (approval 06-110). Experiments were performed on male rats when not otherwise stated.
2.2. Determination of female estrous cycle phases
Estrous cycle was assessed in rats by analysing rat vaginal smears as previously described.39 This screening was performed before the behavioral test; tests were selectively performed in female rats in estrous/proestrous phase to avoid sensitive difference related to the estrous cycle. Moreover, in most studies, this phase is reported to be related to the highest pain sensitivity,45,46,59,60,74 therefore suitable for studying pain-relieving compounds.
2.3. Induction of colitis
Colitis was induced using the method described previously by Fornai et al.30 with minor changes. In brief, during a short period of anaesthesia with isoflurane (2%), 30 mg of DNBS in 0.25 mL of 50% ethanol was administered intrarectally through a polyethylene PE-60 catheter inserted 8 cm proximal to the anus. Control rats received 0.25 mL of saline solution. Subsequent experimental procedures began 14 days after DNBS administration to allow for recovery from the initial colonic inflammation.
2.4. Assessment of visceral sensitivity by visceromotor response and abdominal withdrawal reflex
The visceromotor response (VMR) to colorectal distension (CRD) was used as an objective measure of visceral sensitivity. Visceromotor response assessment was conducted in animals under light anaesthesia (2% isoflurane) 14 and 21 days after DNBS administration. The amplitude of the abdominal contraction consequent to colorectal stimulation (a balloon inflated with 0.5, 1, 2, and 3 mL referred to as distension volume) was quantified by electromyography recordings as reported by Parisio et al.64 Behavioural responses to CRD (0.5, 1, 2, and 3 mL referred to as distension volume) were assessed through abdominal withdrawal reflex (AWR) measurement in conscious animals by using a semiquantitative score (0-4) as described previously.16 The measurements were conducted 14, 21, 28, and 35 days after DNBS administration.
2.5. Upper gastrointestinal transit treatment and harvesting
All experiments were performed as previously described.66,76,95 Before experiment, rats were fasted for 12 hours, maintaining the access to water ad libitum. After 15 minutes had elapsed, rats were then given an oral gavage with fluorescein isothiocyanate dextran (300 μL of 5 mg/mL 70 kDa FITC-dextran; Sigma, Milan, Italy). Forty min after completing the gavage, rats were anaesthetized with isoflurane and whole blood harvested through cardiac puncture. After blood harvest, the upper portion of the gastrointestinal tract was carefully removed, being cut proximally at the pyloric sphincter and distally at the cecum. The intestine was sectioned into 10 equal lengths, which were homogenized in 1 mL 1X PBS and then centrifugated. Fluorescence was determined in the supernatants collected from intestinal lining (10 μL) and content diluted into 90 μL 1X PBS; serum fluorescence was determined after dilution of 10 μL serum into 40 μL 1X PBS using a ClarioStar (BMG Labtech, Milan, Italy) at 483 to 530 nm. Readings were then collected and prepared for statistical analyses.
2.6. Drug administration
The selective A3 AR agonist, 2-chloro-N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (Cl-IB-MECA; Tocris Bioscience, Bristol, United Kingdom), and the selective A3 receptor antagonist, 3-propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate (MRS1523; Sigma-Aldrich, Milan, Italy), were dissolved in 5% DMSO and 5% TWEEN 20 saline solution for in vivo administration. The new, highly selective (10,000-fold vs each of the other 3 receptor subtypes) A3AR agonist, MRS5980, (1S,2R,3S,4R,5S)-4-(2-((5-chlorothiophen-2-yl)ethynyl)-6-(methylamino)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide89 was dissolved in 5% DMSO saline solution for in vivo administration. As already reported,50 the antiallodynic effects of the A3AR agonists used in this work were lost in the A3AR knockout mouse and in the presence of the A3AR antagonist MRS1523.49 N-[[4-(1,1-dimethylethyl)phenyl]methyl]-N-methyl-L-leucyl-N-(1,1-dimethylethyl)-O-(phenylmethyl)-L-tyrosinamide (PD173232) was purchased from Alomone Labs (Jerusalem, Israel) and dissolved in 5% DMSO and 5% TWEEN 20 saline solution for in vivo administration. Both the vehicle and the DNBS control groups received an i.p. injection of the vehicle used for the administration of the respective molecules. The acute injection of the vehicles (5% DMSO or 5% DMSO + 5% TWEEN 20) did not alter visceral pain threshold and did not induce side effects in the animals. The guanylate cyclase-C (GC-C) agonist linaclotide (Allergan, Buckinghamshire, United Kingdom) was dissolved in water and orally administered 1 hour before starting the behavioural tests.25 Cl-IB-MECA, MRS5980, and PD173232 were administered i.p. 15 minutes before the test. MRS1523 was administered i.p. 15 minutes before Cl-IB-MECA and MRS5980. Gastrointestinal transit study: the compound MRS5980 was dissolved in 5% DMSO, and then suspended in saline. MRS5980 and vehicle were administered through i.p. injection to all animals in study at 2.4 μmol·kg−1 body weight (1 mL/kg injection volume). Seventy kDa FITC-dextran (Sigma) was dosed by oral gavage at a concentration of 5 mg/mL, 300 μL per rat. Additional pharmacological characteristics of A3 AR agonists are shown in the Supplemental material (available at http://links.lww.com/PAIN/B10).
2.7. Cell cultures
Primary DRG neurons (related to colon-sensitive innervations: T12, T13, L4, L5, L6, S1, S2) were isolated from vehicle- or DNBS-treated (at 14th day) rats and cultured for 1 to 2 days before being used for experiments as described.19
2.8. Electrophysiology
Whole-cell patch-clamp recordings were performed as previously described.19 Passive membrane properties of DRG neurons isolated from control or DNBS-treated rats were investigated under physiological-like conditions by using the following K-gluconate- based pipette solution (mM): KGlu 130; NaCl 4.8; KCl 10; MgCl2 2; CaCl2 1; Na2-ATP 2; Na2-GTP 0.3; EGTA 3; HEPES 10 (pH 7.4 with KOH). Resting membrane potential (Vm) was measured immediately after seal breakthrough by switching the amplifier to the current-clamp mode. The calculated liquid junction potential for K-gluconate pipettes in our experimental conditions was 15.0 mV, and Vm values reported in the present research have been corrected accordingly.
Voltage-dependent Ca2+ currents (VDCCs) were recorded by using a Cs+-based pipette solution having the following composition (mM): CsCl (130); NaCl (4.8); KCl (10); MgCl2 (2); CaCl2 (1); Na2-ATP (2); Na2-GTP (0.3); EGTA (3); and HEPES (10—pH 7.4 with CsOH). The extracellular solution was (in mM): NaCl (147); CsCl (4); MgCl2 (1); and CaCl2 (5); HEPES (10); D-glucose (10); pH 7.4 with NaOH. Tetrodotoxin (TTX; 1 μM) and 5-(4-butoxy-3-chlorophenyl)-N-[[2-(4-morpholinyl)-3-pyridinyl]methyl]-3-pyridinecarboxamide (A887826; 200 nM) were added to the extracellular solution to block TTX-sensitive Nav1.1, 1.2, 1.3, 1.4, 1.6, 1.7 channels and TTX-resistant Nav1.8, respectively. VDCC currents were evoked by a 0 mV step depolarization (200 ms) once every 30 seconds to minimize Ca2+ current run down. Peak Ca2+ current (ICa peak) was measured as the peak current amplitude reached during the first 50 ms of voltage step. Steady-state Ca2+ current (ICa st-state) was measured as the averaged current amplitude measured between 160 and 190 ms of voltage step. The current-to-voltage relationship (I-V plot) of Ca2+ currents was obtained by eliciting 10 depolarizing voltage steps (200-ms duration, 10-mV increments, 5-second interval) from −50 to +150 mV starting from a holding potential (Vh) of −65 mV.
Data were acquired with an Axopatch 200B amplifier (Axon Instruments, San Jose, CA), low-pass filtered at 10 kHz, and stored and analysed with pClamp 9.2 software (Axon Instruments). Membrane resistance (Rm) and membrane capacitance (Cm) were routinely measured by fast hyperpolarizing voltage pulses (from −60 to −70 mV, 40-ms duration). Averaged currents were normalized to cell capacitance and expressed as pA/pF.
Cell capacitance was used to estimate neuronal diameter by assuming an approximated spherical cell shape according to the calculated Cm for all biological membranes of 1 μF/cm2 and to the equation of the sphere surface: A = 4πr2.
The in vitro concentrations were chosen on the base of our previous work: the A3AR agonists Cl-IB-MECA and MRS5980 were applied at 30 nM, a concentration able to produce maximal inhibition of Ca2+ currents in rat DRG neurons.19
2.9. Statistical analysis
Behavioural measurements were performed on 6 animals for each treatment. All the experimental procedures were performed by a researcher blind to the treatment. Results were expressed as mean ± SEM. The analysis of variance (ANOVA) of behavioural data was performed by one-way ANOVA with Bonferroni’s significant difference procedure used for post hoc comparisons. Statistics of electrophysiological data was performed by Student paired or unpaired t test or by one-way ANOVA followed by Bonferroni’s post hoc test, as appropriate. P values of less than 0.05 were considered significant. Data were analyzed using the “Origin 9” software (OriginLab, Northampton, MA). Data from gastrointestinal transit measures were analyzed in GraphPad Prism 7. Standard curves were fit using a nonlinear, hyperbolic equation for individual plates. A two-way ANOVA with Tukey post hoc test was performed using the variables of GI segment and treatment. Serum samples were compared using an unpaired t test. Data were considered statistically significant when P < 0.05 to detect 20% difference with 80% power (necessary n-values determined using G.Power3.1).
3. Results
3.1. A3AR agonists reduce colitis-induced visceral hypersensitivity in rats
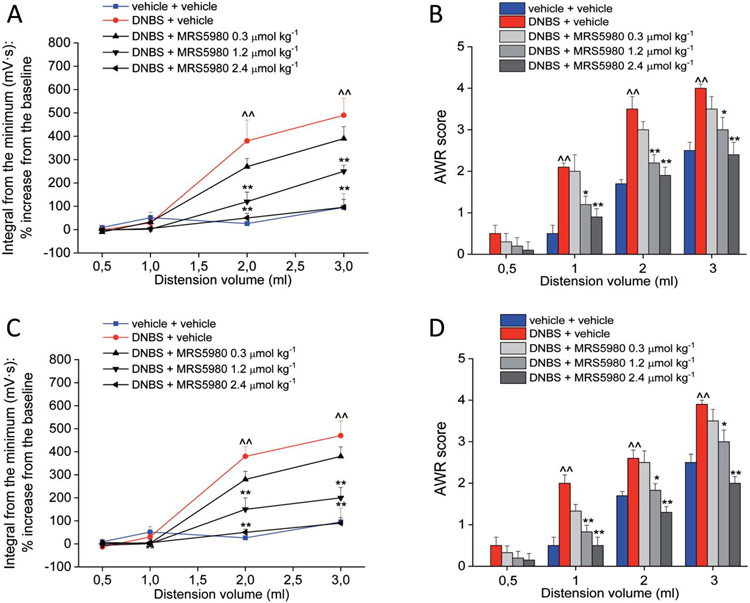
The visceromotor response and the abdominal withdrawal reflex (VMR and AWR) to CRD were measured by using progressively increasing balloon volumes as pressuring stimuli on the colon (highest volume: 3 mL, to avoid tissue damage). Fourteen and 21 days after DNBS injection, both VMR and AWR were significantly higher in comparison to controls (vehicle + vehicle) as shown, respectively, in Figures 1A-B (day 14) and 1C-D (day 21). On day 14, the acute administration of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1; i.p.) dose-dependently reduced the postinflammatory visceral hypersensitivity induced by DNBS; the magnitude of the reduction was similar for VMR and AWR. The highest dose (2.4 μmol·kg−1) completely reversed the sensitivity alteration back to the value of control rats. MRS5980 1.2 μmol·kg−1 partially but significantly reduced the response of the animals to CRD. The lowest dose of MRS5980 (0.3 μmol·kg−1) was ineffective in both tests (Figs. 1A and B). On day 21, the effect of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1) was confirmed (VMR and AWR to CRD, Figs. 1C and D, respectively).
Figure 1.
Effect of MRS5980 on postinflammatory visceral pain induced by DNBS. Effect of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1; i.p.) on visceromotor response (VMR) to CRD (left column) and abdominal withdrawal reflex (right column) on day 14 (A and B) and day 21 (C and D) after DNBS-induced colonic inflammation. Each value is the mean ± SEM of 6 rats per group. ^^P < 0.01 vs vehicle-treated normal controls (blue). *P < 0.05 and **P < 0.01 vs DNBS + vehicle-treated group (red). CRD, colorectal distension.
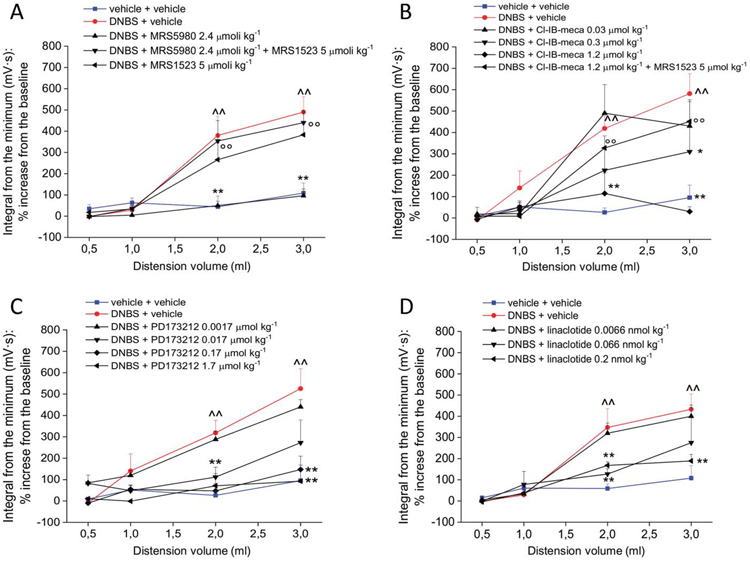
Figure 2A shows the result of the pretreatment with the selective A3AR antagonist MRS152349 on the antihyperalgesic effect of MRS5980 on day 14. MRS1523 (5 μmol kg−1) completely abolished the pain-relieving effect of MRS5980 (2.4 μmol kg−1) confirming a A3AR mechanism of action. Furthermore, the pain-relieving effect evoked by A3AR stimulation was confirmed by using another selective A3AR agonist, Cl-IB-MECA. On day 14, the acute administration of Cl-IB-MECA dose-dependently relieved visceral pain in DNBS-treated animals, reducing their VMR to the value of controls (dosed at 1.2 μmol·kg−1, Fig. 2B). The lowest dose of 0.3 μmol·kg−1 evoked a weaker effect only with the 2 mL stimulus. The Cl-IB-MECA (1.2 μmol·kg−1) effect was blocked by MRS1523 (5 μmol·kg−1, Fig. 2B).
Figure 2.
Evaluation of the role of adenosine A3ARS and N-type voltage-gated calcium channels in visceral pain relief and comparison with the effect of reference drugs. Tests were performed 14 days after DNBS treatment by measuring the visceromotor response (VMR) to colorectal distension (CRD) after compound administration. (A) Effect of pretreatment with the selective A3 antagonist MRS1523 (5 μmol·kg−1 i.p.) on the visceral pain-relieving effect of the highly selective A3AR agonist, MRS5980 (5 μmol kg−1 i.p.). (B) Effect of the selective A3 agonist, Cl-IB-MECA (0.03, 0.3, and 2.4 μmol·kg−1; i.p.). (C) Effect of the selective N-type voltage-gated calcium channel (Cav2.2) blocker, PD173212 (0.0017-1.7 μmol·kg−1 i.p.). (D) Effect of linaclotide (0.0066-0.2 nmol·kg−1 p.o.). Each value is the mean ± SEM of 6 rats per group. ^^P < 0.01 vs vehicle-treated normal controls (blue). *P < 0.05 and **P < 0.01 vs DNBS + vehicle-treated group (red). ∞P < 0.01 vs DNBS + MRS5980 (2.4 μmol·kg−1, black triangles in A) or Cl-IB-MECA (1.2 μmol kg−1, black triangles in B).
3.2. N-type voltage-gated Ca2+ channel block relieves colitis-induced visceral pain in rats
A3AR agonists have been recently reported to inhibit Cav2.2-mediated currents in DRG neurons, thus suggesting a possible mechanism for pain relief.19 To verify this hypothesis, we evaluated the effect of acute administration of the selective N-type Cav2.2 blocker PD17321238 in our visceral pain model. The test (VMR assessment) was performed on day 14 after DNBS injection. As shown in Figure 3C, PD173212 (0.0017-1.7 μmol·kg−1, i.p.) dose-dependently reduced the visceral hypersensitivity induced by DNBS. The compound started to be effective at a dose of 0.017 μmol·kg−1 and completely relieved abdominal pain when administered at a ten-fold higher dose (Fig. 2C).
Figure 3.
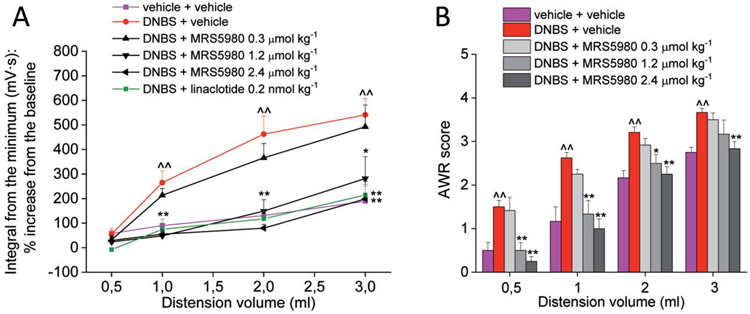
Effect of MRS5980 and linaclotide on visceral pain induced by DNBS in female rats. Effect of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1; i.p.) and linaclotide (0.2 nmol·kg−1 p.o.) on visceromotor response (VMR, A) to CRD on day 14 after DNBS injection. Effect of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1; i.p.) on abdominal withdrawal reflex (AWR, B) to CRD on day 14 after DNBS injection. Each value is the mean ± SEM of 6 rats per group. ^^P < 0.01 vs vehicle-treated normal controls (violet). *P < 0.05 and **P < 0.01 vs DNBS + vehicle-treated group (red). CRD, colorectal distension.
3.3. Comparison between the effects of MRS5980 and linaclotide in male and female rats
The effects of MRS5980 against the postinflammatory hypersensitivity induced by DNBS were evaluated in both male and female rats and compared with the effects of linaclotide, a guanylate cyclase-C (GC-C) agonist that represents the reference drug in the management of pain in patients affected by IBS with predominant constipation (IBS-C).18,97 On day 14, 0.2 and 0.066 nmol kg−1 linaclotide strongly reduced the VMR to CRD in male DNBS-treated animals, whereas the ten-fold lower dose was ineffective (Fig. 2D).
Similar to males, 14 days after DNBS injection in female rats, both VMR and AWR to colorectal distension were significantly higher in comparison to controls (Figs. 3A and B). The increase of visceral sensitivity was even more pronounced in females than in males as DNBS-treated females showed a significantly increased response also when stimulated with the 0.5 mL volume of distension in both the test performed (Figs. 3A and B). The acute i.p. administration of MRS5980 dose-dependently reduced visceral hyperalgesia induced by DNBS in female rats. In particular, the highest doses (1.2 and 2.4 μmol·kg−1) reversed visceral sensitivity alteration back to the value of controls in both VMR and AWR tests (Figs. 3A and B), showing similar efficacy to linaclotide (0.2 nmol·kg−1, Fig. 3A). The lowest dose of MRS5980 (0.3 μmol·kg−1) was ineffective in females like in males (Figs. 3A and B). To note, tests were performed in female animals synchronized for the estrous cycle choosing the proestrous/estrous phases to eliminate any possible confounding factor in the evaluation of A3 receptor agonists’ activity.
3.4. MRS5980 reduces persistent visceral pain
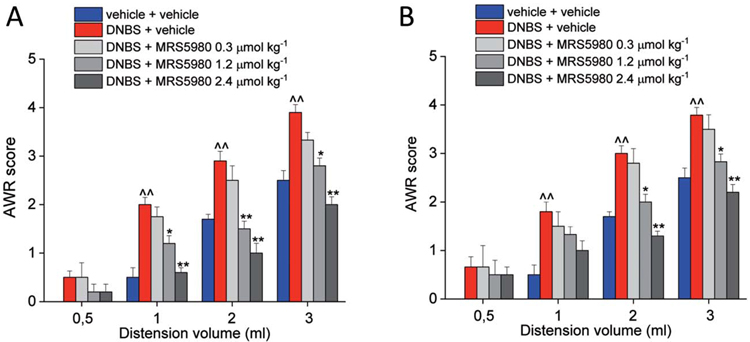
This type of pain has the peculiar tendency to become chronic in patients remitting from an intestinal damage.84,85 Unlike VMR, the assessment of AWR allows the evaluation of DNBS-induced hypersensitivity for a long time permitting the measurement of persistent pain. The behavioural response was still altered 28 and 35 days after the colonic irritation (Figs. 4A and B, respectively). Hence, we used this test to evaluate the effect of MRS5980 on visceral pain in a more delayed phase after the initial insult. On Day 28 and 35, the pain-relieving effect of MRS5980 (0.3, 1.2, and 2.4 μmol·kg−1) was as potent as that seen earlier. The effect on chronic hypersensitivity was dose-dependent, and the highest dose (2.4 μmol·kg−1) was again able to reverse the pain threshold of DNBS-treated animals to the value of controls (Figs. 4A and B).
Figure 4.
Effect of MRS5980 on persistent visceral pain induced by DNBS injection. Effect of MRS5980 (0.3, 1.2, and 2.4 μmol kg−1; i.p.) was observed 28 days (A) and 35 days (B) after DNBS treatment. The compound was administered 15 minutes before the first colorectal distension (CRD). Visceral pain was assessed by measuring the animal abdominal withdrawal reflex (AWR) to CRD. Each value is the mean ± SEM of 6 rats per group. ^^P < 0.01 vs vehicle-treated normal controls (blue). *P < 0.05 and **P < 0.01 vs DNBS + vehicle-treated group (red).
3.5. MRS5980 does not impair gastrointestinal transit
To determine whether the A3AR agonist MRS5980 influenced gut motility, we assessed total gastrointestinal transit. Groups of 6 rats were treated with either vehicle (5% DMSO) or MRS5980 (2.4 μmol·kg−1). Fifteen min later, animals received an oral gavage of 70 kDa FITC dextran (5 mg/mL, 300 mL per rat). Samples were harvested 40 min after 70 kDa FITC-dextran administration. Total fluorescence of 70 kDa FITC-dextran was determined for each of the 10 segments (Fig. 5A). 70 kDa FITC was detected in vehicle-treated samples in segments 5 (8.9 ± 2.5 μg/mL), 6(3.0 ± 2.2 μg/mL), and 8 (6.3 ± 3.2 μg/mL). MRS5980 treatment showed a similar pattern of GI transit of 70 kDa FITC; segment 5:11.17 ± 5.0 μg/mL, segment 6: 11.2 ± 6.3 μg/mL, and segment 8:15.1 ± 14.3 μg/mL. Detection of 70 kDa FITC-dextran in all other segments was <4 μg/mL (2.1-3.7 μg/mL). No significant difference between treatments (P = 0.453) or the interaction of treatment and GI segment (F(9,92) = P = 0.8676) was found. FITC levels were significantly different between the GI segments (P = 0.0010) indicative of intestinal movement as anticipated. These data indicate that A3AR agonism with MRS5980 does not statistically reduce GI transit as compared to vehicle.
Figure 5.
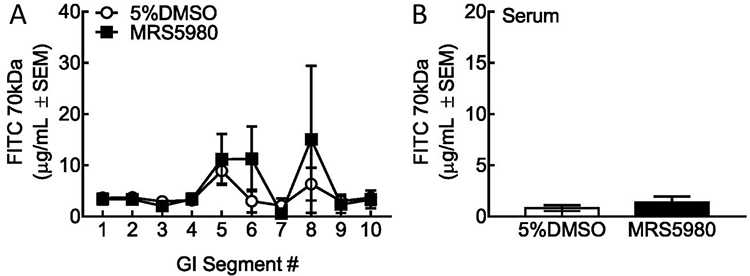
Gastrointestinal transit is not reduced by acute A3AR agonism with MRS5980. (A) Amount of 70 kDA FITC-dextran in small intestine segments 15 minutes after MRS5980 or vehicle dosing and 40 minutes after oral gavage of the marker. Two-way ANOVA, interaction: F(9,92) = 0.8676; P = 0.9553; GI Section: P = 0.0010; Treatment: P = 0.4584 (B) Amount of 70,000 kDa FITC-dextran in serum samples of 15 minutes after MRS5980 or vehicle dosing and 40 minutes after oral gavage of the marker (unpaired t test, P = 0.3955 MRS5980 compared to vehicle.) Each value is the mean ± S.E.M. of 6 rats. ANOVA, analysis of variance.
To determine whether MRS5980 influenced paracellular uptake of 70 kDa from the GI tract, we measured fluorescence in serum collected from the same animals (Fig. 5B). Serum levels of 70 kDa FITC-dextran were minimal (vehicle:0.82 ± 0.27; MRS5980:1.38 ± 0.57) and not significantly different (P = 0.3955). These data suggest that MRS5980 does not influence the paracellular integrity of GI epithelium alone as compared to vehicle.
3.6. A3R agonists inhibit N-type voltage-gated Ca2+ channels in dorsal root ganglia neurons isolated from control or DNBS-treated rats
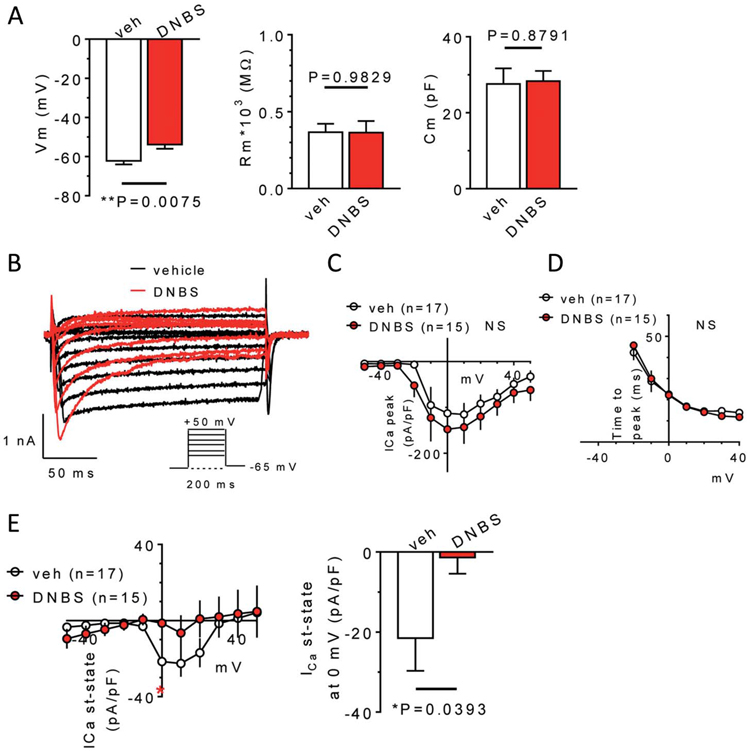
To gain insight into mechanisms underlying the antihyperalgesic role of A3ARs, we explored electrophysiological properties and Cl-IB-MECA effects in DRG primary sensory neurons isolated from vehicle-treated (control group) or DNBS-treated rats (14 days after treatment). Concerning passive membrane properties (Fig. 6A), DNBS neurons presented a more depolarized Vm in comparison to those from the vehicle-treated animals (Fig. 6A, left panel: from −61.4 ± 2.2 mV in vehicle-treated to −52.0 ± 2.6 mV in DNBS group, n = 23 and n = 33, respectively, P = 0.0143, unpaired Student t test), whereas no obvious differences were found in Rm or Cm (Fig. 6A, central and right panels). Cm was, on average, 27.6 ± 4.1 pF in vehicle-treated animals and 28.3 ± 2.7 pF in DNBS-treated animals (Fig. 6A, right panel), corresponding to a cell diameter of 29.9 and 30.7 μm, respectively, thus confirming that present data were collected from small–medium-sized DRG neurons. Of note, VDCCs, activated by a depolarizing voltage step protocol (from −50 to +50 mV, 200 ms: see insert in Fig. 6B), showed some differences in DNBS-treated vs vehicle-treated rats. Although if total peak currents were unchanged, either in amplitude (Fig. 6C) or time to peak (Fig. 6D), Ca2+ currents measured at the steady state in DNBS neurons were significantly smaller in amplitude (Fig. 6E right panel: from −21.5 ± 8.1 pA/pF in vehicle-treated to −1.4 ± 4.0 pA/pF in DNBS group, n = 15 and n = 14, respectively, P = 0.0393, unpaired Student t test).
Figure 6.
Electrophysiological properties of DRG neurons isolated from vehicle- or DNBS-treated rats. (A) Pooled data (mean ± SEM) of passive membrane properties (resting membrane potential: Vm, left panel; membrane resistance: Rm, central panel, and cell capacitance: Cm, right panel) measured in DRG neurons isolated from vehicle- (veh) or DBNS-treated rats (DNBS). Unpaired Student t test, n = 23 and n = 33, respectively. (B) Original Ca2+ current traces elicited in representative DRG neurons isolated from a vehicle- (black traces) or DNBS-treated (red traces) rat by applying a depolarizing voltage step protocol (from −50 to +50 mV, 200 ms duration, Vh = −65 mV: see inset). Averaged I-V plots of peak Ca2+ currents (C), and pooled data of time to peak of Ca2+ currents (D), measured at different step potentials in 17 cells isolated from vehicle-treated rats and 15 cells isolated from DNBS-treated animals. No significant differences were found between groups at any step potential, unpaired Student t test. (E) Left panel: averaged I-V plot of steady-state Ca2+ currents measured in 17 cells isolated from vehicle-treated rats and 15 cells isolated from DNBS-treated animals. Right panel: pooled data of the same currents measured at the 0 mV step. (A) significant reduction of steady-state Ca2+ currents was found in the DNBS-treated group, P = 0.0393, unpaired Student t test. DRG, dorsal root ganglia.
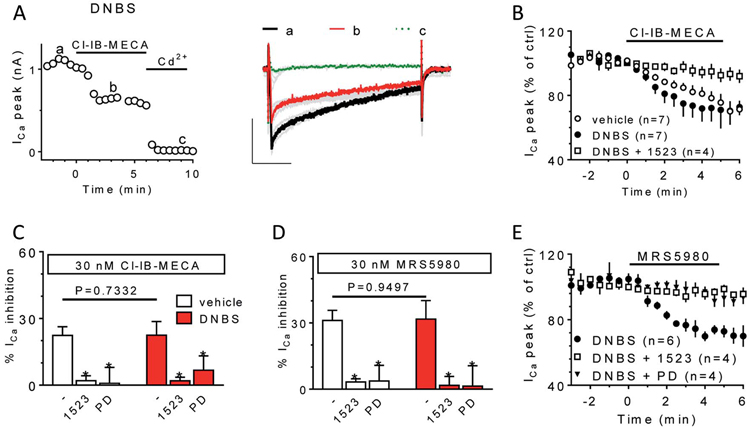
Consistent with our previous work19 and with the above in vivo results (Fig. 2), we confirmed that Cl-IB-MECA (30 nM) inhibited Ca2+ currents in DRG neurons isolated either from vehicle- or DNBS-treated rats (Figs. 7A and B) and the effect was prevented by the A3AR antagonist MRS1523 (100 nM: Fig. 7B) and by the N-type Ca2+ channel blocker PD173232 (1 μM: Figs. 7C-E). No differences were found in the percentage of Cl-IB-MECA-inhibited Ca2+ current in neurons isolated from vehicle- or DNBS-treated rats (20.0 ± 4.1% and 22.5 ± 6.1% of ICa inhibition in Cl-IB-MECA, respectively, n = 7 in both groups; P = 0.7332 unpaired Student t test; Fig. 7C) nor in the percentage of PD173232-blocked currents (72.3 ± 4.8% and 77.7 ± 8.2%, of ICa inhibition in PD173232, respectively; n = 4 in both groups; P = 0.5867 unpaired Student t test, data not shown). Similar results to those obtained with the prototypical A3AR agonist Cl-IB-MECA were recorded with MRS5980 (30 nM): the compound inhibited, to a similar extent, Ca2+ currents either in neurons isolated from vehicle- or DNBS-treated rats and the effect was prevented by MRS1523 and by the N-type channel blocker PD173232 (1 μM: Figs. 7C-E).
Figure 7.
Effects of the adenosine A3R agonists Cl-IB-MECA and MRS5950 on voltage-gated Ca2+ currents in isolated DRG neurons. (A) Time course (left panel) and representative Ca2+ current traces (right panel) measured in a representative DRG neuron isolated from a DNBS-treated rat (DNBS) before and after the application of the A3R agonist Cl-IB-MECA (30 nM) or the Ca2+ channel blocker Cd2+ (100 μM). (B) Averaged time courses of peak Ca2+ currents during Cl-IB-MECA (30 nM) superfusion in vehicle-treated or DNBS-treated animals in the absence or presence of the A3AR antagonist, MRS1523 (1523: 100 nM). Pooled data (mean ± SEM) of % Ca2+ current inhibition induced by Cl-IB-MECA (C) or MRS5980 (D) applied alone or in combination with MRS1523 or PD173232 (PD; 1 μM) in vehicle-treated (white columns) or DNBS-treated (red columns) animals. In all panels: *P <0.05 vs respective control, paired Student t test. (E) Averaged time course of peak Ca2+ currents before or after MRS5980 (30 nM) superfusion DNBS rats in the presence of the A3AR antagonist MRS1523 or the Ca2+ channel blocker PD173232 (1 μM). DRG, dorsal root ganglia.
4. Discussion
In the present work, we studied the role of A3AR as a possible new pharmacological target for relieving visceral pain. The novel highly selective second-generation A3AR agonist, MRS5980, as well as the first-generation A3AR agonist, Cl-IB-MECA, were effective against postinflammatory visceral hypersensitivity in rats. The intracolonic injection of DNBS in rodents induces a long-lasting visceral hypersensitivity,1,32,33 which can be measured with high reproducibility as a lowered sensory threshold to CRD.52,61,82 DNBS-induced visceral hypersensitivity persists after the resolution of the acute inflammatory phase,1,40,53 making this model suitable to investigate visceral pain related not only related to IBD but also to IBS, a chronic disease characterized by a marked abdominal pain in the absence of histopathological explanations.84,90
The administration of A3AR agonists in the model of post-inflammatory visceral pain showed an efficacy equivalent to that of linaclotide, an approved treatment for reducing abdominal pain (and also abdominal bloating and bowel symptoms) in adult patients suffering from IBS with predominant constipation (IBS-C).13,18
The efficacy of MRS5980 and linaclotide was confirmed in female rats. These data have a clinical significance because IBS affects more women than men.12 Abundant evidence from epidemiologic studies clearly demonstrates that women are at substantially greater risk for many clinical pain conditions.5 In accordance, the present results showed a higher visceral hypersensitivity in female animals than in males. This phenomenon is not limited to abdominal/pelvic pain (such as IBS, painful bladder syndrome, and dyspareunia), but also includes conditions associated with neuropathic pain, musculoskeletal pain, orofacial pain, and headache/migraine.27 Women are therefore more likely than men to pursue a variety of treatments for many painful conditions.
The trinitrobenzenesulfonic acid (TNBS)-induced model was used in preclinical studies to identify the potential clinical utility of linaclotide.25 Our results with linaclotide in the DNBS-induced model support previous findings. The analgesic mechanism of linaclotide involves the activation of guanylate cyclase-C (GC-C) expressed on mucosal epithelial cells, resulting in the production and release of cyclic guanosine-3′,5′-monophosphate (cGMP). The extracellular cGMP acts on and inhibits nociceptors, thereby reducing nociception.13 Although linaclotide is an efficacious, well-tolerated treatment option for improving both bowel symptoms and abdominal pain in IBS-C, it acts as a secretagogues and this implies that most patients reported episodes of diarrhea within the first 2 to 4 weeks of treatment.18 This adverse effect also makes linaclotide unsuitable for patients affected by diarrhoea-predominant IBS or IBS with alternating constipation and diarrhoea. In healthy condition, adenosine modulated colonic cholinergic motility through activation of A3 receptors in the myenteric plexus. Antonioli et al. demonstrated that A3 receptor-mediated tonic inhibitory control by adenosine was impaired in inflamed bowel, despite the increased density of functioning and pharmacologically recruitable A3 receptors found in the gut.2 To verify a possible interaction of MRS5980 with gut motility, we performed focused experiments. Our findings using 70 kDa FITC-dextran revealed that MRS5980 does not impair gastrointestinal transit as compared to vehicle. Importantly, these results indicate that the antihyperalgesic efficacy of MRS5980 in visceral pain condition is not associated to motility alteration, suggesting a mechanism related to sensitivity modulation rather than spasmolytic activity. This profile is different, eg, from the opioid analgesic drugs, mainly used in patients affected by IBS-D, that in the same experiment reduce intestinal transit leading to constipation in humans.10,47,66,76 Anyway, the impact of A3 receptor agonists’ repeated administrations on intestinal motility and sensitivity is an aspect that needs to be explored further in the perspective of developing these molecules as drugs and of establishing a therapeutic regime in patients. The lack of significant long-term effects on intestinal motility could privilege A3 adenosine agonists over all the other drugs currently in use. Anyway, in clinical trials investigating the efficacy of A3 receptor agonist on rheumatoid arthritis and psoriasis, no relevant gastrointestinal side effects were reported.20,41,80The efficacy of A3AR agonists against visceral pain is consistent with data showing that the A3AR is involved with multiple pain mechanisms at peripheral, spinal, and supraspinal levels.43,50 There is evidence that A3AR agonists are antihyperalgesic against neuropathic pain through inhibition of the astrocyte-associated neuroinflammatory response in the spinal cord,42,44,96 a phenomenon strongly involved in pain persistence.22,23 A3AR activation has been reported to enhance the formation of anti-inflammatory cytokines36,42,44,96 and the production of glial-derived neuroprotective substances.99 Furthermore, in vitro and in vivo studies demonstrate that A3AR produces its effects by inhibiting the p38 MAPK and NF-κB signaling pathways42,55,92,94 and inflammasome activity.96 All these mechanisms may contribute to the relief of colitis-evoked visceral pain consistently with the reports that A3AR activation reduced colitis-induced tissue injury by modulating the NF-κB signalling pathway and through inhibiting NLRP3 inflammasome activation and pyroptosis in human colonic epithelial cells.70,71
A3ARs may limit excitatory neurotransmission, which is altered in chronic34 as well as in visceral pain.31,48,98 For example, the protective role of A3AR in the first phase of ischemia seems to be at least partly related to a decrease in synaptic transmission.67,68,72 Moreover, A3AR activation protects against the neurotoxic intracellular Ca2+ rise mediated by P2X7 or NMDA receptors.103,104 All the mechanisms described above could account for a therapeutic effect of repeated administrations of A3 receptor agonists, which is another aspect interesting to study. Anyway, the acute efficacy showed by A3 receptor agonists in reducing visceral hypersensitivity can be only partially explained by the mechanisms of action previously cited. This consideration has aroused our interest in elucidating further the pharmacodynamic mechanisms of A3 receptor agonists on pain.
As recently demonstrated,19 selective A3AR stimulation inhibits N-type Cav2.2 opening in isolated rat DRG neurons. Here, we found that both Cl-IB-MECA and MRS5980 significantly inhibited Cav2.2 activation in DRG neurons of DNBS-treated rats. The effect was prevented by the selective A3AR antagonist, MRS1523, and by the Cav2.2 blocker, PD173212, confirming the involvement of Cav2.2 in the A3AR-mediated effect. We did not measure a significant difference between A3AR-mediated inhibition in DRG neurons isolated from DNBS-treated vs vehicle-treated animals. However, alterations in passive membrane properties and steady-state Ca2+ currents were observed between the 2 groups. The resting membrane potential was significantly more depolarized in DRG neurons isolated from DNBS-treated animals, suggesting a hyperexcitable state. Interestingly, steady-state Ca2+ currents, but not peak Ca2+ currents, were markedly reduced in DRG neurons isolated from DNBS animals. In an attempt to explain the relationship between steady-state Ca2+ current reduction in DRG neurons and visceral hypersensitivity, we hypothesize that a decrease in sustained Ca2+ influx into the cell can lead to membrane potential instability and depolarization through a decrease of Ca2+-activated K+ channel (KCa) opening. Ca2+-activated K+ channel is known to stabilize the membrane potential and participate in repolarizing neurons after action potential firing, thus avoiding bursting activity.37,63,102
Moreover, a recent publication demonstrates that Cav2.2 induces a voltage-dependent, but Ca2+-independent ATP secretion from the soma of DRG neurons.15 ATP is a powerful mediator of pain, for example, ATP-induced P2X3 receptor activation is involved in visceral pain, as shown by the ability of P2X3 antagonist A-317491 to potently reduce hypersensitivity.21
The hypothesis that Cav2.2-mediated modulation contributes to A3AR-mediated antihyperalgesia is supported by other evidence. Modulation of Cav2.2 channel activity is implied in the pharmacodynamics of pain-relieving compounds, such as other Gi-coupled receptor agonists, eg, opioids, cannabinoids, neuropeptide Y, and substance P.7,78,81,87,91 Also, the neuropathic pain analgesics gabapentin and pregabalin28 inhibit Cav2.2-mediated synaptic transmission.6,88 Gabapentinoids are effective in reducing visceral pain and preventing spinal neuronal activation associated with CRD in animals62,69,79 and have been suggested to be treatments for IBS.11 A direct inhibitor of Cav2.2 channel activity, ziconotide, is currently used clinically for pain therapy, although it is limited to the intrathecal route.56 In our hands, the i.p. administration of the specific Cav2.2 inhibitor PD17321238,56,73 significantly decreased visceral hypersensitivity in DNBS-treated animals. In conclusion, A3AR agonists seem to be a promising resource for visceral pain management, an unmet medical need that impairs quality of life.
Supplementary Material
Acknowledgments
This research was supported by the Italian Ministry of Instruction, University and Research and by the University of Florence. The authors are grateful to Dr G.J. Bennett (Department of Anesthesiology, University of California, San Diego, La Jolla, CA) for his input in our work and for editorial contribution to the manuscript.
Conflict of interest statement
K.A. Jacobson thanks the NIDDK Intramural Research Program (ZIA DK031117). D. Salvemini is a cofounder of BioIntervene, Inc, which licensed related intellectual property from Saint Louis University. The remaining authors have no conflicts of interest to declare.
Footnotes
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B10.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.painjournalonline.com).
References
- [1].Adam B, Liebregts T, Gschossmann JM, Krippner C, Scholl F, Ruwe M, Holtmann G Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. PAIN 2006;123:179–86. [DOI] [PubMed] [Google Scholar]
- [2].Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Awwad O, Bin A, Zoppellaro C, Castagliuolo I, Gaion RM, Giron MC, Blandizzi C. Control of enteric neuromuscular functions by purinergic A(3) receptors in normal rat distal colon and experimental bowel inflammation. Br J Pharmacol 2010;161:856–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Asano T, Takenaga M. Adenosine A2B receptors: an optional target for the management of irritable bowel syndrome with diarrhea? J Clin Med 2017;6:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Asano T, Tanaka KI, Tada A, Shimamura H, Tanaka R, Maruoka H, Takenaga M, Mizushima T. Aminophylline suppresses stress-induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci Rep 2017;7:40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009;29:4076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beedle AM, Zamponi GW. Modulation of high voltage-activated calcium channels by G protein-coupled receptors. In: McDonough SI, editor. Calcium channel pharmacology. Boston: Springer US, 2004. pp. 331–67. [Google Scholar]
- [8].Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev 2018;98:1591–625. [DOI] [PubMed] [Google Scholar]
- [9].Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol 2014;11:611–27. [DOI] [PubMed] [Google Scholar]
- [10].Burks TF, Fox DA, Hirning LD, Shook JE, Porreca F. Regulation of gastrointestinal function by multiple opioid receptors. Life Sci 1988;43:2177–81. [DOI] [PubMed] [Google Scholar]
- [11].Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017;66:966–74. [DOI] [PubMed] [Google Scholar]
- [12].Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, Cohen MB, MacDougall JE, Lavins BJ, Kurtz CB, Silos-Santiago I, Johnston JM, Currie MG, Blackshaw LA, Brierley SM. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology 2013;145:1334–46.e1331-1311. [DOI] [PubMed] [Google Scholar]
- [14].Cervero F, Laird JM. Spinal mechanisms of visceral pain and hyperalgesia. In: Synaptic plasticity in pain. New York: Springer, 2009. pp. 289–306. [Google Scholar]
- [15].Chai Z, Wang C, Huang R, Wang Y, Zhang X, Wu Q, Wang Y, Wu X, Zheng L, Zhang C, Guo W, Xiong W, Ding J, Zhu F, Zhou Z. CaV2.2 gates calcium-independent but voltage-dependent secretion in mammalian sensory neurons. Neuron 2017;96:1317–26.e1314. [DOI] [PubMed] [Google Scholar]
- [16].Chen Y, Lin C, Tang Y, Chen AQ, Liu CY, Lu DL. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J Gastroenterol 2014;20:2091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen Z, Janes K, Chen C, Doyle T, Bryant L, Tosh DK, Jacobson KA, Salvemini D. Controlling murine and rat chronic pain through A3 adenosine receptor activation. FASEB J 2012;26:1855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chey WD, Lembo AJ, Lavins BJ, Shift SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA, Baird MJ, Schneier HA, Johnston JM. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012;107:1702–12. [DOI] [PubMed] [Google Scholar]
- [19].Coppi E, Cherchi F, Fusco I, Failli P, Vona A, Dettori I, Gaviano L, Lucarini E, Jacobson KA, Tosh DK, Salvemini D, Ghelardini C, Pedata F, Cesare Di Mannelli L, Pugliese AM. Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. PAIN 2019;160:1103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].David M, Gospodinov DK, Gheorghe N, Mateev GS, Rusinova MV, Hristakieva E, Solovastru LG, Patel RV, Giurcaneanu C, Hitova MC, Purcaru AI, Horia B, Tsingov II, Yankova RK, Kadurina MI, Ramon M, Rotaru M, Simionescu O, Benea V, Demerdjieva ZV, Cosgarea MR, Morariu HS, Michael Z, Cristodor P, Nica C, Silverman MH, Bristol DR, Harpaz Z, Farbstein M, Cohen S, Fishman P. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol 2016;15:931–8. [PubMed] [Google Scholar]
- [21].Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One 2015;10:e0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphologic features and glial activation in rat oxaliplatin-dependent neuropathic pain. J Pain 2013;14:1585–600. [DOI] [PubMed] [Google Scholar]
- [23].Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol 2014;261:22–33. [DOI] [PubMed] [Google Scholar]
- [24].Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilic-Stojanovic M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, Fioramonti J, Cohen M, Bryant AP, Kurtz C, Currie MG, Mayer EA, Bueno L. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 2010;22:312–e84. [DOI] [PubMed] [Google Scholar]
- [26].Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis 2006;12:38–46. [DOI] [PubMed] [Google Scholar]
- [27].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL III. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today 2012;17:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fornai M, Blandizzi C, Antonioli L, Colucci R, Bernardini N, Segnani C, De Ponti F, Del Tacca M. Differential role of cyclooxygenase 1 and 2 isoforms in the modulation of colonic neuromuscular function in experimental inflammation. J Pharmacol Exp Ther 2006;317:938–45. [DOI] [PubMed] [Google Scholar]
- [31].Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol 2016;6:1609–33. [DOI] [PubMed] [Google Scholar]
- [32].Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 2015;308:G885–903. [DOI] [PubMed] [Google Scholar]
- [33].Gschossmann JM, Liebregts T, Adam B, Buenger L, Ruwe M, Gerken G, Holtmann G. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 2004;49:96–101. [DOI] [PubMed] [Google Scholar]
- [34].Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol 2012;234:362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82. [DOI] [PubMed] [Google Scholar]
- [36].Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996;157:4634–40. [PubMed] [Google Scholar]
- [37].Hogan QH. Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain. Croat Med J 2007;48:9–21. [PMC free article] [PubMed] [Google Scholar]
- [38].Hu LY, Ryder TR, Rafferty MF, Dooley DJ, Geer JJ, Lotarski SM, Miljanich GP, Millerman E, Rock DM, Stoehr SJ, Szoke BG, Taylor CP, Vartanian MG. Structure-activity relationship of N-methyl-N-aralkyl-peptidylamines as novel N-type calcium channel blockers. Bioorg Med Chem Lett 1999;9:2151–6. [DOI] [PubMed] [Google Scholar]
- [39].Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem 2005;80:79–87. [DOI] [PubMed] [Google Scholar]
- [40].Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 2009;58:1333–41. [DOI] [PubMed] [Google Scholar]
- [41].Jacobson KA, Merighi S, Varani K, Borea PA, Baraldi S, Aghazadeh Tabrizi M, Romagnoli R, Baraldi PG, Ciancetta A, Tosh DK, Gao ZG, Gessi S. A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med Res Rev 2018;38:1031–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA, Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. PAIN 2014;155:2560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Janes K, Symons-Liguori AM, Jacobson KA, Salvemini D. Identification of A3 adenosine receptor agonists as novel non-narcotic analgesics. Br J Pharmacol 2016;173:1253–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Janes K, Wahlman C, Little JW, Doyle T, Tosh DK, Jacobson KA, Salvemini D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun 2015;44:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci 2003;23:3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 2008;154:1562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jiang Q, Sheldon RJ, Porreca F. A comparison of the central gastrointestinal antitransit effects of morphine and bombesin in the mouse. Life Sci 1987;41:2455–61. [DOI] [PubMed] [Google Scholar]
- [48].Lapointe TK, Basso L, Iftinca MC, Flynn R, Chapman K, Dietrich G, Vergnolle N, Altier C. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol 2015;309:G87–99. [DOI] [PubMed] [Google Scholar]
- [49].Li AH, Moro S, Melman N, Ji XD, Jacobson KA. Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J Med Chem 1998;41:3186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Little JW, Ford A, Symons-Liguori AM, Chen Z, Janes K, Doyle T, Xie J, Luongo L, Tosh DK, Maione S, Bannister K, Dickenson AH, Vanderah TW, Porreca F, Jacobson KA, Salvemini D. Endogenous adenosine A3 receptor activation selectively alleviates persistent pain states. Brain 2015;138:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- [52].Lowen MB, Mayer E, Tillisch K, Labus J, Naliboff B, Lundberg P, Thorell LH, Strom M, Engstrom M, Walter S. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterol Motil 2015;27:646–55. [DOI] [PubMed] [Google Scholar]
- [53].Lucarini E, Di Cesare Mannelli L, Micheli L, Trallori E, Antonioli L, Fornai M, Blandizzi C, Ghelardini C. P060 Post-inflammatory visceral pain induced by DNBS: preclinical features for novel therapeutics. J Crohn’s Colitis 2018;12(suppl_1):S123. [Google Scholar]
- [54].Luongo L, Petrelli R, Gatta L, Giordano C, Guida F, Vita P, Franchetti P, Grifantini M, de Novellis V, Cappellacci L, Maione S. 5’-Chloro-5’-deoxy-(+/−)-ENBA, a potent and selective adenosine A(1) receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules 2012;17:13712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer F, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. J Rheumatol 2007;34:20–6. [PubMed] [Google Scholar]
- [56].McDowell GC II, Pope JE. Intrathecal ziconotide: dosing and administration strategies in patients with refractory chronic pain. Neuromodulation 2016;19:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 2015;172:3189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Minderhoud IM, Oldenburg B, Wismeijer JA, van Berge Henegouwen GP, Smout AJ. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci 2004;49:469–74. [DOI] [PubMed] [Google Scholar]
- [59].Moloney RD, Sajjad J, Foley T, Felice VD, Dinan TG, Cryan JF, O’Mahony SM. Estrous cycle influences excitatory amino acid transport and visceral pain sensitivity in the rat: effects of early-life stress. Biol Sex Differ 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ness TJ. Cardiovascular and visceromotor responses to noxious visceral stimuli vary with the estrous cycle in rats. Reg Anesth Pain Med 1999;24(suppl 1):26. [Google Scholar]
- [61].Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 1988;450:153–69. [DOI] [PubMed] [Google Scholar]
- [62].Ohashi K, Kawai M, Ninomiya N, Taylor C, Kurebayashi Y. Effect of a new alpha 2 delta ligand PD-217014 on visceral hypersensitivity induced by 2,4,6-trinitrobenzene sulfonic acid in rats. Pharmacology 2008;81:144–50. [DOI] [PubMed] [Google Scholar]
- [63].Pagadala P, Park CK, Bang S, Xu ZZ, Xie RG, Liu T, Han BX, Tracey WD, Wang F, Ji RR, Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca2+-activated small conductance potassium channels. J Neurosci 2013;33:13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Parisio C, Lucarini E, Micheli L, Toti A, Di Cesare Mannelli L, Antonini G, Panizzi E, Maidecchi A, Giovagnoni E, Lucci J, Ghelardini C. Researching new therapeutic approaches for abdominal visceral pain treatment: preclinical effects of an assembled system of molecules of vegetal origin. Nutrients 2019;12:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Petrelli R, Scortichini M, Belardo C, Boccella S, Luongo L, Capone F, Kachler S, Vita P, Del Bello F, Maione S, Lavecchia A, Klotz KN, Cappellacci L. Structure-based design, synthesis, and in vivo antinociceptive effects of selective A1 adenosine receptor agonists. J Med Chem 2018;61:305–18. [DOI] [PubMed] [Google Scholar]
- [66].Porreca F, Burks TF. The spinal cord as a site of opioid effects on gastrointestinal transit in the mouse. J Pharmacol Exp Ther 1983;227:22–7. [PubMed] [Google Scholar]
- [67].Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol 2006;147:524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pugliese AM, Latini S, Corradetti R, Pedata F. Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br J Pharmacol 2003;140:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ravnefjord A, Brusberg M, Larsson H, Lindstrom E, Martinez V. Effects of pregabalin on visceral pain responses and colonic compliance in rats. Br J Pharmacol 2008;155:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ren T, Tian T, Feng X, Ye S, Wang H, Wu W, Qiu Y, Yu C, He Y, Zeng J, Cen J, Zhou Y. An adenosine A3 receptor agonist inhibits DSS-induced colitis in mice through modulation of the NF-kappaB signaling pathway. Sci Rep 2015;5:9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ren T, Zhou Y, Wu W. Su1831—activation of adenosine A3 receptor inhibits Nlrp3 inflammasome and pyroptosis of colonic epithelial cells of patients with ulcerative colitis. Gastroenterology 2019;156:S–627. [Google Scholar]
- [72].Rivera-Oliver M, Diaz-Rios M. Using caffeine and other adenosine receptor antagonists and agonists as therapeutic tools against neurodegenerative diseases: a review. Life Sci 2014;101:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ryder TR, Hu LY, Rafferty MF, Millerman E, Szoke BG, Tarczy-Hornoch K Multiple parallel synthesis of N,N-dialkyldipeptidylamines as N-type calcium channel blockers. Bioorg Med Chem Lett 1999;9:1813–18. [DOI] [PubMed] [Google Scholar]
- [74].Sajjad J, Felice VD, Golubeva AV, Cryan JF, O’Mahony SM. Sex-dependent activity of the spinal excitatory amino acid transporter: role of estrous cycle. Neuroscience 2016;333:311–19. [DOI] [PubMed] [Google Scholar]
- [75].Salvemini D, Jacobson KA. Highly selective A3 adenosine receptor agonists relieve chronic neuropathic pain. Expert Opin Ther Pat 2017;27:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Santos FA, Rao VS. Quinine-induced inhibition of gastrointestinal transit in mice: possible involvement of endogenous opioids. Eur J Pharmacol 1999;364:193–7. [DOI] [PubMed] [Google Scholar]
- [77].Sawynok J Adenosine receptor targets for pain. Neuroscience 2016;338:1–18. [DOI] [PubMed] [Google Scholar]
- [78].Shapiro MS, Hille B. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G protein pathways. Neuron 1993;10:11–20. [DOI] [PubMed] [Google Scholar]
- [79].Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care 2012;6:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, Rosner I, Rozenbaum M, Mader R, Adawi M, Caspi D, Tishler M, Langevitz P, Rubinow A, Friedman J, Green L, Tanay A, Ochaion A, Cohen S, Kerns WD, Cohn I, Fishman-Furman S, Farbstein M, Yehuda SB, Fishman P. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol 2008;35:41–8. [PubMed] [Google Scholar]
- [81].Soldo BL, Moises HC. mu-opioid receptor activation inhibits N- and P-type Ca2+ channel currents in magnocellular neurones of the rat supraoptic nucleus. J Physiol 1998;513:787–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Spaziani R, Bayati A, Redmond K, Bajaj H, Mazzadi S, Bienenstock J, Collins SM, Kamath MV. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol Motil 2008;20:336–42. [DOI] [PubMed] [Google Scholar]
- [83].Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis 2009;41:844–9. [DOI] [PubMed] [Google Scholar]
- [84].Spiller R, Major G. IBS and IBD—separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol 2016;13:613–21. [DOI] [PubMed] [Google Scholar]
- [85].Srinath A, Young E, Szigethy E. Pain management in patients with inflammatory bowel disease: translational approaches from bench to bedside. Inflamm Bowel Dis 2014;20:2433–49. [DOI] [PubMed] [Google Scholar]
- [86].Srinath AI, Walter C, Newara MC, Szigethy EM. Pain management in patients with inflammatory bowel disease: insights for the clinician. Therap Adv Gastroenterol 2012;5:339–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sun L, Miller RJ. Multiple neuropeptide Y receptors regulate K+ and Ca2+ channels in acutely isolated neurons from the rat arcuate nucleus. J Neurophysiol 1999;81:1391–403. [DOI] [PubMed] [Google Scholar]
- [88].Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol 2002;135:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tosh DK, Finley A, Paoletta S, Moss SM, Gao ZG, Gizewski ET, Auchampach JA, Salvemini D, Jacobson KA. In vivo phenotypic screening for treating chronic neuropathic pain: modification of C2-arylethynyl group of conformationally constrained A3 adenosine receptor agonists. J Med Chem 2014;57:9901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tsang SW, Auyeung KKW, Bian ZX, Ko JKS. Pathogenesis, experimental models and contemporary pharmacotherapy of irritable bowel syndrome: story about the brain-gut Axis. Curr Neuropharmacol 2016;14:842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol 1997;78:43–50. [DOI] [PubMed] [Google Scholar]
- [92].Varani K, Maniero S, Vincenzi F, Targa M, Stefanelli A, Maniscalco P, Martini F, Tognon M, Borea PA. A(3) receptors are overexpressed in pleura from patients with mesothelioma and reduce cell growth via Akt/nuclear factor-kappaB pathway. Am J Respir Crit Care Med 2011;183:522–30. [DOI] [PubMed] [Google Scholar]
- [93].Varani K, Vincenzi F, Targa M, Paradiso B, Parrilli A, Fini M, Lanza G, Borea PA. The stimulation of A(3) adenosine receptors reduces bone-residing breast cancer in a rat preclinical model. Eur J Cancer 2013;49:482–91. [DOI] [PubMed] [Google Scholar]
- [94].Varani K, Vincenzi F, Tosi A, Targa M, Masieri FF, Ongaro A, De Mattei M, Massari L, Borea PA Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes. Br J Pharmacol 2010;160:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vilz TO, Overhaus M, Stoffels B, Websky M, Kalff JC, Wehner S. Functional assessment of intestinal motility and gut wall inflammation in rodents: analyses in a standardized model of intestinal manipulation. J Vis Exp 2012;67:4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wahlman C, Doyle TM, Little JW, Luongo L, Janes K, Chen Z, Esposito E, Tosh DK, Cuzzocrea S, Jacobson KA, Salvemini D. Chemotherapy-induced pain is promoted by enhanced spinal adenosine kinase levels through astrocyte-dependent mechanisms. PAIN 2018;159:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 2018;67:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology 2004;126:683–92. [DOI] [PubMed] [Google Scholar]
- [99].Wittendorp MC, Boddeke HW, Biber K. Adenosine A3 receptor-induced CCL2 synthesis in cultured mouse astrocytes. Glia 2004;46:410–18. [DOI] [PubMed] [Google Scholar]
- [100].Wu WP, Hao JX, Halldner-Henriksson L, Xu XJ, Jacobson MA, Wiesenfeld-Hallin Z, Fredholm BB. Decreased inflammatory pain due to reduced carrageenan-induced inflammation in mice lacking adenosine A3 receptors. Neuroscience 2002;114:523–7. [DOI] [PubMed] [Google Scholar]
- [101].Yoon MH, Bae HB, Choi JI. Antinociception of intrathecal adenosine receptor subtype agonists in rat formalin test. Anesth Analg 2005;101:1417–21. [DOI] [PubMed] [Google Scholar]
- [102].Zahn PK, Straub H, Wenk M, Pogatzki-Zahn EM. Adenosine A1 but not A2a receptor agonist reduces hyperalgesia caused by a surgical incision in rats: a pertussis toxin-sensitive G protein-dependent process. Anesthesiology 2007;107:797–806. [DOI] [PubMed] [Google Scholar]
- [103].Zhang M, Hu H, Zhang X, Lu W, Lim J, Eysteinsson T, Jacobson KA, Laties AM, Mitchell CH. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int 2010;56:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhang X, Zhang M, Laties AM, Mitchell CH. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J Neurochem 2006;98:566–75. [DOI] [PubMed] [Google Scholar]
- [105].Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med 2011;17:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.