Abstract
Genetic abnormalities, whether occurring in the conceptus or the parents, can predispose to sporadic or recurrent pregnancy loss (RPL). Abnormalities in the conceptus include aneuploidy, copy number changes, skewed X inactivation, and single gene disorders or mutations. Among parents who suffer RPL, the best studied genetic cause is balanced chromosomal translocations. For evaluation of genetic abnormalities in cases of pregnancy loss, chromosomal microarray is more likely to yield interpretable results than karyotype due to cell culture failure. For parents, karyotype remains the standard since microarray may not detect truly balanced translocations. For those with an identified underlying genetic abnormality, preimplantation genetic testing has been proposed to optimize the live birth rate. This approach shows promise, but currently lacks supporting evidence. In summary, various genetic causes for recurrent pregnancy loss are known, but when such a cause is identified, the implications for management remain unclear.
Introduction
Pregnancy loss is common, occurring in 30% of conceptions and 10% of clinically recognized pregnancies.1 While most losses are sporadic (rather than recurrent), significant overlap exists between the genetic causes of sporadic and recurrent pregnancy loss. The causes of pregnancy loss vary based on the timing of the loss, with an increased likelihood of a genetic cause in early pregnancy. Therefore, it is important to categorize losses precisely with regard to gestational age and developmental stage at the time of the loss. Losses can be preimplantation, pre-embryonic (post implantation but embryo not visible on ultrasound), embryonic (embryo visible on ultrasound and < 10 weeks gestation), early fetal (10 – 13 weeks), late fetal (14–19 weeks), or stillbirth (≥ 20 weeks).2 For this categorization, documenting the developmental stage rather than the gestational age at the time a loss is diagnosed is important because there is often a substantial lag between demise and onset of symptoms of pregnancy loss and / or diagnosis.
Many clinicians choose not to pursue genetic testing in cases of pregnancy losses because the benefit is not apparent. It is costly, presumed to be of little value for future pregnancy planning, and does not alleviate the acute emotional sequelae facing a couple once a loss has occurred. Indeed, most professional societies do not endorse genetic testing for sporadic early losses. There is more enthusiasm and justification for genetic testing in cases of recurrent early loss and sporadic late loss. In these instances, identifying a genetic cause provides important information on recurrence risk and helps avoid other types of potentially unnecessary evaluation and experimental treatments. In some cases, genetic abnormalities can influence care and hopefully, eventual breakthroughs may render some genetic causes treatable.
Genetic Abnormalities in the Conceptus: Aneuploidy and Copy Number Changes
Aneuploidy is a common finding in both sporadic and recurrent losses. The overall incidence of cytogenetic abnormalities is somewhat lower in recurrent than in sporadic losses (30–50% vs 70%, respectively). This is because RPL is often attributable to a non-genetic cause.3 Although chromosomal abnormalities are generally similar in recurrent and sporadic losses, trisomies are less common in RPL.3 Given that aneuploidy in the conceptus is usually a sporadic occurrence, it follows that when a prior loss occurs due to aneuploidy, a subsequent pregnancy is more likely to be uncomplicated than when the prior loss is cytogenetically normal. This has been borne out in epidemiologic studies.3
Nonetheless, aneuploidy accounts for a meaningful proportion of recurrent losses. Among those with RPL and prior chromosomally normal losses, subsequent losses are also likely to be chromosomally normal. When prior losses are attributable to aneuploidy, subsequent losses are also likely to be aneuploid.4 Cases of chromosomally abnormal RPL may be related to a yet undiscovered meiotic regulation defect. While therapeutic options are limited, in vitro fertilization (IVF) with preimplantation genetic testing may be useful in cases of recurrent aneuploidy loss (see below).
A variety of factors and characteristics are associated with aneuploidies. Trisomies are known to be associated with increasing maternal age.5 Chromosomal abnormalities are common in the presence of congenital malformations, occurring in two thirds of anomalous embryos and one third of anomalous fetuses.6 The earlier in gestation the loss, the higher the rate of chromosomal abnormalities: 90% in pre-embryonic, 50% of losses at 8–11 weeks, and 30% at 16–19 weeks.7 Only 6–12% of stillbirths (≥ 20 weeks) are associated with chromosomal anomalies.8,9
Accounting for 70% of first trimester losses, aneuploidy represents the most common etiology of sporadic loss.3 The most common type of aneuploidy is trisomy (60% of aneuploidies), of which the most common is trisomy 16 (20–30% of trisomies, Fig. 1). The most common single aneuploidy overall is Monosomy X, which accounts for 20% of all aneuploidies (Fig 2). The trisomies that are most prevalent in live births (trisomies 21, 18, 13) are the most common among late fetal losses and stillbirths.
Figure 1.
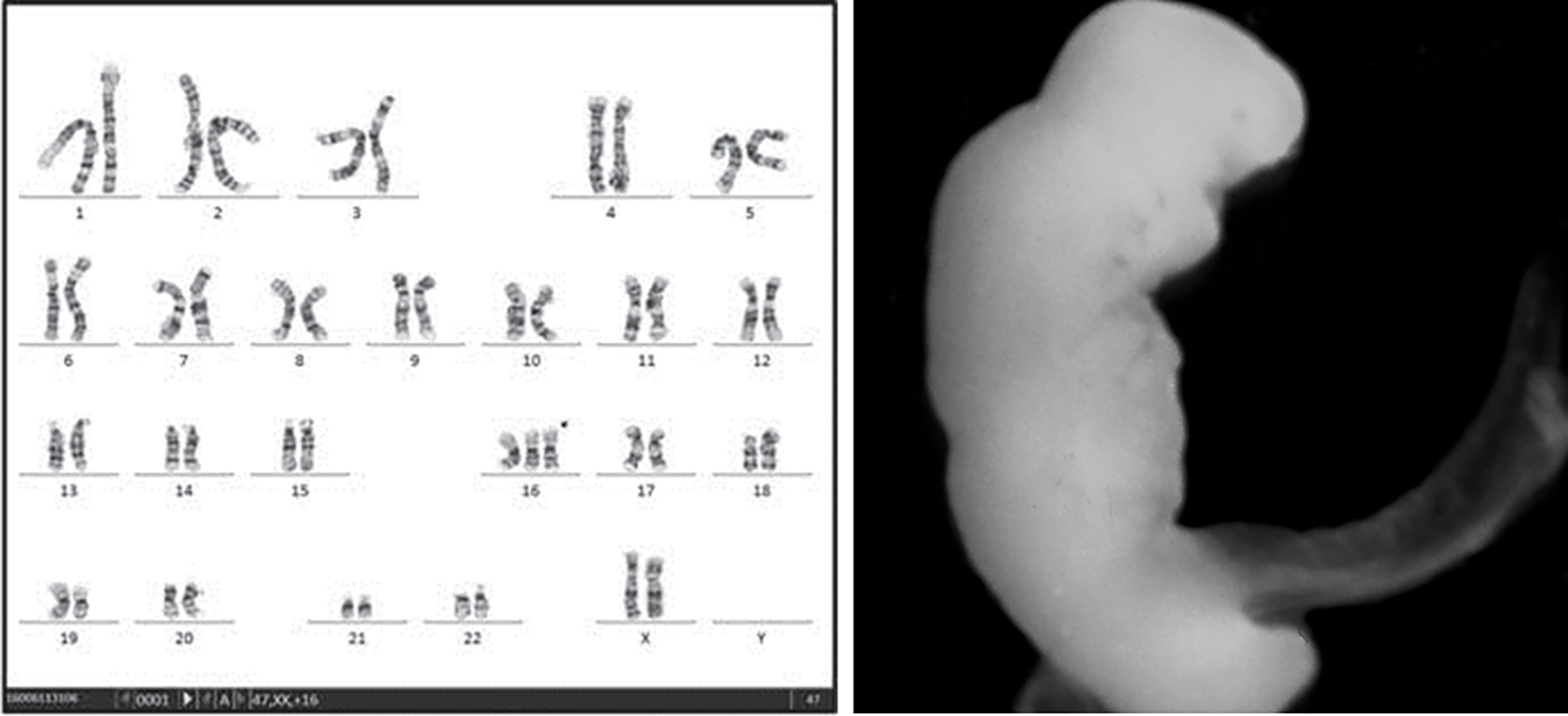
Karyotype and embryo phenotype of trisomy 16. Karyotype reprinted with permission.(53)
Figure 2.
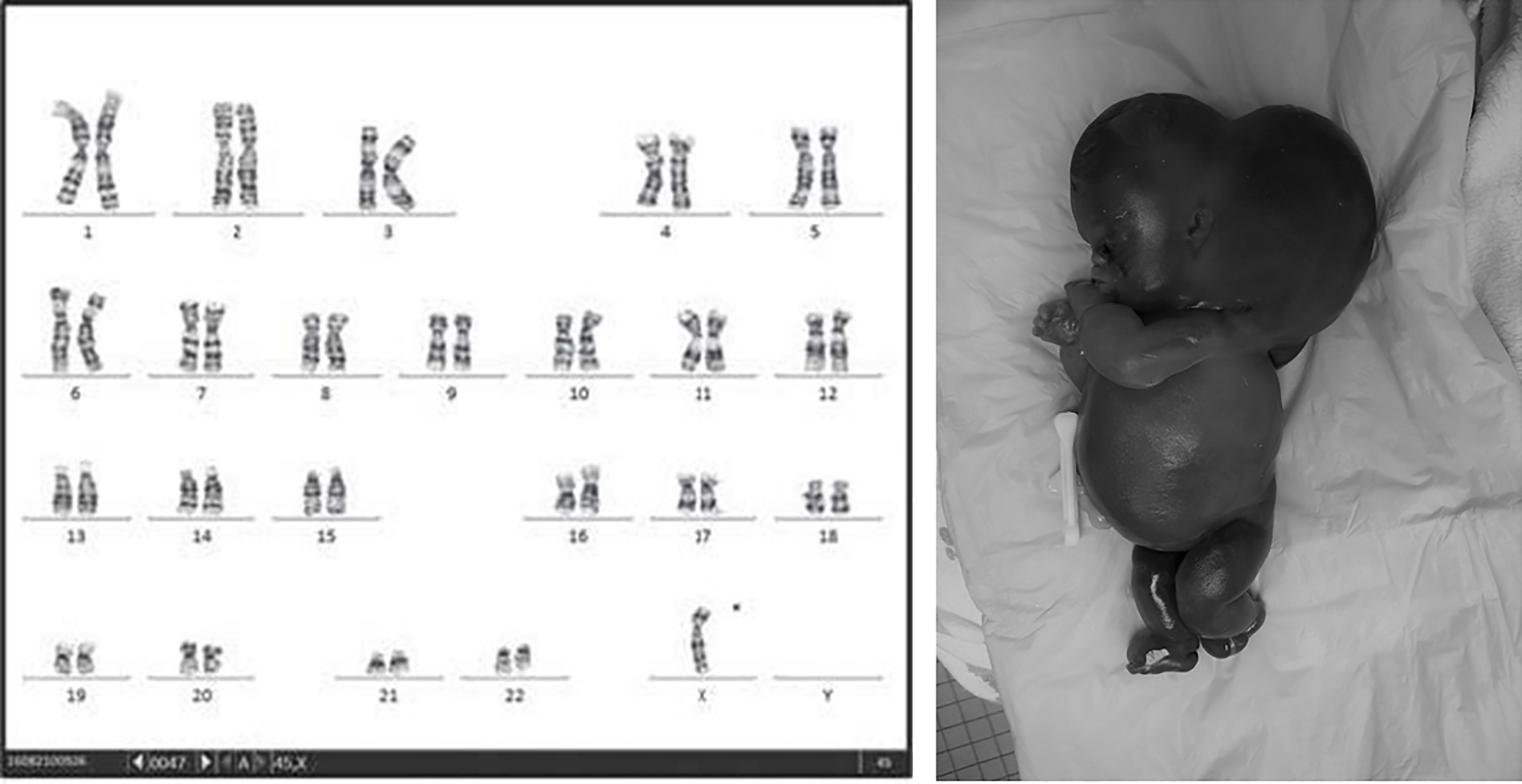
Karyotype and phenotype of 45X. Karyotype reprinted with permission.(53)
Whereas aneuploidy is defined as the gain or loss of a chromosome, genetic material can be gained or lost in smaller quantities as well. Deletions and duplications, when larger than approximately five million bases, can be identified using karyotype, with smaller gains or losses (microdeletions/microduplications) detectable only using microarray. These smaller changes are referred to as copy number changes (CNCs). Because not all CNCs are pathologic, one disadvantage of microarray is that the clinical significance of identified CNCs is not always clear. However, certain CNC characteristics are more likely to represent a clinically meaningful potential cause of pregnancy loss. These include large CNC size, deletions (rather than duplications), CNCs including genes described in the Online Mendelian Inheritance in Man (OMIM) database, and changes not described in databases of normal individuals.
CNCs of undetermined significance are less likely associated with pregnancy loss. Examples include CNCs that are small (<400 kb) and have not been demonstrated to be pathologic. In a large series of more than 500 stillbirths analyzed by microarray, Reddy et al identified multiple CNCs of potential relevance to pregnancy loss. However, the only ones that occurred in more than one case were those in the chromosome 22 region associated with DiGeorge syndrome.9 The need for further research into the significance of newly identified CNCs is illustrated by an analysis of array comparative genomic hybridization in 26 couples with RPL. Rajcan-Separaovic et al identified 11 unique CNCs in 13 of the losses from 8 couples. Two of the CNCs involved the TIMP2 and CTNNA3 genes, which are imprinted and usually only expressed in the placenta.10 Whether these are related to pathways relevant to pregnancy loss or RPL is still unknown.
Genetic causes in the conceptus: Confined Placental Mosaicism
Confined placental mosaicism (CPM) is defined as the presence of genetically abnormal (usually referring to aneuploidy) cell lines coexisting with normal cell lines in the placenta in the setting of a genetically normal fetus. Several mechanisms exist for CPM. Errors in meiosis during gametogenesis may cause trisomy that then undergoes trisomic rescue in only part of the cell lines, with the aneuploid lines ending up in the placenta. Alternatively, the early morula can undergo postzygotic mitotic nondisjunction, and if the aneuploid cells segregate to what is ultimately the placenta, CPM results.
Just as fetal aneuploidy can lead to changes in organ function, the aneuploid placenta is also frequently dysfunctional, resulting in growth restriction and even death from placental insufficiency. It follows that a partially aneuploid placenta would also have some degree of dysfunction, though perhaps to a lesser degree. The outcome of CPM depends on a variety of factors, including the proportion of abnormal placental cells, whether or how many abnormal cells persist late into pregnancy, and the particular genetic abnormality. So far, early case control studies have found CPM in more than 15% of growth restricted fetuses.11
CPM can be a challenging diagnosis to make. Prenatally identifying CPM is difficult because it requires analysis of both the fetus and the placenta, and commonly used screening and diagnostic tests for aneuploidy analyze one or the other, not both. In the setting of a loss, genetic analysis of both the fetal and placental compartments is more costly since two analyses are required rather than one. For early losses, sampling the appropriate tissue can be challenging if placental and embryonic tissue are difficult to grossly distinguish. Further, CPM may go unidentified if the placental specimen analyzed does not include the abnormal portion, such that several placental samples must be assessed.Clarifying the incidence and role of CPM in RPL will be progressively more feasible as the cost and availability of genetic analyses such as microarray increase.
Genetic causes in the conceptus: Skewed X inactivation
While all female cells have two X chromosomes, one of them is inactivated during the embryonic period, leading to one functional X chromosome in all cells throughout life. In typical circumstances, the inactivation is random so that each X chromosome is active in approximately half of a woman’s cells. This random process can become preferential, however, resulting in a disproportionate representation, termed “skewed X inactivation”. This can be problematic if the X chromosome that remains active has mutations, the expression of which can lead to disease. Expression of a lethal mutation on the X chromosome that is selected for preferential activity could be one mechanism by which skewed X inactivation could contribute to RPL. It is noteworthy, however, that this “lethal mutation hypothesis” has not yet been documented in human pregnancy. In addition to being a potential direct cause for RPL, skewed X inactivation may simply be a marker for increased risk of RPL. For example, X inactivation may be more likely to occur in the setting of an abnormally small number of embryonic cells, which would lead to both skewed X inactivation and pregnancy loss. Whether skewed X inactivation is associated with RPL is uncertain, as some studies report an associated while others do not.12,13 The contribution of skewed X inactivation to RPL remains unclear.
Genetic causes in the conceptus: Single Gene disorders
RPL has been associated with several single gene disorders. These include inborn errors of metabolism, hemoglobinopathies, and X-linked diseases. While most hemoglobinopathies in the conceptus would not be severe enough to cause loss, untreated alpha thalassemia major (Bart syndrome), leads almost universally to fetal hydrops and stillbirth due to all four α-globin genes being defective. Inborn errors of metabolism are primarily transmitted via autosomal recessive inheritance, a few examples of which lysosomal or amino acid disorders, mucolipidoses, critical enzymatic deficiencies, and defects in mitochondrial function, glycogen storage, or fatty acid oxidation. X-linked disorders could contribute to recurrent losses when the conceptus is male, such as occurs in Rett syndrome, where the defective gene (MECP2) is on the X chromosome. Until recently, single gene disorders have been difficult to identify prenatally or in losses as they are not detected by either karyotype or microarray. Whole exome and genome sequencing are capable of detecting single gene abnormalities and have the potential to increase the diagnostic yield of pregnancy loss testing.
One single-gene disorder of potential relevance is Long QT syndrome. Long QT syndrome is a condition characterized by sudden cardiac death, usually in the setting of an anatomically normal heart. It appears to occur more frequently in stillbirths than the general population (3% vs < 0.05%, respectively) and has been associated with sporadic pregnancy loss, but has not been studied as a potential cause of RPL.14
It is almost a certainty that more single gene mutations contribute to RPL than are known. This is supported by collective experience with transgenic mice in which various genes have been experimentally disrupted, triggering recurrent losses in these mice. These genes are often related to placental or vascular function, such as tropomodulin-3, a defect in which leads to mid-gestation death in mice because of defective erythropoiesis.15 The presence of analogous human genetic defects is likely.
Beyond catastrophic single gene mutations, it is likely that single gene polymorphisms exist which do not guarantee loss but simply increase its likelihood. If such polymorphisms only marginally increase the risk of loss but still frequently allow for normal pregnancy to continue, they might occur commonly in the general population and be very difficult to identify. Additionally, it may be that such genetic variations require interaction with other exposures or factors in order to cause a loss, as is the case with contributing polymorphisms in multi-factorial conditions such as hypertension.
Recently, the ability to systematically assess single gene mutations in RPL has expanded with advancing molecular technology. This has resulted in the identification of hundreds of genes that may contribute to pregnancy loss.16,17 While comparison across studies is difficult due to differences in methodology, genes of interest can be categorized according to potential mechanisms and pathways: aneuploidy or chromosomal anomalies, autoimmune conditions, angiogenesis, human leukocycte antigen defects, maternal immunologic dysregulation and hyper-immunity, defects in placental development, and thrombophilias.16 Screening for these genes is not clinically helpful at present because the reported associations are generally weak or moderate, but they may be useful in guiding future research into mechanisms of RPL.17 It is also possible that applying more integrated assessments of many potential gene defects to larger study cohorts will clarify their relationships and interactions, offering insights into potential management and treatment prospects. Future studies of single gene polymorphisms should include analyses of the full trio (mother, father, conceptus).17
For a time, couples with RPL were advised to undergo testing for single gene disorders related to inherited thrombophilias. These consist of a variety of conditions that increase the risk of venous thromboembolism (VTE), usually by reducing the production of anticoagulant proteins or increasing the production of procoagulant proteins, leading to pathologic clotting. The most frequently occurring mutations linked to RPL are for the prothrombin 20210A gene, the methylenetetrahydrofolate reductase (MTHFR) gene (C677T variant), and factor V Leiden (FVL) gene. Even in cases of homozygosity, FVL does not appear to increase the risk of RPL and so its assessment is not recommended.18 Additional thrombophilias occurring with less frequency include protein C, S, and antithrombin deficiencies, all of which are inherited in an autosomal dominant fashion. Testing for these thrombophilias are also not advised in the evaluation of RPL.
The associations of thrombophilias with RPL (especially loss beyond 10 weeks gestation) and other poor pregnancy outcomes have been supported by meta-analyses of case-control and retrospective cohort studies.19,20 Subsequent prospective studies could not reproduce these results, though the inclusion of women later in pregnancy may have limited their ability to assess for associations with earlier loss.21–23 Integrating these data, inherited thrombophilias do not appear to cause RPL, but could be considered a minor risk factor. One final reason that testing for these thrombophilias is no longer recommended is that randomized, controlled trials assessing maternal treatment with anticoagulation in subsequent pregnancies did not demonstrate any benefit.24
Parental Genetic Causes: Balanced Chromosome Abnormalities
Approximately 3–5% of couples with RPL are estimated to have a balanced parental chromosome rearrangement, a 5–10 fold increase compared to the general population. Indeed, the presence of a parental chromosomal abnormality is associated with a higher loss recurrence rate than among couples with normal karyotypes.25,26 In couples with RPL, balanced parental abnormalities, usually balanced reciprocal translocations, are more common in women than men. Other anomalies such as inversions, sex chromosome mosaicisms, or Robertsonian translocations, occur less frequently. The rate of live births from couples with a balanced parental rearrangement is higher than would be expected using mathematical modeling. While rates differ somewhat by specific abnormality, the likelihood of a successful pregnancy approaches 70% in some couples, with fewer than 1% of live births complicated by a chromosomal abnormality.26–28
Testing the conceptus
There are several options to genetically evaluate products of conception after a loss. Approaches range from long established options of fluorescence in situ hybridization (FISH) and karyotype to newer molecular methods that offer much more in-depth assessments, such as chromosomal microarray and whole exome or whole genome sequencing. The most commonly used methods, however, are karyotype and chromosomal microarray.
Chromosomal microarray has several advantages over karyotype when working up RPL. The principal benefit of microarray is that cultured live cells are not required. This confers a higher likelihood of obtaining interpretable results than with karyotype, which has a failure rate up to 40%. Additionally, karyotype is prone to overgrowth of maternal cells and preferential growth of chromosomally normal cell lines, obscuring the true diagnosis. Not only does microarray fail less frequently, but it can be performed on both fresh tissue as well as non-viable tissue. It can even be done after tissue has been fixed in formalin and embedded in paraffin. Lastly, the higher resolution of the microarray assessment can identify smaller losses or gains in genetic material, termed copy number changes (CNCs, microduplications or microdeletions), than can be identified using karyotype.
Since microarray has become widely available, it has proven to be highly reliable. Studies of early losses demonstrated that microarray yielded interpretable results in 91–99% of cases, albeit with maternal cell contamination rates of anywhere from 3–22%.29,30 In one series of more than 8,000 specimens undergoing microarray for both sporadic and recurrent losses, 86.4% of 1,823 formalin-fixed/paraffin-embedded samples yielded interpretable results.31 Microarray also performed better than karyotype in the evaluation of a large cohort of stillbirths (> 20w gestation), with microarray providing interpretable results in 86% of those with failed karyotypes and identifying aneuploidy or a pathogenic variant in a significant number of both anomalous and nonanomalous fetuses (Table 1).9 The significant rates of maternal cell contamination in cases of microarray with early pregnancy loss illustrate the importance of ruling out maternal cell contamination whenever microarray is used to analyze products of conception, especially in the presence of a female fetus. This important step is easy and only requires concurrent analysis of maternal DNA.
Tables 1:
Results of microarray among stillbirths with either failed or normal karyotype
| Interpretable result, n (%) | Pathogenic variant or aneuploidy, n (%) | Variant of unknown significance, n (%) | |
|---|---|---|---|
| Anomalous n=54 | 47 (87.0) | 7 (13.0) | 3 (5.6) |
| Nonanomalous n=393 | 345 (87.8) | 9 (2.3) | 20 (5.1) |
Adapted from Reddy et al.(9)
The utility of microarray was further demonstrated in a recent prospective observational study of 100 women with at least two early losses who underwent the RPL evaluation recommended by the American Society for Reproductive Medicine (ASRM).32 In addition to the standard tests, 24-chromosome microarray analysis of products of conception was also performed. Of the 100, 45 had a parental abnormality identified by the RPL workup (parental karyotype along with maternal anatomic, endocrinologic, and autoimmune evaluation). Of the remaining 55, 50 (90%) had a likely cause identified using microarray.33 A total of 67/100 had a clinically relevant abnormality identified by microarray. Of note, the study excluded cases with significant maternal cell contamination. In another study of second trimester losses that had undergone either failed karyotype or had a normal karyotype, microarray identified potentially significant copy number changes (CNCs) in an additional 13% of cases.34
While Fluorescent in situ hybridization (FISH) is not the currently preferred diagnostic platform, it deserves mention. FISH makes use of fluorescent DNA probes, which bind specific regions of the genome and indicate whether the region of interest is present or absent under fluorescent microscopy. Although it is not preferred because it can only assess a limited portion of the genome at a time, it offers two distinct advantages. First, it can provide time-sensitive results, usually within 2–3 days. Second, it can be used to identify microdeletions (such as those that cause DiGeorge syndrome) for regions too small to assess on karyotype. Because of the fast turn-around, it is still often used when the results are time-sensitive. More definitive methods are used concurrently for confirmation of FISH results.
Parental testing
Assessment of parental karyotype is routinely advised in the evaluation of RPL, but the utility is unclear. Although preimplantation genetic diagnosis in couples with balanced translocations offers theoretical benefit, no proven treatment exists for parental chromosome abnormalities. Also, treatment may not be truly needed since the live birth rate is relatively similar between couples with RPL who do and do not have parental translocations.35 This is noteworthy because the cost of a parental karyotype is considerable and is often not covered by some payers in the United States. Even so, a couple with RPL may derive psychological benefit from knowledge of the underlying cause of their losses. Despite the lack of clear clinical benefit, potential benefits include psychological value of identifying the underlying cause, individualized counseling for specific translocations for which data exist, and the avoidance of experimental therapies that would otherwise be considered for idiopathic RPL.
One final domain of parental testing is for genetic abnormalities in the sperm. Previous studies associating abnormal sperm DNA fragmentation with RPL have not been consistently replicated. Thus, screening for it is not recommended by the American Society for Reproductive Medicine.32 Several small case-control studies have now associated sperm aneuploidy with recurrent pregnancy loss, but the clinical significance of this has not been investigated adequately to recommend screening or guide management.36–39 It is a fertile are for ongoing investigation.
Treatment: IVF with preimplantation genetic testing
Preimplantation genetic testing (PGT), which can be used to identify genetic abnormalities prior to embryo transfer, holds promise for couples with genetic causes for RPL. Previously referred to as either preimplantation genetic screening (PGS) or diagnosis (PGD), PGT is now categorized based on the type of condition it tests for: aneuploidy (PGT-A) or monogenic defects (PGT-M).40,41 In the few years since it became widely available, PGT is now widely used. Indeed, it is so potentially attractive that a worldwide survey of 386 IVF units from 70 countries found that clinics offering PGT use it in couples with RPL as often as for any other indication.42 Evidence supporting its utility for this purpose does not yet exist, however.40
PGT for parental balanced translocations
A couple with RPL and a parental balanced translocation presents a potential indication for IVF and PGT. Preliminary studies of couples with Robertsonian or reciprocal translocations noted improved outcomes when IVF and PGT-A are used in this setting, with per-cycle loss rates of only 10–13% and per-cycle live birth rates of 20–38%.43–45 Higher rates live birth rates occur once embryo transfer actually takes place.43–45 Each of these studies compared outcomes to those of historical controls and so the ability to draw conclusions is limited. This is especially relevant given the findings of a systematic review comparing outcomes of natural conception to IVF-PGT-A in couples with RPL and balanced translocations. Live birth rates in 469 couples pursuing natural conception (55.5%, range 33–60%) were not significantly different from that of 126 couples undergoing IVF-PGT-A (34%, range 0–100%). The authors conclude that evidence does not yet support the use of IVF-PGT for this indication.46
A more recent prospective cohort study of 89 patients with RPL and a known translocation who chose either natural conception (n=52) or IVF-PGT (n=37) found that live birth rates were not statistically different either once a pregnancy was achieved (37.8% for PDT-A, 53.8% for natural conception) or when analyzing cumulative live birth rates (67.6% vs 65.4%). Additionally, they found that the time required to achieve pregnancy was similar between groups, with PDT-A associated with higher rates of twins and higher cost.47 PGT-A has been proposed as a method to increase the live birth rate in couples with sperm aneuploidy,48 but as of yet the efficacy of IVF-PGS for this indication has not been validated. This may represent an opportunity for future study.
PGT for idiopathic RPL
Some authors have proposed that in cases of idiopathic RPL, especially when prior losses were chromosomally abnormal, IVF-PGT-A may be useful. While case series report that IVF-PGT-A in such cases is associated with loss rates of <10%, no studies with comparable controls or randomized trials have been performed.49,50 Even so, early results appear promising. A case series of 287 IVF-PGT-A cycles resulted in 94 pregnancies that continued either to delivery or beyond the 2nd trimester.51 In this same series, only 6.4% embryos successfully implanted following IVF-PGT-A led to miscarriage, a lower rate than the expected 33.5% in couples with idiopathic RPL. It is important to recognize that that this low loss rate applies only after a successful embryo transfer is confirmed.
Considerations for studies of PGT and pregnancy loss
Several additional elements are important in the ongoing evaluation of IVF-PGT. Studies differ in which days embryo biopsies and transfers were executed, along with whether biopsies were taken from trophoectoderm, blastocysts, or polar bodies. Evolving technology has also led to variations in reported genetic techniques, which include genomic sequencing, microarray, FISH, polymerase chain reaction, and comparative genomic hybridization. While new technologies may lead to more discriminatory embryo selection, it follows that this may equate to fewer normal embryo transfers per cycle, and hence fail to yield a net increase in the number of live births per IVF cycle. This matters because the emotional and financial costs of failed IVF cycles are considerable. A retrospective cohort study and cost-effectiveness analysis of PGT-A in idiopathic RPL found that IVF-PGT-A and expectant management had live birth rates of 53% and 67%, respectively, and loss rates of 7% and 24%, respectively. The cost of IVF-PGT-A was $45,300 per live birth, nearly 100 times the cost of a live birth following expectant management.52
Given these complexities, deciding whether to routine use IVF-PGT in RPL is difficult as the efficacy and benefit remain unclear. Evidence for IVF-PGT in RPL is both promising and limited. It is therefore important for clinicians and couples to be aware that expectant management is usually associated with a good prognosis, and that other viable options exist, such as the use of a donor egg or sperm, and adoption.
Summarizing the evidence
Quality evidence is lacking to generate strong recommendations, but certain considerations are worthwhile:
In both early recurrent losses and late sporadic losses, consider genetic evaluation of the products of conception.
Use chromosomal microarray rather than karyotype. Ruling out maternal cell contamination is important.
Couples with RPL should be offered parental karyotype, with genetic counseling if abnormalities are found. In cases of balanced parental translocations, live birth rates are good with expectant management, though IVF with PGT can be considered.
RPL in the setting of known parental autosomal recessive mutations requires genetic counseling. IVF with PGT-M may be appropriate in these cases.
Idiopathic RPL alone is not an evidence-based indication for IVF-PGT.
Future directions
Many important questions regarding genetic causes of RPL remain unanswered. Newer techniques such as whole genome sequencing will uncover additional genetic contributors to RPL and will likely lead to new therapeutic approaches. As newer approaches become less costly and more widely available, they should undergo rigorous evaluation before being widely adopted. Evaluation of both efficacy and cost-effectiveness should be research priorities.
Acknowledgments
Grant support: This work was funded in part by NICHD (WRHR K12, 1K12 HD085816)
Footnotes
Disclosure statement: The authors have no conflicts of interest to report.
Contributor Information
Nathan R. Blue, University of Utah Health, Dept. of Obstetrics and Gynecology, Maternal-Fetal Medicine. Salt Lake City, Utah..
Jessica M. Page, University of Utah Health, Dept. of Obstetrics and Gynecology, Maternal-Fetal Medicine; Intermountain Healthcare, Salt Lake City, Utah..
Robert M. Silver, University of Utah Health, Dept. of Obstetrics and Gynecology, Maternal-Fetal Medicine. Salt Lake City, Utah..
References:
- 1.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–194. doi: 10.1056/NEJM198807283190401 [DOI] [PubMed] [Google Scholar]
- 2.Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA. Nomenclature for pregnancy outcomes: time for a change. Obstet Gynecol. 2011;118(6):1402–1408. doi: 10.1097/AOG.0b013e3182392977 [DOI] [PubMed] [Google Scholar]
- 3.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–304. http://www.ncbi.nlm.nih.gov/pubmed/10685533. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104(4):784–788. doi: 10.1097/01.AOG.0000137832.86727.e2 [DOI] [PubMed] [Google Scholar]
- 5.Eiben B, Bartels I, Bähr-Porsch S, et al. Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage. Am J Hum Genet. 1990;47(4):656–663. http://www.ncbi.nlm.nih.gov/pubmed/2220806%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1683793. [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne J, Warburton D, Kline J, Blanc W, Stein Z. Morphology of early fetal deaths and their chromosomal characteristics. Teratology. 1985;32(2):297–315. doi: 10.1002/tera.1420320218 [DOI] [PubMed] [Google Scholar]
- 7.Geraedts J Chromosomal anomalies and recurrent miscarriage. Infertil Reprod Med Clin North Am. 1996;7:677–688. [Google Scholar]
- 8.Korteweg FJ, Bouman K, Erwich JJHM, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111(4):865–874. doi: 10.1097/AOG.0b013e31816a4ee3 [DOI] [PubMed] [Google Scholar]
- 9.Reddy UM, Page GP, Saade GR, et al. Karyotype versus microarray testing for genetic abnormalities after stillbirth. N Engl J Med. 2012;367(23):2185–2193. doi: 10.1056/NEJMoa1201569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajcan-Separovic E, Diego-Alvarez D, Robinson WP, et al. Identification of copy number variants in miscarriages from couples with idiopathic recurrent pregnancy loss. Hum Reprod. 2010;25(11):2913–2922. doi: 10.1093/humrep/deq202 [DOI] [PubMed] [Google Scholar]
- 11.Wilkins-Haug L, Quade B, Morton CC. Confined placental mosaicism as a risk factor among newborns with fetal growth restriction. Prenat Diagn. 2006;26(5):428–432. doi: 10.1002/pd.1430 [DOI] [PubMed] [Google Scholar]
- 12.Robinson WP, Beever C, Brown CJ, Stephenson MD. Skewed X inactivation and recurrent spontaneous abortion. Semin Reprod Med. 2001;19(2):175–181. doi: 10.1055/s-2001-15397 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan AE, Lewis T, Stephenson M, et al. Pregnancy outcome in recurrent miscarriage patients with skewed X chromosome inactivation. Obstet Gynecol. 2003;101(6):1236–1242. http://www.ncbi.nlm.nih.gov/pubmed/12798530. [DOI] [PubMed] [Google Scholar]
- 14.Crotti L, Tester DJ, White WM, et al. Long QT syndrome-associated mutations in intrauterine fetal death. JAMA. 2013;309(14):1473–1482. doi: 10.1001/jama.2013.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui Z, Nowak RB, Bacconi A, et al. Tropomodulin3-null mice are embryonic lethal with anemia due to impaired erythroid terminal differentiation in the fetal liver. Blood. 2014;123(5):758–767. doi: 10.1182/blood-2013-03-492710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaiman D Genetic regulation of recurrent spontaneous abortion in humans. Biomed J. 38(1):11–24. doi: 10.4103/2319-4170.133777 [DOI] [PubMed] [Google Scholar]
- 17.Pereza N, Ostojić S, Kapović M, Peterlin B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril. 2017;107(1):150–159.e2. doi: 10.1016/j.fertnstert.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 18.ACOG Practice Bulletin No. 197: Inherited Thrombophilias in Pregnancy. Obstet Gynecol. 2018;132(1):e18–e34. doi: 10.1097/AOG.0000000000002703 [DOI] [PubMed] [Google Scholar]
- 19.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet (London, England). 2003;361(9361):901–908. doi: 10.1016/S0140-6736(03)12771-7 [DOI] [PubMed] [Google Scholar]
- 20.Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132(2):171–196. doi: 10.1111/j.1365-2141.2005.05847.x [DOI] [PubMed] [Google Scholar]
- 21.Dizon-Townson DS, Nelson LM, Jang H, Varner MW, Ward K. The incidence of the factor V Leiden mutation in an obstetric population and its relationship to deep vein thrombosis. Am J Obstet Gynecol. 1997;176(4):883–886. http://www.ncbi.nlm.nih.gov/pubmed/9125615. [DOI] [PubMed] [Google Scholar]
- 22.Lindqvist PG, Svensson PJ, Marsaál K, Grennert L, Luterkort M, Dahlbäck B. Activated protein C resistance (FV:Q506) and pregnancy. Thromb Haemost. 1999;81(4):532–537. http://www.ncbi.nlm.nih.gov/pubmed/10235434. [PubMed] [Google Scholar]
- 23.Silver RM, Zhao Y, Spong CY, et al. Prothrombin gene G20210A mutation and obstetric complications. Obstet Gynecol. 2010;115(1):14–20. doi: 10.1097/AOG.0b013e3181c88918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): A multinational open-label randomised trial. Lancet. 2014;384(9955):1673–1683. doi: 10.1016/S0140-6736(14)60793-5 [DOI] [PubMed] [Google Scholar]
- 25.Sugiura-Ogasawara M, Ozaki Y, Sato T, Suzumori N, Suzumori K. Poor prognosis of recurrent aborters with either maternal or paternal reciprocal translocations. Fertil Steril. 2004;81(2):367–373. doi: 10.1016/j.fertnstert.2003.07.014 [DOI] [PubMed] [Google Scholar]
- 26.Sugiura-Ogasawara M, Aoki K, Fujii T, et al. Subsequent pregnancy outcomes in recurrent miscarriage patients with a paternal or maternal carrier of a structural chromosome rearrangement. J Hum Genet. 2008;53(7):622–628. doi: 10.1007/s10038-008-0290-2 [DOI] [PubMed] [Google Scholar]
- 27.Engels H, Eggermann T, Caliebe A, et al. Genetic counseling in Robertsonian translocations der(13;14): frequencies of reproductive outcomes and infertility in 101 pedigrees. Am J Med Genet A. 2008;146A(20):2611–2616. doi: 10.1002/ajmg.a.32500 [DOI] [PubMed] [Google Scholar]
- 28.Keymolen K, Staessen C, Verpoest W, et al. A proposal for reproductive counselling in carriers of Robertsonian translocations: 10 years of experience with preimplantation genetic diagnosis. Hum Reprod. 2009;24(9):2365–2371. doi: 10.1093/humrep/dep201 [DOI] [PubMed] [Google Scholar]
- 29.Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124(2 Pt 1):202–209. doi: 10.1097/AOG.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Cheng Q, Meng L, et al. Clinical application of SNP array analysis in first-trimester pregnancy loss: a prospective study. Clin Genet. 2017;91(6):849–858. doi: 10.1111/cge.12926 [DOI] [PubMed] [Google Scholar]
- 31.Sahoo T, Dzidic N, Strecker MN, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19(1):83–89. doi: 10.1038/gim.2016.69 [DOI] [PubMed] [Google Scholar]
- 32.Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi: 10.1016/j.fertnstert.2012.06.048 [DOI] [PubMed] [Google Scholar]
- 33.Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33(4):579–587. doi: 10.1093/humrep/dey021 [DOI] [PubMed] [Google Scholar]
- 34.Warren JE, Turok DK, Maxwell TM, Brothman AR, Silver RM. Array comparative genomic hybridization for genetic evaluation of fetal loss between 10 and 20 weeks of gestation. Obstet Gynecol. 2009;114(5):1093–1102. doi: 10.1097/AOG.0b013e3181bc6ab0 [DOI] [PubMed] [Google Scholar]
- 35.Franssen MTM, Korevaar JC, van der Veen F, Leschot NJ, Bossuyt PMM, Goddijn M. Reproductive outcome after chromosome analysis in couples with two or more miscarriages: index [corrected]-control study. BMJ. 2006;332(7544):759–763. doi: 10.1136/bmj.38735.459144.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramasamy R, Scovell JM, Kovac JR, Cook PJ, Lamb DJ, Lipshultz LI. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril. 2015;103(4):906–909.e1. doi: 10.1016/j.fertnstert.2015.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neusser M, Rogenhofer N, Dürl S, et al. Increased chromosome 16 disomy rates in human spermatozoa and recurrent spontaneous abortions. Fertil Steril. 2015;104(5):1130–7.e1–10. doi: 10.1016/j.fertnstert.2015.07.1160 [DOI] [PubMed] [Google Scholar]
- 38.Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016;105(1):58–64. doi: 10.1016/j.fertnstert.2015.09.041 [DOI] [PubMed] [Google Scholar]
- 39.Esquerré-Lamare C, Walschaerts M, Chansel Debordeaux L, et al. Sperm aneuploidy and DNA fragmentation in unexplained recurrent pregnancy loss: a multicenter case-control study. Basic Clin Androl. 2018;28:4. doi: 10.1186/s12610-018-0070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Electronic address: ASRM@asrm.org, Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–436. doi: 10.1016/j.fertnstert.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 41.Ethics Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org, Ethics Committee of the American Society for Reproductive Medicine. Use of preimplantation genetic testing for monogenic defects (PGT-M) for adult-onset conditions: an Ethics Committee opinion. Fertil Steril. 2018;109(6):989–992. doi: 10.1016/j.fertnstert.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 42.Weissman A, Shoham G, Shoham Z, Fishel S, Leong M, Yaron Y. Preimplantation genetic screening: results of a worldwide web-based survey. Reprod Biomed Online. 2017;35(6):693–700. doi: 10.1016/j.rbmo.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 43.Fischer J, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94(1):283–289. doi: 10.1016/j.fertnstert.2009.02.060 [DOI] [PubMed] [Google Scholar]
- 44.Scriven PN, Flinter FA, Khalaf Y, Lashwood A, Mackie Ogilvie C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur J Hum Genet. 2013;21(10):1035–1041. doi: 10.1038/ejhg.2013.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idowu D, Merrion K, Wemmer N, et al. Pregnancy outcomes following 24-chromosome preimplantation genetic diagnosis in couples with balanced reciprocal or Robertsonian translocations. Fertil Steril. 2015;103(4):1037–1042. doi: 10.1016/j.fertnstert.2014.12.118 [DOI] [PubMed] [Google Scholar]
- 46.Franssen MTM, Musters AM, van der Veen F, et al. Reproductive outcome after PGD in couples with recurrent miscarriage carrying a structural chromosome abnormality: a systematic review. Hum Reprod Update. 17(4):467–475. doi: 10.1093/humupd/dmr011 [DOI] [PubMed] [Google Scholar]
- 47.Ikuma S, Sato T, Sugiura-Ogasawara M, Nagayoshi M, Tanaka A, Takeda S. Preimplantation Genetic Diagnosis and Natural Conception: A Comparison of Live Birth Rates in Patients with Recurrent Pregnancy Loss Associated with Translocation. PLoS One. 2015;10(6):e0129958. doi: 10.1371/journal.pone.0129958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohn TP, Kohn JR, Darilek S, Ramasamy R, Lipshultz L. Genetic counseling for men with recurrent pregnancy loss or recurrent implantation failure due to abnormal sperm chromosomal aneuploidy. J Assist Reprod Genet. 2016;33(5):571–576. doi: 10.1007/s10815-016-0702-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musters AM, Repping S, Korevaar JC, et al. Pregnancy outcome after preimplantation genetic screening or natural conception in couples with unexplained recurrent miscarriage: a systematic review of the best available evidence. Fertil Steril. 2011;95(6):2153–2157, 2157.e1–3. doi: 10.1016/j.fertnstert.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 50.Shahine LK, Lathi RB. Embryo selection with preimplantation chromosomal screening in patients with recurrent pregnancy loss. Semin Reprod Med. 2014;32(2):93–99. doi: 10.1055/s-0033-1363550 [DOI] [PubMed] [Google Scholar]
- 51.Hodes-Wertz B, Grifo J, Ghadir S, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril. 2012;98(3):675–680. doi: 10.1016/j.fertnstert.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 52.Murugappan G, Ohno MS, Lathi RB. Cost-effectiveness analysis of preimplantation genetic screening and in vitro fertilization versus expectant management in patients with unexplained recurrent pregnancy loss. Fertil Steril. 2015;103(5):1215–1220. doi: 10.1016/j.fertnstert.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 53.Page JM, Silver RMS. Genetic causes of Recurrent Pregnancy Loss. Clin Obstet Gyncol. 2016;59(3):498–508. [DOI] [PubMed] [Google Scholar]


