Abstract
COVID-19 vaccination was launched in the United States in mid-December 2020. There are limited data on the risk of thrombotic events related to COVID-19 vaccines. In conclusion, we report 2 cases of acute myocardial infarction with onset <24 hours after the first dose of a COVID-19 vaccine in patients presenting with shoulder pain.
The United States launched the COVID-19 vaccination campaign in mid-December 2020 as a measure to contain the pandemic. Although there are limited data on the risk of thrombotic events related to the vaccines currently in use in the United States, there have been reports on thrombotic events after COVID-19 vaccines in Europe recently. We report 2 cases of acute myocardial infarction with onset less than 24 hours after the first dose of a COVID-19 vaccine in the United States. Early recognition of this acute cardiovascular condition could be challenging, as injection site soreness may lead to ischemic symptoms being overlooked and result in delayed presentations. This report should in no way diminish enthusiasm about vaccination.
Patient 1
In the first case, the patient experienced gradual onset of left shoulder pain within 1 day after her first dose of COVID-19 vaccine (mRNA-1273) that later progressed to left-sided chest pain. She initially attributed her symptoms to the vaccine. She presented to the emergency department (ED) approximately two hours after chest pain (Table 1 ). Bedside cardiac ultrasound showed left ventricular ejection fraction of 50% and hypokinesis of the anterolateral and inferolateral walls. The patient tested negative for SARS-CoV-2 by PCR on 2 separate nasopharyngeal aspirates. Emergency coronary angiography revealed an occluded proximal segment of the left circumflex (LC) artery with a globular thrombus and TIMI 0 flow (Figure 1 & Video 1).
Table 1.
| Case #1 | Case #2 | |
|---|---|---|
| Age (years) | 68 | 42 |
| Sex | Female | Male |
| BMI (kg/m2) | 46.6 | 42.8 |
| Smoker | Current | Never |
| Hypertension | Yes | No |
| Hyperlipidemia | Yes | Yes |
| Diabetes Mellitus | No | No |
| Prior Coronary Artery Disease | Yes | No |
| Family History of Premature Coronary Artery Disease | No | Yes |
| Interval (hours) Between Vaccine Injection and Chest Pain | <24 | <12 |
| Peak Troponin (ng/L) | 4714 | 1763 |
| Ischemic Changes on ECG | Inferior ST elevation | Nil |
| Coronary Angiogram | LC 100% | LC 90% |
| LVEF (%) | 50 | 60 |
| PCI Vessel | LC | LC |
Figure 1.
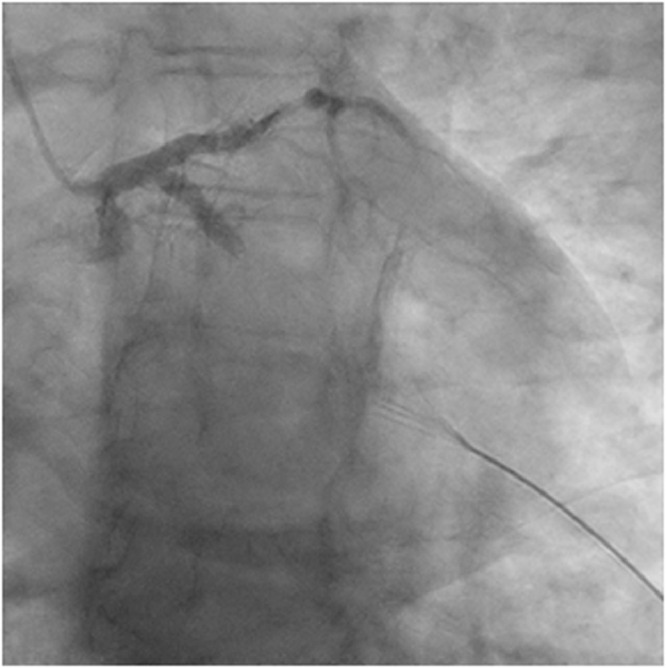
Preintervention image of left coronary system of patient 1.
Emergency percutaneous coronary intervention (PCI) was performed to the LC artery. A workhorse wire (Asahi 0.014” Sion Blue) was used to cross the lesion. The culprit lesion was predilated with a 2.5 mm × 12 mm semicompliant balloon. A 4.0 mm × 18 mm everolimus-eluting stent was placed in the proximal segment of the left circumflex artery and was optimized with a 4.0 mm × 15 mm noncompliant balloon. The final angiogram showed an excellent result with TIMI III flow (Video 2). The patient was free of chest pain after PCI. Formal cardiac ultrasound one day after PCI showed a left ventricular ejection fraction of 60% with only mild hypokinesis of the anterolateral and inferolateral walls. She was discharged home 1 week after admission.
Patient 2
The patient in the second case presented with 4 days of chest and shoulder pain. Symptoms started the night after he had his first dose of COVID-19 vaccine (mRNA-1273). He woke up from sleep with sharp, intense chest pain that radiated to the jaw. Symptoms slightly improved but remained for 4 more days, and he finally presented to an outside hospital. He also reported injection site pain from the vaccination but was otherwise asymptomatic. At the emergency department, he was found to have elevated troponin with no significant ECG changes. He was then transferred to our center, and a CT coronary angiogram revealed complete occlusion of the LC artery. In view of this finding, he was transferred to the catheterization laboratory (Table 1).
Ultrasensitive troponin-I at the outside hospital was 9,200 ng/L (normal: 0 to 47 ng/L). After transfer to our center, the high-sensitivity troponin-T rose from 723 ng/L (normal: 0 to 14 ng/L) to 1763 ng/L. Patient tested negative for SARS-CoV-2 by PCR first at the outside hospital and tested negative again at our center. Cardiac ultrasound showed left ventricular ejection fraction of 60%, with hypokinetic inferior and inferolateral walls. CT coronary angiography showed a large amount of noncalcified plaque in the proximal LC artery, resulting in complete focal occlusion just proximal to the origin of first obtuse marginal artery. Coronary angiography in the catheterization laboratory revealed a 90% stenosis in the proximal LC artery with TIMI 1 flow (Figure 2 & Video 3). The rest of the coronary arteries were free of significant disease.
Figure 2.
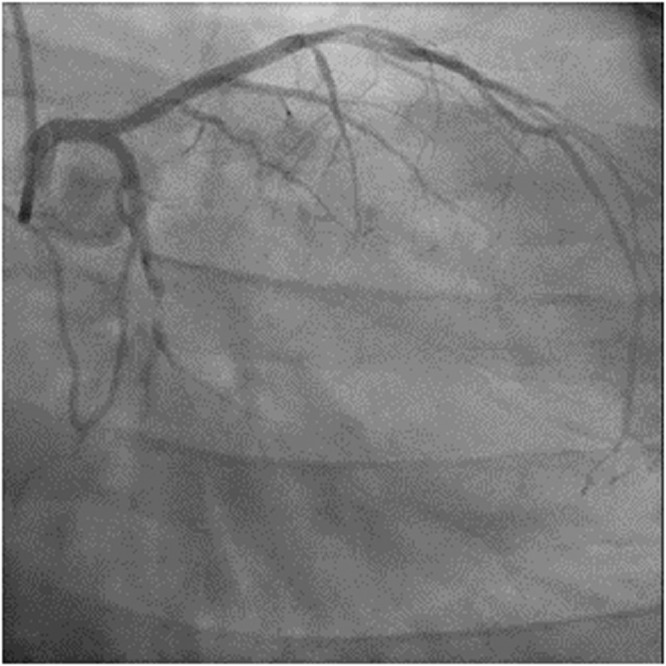
Preintervention image of left coronary system of patient 2.
Emergency PCI of the LC artery was performed. A workhorse wire (Asahi 0.014” Prowater) was used to cross the lesion. The lesion was dilated with a 2.5 mm × 12 mm semicompliant balloon. A 3.0 mm × 18 mm zotarolimus-eluting stent was deployed to treat the lesion and was optimized with a 3.5 mm × 12 mm noncompliant balloon. The final angiogram showed an excellent result with TIMI III flow (Video 4). The patient had no more chest pain after PCI and remained hemodynamically stable. He was initiated on guideline-directed medical therapy and discharged home 2 days later.
Discussion
We report 2 cases of AMI that occurred within 24 hours after the first dose of COVID-19 vaccination. The angiographic features of both cases were suggestive of acute thrombotic events as the underlying mechanism. Both cases were notable for the variable degree of delay in presentation, as patients attributed the chest pain to local effects of the vaccine injection. Both patients received the same COVID-19 vaccine (mRNA-1273). Acute myocardial infarction was not reported in the original study of this vaccine.1, 2, 3 There was no significant difference in thrombotic events between the vaccinated group and placebo group.4 A search yielded only a single case report of AMI early after injection of the same vaccine.5 On the other hand, as of March 10, 2021, among the 5 million people vaccinated with the ChAdOx1 nCoV-19 vaccine in the European Economic Area, 30 cases of thromboembolic events have been reported.6 It is worth noting that the ChAdOx1 nCoV-19 vaccine triggers immune response by nCoV-19 spike protein,7 whereas the mRNA-1273 vaccine induces antibody response with a lipid-nanoparticle-encapsulated mRNA.
As the COVID-19 vaccination campaign moves toward a new phase around the world and vaccines become available to the general public, more data will be available on safety and potential side effects. More data on the nationwide trends of myocardial infarction or other thrombotic events before and after vaccination launch should be carefully obtained and interpreted before commenting on any possible causal relationship. Furthermore, these 2 case reports should not dampen enthusiasm for vaccination but should raise awareness of the possibility of myocardial ischemia in patients having shoulder pain after vaccination.
Disclosures
Dr. Deepak L. Bhatt discloses the following relationships: Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, 89Bio.
Video 1: Preintervention image of left coronary system
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2021.06.047.
Appendix. Supplementary materials
Video 2: Postintervention image of left coronary system
Video 3: Preintervention image of left coronary system
Video 4: Postintervention image of left coronary system
References
- 1.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O'Dell S, Schmidt SD, Swanson PA, Padilla M, Mascola J, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, Schmidt SD, Corbett KS, Swanson PA, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moderna. Clinical trial results. 2021. Accessed March 14, 2021. Available at:https://www.modernatx.com/covid19vaccine-eua/providers/clinical-trial-data
- 5.Boivin Z, Martin J. Untimely myocardial infarction or COVID-19 vaccine side effect. Cureus. March 02, 2021;13(3):e13651. doi: 10.7759/cureus.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Medicines Agency. COVID-19 vaccine AstraZeneca: PRAC investigating cases of thromboembolic events—vaccine's benefits currently still outweigh risks. 2021. Available at:https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-cases-thromboembolic-events-vaccines-benefits. Accessed May 3, 2021.
- 7.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 2: Postintervention image of left coronary system
Video 3: Preintervention image of left coronary system
Video 4: Postintervention image of left coronary system


