ABSTRACT
Cilia are essential organelles required for cell signaling and motility. Nearly all motile cilia have a ‘9+2’ axoneme composed of nine outer doublet microtubules plus two central microtubules; the central microtubules together with their projections are termed the central apparatus (CA). In Chlamydomonas reinhardtii, a model organism for studying cilia, 30 proteins are known CA components, and ∼36 more are predicted to be CA proteins. Among the candidate CA proteins is the highly conserved FAP70 (CFAP70 in humans), which also has been reported to be associated with the doublet microtubules. Here, we determined by super-resolution structured illumination microscopy that FAP70 is located exclusively in the CA, and show by cryo-electron microscopy that its N-terminus is located at the base of the C2a projection of the CA. We also found that fap70-1 mutant axonemes lack most of the C2a projection. Mass spectrometry revealed that fap70-1 axonemes lack not only FAP70 but two other conserved candidate CA proteins, FAP65 (CFAP65 in humans) and FAP147 (MYCBPAP in humans). Finally, FAP65 and FAP147 co-immunoprecipitated with HA-tagged FAP70. Taken together, these data identify FAP70, FAP65 and FAP147 as the first defining components of the C2a projection.
KEY WORDS: Flagella, Axonemal central apparatus, FAP65, FAP147, CFAP70, MYCBP, MYCBPAP, FAP174, ASH domains
Summary: The central apparatus is essential for motility of ‘9+2’ cilia. The conserved proteins FAP70, FAP65 and FAP147 are components of the previously uncharacterized central apparatus projection C2a.
INTRODUCTION
Cilia and flagella (terms used interchangeably here) are important organelles required for motility and signal transduction. Defects in cilia cause a constellation of diseases collectively termed ciliopathies (Anvarian et al., 2019; Brown and Witman, 2014). Defects specifically affecting ciliary motility cause primary ciliary dyskinesia (PCD) (Legendre et al., 2021; Wallmeier et al., 2020). Most motile cilia have a typical 9+2 axoneme comprised of nine outer doublet microtubules (DMTs) plus two central microtubules. Attached to the DMTs are substructures, including outer and inner dynein arms, nexin-dynein regulatory complexes and radial spokes. Attached to the two central microtubules is a complex array of interconnected projections; the central microtubules together with their projections are termed the central apparatus (CA). The DMT substructures and the CA work together to generate and regulate ciliary motility.
To date, much of our knowledge of the structure, function and composition of axonemes of motile cilia has come from the model organism Chlamydomonas reinhardtii (Grossman-Haham et al., 2021; Gui et al., 2021; King, 2018; Ma et al., 2019; Witman, 2009). A 2005 proteomics analysis of the Chlamydomonas cilium revealed that its axoneme contains about 400 proteins (Pazour et al., 2005), of which fewer than 100 had previously been characterized at the molecular level. Most of these proteins are conserved in other organisms, including humans, and much research since then has been aimed at determining where in the axoneme the previously uncharacterized proteins are located and what their functions are.
One such highly conserved axonemal protein that is of considerable interest, but which has received attention only recently, is FAP70. Chlamydomonas FAP70 is a 111-kDa protein that is removed from the axoneme by treatment with 0.6 M KCl (Pazour et al., 2005). The mammalian ortholog is CFAP70. Shamoto et al. (2018) detected CFAP70 mRNA in numerous mouse tissues that have motile cilia, but not in tissues with only non-motile cilia; immunofluorescence localization of the CFAP70 protein in these tissues confirmed that it is present in epithelial cilia and sperm flagella. Shamoto et al. (2018) demonstrated that CFAP70, like Chlamydomonas FAP70, is an axonemal protein solubilized by treatment with 0.6 M KCl, a property shared with outer arm dynein and some inner arm dyneins. To further investigate the function of this protein, Shamoto et al. (2018) used insertional mutagenesis to create FAP70 null mutants (fap70-1 and fap70-2) in Chlamydomonas; compared to controls, swimming speed of the fap70 cells was reduced by ∼60% and ciliary beat frequency was reduced by about the same amount, with little effect on ciliary waveform. This phenotype, which demonstrated that FAP70 is important for ciliary motility, was similar to that previously observed for mutants with defects in the outer dynein arms. Shamoto et al. (2018) then used cryo-electron tomography (cryo-ET) to investigate the location of FAP70 within the axoneme. Close examination of the fap70 DMTs found no obvious changes. However, when the mutant was rescued by expression of FAP70 fused to biotin carboxyl carrier protein (FAP70-N-BCCP), which forms a tight complex with streptavidin that can be detected by cryo-ET (Oda and Kikkawa, 2013), streptavidin signal was present at the base of the outer dynein arm, as well as associated with the nexin-dynein regulatory complex. Taking all of the above into consideration, they concluded that FAP70 is located on the DMTs, at the base of the outer arms.
However, more recently, FAP70 was identified as a candidate CA protein in two separate proteomic studies of the Chlamydomonas CA (Dai et al., 2020; Zhao et al., 2019). Prior to these studies, 22 proteins had been identified as components of the CA; the proteomics analyses identified over three dozen additional proteins, including FAP70, as candidate CA components. The two central microtubules, which are held together by ‘bridge’ structures and can be distinguished from each other by their projections and stability properties, are termed C1 and C2. Cryo-ET has revealed that C1 has six highly interconnected projections termed C1a to C1f (see Fig. 4A) (Carbajal-González et al., 2013); to date, approximately two dozen proteins have been localized to specific projections of the C1 microtubule (Brown et al., 2012; DiPetrillo and Smith, 2010; Dutcher et al., 1984; Fu et al., 2019; Mitchell et al., 2005; Mitchell and Sale, 1999; Wargo et al., 2005; Zhang and Mitchell, 2004; Cai et al., 2021 preprint). The C2 microtubule, which in isolated axonemes is less stable than C1 and can be selectively solubilized by 0.6 M KCl (Dutcher et al., 1984; Lechtreck and Witman, 2007; Mitchell and Sale, 1999), has five projections termed C2a to C2e. To date, only three proteins have been localized to specific C2 projections (Bernstein et al., 1994; Lechtreck and Witman, 2007; Rao et al., 2016; Yokoyama et al., 2004). The proteomics studies did not definitively localize FAP70 in the CA, but based on mutant analyses and protein solubility studies, it was predicted to be associated with the C2 microtubule (Dai et al., 2020; Zhao et al., 2019).
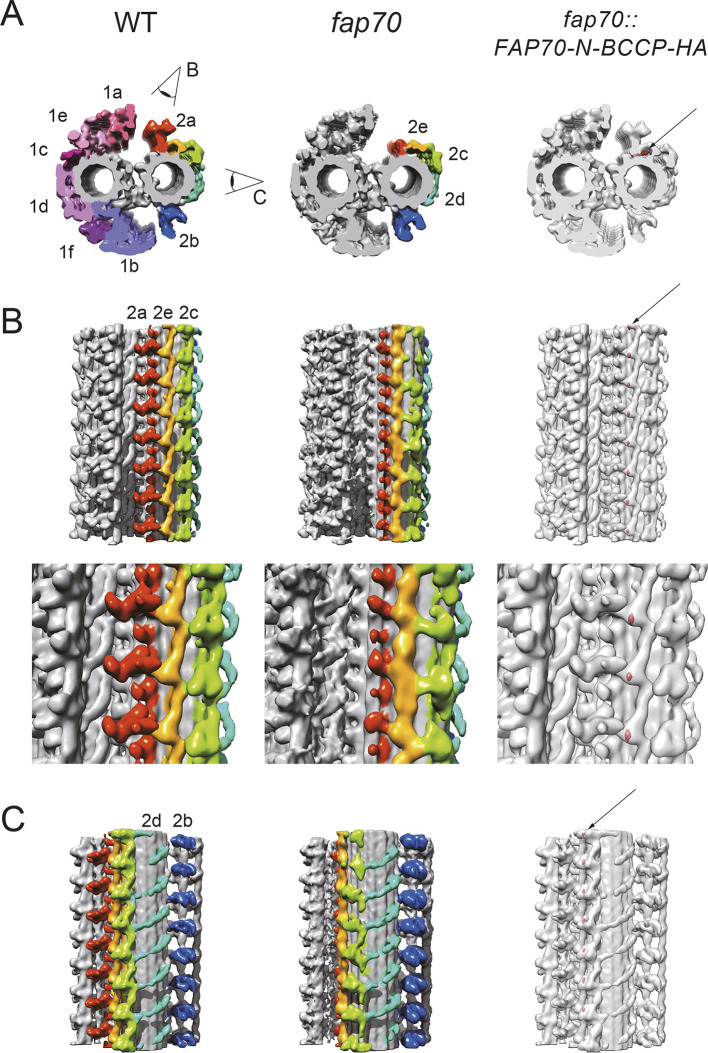
Fig. 4.
Cryo-electron tomography confirms ablation of the C2a projection in fap70 axonemes and localizes FAP70 within the CA. (A-C) Averaged subtomograms of the CA. Wild-type (WT) and fap70-1 CAs are shown in cross-sectional (A, left and middle subtomograms, respectively) and longitudinal (B and C, left and middle subtomograms, respectively) views. In A, the CA is viewed from base to tip. In B and C, the proximal end of the CA is at the bottom. Most of the C2a projection is absent in the fap70-1 CA. Red, C2a; yellow, C2e; green, C2c; cyan, C2d; and blue, C2b. Eye symbols indicate the directions of the views in B and C. (A-C, right) Three-dimensional-localization of the N-terminus of FAP70-N-BCCP-HA. Tag densities (red, arrows) were visualized by comparing the streptavidin-treated wild-type and streptavidin-labeled fap70::FAP70-N-BCCP-HA CAs. The N-terminus of FAP70 is located at the base of the C2a projection below the C2e arcade. The top row of panel B shows seven 16-nm repeat units of the C2a, C2e and C2c projections. The bottom row of panel B shows magnified views of three of the repeat units. Panel C shows seven 16-nm repeats of the C2d and C2b projections.
Resolution of where in the axoneme FAP70/CFAP70 is located is very important. First, that information is needed to understand the fundamental role of the protein in ciliary motility. Second, its localization is of clinical relevance for the diagnosis of patients who have mutations in CFAP70, because disruption of a protein associated with the DMTs is likely to result in a different clinical presentation than disruption of a protein associated with the CA. Specifically, patients with a defect in a CA-specific protein would not be expected to have laterality defects, which are a key indicator for the screening of potential PCD patients (Shapiro et al., 2018; Zhao et al., 2019).
To resolve the question of where in the axoneme FAP70 is located, as well as to learn more about the protein, we have re-examined its location by immunofluorescence microscopy, conventional transmission electron microscopy (TEM) and cryo-ET. Our results show that FAP70 is a subunit of the C2a projection and that its absence greatly affects the integrity of the C2a projection, with lesser effect on other nearby projections of the C2 microtubule. Furthermore, we compared the proteome of the fap70-1 null mutant axoneme with that of wild-type axonemes by label-free quantitative mass spectrometry (MS) and identified two additional proteins, FAP65 (CFAP65 in humans) and FAP147 (MYCBPAP in humans), which are greatly reduced in fap70-1 axonemes. These two proteins previously were designated candidate CA proteins (Dai et al., 2020; Zhao et al., 2019); interestingly, they both contain ASH domains, which have been proposed to have a microtubule-binding function (Ponting, 2006). We also found that these same two proteins were specifically co-immunoprecipitated with hemagglutinin (HA)-tagged FAP70 expressed in a fap70-1 null background. We conclude that FAP65 and FAP147 are associated with FAP70 in the C2a projection, making C2a at least the fifth projection known to be associated with an ASH-domain protein. This is the first time that components of this projection have been identified. The fact that the fap70 mutation reduces flagellar beat frequency with little effect on waveform (Shamoto et al., 2018) raises the possibility that the C2a projection has a specific role in the regulation of the outer dynein arms. Finally, the confirmation that FAP65, FAP70 and FAP147 are CA proteins will facilitate molecular genetic testing to diagnose suspected PCD patients that may have defects in the human homologs of these proteins.
RESULTS
FAP70 localizes exclusively to the CA
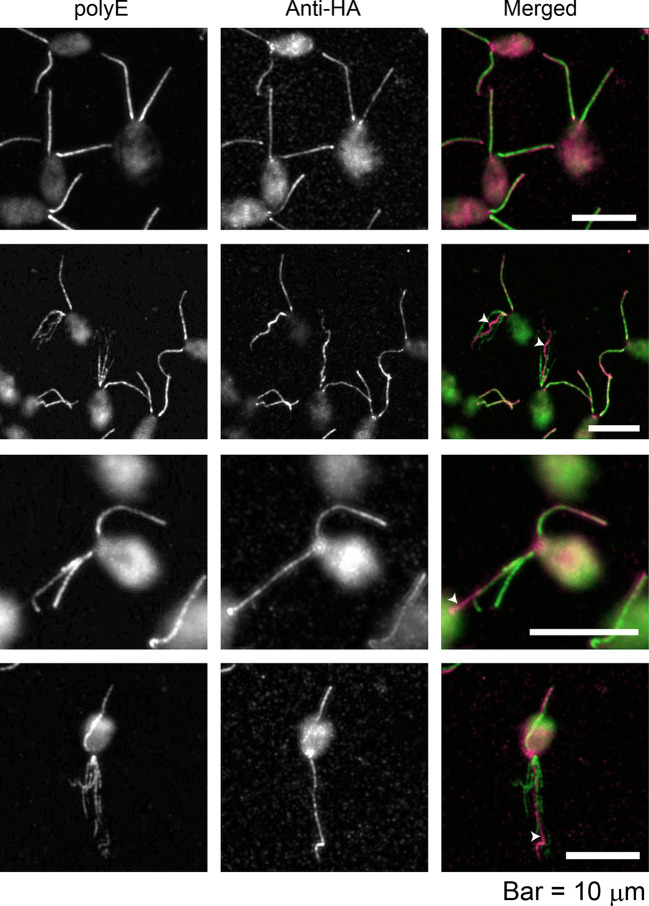
As an initial step to determine the location of FAP70 in the axoneme, we treated demembranated cell models of the fap70::FAP70-N-BCCP-HA strain [fap70-1 cells rescued with a construct expressing FAP70 tagged with biotin carboxyl carrier protein (BCCP) and HA] to induce partial axonemal fraying, and then immunolabeled the specimens with antibodies against HA and against polyglutamate side chains (anti-polyE), which are present only on the B-tubule of the DMTs (Kubo et al., 2010; Lechtreck and Geimer, 2000). Labeling by anti-polyE revealed numerous cells in which one or both axonemes had frayed into two or more bundles of microtubules (Fig. 1). In all cases, only one of the bundles was labeled by anti-HA, suggesting that FAP70 is associated either with a small subset of DMTs or with the CA.
Fig. 1.
FAP70 localizes to only one microtubule bundle in frayed axonemes. fap70::FAP70-N-BCCP-HA cells were double labeled with anti-HA antibody and anti-polyE antibody, which exclusively labels DMTs. The upper row shows cells with intact axonemes; the other rows show cells with frayed axonemes. In the merged images, green is anti-polyE labeling, and magenta is anti-HA labeling. As reported previously, there is a gradient of polyE labeling of intact axonemes, with the base being more strongly labeled than the tip (upper row); this is less apparent in frayed axonemes (Kubo et al., 2010). Anti-HA labeling is more uniform; in those frayed axonemes in which it is brighter toward the tip (third row), it may reflect partial extrusion of the CA, or increased antibody accessibility to the tip region, where axonemal fraying begins. The anti-HA antibody brightly labels only one microtubule bundle in each frayed axoneme (white arrowheads).
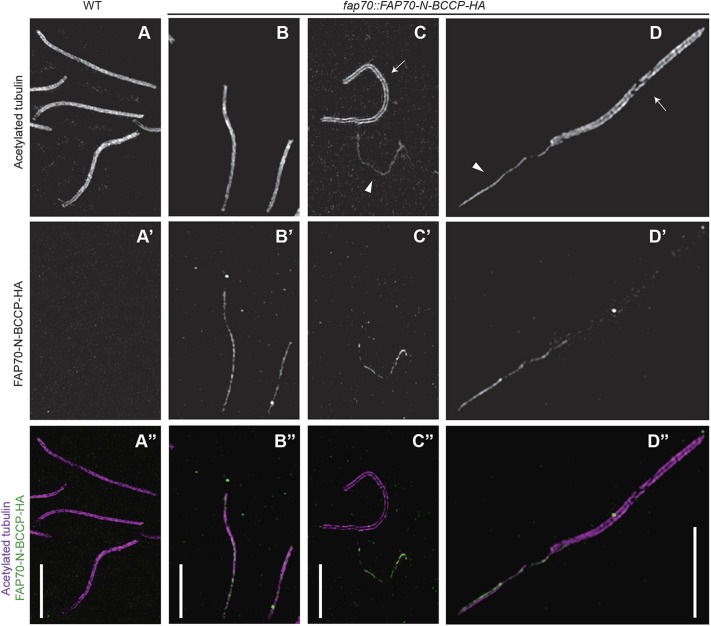
To distinguish between these two possibilities, we next isolated axonemes from wild-type and fap70::FAP70-N-BCCP-HA cells, labeled them with anti-HA and anti-acetylated tubulin antibodies and examined them by super-resolution structured-illumination microscopy (Fig. 2). The antibody to the HA tag labeled a thin structure that is centered in and runs the entire length of the fap70::FAP70-N-BCCP-HA axoneme (column B in Fig. 2); no HA signal was detected in wild-type control axonemes (column A in Fig. 2). Next, the CA was completely (column C in Fig. 2) or partially (column D in Fig. 2) extruded from the axoneme by addition of ATP, followed by immunolabeling (Lechtreck and Witman, 2007; Mitchell and Nakatsugawa, 2004; Zhao et al., 2019). In this case, the HA signal was clearly associated with the CA, but not with the DMTs in the portion of the axoneme from which the CA had been extruded. Therefore, within the axoneme, FAP70 is located exclusively in the CA.
Fig. 2.
FAP70 localizes exclusively to the CA, as demonstrated by super-resolution structured-illumination microscopy. (A-D) Axonemes from wild-type (WT, column A) or fap70::FAP70-N-BCCP-HA (columns B to D) cells were double labeled with anti-acetylated tubulin antibody (upper row, A-D) and anti-HA antibody (middle row, A′-D′). The lower row (A″-D″) shows merged images with labeling by anti-acetylated tubulin in magenta and anti-HA in green. In column A, wild-type axonemes are intact with the CA still inside the axonemes. As expected, the acetylated tubulin label covers the entire width of the axoneme, and there is no HA signal. (Column B) Two intact fap70::FAP70-N-BCCP-HA axonemes. The HA signal colocalizes with the acetylated tubulin signal but is thinner, suggesting that it emanates from the CA. (Column C) fap70::FAP70-N-BCCP-HA axoneme (arrow in panel C) from which the CA has been completely extruded, and a nearby extruded CA (arrowhead in panel C). The HA signal is associated exclusively with the CA. Only the edges of the axoneme are labeled by the antibody to acetylated tubulin, revealing the void that was formerly occupied by the CA. (Column D) fap70::FAP70-N-BCCP-HA axoneme (arrow in panel D) from which the CA (arrowhead in panel D) has been partially extruded. Anti-HA strongly labels the extruded CA but not the DMTs in the portion of the axoneme from which the CA has been extruded. Therefore, FAP70 is located exclusively in the CA. Scale bars: 5 µm.
Loss of FAP70 disrupts the C2a projection
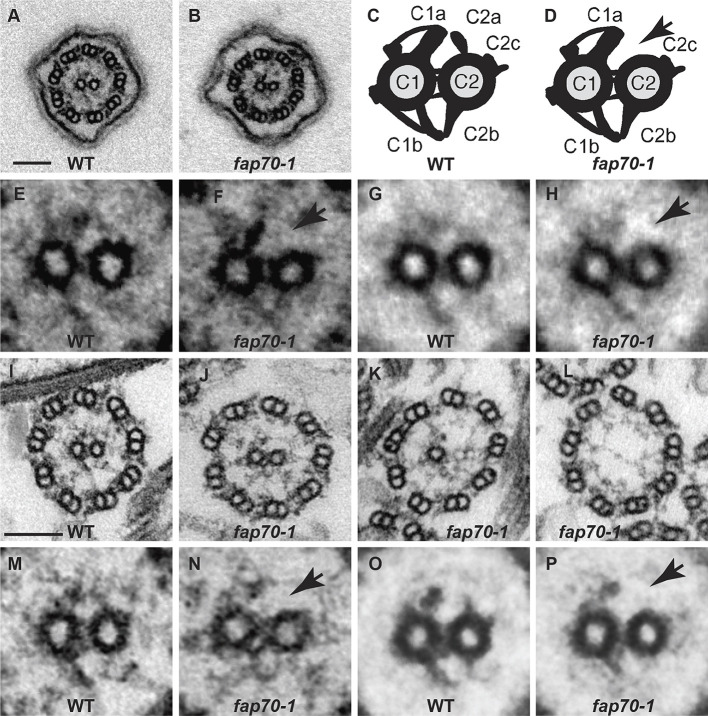
To determine how loss of FAP70 affected axonemal structure, we first compared intact flagella of fixed cells of the wild-type and fap70-1 strains by conventional TEM (Fig. 3A,B, respectively). The ring of nine DMTs appeared normal in the fap70-1 cells, consistent with the observations of Shamoto et al. (2018); in addition, both central microtubules were present in all cross-sections of both wild-type (n=47) and fap70-1 flagella (n=55). To more clearly observe minor structural changes, we also examined isolated demembranated axonemes, which lack the flagellar matrix that tends to obscure structural details in TEM. Almost all cross-sections of wild-type axonemes (Fig. 3I) contained both C1 and C2 microtubules (96% had two microtubules, 4% had zero microtubules, n=271). However, one or both central microtubules were more frequently absent from cross-sections of purified fap70-1 axonemes: among 236 cross-sections scored, 56% had both CA microtubules, 15% had one and 29% had none (Fig. 3J-L, respectively). As viewed in cross-section, the C1 microtubule has larger projections than the C2 microtubule (Fig. 3C). When fap70-1 axonemal cross-sections with both CA microtubules were closely compared with wild-type axonemal cross-sections, the former appeared to be lacking the C2a projection (cf. Fig. 3M,N). This was confirmed by averaging images of the wild-type and fap70-1 CA (Fig. 3O,P, respectively). The difference is illustrated diagrammatically in Fig. 3C,D. As best as can be assessed by conventional TEM, the other projections of the C2 microtubule were unaffected. Therefore, the assembly of C2a is dependent on FAP70.
Fig. 3.
Loss of FAP70 causes ablation of the C2a projection. (A,B) Representative cross-sections of intact in situ flagella of wild-type (WT) and fap70-1 cells, respectively, as imaged by conventional TEM. All flagella of both strains had axonemes with nine DMTs and two central microtubules. (C,D) Diagrams of cross-sections of CAs of wild type and fap70-1, respectively, illustrating the C1 and C2 microtubules connected by bridge structures and with major projections (C1a, C1b, C2a, C2c and C2b) normally visible by conventional TEM labeled. The fap70-1 CA appears to lack the C2a projection (arrow in D). (E,F) Enlargement of CAs from panels A and B, respectively. The C2a projection appears to be absent from the fap70-1 CA (arrow in F). (G,H) Image averages based on six wild-type (G) and six fap70-1 (H) CAs of intact in situ flagella showing the apparent absence of the C2a projection in the fap70-1 flagellum (arrow in H). (I-L) Representative cross-sections of isolated axonemes from wild type (I) and fap70-1 (J-L). Some cross-sections of fap70-1 axonemes lacked one (K) or both (L) central microtubules. (M,N) Enlargement of CAs from panels I and J, respectively. The C2a projection appears to be absent from the fap70-1 CA (arrow in N). (O,P) Image averages based on six wild-type (O) and six fap70-1 (P) CAs of isolated axonemes showing the apparent absence of the C2a projection in the fap70-1 axoneme (arrow in P). Scale bars: 100 nm.
The observation that both central microtubules are present in whole flagella but only in about half of isolated axonemes of fap70-1 indicates that loss of the central microtubule(s) is an artifact that arises during axonemal isolation. However, close examination of in situ flagella of fap70-1 revealed that the C2a projection was missing even in intact flagella (Fig. 3E-H). Apparently, loss of the C2a projection destabilizes the C2 microtubule and then the C1 microtubule, possibly through a domino effect propagated through connections between C2a and C1a, C2b and C1b, and the bridges between the two microtubules (Fig. 3C). A similar loss of one or both central microtubules in axonemes but not whole flagella has been observed for the mutant pf16, which lacks the C1a projection (Dutcher et al., 1984; Fu et al., 2019).
Localization of FAP70 within the CA
To more clearly determine the effect of loss of FAP70 on the C2 microtubule, we compared isolated axonemes of fap70-1 and wild type by cryo-ET (Fig. 4; Movies 1, 2). Owing to the structural heterogeneity of the C2 projections, we aligned the C1 microtubule and the C2a, C2b, C2e plus C2c, and C2d projection regions separately, and generated a composite map by merging the five structures (Fig. S1). In the fap70-1 mutant, there was substantial structural loss in the outermost regions of C2a (Fig. 4), in good agreement with our observations on fixed material. In addition, slight structural changes were apparent in C2e and C2c, suggesting instability or abnormal flexibility of these projections due to the absence of FAP70.
To determine where in the CA FAP70 is located, we treated wild-type and fap70::FAP70-N-BCCP-HA axonemes with streptavidin and biotinylated cytochrome c (Oda et al., 2015), and then compared them using cryo-ET. Surface rendering of the difference map put the densities of the streptavidin label adjacent to the base of C2a, beneath the arcade of C2e, with a repeat of 16 nm (Fig. 4, right; Movie 3). This localization of the FAP70 N-terminus is consistent with the structural defects in the fap70-1 CA.
Proteins missing or reduced in fap70 axonemes
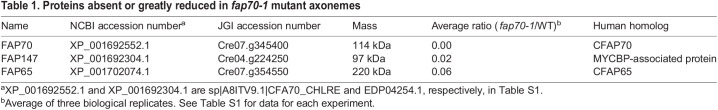
To identify proteins missing in fap70-1 axonemes, we used label-free quantitative MS to compare the axonemal proteome of the mutant with that of wild type in three biological replicates (Table S1). Among the proteins that in wild type had an abundance greater than one-tenth that of FAP70, we identified three proteins that in fap70-1 were either missing or reduced to less than 15% of their wild-type levels (Table 1). One was FAP70, which was not detected in any of the fap70-1 samples, consistent with fap70-1 being a null mutant (Shamoto et al., 2018). The other proteins were FAP65 and FAP147, which previously were identified as candidate CA proteins, and were predicted to be associated with the C2 microtubule (Dai et al., 2020; Zhao et al., 2019). These results indicate that FAP65 and FAP147 are dependent on FAP70 for assembly into the CA.
Table 1.
Proteins absent or greatly reduced in fap70-1 mutant axonemes
Interacting partners of FAP70
We next used immunoprecipitation coupled with MS to identify possible interacting partners for FAP70. Briefly, axonemes from wild type (control) and the fap70::FAP70-N-BCCP-HA strain were isolated and extracted with 0.6 M KCl to solubilize most CA proteins (Zhao et al., 2019). The extracts were subjected to immunoprecipitation with anti-HA antibodies, and the immunoprecipitates analyzed by label-free quantitative MS. In two biological replicates, a sum of 188 proteins were identified by MS (Table S2). For each protein detected, the specificity of co-immunoprecipitation was assessed based on the abundance of the protein relative to FAP70, and on the enrichment of the protein in the FAP70-N-BCCP-HA immunoprecipitate relative to the wild-type immunoprecipitate. Using as criteria (1) an abundance of at least 1/100 of FAP70 in the FAP70-N-BCCP-HA immunoprecipitate, and (2) an enrichment of at least 100-fold in the FAP70-N-BCCP-HA immunoprecipitate compared with the wild-type immunoprecipitate, the proteins BCC1, FAP65 and FAP147 were specifically co-immunoprecipitated with FAP70-N-BCCP-HA (Table 2). BCC1 is acetyl-CoA biotin carboxyl carrier protein, which is the BCCP tag in the FAP70-N-BCCP-HA protein. This result shows that FAP70, FAP65 and FAP147 are in the same complex and likely associated with each other in the C2a projection.
Table 2.
FAP65 and FAP147 are in the same complex as FAP70, as shown by MS analysis of immunoprecipitates
DISCUSSION
FAP70 is located exclusively in the CA and is a C2a protein
Our immunofluorescence localization of tagged FAP70, in both frayed axonemes and axonemes from which the CA had been partially or completely extruded, revealed that FAP70 is located exclusively in the CA, and not on the DMTs. This is consistent with previous MS analyses comparing wild-type and CA-less axonemes (Dai et al., 2020; Zhao et al., 2019). Specifically, in a comparison of axonemes of wild type and the CA-less mutant pf18, FAP70, which in wild type is present at a level similar to that of other CA proteins, was present in pf18 at only 0.1-0.2% of its wild-type levels (Zhao et al., 2019). Such a great reduction in the ‘9+0’ axoneme would not be expected if FAP70 were located in both the CA and DMTs, even if it were present in only one of the nine DMTs. Therefore, within the axoneme, FAP70 is specific to the CA.
In our conventional TEM comparison of wild-type versus fap70-1 flagella and axonemes, the C2a projection appeared to be absent from both in situ flagella and isolated axonemes. In good agreement with this, cryo-ET showed that C2a was severely truncated in fap70-1 axonemes, with lesser structural changes – possibly rearrangements – observed in the C2e and C2c projections. Therefore, FAP70 is required for the assembly of a complete C2a projection. Our conventional TEM analysis also showed that loss of FAP70 results in destabilization of the C2 microtubule, so that C2 is frequently lost from isolated axonemes of fap70-1 but not wild type. The structural changes to the C2e and C2c projections in the absence of most of C2a could be caused by changes in the interactions between the highly interconnected projections of the C2 microtubule. A similar phenomenon has been observed for projections on the C1 microtubule, where loss of portions of the C1b projection causes reduced occupancy and positional flexibility in the adjacent and interacting C1f projection (Cai et al., 2021 preprint). Alternatively, the changes observed in the C2e and C2c projections of the fap70-1 axonemes could reflect an early stage in the destabilization of the C2 microtubule as a result of axoneme preparation.
Within the CA, the N-terminus of FAP70-N-BCCP-HA was localized by cryo-ET to the C2 microtubule, at the base of the C2a projection, but also close to the C2e projection. This localization, plus the severe effect of loss of FAP70 on the C2a projection, strongly argue that FAP70 is a C2a protein. However, FAP70 is a relatively large protein (1104 amino acids), so it easily could extend from C2a to C2e or further. If so, it could explain the structural changes to C2e and C2c in the absence of FAP70. Additional experiments, such as localization of the C-terminus of FAP70 or higher resolution single-particle cryo-electron microscopy, will be necessary to delimit FAP70 within the C2 microtubule.
FAP65 and FAP147 interact with FAP70 and are dependent on FAP70 for assembly into the axoneme
Our comparison of isolated axonemes of wild type versus fap70-1 by MS revealed only three proteins that were greatly reduced in the later: FAP70, FAP65 and FAP147. Therefore, assembly of FAP65 and FAP147 into the axoneme is dependent on FAP70. Moreover, when FAP70-N-BCCP-HA was immunoprecipitated from an axonemal extract by anti-HA antibodies, FAP65 and FAP147 were the only proteins that met our criteria for being specifically co-immunoprecipitated. Therefore, FAP70, FAP65 and FAP147 are interacting proteins and part of the same complex. Given that the greatest structural defect in fap70-1 axonemes is loss of a large part of the C2a projection, it is likely that FAP65 and FAP147 also are components of C2a. The mass of the C2a projection has been estimated to be 1750 kDa based on calculations of volume as determined by cryo-ET (Carbajal-González et al., 2013), whereas the sum of the masses of FAP70 (111 kDa), FAP65 (220 kDa) and FAP147 (97 kDa), as predicted from their sequences, is 431 kDa. However, this comparison should be taken warily, because calculation of mass by cryo-ET can be difficult (Oda, 2017), and the stoichiometries of the subunits in C2a cannot be determined from low-resolution cryo-ETs. Other methods, such as high-resolution single-particle cryo-electron microscopy, will be necessary to determine how many copies of each protein identified here are contained in each C2a projection, and what additional proteins contribute to the architecture of C2a.
It is of interest that FAP65 and FAP147 are both ASH-domain proteins. The Chlamydomonas flagellum contains a total of eight ASH-domain proteins (Zhao et al., 2020). Six were previously known to be CA components; the current work, by confirming that FAP65 and FAP147 also are CA proteins, shows that all eight are localized to the CA. This suggests that ASH domains have special significance for CA assembly or function. It has been proposed that ASH domains function in microtubule binding (Ponting, 2006), and there is experimental evidence to support this (Schou et al., 2014). It may be relevant that at least five CA projections are associated with an ASH-domain protein (Fu et al., 2019; Zhao et al., 2020).
It should be noted that a few additional proteins were substantially reduced in fap70-1 axonemes in one or two of our biological replicates (Table S1), but did not meet our stringent requirement that a protein had to be reduced to less than 0.15 of the wild-type level in all three experiments. This variability could reflect different degrees of disintegration of the C2 microtubule in the different preparations. One of these is KLP1, a known C2c protein (Bernstein et al., 1994; Yokoyama et al., 2004). Therefore, proteins with abundances similar to that of other CA proteins and substantially reduced in one or more of our preparations of fap70-1 axonemes are strong candidates for being C2 proteins.
The FAP70 complex may include FAP174
Our finding that FAP65, FAP70 and FAP147 are in a complex associated with the C2a projection ties together and sheds new light on several previous observations. The human homolog of FAP147 is MYCBPAP, which binds MYCBP (Yukitake et al., 2002), a small protein of 12 kDa; these proteins were implicated in spermatogenesis but their functions were not determined. The Chlamydomonas homolog of MYCBP is FAP174, a similarly small protein (Rao et al., 2016). Rao et al. (2016) showed by blot overlay assays that FAP174 binds a protein then known as AKAP240 (Gaillard et al., 2001) and subsequently predicted to be FAP65 (Zhao et al., 2019). Based on mutant analysis, both FAP174 and AKAP240 were predicted to be associated with the C2 microtubule (Gaillard et al., 2001; Rao et al., 2016), although they were not localized to a specific projection. In our experiments to identify proteins that interact with FAP70, FAP174 was enriched over 105-fold in one of our FAP70-N-BCCP-HA immunoprecipitates, but was enriched only 98-fold in the other immunoprecipitate, and so just failed to meet our criterion that a protein had to be enriched at least 100-fold in both experiments to be considered a FAP70 interactor (see ‘Interacting partners of FAP70’ above and Table S2). Nevertheless, considering all of the above, it seems likely that FAP174 is located in the C2a projection, in contact with both FAP65 and FAP147. Intriguingly, Zhao et al. (2019) reported that FAP174 was associated with the C1b projection, so it may be located in more than one place in the CA. If it is present in multiple locations in the CA, it would explain the only modest decrease of FAP174 in fap70-1 axonemes relative to wild-type axonemes (Table S1). Further studies will be necessary to confirm the localization of FAP174 to these projections.
Function of the C2a projection
The fap70 mutant has a slow-swimming phenotype resembling that of outer dynein arm-less mutants, suggesting that FAP70 is important for the regulation of outer arm dyneins (Shamoto et al., 2018). Further evidence that FAP70 is involved in the control of outer arm activity comes from observations that a double mutant of fap70 and oda2 (lacking all outer arms) has a flagellar beat frequency identical to that of the oda2 parent, and that a double mutant of fap70 and ida4 (lacking several inner arm dyneins) is completely immotile, a phenotype observed when both outer and inner arms are defective (Kamiya et al., 1991; Shamoto et al., 2018). Our finding that FAP70 is a component of the C2a projection requires consideration of how it might function in such control.
Chlamydomonas mutants completely lacking the CA generally have paralyzed flagella (Mitchell, 2009), emphasizing the importance of the CA to flagellar motility. However, under certain conditions, these flagella or their isolated axonemes can be induced to beat, indicating that the DMTs contain all of the machinery necessary for coordinated flagellar movement (Huang et al., 1982; Yagi and Kamiya, 2000; Yagi and Nishiyama, 2020). Therefore, the CA likely overrides the regulatory elements located in the DMTs to add another layer of control. This is believed to be accomplished, at least in part, by non-specific mechanical interactions between the CA and the radial spoke heads, generating signals that are then relayed via the radial spokes to the DMTs to control the activity of the dynein arms, which must be turned on and off on subsets of DMTs at regular times in the flagellar beat cycle (Oda et al., 2013).
In Chlamydomonas, the CA rotates during flagellar beating, so that the same CA projections are apposed to different radial spoke heads at different times in the beat cycle (Kamiya, 1982; Mitchell, 2003). Moreover, because the Chlamydomonas CA also is twisted (Mitchell, 2003), C2a projections at different locations along the length of the axoneme have the potential to send signals to different subsets of DMTs at any given time in the beat cycle. In cross-sections of the CA, each projection extends just far enough from the microtubule so that the profile of the CA is round with a relatively smooth circumference, as appropriate for a CA that rotates, and in contrast to the profile of the sea urchin CA, which does not rotate and is more oval in cross-section (Carbajal-González et al., 2013). In our cryo-ETs, the C2a projection extends to within ∼1 nm of the circumference of a circle centered on the CA and just touching the majority of the C1 projections (Fig. S2), which are separated from the radial spoke heads by a gap of ∼5 nm (Oda et al., 2014). Because of this slightly larger gap, it is possible that C2a interacts with radial spoke heads less forcefully than projections such as C2c that extend further out (Fig. S2), thus modulating the signal sent to the DMTs. In this case, C2a would generate a localized mechanical signal that could regulate the outer arms on different subsets of DMTs at different times in the flagellar beat cycle.
In outer arm-less mutants, flagellar beat frequency is reduced to ∼0.3 that of wild type, whereas in the fap70 mutant flagellar beat frequency is reduced to only ∼0.6 of wild type (Shamoto et al., 2018). This is consistent, in the fap70 mutant, with an abnormal uncoupling of outer arm activity from motility in only a subset of the DMTs that are actively generating force, as would be expected if interaction of C2a with specific radial spoke heads produced a localized mechanical signal at specific times in the flagellar beat cycle.
Another Chlamydomonas mutant, cpc1, which lacks the C1b projection, also exhibits a reduction in flagellar beat frequency to ∼0.6 of wild type, with no major effect on flagellar waveform (Mitchell and Sale, 1999). In this case, most of the reduction in flagellar beat frequency has been attributed to reduced intraflagellar ATP concentration due to loss of enolase, which is a subunit of the C1b projection (Mitchell et al., 2005; Zhang and Mitchell, 2004). This is unlikely to be the case for the fap70 mutant, as we found that axonemal enolase was only modestly reduced in fap70-1 relative to wild type (Table S1). Moreover, the cpc1 mutation causes a similar reduction in beat frequency in a wild-type background (30% reduction) and in the mutant pf28 lacking outer dynein arms (30% reduction), suggesting that the reduced beat frequency is not due solely to an effect on outer arm dynein activity (Mitchell and Sale, 1999).
Recent cryo-electron microscopy studies have shown that the radial spoke head surface facing the CA is negatively charged (Grossman-Haham et al., 2021; Gui et al., 2021), and it has been proposed that if the projections of the CA are similarly negatively charged it would prevent sticking of the radial spokes to the CA projections when the two collide (Grossman-Haham et al., 2021). It may be relevant that all three of the C2a proteins, like the radial spoke head proteins (Yang et al., 2006), are acidic proteins.
C2a proteins and PCD
Our finding that FAP70 and its interacting partners are CA proteins suggests certain challenges for the identification of patients that have defects in the genes encoding the human homologs of these proteins. Mutations in at least three genes encoding CA proteins are known to cause PCD (Legendre et al., 2021; Wallmeier et al., 2020), but patients with suspected PCD who have mutations in such genes can be difficult to confirm by traditional approaches. When present, one of the most important clinical manifestations of PCD is the presence of left-right laterality defects (Shapiro et al., 2018). Such defects arise from faults in the nodal cilia, the beating of which initiates left-right asymmetry in the early embryo. However, nodal cilia are unusual among motile cilia in that they lack the CA; hence defects in the CA do not affect them and do not cause laterality abnormalities (Bustamante-Marin et al., 2019; Cindrić et al., 2020; Olbrich et al., 2012). In the absence of this characteristic clinical feature, a diagnosis of PCD is often based on the finding of ultrastructural defects in the ciliary axoneme of respiratory epithelial cells; however, defects in CA projections may not be readily detected by conventional TEM, thus rendering less useful this tool that for many decades was the ‘gold standard’ for PCD diagnosis. High-speed video microscopy to assess ciliary beat frequency and waveform of respiratory epithelial cells also may be helpful for diagnosis of PCD, but so far mutations in known CA components cause subtle changes in ciliary beating that require specialized equipment and training to document (Horani and Ferkol, 2018; Legendre et al., 2021; Wallmeier et al., 2020), and this is also expected to be the case for CFAP70 (Shamoto et al., 2018). Immunofluorescence microscopy using antibodies against specific axonemal proteins can be a rapid and very sensitive tool for diagnosing PCD by demonstrating that a protein, or axonemal structure containing that protein, are missing from cilia or sperm flagella of patients (Wallmeier et al., 2020). Antibodies against CFAP65 and CFAP70 are commercially available (Sigma-Aldrich, HPA055156, and Sigma-Aldrich, HPA037582, respectively); however, at the present time this approach is better suited for the confirmation of a suspected loss of a protein or structure than determining which one of hundreds of axonemal proteins might be missing in a patient. Thus, at present, molecular genetic testing may be the most efficient and accurate means for identifying patients that have mutations in genes encoding the C2a proteins.
Indeed, whole-exome sequencing of a large cohort of patients with multiple morphological abnormalities of the sperm flagellum (MMAF) characterized by severe asthenozoospermia recently identified two unrelated patients with apparently deleterious homozygous mutations in the CFAP70 gene (Beurois et al., 2019). Other than primary infertility due to MMAF, neither patient exhibited clinical features that would support a PCD diagnosis. In the first patient, the mutation altered a consensus splice acceptor site of CFAP70. Immunofluorescence microscopy using the Sigma-Aldrich anti-CFAP70 antibody detected CFAP70 in the flagella of control sperm but not in sperm of the patient. Interestingly, immunofluorescence microscopy also revealed that the sperm flagella of the patient lacked SPAG6 (a component of the C1a projection) and lacked, or had reduced amounts of, DNAI2 (an outer arm dynein intermediate chain), consistent with previous reports that defects in CA proteins that have minor effects on respiratory cilia can have major effects on sperm flagella (Lee et al., 2008; McKenzie et al., 2015; Sapiro et al., 2002; Sironen et al., 2011; Teves et al., 2014), possibly through a quality control mechanism that triggers the abortion of spermiogenesis when the spermatids are defective (McKenzie et al., 2015). The second patient had a missense variant in the CFAP70 gene that was predicted to be deleterious; limited sample availability from this patient precluded analysis by immunofluorescence microscopy. Unfortunately, also owing to the low number of sperm cells, it was not possible to examine the sperm of either patient by TEM.
Whole-exome sequencing also has identified mutations in CFAP65 in patients with MMAF (Li et al., 2020; Wang et al., 2019; Zhang et al., 2019). In one of the studies, six patients with bi-allelic null mutations in CFAP65 were identified in a cohort of 88 Han Chinese probands with MMAF but no other symptoms of PCD (Zhang et al., 2019). In another study, three patients with bi-allelic loss-of-function mutations in CFAP65 were identified in a study of 47 Chinese men with severe asthenozoospermia; two of the patients had typical PCD symptoms (Wang et al., 2019). Importantly, in both studies, cross-sections of sperm flagella of patients with CFAP65 mutations lacked the CA when examined by conventional TEM.
As additional patients with mutations in CFAP65 and CFAP70 are identified, it will be of interest to determine how the ultrastructure of their cilia and flagella are affected, and whether they have clinical features of PCD despite the absence of laterality defects. In light of the above findings, MYCBPAP, the human homolog of Chlamydomonas FAP147, should also be considered a candidate disease gene for PCD and MMAF.
MATERIALS AND METHODS
Strains and culture conditions
The C. reinhardtii wild-type strain used in this study was CC-125 (Chlamydomonas Resource Center, www.chlamycollection.org/). The fap70-1 (containing an insert in FAP70 exon 1) and fap70::FAP70-N-BCCP-HA strains were generated previously (Shamoto et al., 2018) and are available from the Chlamydomonas Resource Center. Cells were grown in modified M medium I (Witman, 1986) at 23°C with aeration of 5% CO2 and a light/dark cycle of 14/10 h.
Flagella preparation and fractionation
Flagella were isolated as described previously (Witman, 1986), and demembranated by resuspension in HMDEK (30 mM HEPES, 5 mM MgSO4, 1 mM DTT, 0.5 mM EGTA, 25 mM KCl) containing 0.5% NP-40 (Calbiochem) for 10 min at 4°C. The resulting axonemes were collected by centrifugation (30,000 g for 20 min) at 4°C.
Immunofluorescence microscopy and conventional TEM
For analysis of frayed axonemes, immunofluorescence microscopy was carried out according to Sanders and Salisbury (1995) with a slight modification. Because the presence of a cell wall often causes strong autofluorescence, the cells were treated with autolysin for 60 min to remove the cell wall. Autolysin-treated cells were adhered to a polyethylenimine-coated 8-well slide and treated with 1 mM ATP to induce disintegration of the axonemes. Then the cells were fixed with −20°C methanol and acetone for 5 min each. Fixed cells were incubated with primary antibodies [mouse monoclonal anti-HA antibody, Sigma-Aldrich clone 12CA5, 1:200; rabbit polyclonal anti-polyglutamylated tubulin antibody (polyE), 1:200 (Kubo and Oda, 2017)], followed by incubation with secondary antibodies [goat anti-mouse IgG Alexa Fluor 594 (Invitrogen A-11005)/goat anti-rabbit IgG Alexa 488 (Invitrogen A-27034)]. The cells were mounted in an antifade mountant (SlowFade Diamond, Thermo Fisher Scientific) and examined with an Olympus BX53 microscope equipped with a 100× UPlan FL N 1.30 numerical aperture objective. Images were obtained with an ORCA-Flash4.0 sCMOS camera (Hamamatsu) and analyzed by ImageJ.
Central pair extrusion; labeling of samples with rat monoclonal anti-HA antibody, 1:150 (Roche Holding AG, clone 3F10, 11 867 423 001) and mouse monoclonal anti-acetylated tubulin antibody, 1:1000 (Sigma-Aldrich, clone 6-11B-1, T-6793); and structured illumination microscopy were carried out as described previously (Zhao et al., 2019).
Whole cells and isolated axonemes were fixed and embedded for conventional TEM as described by Hoops and Witman (1983) and Zhao et al. (2019), respectively. TEM was performed in the Electron Microscopy Facility of the University of Massachusetts Medical School (UMMS).
Immunoprecipitation
Immunoprecipitations from 0.6 M KCl extracts of fap70::FAP70-N-BCCP-HA and wild-type axonemes were carried out using anti-HA antibody as described previously (Zhao et al., 2019), except that 40 µg instead of 77 µg of total proteins were used for each immunoprecipitation.
Mass spectrometry
Sample preparation and liquid chromatography (LC)-MS/MS for comparison of wild-type versus mutant axonemes, and for analysis of immunoprecipitates were performed as described previously (Zhao et al., 2019). LC-MS/MS was performed in the Mass Spectrometry Facility of UMMS.
Label-free MS quantification
MS data loaded in Scaffold (version 4.8; Proteome Software) were filtered with the peptide false discovery rate set at 1%, number of peptides identified for each protein set at two or more, and protein threshold set at 90%. Proteins thus identified together with their iBAQ values (Schwanhäusser et al., 2011) were exported to Microsoft Excel for further analysis.
To identify proteins missing or greatly reduced in fap70-1 axonemes, we performed MS analysis on three sets of biological samples using iBAQ values without normalization. All the proteins identified from each biological sample set were filtered using two criteria: (1) the iBAQ value of the protein in the wild-type sample must be greater than 10% of that of FAP70 in the wild-type sample, and (2) the ratio of the iBAQ value of the protein in the fap70-1 sample to that of the same protein in the wild-type sample must be less than 0.15. Only proteins that met both criteria in all three biological sample sets were considered positive results.
To identify proteins that were specifically immunoprecipitated from the fap70::FAP70-N-BCCP-HA axonemal extract by the anti-HA antibody, we carried out two independent sets of immunoprecipitation experiments coupled with MS analysis using iBAQ values. All the proteins identified by each experiment were filtered using two criteria: (1) the iBAQ value of the protein in the fap70::FAP70-N-BCCP-HA immunoprecipitate must be greater than 1/100 that of FAP70 in the same sample, and (2) the iBAQ value of the protein in the fap70::FAP70-N-BCCP-HA immunoprecipitate must be 100× greater than that of the same protein in the wild-type control immunoprecipitate. In cases in which a protein was not detected in the wild-type immunoprecipitate, or no value was reported (e.g. because protein abundance was too low for quantification) for the wild-type control, the protein in that sample was arbitrarily assigned an iBAQ value of 1 for calculation purposes. Only proteins that met both criteria in both immunoprecipitation experiments were considered positive results.
Sample preparation for cryo-electron tomography
For localization of the FAP70 N-terminus, isolated wild-type and fap70::FAP70-N-BCCP-HA axonemes were incubated with 0.05 mg/ml streptavidin for 15 min at 4°C in HMDEK supplemented with 1 mg/ml bovine serum albumin. After washing in HMDEK three times, axonemes were incubated with 0.05 mg/ml biotinylated cytochrome c. This sequence was repeated once.
In all cases, axonemes were resuspended in HMDEK at a concentration of 0.02 mg/ml, and mixed with cytochrome c-stabilized 15-nm colloidal gold (BBI Solutions, Cardiff, UK) to serve as fiducial markers. Both sides of homemade holey carbon grids were glow-discharged for 1 min. Suspended axonemes were loaded onto the grids and plunge-frozen in liquid ethane at −180°C using a Vitrobot Mark IV (Thermo Fisher Scientific, Waltham, MA, USA).
Image acquisition
Grids were transferred to a JEM-3100FEF TEM (JEOL, Tokyo, Japan) with a Gatan 914 high-tilt liquid nitrogen cryo-transfer holder (Gatan Inc., Pleasanton, CA, USA). Tilt-series images were recorded using a Gatan K2 Summit direct detector. Automated acquisition was performed using SerialEM software (Mastronarde, 2005) in the range of ±60 degrees with 2° increments. The total electron dose was ∼100 e−/Å2. Images were recorded at 300 keV, with 6-8 µm defocus at a magnification of 7100 and a pixel size of 7 Å, using an in-column omega energy filter with a slit width of 40 eV.
Image processing
Tilt-series images were aligned and back-projected using IMOD software (Kremer et al., 1996). Subtomogram averaging was conducted using custom Ruby-Helix scripts (Metlagel et al., 2007) and the PEET software suite (Nicastro et al., 2006). As C1 and C2 projections are structurally uncoupled from one another, we aligned the C1 microtubule and the C2a, C2b, C2c plus C2e, and C2d projections separately by making Gaussian-smoothened masks (Fig. S1A). The numbers of subtomograms averaged were as follows: 934 (wild type); 1022 (fap70-1); and 1196 (fap70::FAP70-N-BCCP-HA). The effective resolutions were estimated to be 3.3-3.9 nm by Fourier shell correlation with a cutoff value of 0.5 (Fig. S1B). For identification of the label densities, we applied Student's t-test to compare streptavidin- plus cytochrome c-treated wild-type and fap70::FAP70-N-BCCP-HA axonemes (Oda and Kikkawa, 2013; Oda et al., 2014). The isosurface threshold values were t>9.8, with a one-tailed probability of <0.1%. The maps of the averaged subtomograms are available at the EM Data Bank under the following accession numbers: EMD-31143 and EMD-31144.
Supplementary Material
Acknowledgements
We thank Drs G. Hendricks and L. Strittmatter and Mr K. Reddig of the Electron Microscopy Facility at UMMS for expert assistance with conventional TEM; Drs X. Li, R. Aslebagh and S. Shaffer at the Mass Spectrometry Facility at UMMS for expert assistance with MS; Dr C. Baer of the Sanderson Center for Optical Experimentation Imaging Facility at UMMS for expert assistance with structured illumination microscopy; and Dr H. Yanagisawa at the University of Tokyo for expert assistance with cryo-TEM. We also thank Dr David Mitchell (The State University of New York Upstate Medical University, Syracuse, New York, NY, USA) and anonymous reviewers for insightful suggestions for improving the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.H., L.Z., T.O., G.B.W.; Methodology: Y.H., L.Z., T.K., T.O., G.B.W.; Validation: Y.H., L.Z., T.K., T.O., G.B.W.; Formal analysis: Y.H., L.Z., T.K., T.O.; Investigation: Y.H., L.Z., T.K., X.C., N.M., T.O.; Resources: T.O., G.B.W.; Data curation: Y.H., L.Z., T.K., T.O.; Writing - original draft: Y.H., L.Z., T.K., T.O., G.B.W.; Writing - review & editing: Y.H., L.Z., T.K., T.O., G.B.W.; Visualization: Y.H., L.Z., T.K., T.O., G.B.W.; Supervision: T.O., G.B.W.; Project administration: T.O., G.B.W.; Funding acquisition: T.O., G.B.W.
Funding
This work was supported by the National Institutes of Health (NIH; R37 GM030626 and R35 GM122574 to G.B.W.); the Robert W. Booth Endowment at the University of Massachusetts Medical School (to G.B.W.); the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research) from the Japan Agency for Medical Research and Development (JP19am0101115); the Takeda Science Foundation (to T.O.); the Naito Foundation (to T.O.); the Daiichi Sankyo Foundation of Life Science (to T.O.); the Japan Society for the Promotion of Science (KAKENHI Grant number JP21H02654 to T.O.); and a Multidisciplinary Research Grant from the University of Yamanashi (to T.O.). Molecular graphics and analyses were performed with University of California, San Francisco (UCSF) ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the UCSF, with support from NIH grant R01 GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases. Deposited in PMC for release after 12 months.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.258540
References
- Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B. and Christensen, S. T. (2019). Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199-219. 10.1038/s41581-019-0116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, M., Beech, P. L., Katz, S. G. and Rosenbaum, J. L. (1994). A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J. Cell Biol. 125, 1313-1326. 10.1083/jcb.125.6.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurois, J., Martinez, G., Cazin, C., Kherraf, Z. E., Amiri-Yekta, A., Thierry-Mieg, N., Bidart, M., Petre, G., Satre, V., Brouillet, S.et al. (2019). CFAP70 mutations lead to male infertility due to severe astheno-teratozoospermia. A Case Report. Hum. Reprod 34, 2071-2079. 10.1093/humrep/dez166 [DOI] [PubMed] [Google Scholar]
- Brown, J. M. and Witman, G. B. (2014). Cilia and diseases. Bioscience 64, 1126-1137. 10.1093/biosci/biu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. M., DiPetrillo, C. G., Smith, E. F. and Witman, G. B. (2012). A FAP46 mutant provides new insights into the function and assembly of the C1d complex of the ciliary central apparatus. J. Cell Sci. 125, 3904-3913. 10.1242/jcs.107151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante-Marin, X. M., Yin, W.-N., Sears, P. R., Werner, M. E., Brotslaw, E. J., Mitchell, B. J., Jania, C. M., Zeman, K. L., Rogers, T. D., Herring, L. E.et al. (2019). Lack of GAS2L2 causes PCD by impairing cilia orientation and mucociliary clearance. Am. J. Hum. Genet. 104, 229-245. 10.1016/j.ajhg.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, K., Zhao, Y., Zhao, L., Phan, N., Witman, G. B. and Nicastro, D. (2021). Structural organization of the C1b projection within the ciliary central apparatus. bioRxiv 2021.06.16.448709. 10.1101/2021.06.16.448709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal-González, B. I., Heuser, T., Fu, X., Lin, J., Smith, B. W., Mitchell, D. R. and Nicastro, D. (2013). Conserved structural motifs in the central pair complex of eukaryotic flagella. Cytoskeleton (Hoboken) 70, 101-120. 10.1002/cm.21094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrić, S., Dougherty, G. W., Olbrich, H., Hjeij, R., Loges, N. T., Amirav, I., Philipsen, M. C., Marthin, J. K., Nielsen, K. G., Sutharsan, S.et al. (2020). SPEF2- and HYDIN-mutant cilia lack the central pair-associated protein SPEF2, aiding primary ciliary dyskinesia diagnostics. Am. J. Respir. Cell Mol. Biol. 62, 382-396. 10.1165/rcmb.2019-0086OC [DOI] [PubMed] [Google Scholar]
- Dai, D., Ichikawa, M., Peri, K., Rebinsky, R. and Huy Bui, K. (2020). Identification and mapping of central pair proteins by proteomic analysis. Biophys. Physicobiol. 17, 71-85. 10.2142/biophysico.BSJ-2019048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo, C. G. and Smith, E. F. (2010). Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motility. J. Cell Biol. 189, 601-612. 10.1083/jcb.200912009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher, S. K., Huang, B. and Luck, D. J. (1984). Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J. Cell Biol. 98, 229-236. 10.1083/jcb.98.1.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, G., Zhao, L., Dymek, E., Hou, Y., Song, K., Phan, N., Shang, Z., Smith, E. F., Witman, G. B. and Nicastro, D. (2019). Structural organization of the C1a-e-c supercomplex within the ciliary central apparatus. J. Cell Biol. 218, 4236-4251. 10.1083/jcb.201906006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, A. R., Diener, D. R., Rosenbaum, J. L. and Sale, W. S. (2001). Flagellar radial spoke protein 3 is an A-kinase anchoring protein (AKAP). J. Cell Biol. 153, 443-448. 10.1083/jcb.153.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman-Haham, I., Coudray, N., Yu, Z., Wang, F., Zhang, N., Bhabha, G. and Vale, R. D. (2021). Structure of the radial spoke head and insights into its role in mechanoregulation of ciliary beating. Nat. Struct. Mol. Biol. 28, 20-28. 10.1038/s41594-020-00519-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui, M., Ma, M., Sze-Tu, E., Wang, X., Koh, F., Zhong, E. D., Berger, B., Davis, J. H., Dutcher, S. K., Zhang, R.et al. (2021). Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 28, 29-37. 10.1038/s41594-020-00530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops, H. J. and Witman, G. B. (1983). Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J. Cell Biol. 97, 902-908. 10.1083/jcb.97.3.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horani, A. and Ferkol, T. W. (2018). Advances in the genetics of primary ciliary dyskinesia: clinical implications. Chest 154, 645-652. 10.1016/j.chest.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, B., Ramanis, Z. and Luck, D. J. L. (1982). Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell 28, 115-124. 10.1016/0092-8674(82)90381-6 [DOI] [PubMed] [Google Scholar]
- Kamiya, R. (1982). Extrusion and rotation of the central-pair microtubules in detergent-treated Chlamydomonas flagella. Cell Motility 1, 169-173. 10.1002/cm.970020732 [DOI] [PubMed] [Google Scholar]
- Kamiya, R., Kurimoto, E. and Muto, E. (1991). Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J. Cell Biol. 112, 441-447. 10.1083/jcb.112.3.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S. M. (2018). Turning dyneins off bends cilia. Cytoskeleton (Hoboken) 75, 372-381. 10.1002/cm.21483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, J. R., Mastronarde, D. N. and McIntosh, J. R. (1996). Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71-76. 10.1006/jsbi.1996.0013 [DOI] [PubMed] [Google Scholar]
- Kubo, T. and Oda, T. (2017). Electrostatic interaction between polyglutamylated tubulin and the nexin-dynein regulatory complex regulates flagellar motility. Mol. Biol. Cell 28, 2260-2266. 10.1091/mbc.e17-05-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, T., Yanagisawa, H. A., Yagi, T., Hirono, M. and Kamiya, R. (2010). Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 20, 441-445. 10.1016/j.cub.2009.12.058 [DOI] [PubMed] [Google Scholar]
- Lechtreck, K. F. and Geimer, S. (2000). Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil. Cytoskeleton 47, 219-235. 10.1002/1097-0169(200011)47:3<219::AID-CM5>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Lechtreck, K. F. and Witman, G. B. (2007). Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J. Cell Biol. 176, 473-482. 10.1083/jcb.200611115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, L., Campagna, D. R., Pinkus, J. L., Mulhern, H., Wyatt, T. A., Sisson, J. H., Pavlik, J. A., Pinkus, G. S. and Fleming, M. D. (2008). Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol. Cell. Biol. 28, 949-957. 10.1128/MCB.00354-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre, M., Zaragosi, L. E. and Mitchison, H. M. (2021). Motile cilia and airway disease. Semin. Cell Dev. Biol. 110, 19-33. 10.1016/j.semcdb.2020.11.007 [DOI] [PubMed] [Google Scholar]
- Li, W., Wu, H., Li, F., Tian, S., Kherraf, Z. E., Zhang, J., Ni, X., Lv, M., Liu, C., Tan, Q.et al. (2020). Biallelic mutations in CFAP65 cause male infertility with multiple morphological abnormalities of the sperm flagella in humans and mice. J. Med. Genet. 57, 89-95. 10.1136/jmedgenet-2019-106344 [DOI] [PubMed] [Google Scholar]
- Ma, M., Stoyanova, M., Rademacher, G., Dutcher, S. K., Brown, A. and Zhang, R. (2019). Structure of the decorated ciliary doublet microtubule. Cell 179, 909-922.e12. 10.1016/j.cell.2019.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde, D. N. (2005). Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36-51. 10.1016/j.jsb.2005.07.007 [DOI] [PubMed] [Google Scholar]
- McKenzie, C. W., Craige, B., Kroeger, T. V., Finn, R., Wyatt, T. A., Sisson, J. H., Pavlik, J. A., Strittmatter, L., Hendricks, G. M., Witman, G. B.et al. (2015). CFAP54 is required for proper ciliary motility and assembly of the central pair apparatus in mice. Mol. Biol. Cell 26, 3140-3149. 10.1091/mbc.e15-02-0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlagel, Z., Kikkawa, Y. S. and Kikkawa, M. (2007). Ruby-Helix: an implementation of helical image processing based on object-oriented scripting language. J. Struct. Biol. 157, 95-105. 10.1016/j.jsb.2006.07.015 [DOI] [PubMed] [Google Scholar]
- Mitchell, D. R. (2003). Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil. Cytoskeleton 56, 120-129. 10.1002/cm.10142 [DOI] [PubMed] [Google Scholar]
- Mitchell, D. R. (2009). Chapter 8: The flagellar central pair apparatus. In The Chlamydomonas Sourcebook Second Edition, Vol. 3 (ed. Witman G. B.), pp. 235-252: Amsterdam: Academic Press. [Google Scholar]
- Mitchell, D. R. and Nakatsugawa, M. (2004). Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J. Cell Biol. 166, 709-715. 10.1083/jcb.200406148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. R. and Sale, W. S. (1999). Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J. Cell Biol. 144, 293-304. 10.1083/jcb.144.2.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, B. F., Pedersen, L. B., Feely, M., Rosenbaum, J. L. and Mitchell, D. R. (2005). ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol. Biol. Cell 16, 4509-4518. 10.1091/mbc.e05-04-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro, D., Schwartz, C., Pierson, J., Gaudette, R., Porter, M. E. and McIntosh, J. R. (2006). The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944-948. 10.1126/science.1128618 [DOI] [PubMed] [Google Scholar]
- Oda, T. (2017). Three-dimensional structural labeling microscopy of cilia and flagella. Microscopy (Oxf) 66, 234-244. 10.1093/jmicro/dfx018 [DOI] [PubMed] [Google Scholar]
- Oda, T. and Kikkawa, M. (2013). Novel structural labeling method using cryo-electron tomography and biotin-streptavidin system. J. Struct. Biol. 183, 305-311. 10.1016/j.jsb.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Oda, T., Yagi, T., Yanagisawa, H. and Kikkawa, M. (2013). Identification of the outer-inner dynein linker as a hub controller for axonemal dynein activities. Curr. Biol. 23, 656-664. 10.1016/j.cub.2013.03.028 [DOI] [PubMed] [Google Scholar]
- Oda, T., Yanagisawa, H., Yagi, T. and Kikkawa, M. (2014). Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J. Cell Biol. 204, 807-819. 10.1083/jcb.201312014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda, T., Yanagisawa, H. and Kikkawa, M. (2015). Detailed structural and biochemical characterization of the nexin-dynein regulatory complex. Mol. Biol. Cell 26, 294-304. 10.1091/mbc.E14-09-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich, H., Schmidts, M., Werner, C., Onoufriadis, A., Loges, N. T., Raidt, J., Banki, N. F., Shoemark, A., Burgoyne, T., Al Turki, S.et al. (2012). Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am. J. Hum. Genet. 91, 672-684. 10.1016/j.ajhg.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G. J., Agrin, N., Leszyk, J. and Witman, G. B. (2005). Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103-113. 10.1083/jcb.200504008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C. P. (2006). A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics 22, 1031-1035. 10.1093/bioinformatics/btl022 [DOI] [PubMed] [Google Scholar]
- Rao, V. G., Sarafdar, R. B., Chowdhury, T. S., Sivadas, P., Yang, P., Dongre, P. M. and D'Souza, J. S. (2016). Myc-binding protein orthologue interacts with AKAP240 in the central pair apparatus of the Chlamydomonas flagella. BMC Cell Biol. 17, 24. 10.1186/s12860-016-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, M. A. and Salisbury, J. L. (1995). Immunofluorescence microscopy of cilia and flagella. Methods Cell Biol. 47, 163-169. 10.1016/S0091-679X(08)60805-5 [DOI] [PubMed] [Google Scholar]
- Sapiro, R., Kostetskii, I., Olds-Clarke, P., Gerton, G. L., Radice, G. L. and Strauss, I. J. (2002). Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol. Cell. Biol. 22, 6298-6305. 10.1128/MCB.22.17.6298-6305.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou, K. B., Morthorst, S. K., Christensen, S. T. and Pedersen, L. B. (2014). Identification of conserved, centrosome-targeting ASH domains in TRAPPII complex subunits and TRAPPC8. Cilia 3, 6. 10.1186/2046-2530-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhäusser, B., Busse, D., Li, N., Dittmar, G., Schuchhardt, J., Wolf, J., Chen, W. and Selbach, M. (2011). Global quantification of mammalian gene expression control. Nature 473, 337-342. 10.1038/nature10098 [DOI] [PubMed] [Google Scholar]
- Shamoto, N., Narita, K., Kubo, T., Oda, T. and Takeda, S. (2018). CFAP70 Is a novel axoneme-binding protein that localizes at the base of the outer dynein arm and regulates ciliary motility. Cells 7, 124. 10.3390/cells7090124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, A. J., Davis, S. D., Polineni, D., Manion, M., Rosenfeld, M., Dell, S. D., Chilvers, M. A., Ferkol, T. W., Zariwala, M. A., Sagel, S. D.et al. (2018). Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care. Med. 197, e24-e39. 10.1164/rccm.201805-0819ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironen, A., Kotaja, N., Mulhern, H., Wyatt, T. A., Sisson, J. H., Pavlik, J. A., Miiluniemi, M., Fleming, M. D. and Lee, L. (2011). Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol. Reprod. 85, 690-701. 10.1095/biolreprod.111.091132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves, M. E., Sears, P. R., Li, W., Zhang, Z., Tang, W., van Reesema, L., Costanzo, R. M., Davis, C. W., Knowles, M. R., Strauss, J. F., III et al. (2014). Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: polarity, density, and beat. PLoS ONE 9, e107271. 10.1371/journal.pone.0107271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmeier, J., Nielsen, K. G., Kuehni, C. E., Lucas, J. S., Leigh, M. W., Zariwala, M. A. and Omran, H. (2020). Motile ciliopathies. Nat. Rev. Dis. Primers 6, 77. 10.1038/s41572-020-0209-6 [DOI] [PubMed] [Google Scholar]
- Wang, W., Tu, C., Nie, H., Meng, L., Li, Y., Yuan, S., Zhang, Q., Du, J., Wang, J., Gong, F.et al. (2019). Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J. Med. Genet. 56, 750-757. 10.1136/jmedgenet-2019-106031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo, M. J., Dymek, E. E. and Smith, E. F. (2005). Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J. Cell Sci. 118, 4655-4665. 10.1242/jcs.02585 [DOI] [PubMed] [Google Scholar]
- Witman, G. B. (1986). Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 134, 280-290. 10.1016/0076-6879(86)34096-5 [DOI] [PubMed] [Google Scholar]
- Witman, G. B. (2009). The Chlamydomonas Sourcebook. Vol. 3. Cell Motility and Behavior. 2nd edn, pp. 1-501. Amsterdam: Academic Press. [Google Scholar]
- Yagi, T. and Kamiya, R. (2000). Vigorous beating of Chlamydomonas axonemes lacking central pair/radial spoke structures in the presence of salts and organic compounds. Cell Motil. Cytoskeleton 46, 190-199. [DOI] [PubMed] [Google Scholar]
- Yagi, T. and Nishiyama, M. (2020). High hydrostatic pressure induces vigorous flagellar beating in Chlamydomonas non-motile mutants lacking the central apparatus. Sci. Rep. 10, 2072. 10.1038/s41598-020-58832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, P., Diener, D. R., Yang, C., Kohno, T., Pazour, G. J., Dienes, J. M., Agrin, N. S., King, S. M., Sale, W. S., Kamiya, R.et al. (2006). Radial spoke proteins of Chlamydomonas flagella. J. Cell Sci. 119, 1165-1174. 10.1242/jcs.02811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, R., O'Toole, E., Ghosh, S. and Mitchell, D. R. (2004). Regulation of flagellar dynein activity by a central pair kinesin. Proc. Natl. Acad. Sci. USA 101, 17398-17403. 10.1073/pnas.0406817101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukitake, H., Furusawa, M., Taira, T., Iguchi-Ariga, S. M. and Ariga, H. (2002). AMAP-1, a novel testis-specific AMY-1-binding protein, is differentially expressed during the course of spermatogenesis. Biochim. Biophys. Acta 1577, 126-132. 10.1016/S0167-4781(02)00411-6 [DOI] [PubMed] [Google Scholar]
- Zhang, H. and Mitchell, D. R. (2004). Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J. Cell Sci. 117, 4179-4188. 10.1242/jcs.01297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Shen, Y., Wang, X., Yuan, G., Zhang, C. and Yang, Y. (2019). A novel homozygous CFAP65 mutation in humans causes male infertility with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 96, 541-548. 10.1111/cge.13644 [DOI] [PubMed] [Google Scholar]
- Zhao, L., Hou, Y., Picariello, T., Craige, B. and Witman, G. B. (2019). Proteome of the central apparatus of a ciliary axoneme. J. Cell Biol. 218, 2051-2070. 10.1083/jcb.201902017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L., Hou, Y., McNeill, N. A. and Witman, G. B. (2020). The unity and diversity of the ciliary central apparatus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190164. 10.1098/rstb.2019.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.