Abstract
Objective
To perform a geospatial and temporal trend analysis for coronavirus disease 2019 (COVID-19) in a Midwest community to identify and characterize hot spots for COVID-19.
Participants and Methods
We conducted a population-based longitudinal surveillance assessing the semimonthly geospatial trends of the prevalence of test confirmed COVID-19 cases in Olmsted County, Minnesota, from March 11, 2020, through October 31, 2020. As urban areas accounted for 84% of the population and 86% of all COVID-19 cases in Olmsted County, MN, we determined hot spots for COVID-19 in urban areas (Rochester and other small cities) of Olmsted County, MN, during the study period by using kernel density analysis with a half-mile bandwidth.
Results
As of October 31, 2020, a total of 37,141 individuals (30%) were tested at least once, of whom 2433 (7%) tested positive. Testing rates among race groups were similar: 29% (black), 30% (Hispanic), 25% (Asian), and 31% (white). Ten urban hot spots accounted for 590 cases at 220 addresses (2.68 cases per address) as compared with 1843 cases at 1292 addresses in areas outside hot spots (1.43 cases per address). Overall, 12% of the population residing in hot spots accounted for 24% of all COVID-19 cases. Hot spots were concentrated in neighborhoods with low-income apartments and mobile home communities. People living in hot spots tended to be minorities and from a lower socioeconomic background.
Conclusion
Geographic and residential risk factors might considerably account for the overall burden of COVID-19 and its associated racial/ethnic and socioeconomic disparities. Results could geospatially guide community outreach efforts (eg, testing/tracing and vaccine rollout) for populations at risk for COVID-19.
Abbreviations and Acronyms: APT, apartment; COVID-19, coronavirus disease 2019; MHC, mobile home community; REP, Rochester Epidemiology Project; SDHs, social determinants of health; SES, socioeconomic status; SFH, single family house
The fast spread of the infectious coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2, has created a worldwide pandemic with high morbidity and mortality rates since January 2020.1 Current research on COVID-19 largely focuses on clinical and biological factors for the risk of COVID-19, whereas public communications, community health interventions, and allocation of resources could benefit from community-based contextual data of patients and populations such as precise geographic distributions and residential units, given the well-recognized health effects of the places in which people live,2 and other social determinants of health (SDHs).3, 4, 5 For example, despite the reported geographic clusters nationally4,5 and limited access to centralized COVID-19 testing facilities, testing and tracing efforts could be guided by more precise geospatial clusters of COVID-19 cases and their associated characteristics. Because COVID-19 vaccines are now available, how best to prioritize and reach out to populations with disproportionate burdens of COVID-19 is critical.
Some surveillance research performed geospatial analysis for COVID-19 at either county or state levels in the United States6, 7, 8, 9; however, to date, no studies have performed longitudinal geospatial analysis to identify hot spots (high geographic clusters of COVID-19 cases) at a community level or to characterize population characteristics of those residing in identified hot spots on the basis of contextual factors, for example, type of residential unit and socioeconomic environment. This information could help us better understand racial/ethnic disparities of the burden of COVID-1910, 11, 12, 13 and the extent to which SDHs (socioeconomic, geospatial, and residential building features) account for such disparities. To address these gaps, we performed a longitudinal geospatial analysis for COVID-19 in Olmsted County, Minnesota, a community of the Midwestern region.
Participants and Methods
Study Setting
Olmsted County, MN, contains both urban and rural areas defined by the US census. The city of Rochester has low white/African American dissimilarity index of 29.5 in 2010 (vs 82.5 for Chicago, Illinois).14,15 According to the 2010 census, the population of Olmsted County was 86% white, 5% African American, 5% Asian, and 4% Hispanic.16 Olmsted County has a higher median family income ($66,252 in 2009-2013) than the national average ($53,046).16 Olmsted County, MN, is an excellent setting to conduct a longitudinal population-based study because medical care is virtually self-contained within the community. Medical records–based COVID-19 research of the Olmsted County population is possible through access to Mayo Clinic COVID-19 testing laboratory data and the Rochester Epidemiology Project (REP). The REP, a National Institutes of Health–funded medical record linkage system including virtually all Olmsted County residents, contains all inpatient and outpatient clinical diagnoses as well as address information of Olmsted County residents. More than 95% of residents authorize their medical information for research use.17
The United States declared a national emergency for the COVID-19 pandemic on March 13, 2020, and the state of Minnesota issued a shelter-in-place (lockdown) order from March 27, 2020, to May 13, 2020. The first COVID-19 case in Olmsted County was reported on March 11, 2020. Since then, as of the end of October (study period), the total number of COVID-19 cases were 142,311 in the state of Minnesota and 3402 in Olmsted County (as of October 29, 2020).18 The study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards.
Study Design and Cohort
This is a population-based longitudinal surveillance that assessed the temporal (semimonthly) and geospatial trends of the prevalence of test confirmed COVID-19 cases in Olmsted County, MN, from March 11, 2020, through October 31, 2020. Severe acute respiratory syndrome coronavirus 2 polymerized chain reaction test results for Olmsted County (n=43,750) were obtained from the REP. Testing for severe acute respiratory syndrome coronavirus 2 was performed at the Department of Laboratory Medicine and Pathology at Mayo Clinic. For people tested multiple times, the date of the first negative test result was retained for temporal analysis purposes, unless superseded by a positive test result. In that case, the date of the first positive test result was used for temporal analysis. The unit of analysis was persons tested, not tests. For calculating the prevalence of COVID-19 cases, we used the REP census for Olmsted County urban population (denominator) (n=123,939). Case density was weighted as described in the Geospatial Analysis section below by following our previously reported geographic information system analysis methods.19,20
Geospatial Analyses
1. Geocoding: The addresses of all persons who were tested during the study period were geocoded using parcel-based geocoding methods. Doing so enabled us to identify the precise individual and household location, including housing characteristics (eg, apartment [APT], mobile home community [MHC], or single family house [SFH]) and neighborhood characteristics, in relation to the epidemiology of COVID-19.
2. Weighting: To account for undertesting of some populations or neighborhoods, persons testing positive were weighted by a factor derived using the formula W=(BGpop/totpop)/(BGTP/TotTP), in which W is the weight, BGpop is the census block group population, totpop is the total county population, BGTP is the number of tested persons in the census block group, and TotTP is the number of tested persons in the county. The resulting weights were then applied to each positive test in subsequent analysis steps. This procedure was applied for each semimonthly period and cumulatively for the March through the end of the study period. For the combined analysis for the entire study period (March through October 2020), we determined relative hot spots in urban areas over the period from the first positive test result through the end of the analysis by using kernel density analysis with a half-mile bandwidth. Urban areas had a population density sufficiently high to enable using a half-mile bandwidth in kernel density analysis, enabling more precise location of hot spots. We limited the analysis to geocoded cases. Hot spots for the combined analysis (March through October) were defined as case concentrations that (1) were in the 95th percentile of case density and (2) had a relative difference equivalent to at least 33% higher than the expected case density. The relative difference was derived using the formula RD=(OCDw−ECD)/ECD, in which RD is the “relative density”, OCDw is the weighted observed case density, and ECD is the expected case density based on the average incidence applied to the REP population. In many rural areas of Olmsted County, the population density was low enough that the expected case density would be close to zero. Focusing on the cumulative time period from March 11 through October 31 limited hot spots to 10 areas, all within the city of Rochester. Thus, we focused our geospatial analysis on COVID-19 epidemiology in the city of Rochester.
3. Temporal trend analysis: To determine temporal differences in the spatial locations of hot spots, we mapped urban hot spots. We examined cases and testing over the following periods: all of March 2020 (March 3 to March 31), early April (April 1 to April 15), late April, early May, late May, and so on. For urban areas (operationalized as areas within municipal boundaries), we mapped the kernel density of weighted positive cases by using a half-mile bandwidth and identified hot spots as defined above.
Data Analyses
Apart from the geospatial and temporal trend analysis for COVID-19 cases in the community, we compared study individuals who were tested for COVID-19 and those with positive COVID-19 test results by using descriptive analysis. Similarly, we compared populations residing within and outside hot spots in the community. We characterized study individuals with age, sex, race/ethnicity, and a validated individual level socioeconomic measure called Housing-Based Socioeconomic Status index21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 with regard to their COVID-19 test status (Table 1) and residence status in hot spots (Table 2). Geospatial analysis was performed using ArcMap 10.4.1 (produced by Esri).
Table 1.
| Characteristic | Not tested (n=86,798) | Negative (n=34,708) | Positive (n=2433) | Total (N=123,939) | P value |
|---|---|---|---|---|---|
| Age at the laboratory test date | <.001d | ||||
| N | 86,798 | 34,708 | 2433 | 123,939 | |
| Mean ± SD | 39.9±24.0 | 41.4±22.8 | 36.7±18.8 | 40.3±23.6 | |
| Median | 37.9 | 38.2 | 33.6 | 37.9 | |
| Q1, Q3 | 18.9, 59.1 | 24.8, 59.2 | 22.4, 49.6 | 21.0, 59.0 | |
| Range | 1.8-119.8 | 0.0-107.2 | 0.5-96.5 | 0.0-119.8 | |
| Age group | <.001e | ||||
| 0-5 y | 5341 (6.2) | 1897 (5.5) | 57 (2.3) | 7295 (5.9) | |
| 6-19 y | 17,366 (20.0) | 4459 (12.8) | 394 (16.2) | 22,219 (17.9) | |
| 20-44 y | 28,672 (33.0) | 14,253 (41.1) | 1226 (50.4) | 44,151 (35.6) | |
| 45-64 y | 20,166 (23.2) | 7735 (22.3) | 541 (22.2) | 28,442 (22.9) | |
| ≥65 y | 15,253 (17.6) | 6364 (18.3) | 215 (8.8) | 21,832 (17.6) | |
| Female/not female | <.001e | ||||
| Female | 44,611 (51.4) | 19,858 (57.2) | 1291 (53.1) | 65,760 (53.1) | |
| Not female | 42,187 (48.6) | 14,850 (42.8) | 1142 (46.9) | 58,179 (46.9) | |
| Race | <.001e | ||||
| AI/H/PI/Oth/Mix | 6179 (7.1) | 2118 (6.1) | 232 (9.5) | 8529 (6.9) | |
| African American | 7359 (8.5) | 2342 (6.7) | 642 (26.4) | 10,343 (8.3) | |
| Asian | 6028 (6.9) | 1881 (5.4) | 149 (6.1) | 8058 (6.5) | |
| Refusal/unknown | 1374 (1.6) | 639 (1.8) | 56 (2.3) | 2069 (1.7) | |
| White | 65,858 (75.9) | 27,728 (79.9) | 1354 (55.7) | 94,940 (76.6) | |
| Ethnicity | <.001e | ||||
| Hispanic | 6278 (7.2) | 2357 (6.8) | 275 (11.3) | 8910 (7.2) | |
| Non-Hispanic | 80,520 (92.8) | 32,351 (93.2) | 2158 (88.7) | 115,029 (92.8) | |
| Housing-Based Socioeconomic Status index quartile | <.001e | ||||
| Q1 | 26,678 (30.7) | 7947 (22.9) | 687 (28.2) | 35,312 (28.5) | |
| Q2 | 17,577 (20.3) | 6032 (17.4) | 334 (13.7) | 23,943 (19.3) | |
| Q3 | 20,225 (23.3) | 7191 (20.7) | 497 (20.4) | 27,913 (22.5) | |
| Q4 | 20,577 (23.7) | 7789 (22.4) | 423 (17.4) | 28,789 (23.2) | |
| N/A | 1741 (2.0) | 5749 (16.6) | 492 (20.2) | 7982 (6.4) |
AI/H/PI/Oth/Mix, American Indian, Hawaiian, Pacific Islander, Other, and Two or More Races; N/A, non-applicable (e.g., PO box addresses); Q1, Q2, Q3, Q4, quartiles 1, 2, 3, 4.
Data are presented as No. (percentage) unless indicated otherwise.
Report generated on January 6, 2021.
Kruskal-Wallis test.
Chi-square test.
Table 2.
| Characteristic | Does not live in hotspots (n=109,288) | Lives in hotspots (n=14,651) | Total (N=123,939) | P value |
|---|---|---|---|---|
| Test result | <.001d | |||
| N/A test | 76,195 (69.7) | 10,603 (72.4) | 86,798 (70.0) | |
| Negative | 31,250 (28.6) | 3458 (23.6) | 34,708 (28.0) | |
| Positive | 1843 (1.7) | 590 (4.0) | 2433 (2.0) | |
| Age at the laboratory date | <.001e | |||
| N | 109,288 | 14,651 | 123,939 | |
| Mean ± SD | 40.9±23.8 | 35.3±21.9 | 40.3±23.6 | |
| Median | 38.7 | 32.3 | 37.9 | |
| Q1, Q3 | 21.6, 59.8 | 17.2, 51.3 | 21.0, 59.0 | |
| Range | 0.0-119.8 | 0.0-117.8 | 0.0-119.8 | |
| Female/not female | .088d | |||
| Female | 58,083 (53.1) | 7677 (52.4) | 65,760 (53.1) | |
| Not female | 51,205 (46.9) | 6974 (47.6) | 58,179 (46.9) | |
| Race | <.001d | |||
| AI/H/PI/Oth/Mix | 6619 (6.1) | 1910 (13.0) | 8529 (6.9) | |
| African American | 6353 (5.8) | 3990 (27.2) | 10,343 (8.3) | |
| Asian | 6883 (6.3) | 1175 (8.0) | 8058 (6.5) | |
| Refusal/unknown | 1722 (1.6) | 347 (2.4) | 2069 (1.7) | |
| White | 87,711 (80.3) | 7229 (49.3) | 94,940 (76.6) | |
| Ethnicity | <.001d | |||
| Hispanic | 6864 (6.3) | 2046 (14.0) | 8910 (7.2) | |
| Non-Hispanic | 102,424 (93.7) | 12,605 (86.0) | 115,029 (92.8) | |
| Housing-Based Socioeconomic Status index quartile | <.001d | |||
| Q1 (lowest SES) | 27,316 (25.0) | 7996 (54.6) | 35,312 (28.5) | |
| Q2 | 21,394 (19.6) | 2549 (17.4) | 23,943 (19.3) | |
| Q3 | 25,418 (23.3) | 2495 (17.0) | 27,913 (22.5) | |
| Q4 (highest SES) | 28,363 (26.0) | 426 (2.9) | 28,789 (23.2) | |
| N/A | 6797 (6.2) | 1185 (8.1) | 7982 (6.4) |
AI/H/PI/Oth/Mix, American Indian, Hawaiian, Pacific Islander, Other, and Two or More Races; N/A, non-applicable (e.g., PO box addresses); Q1, Q2, Q3, Q4, quartiles 1, 2, 3, 4; SES, socioeconomic status.
Data are presented as No. (percentage) unless indicated otherwise.
Report generated on January 6, 2021.
Chi-square test
Kruskal-Wallis test.
Results
Characteristics of Study Individuals
Of 123,939 Olmsted County urban residents included in the analysis, 65,760 (53%) were women and the mean age was 40.3±23.6 years (Table 1). On the basis of self-report, 94,940 (77%) were white, 10,343 (8%) African American, and 8058 (7%) Asian; 8910 (7%) reported Hispanic ethnicity. Addresses were successfully geocoded for 97% of the full county sample (n=140,829). The populations residing in urban areas (the city of Rochester and other small cities) in Olmsted County, MN, account for 88% of the Olmsted County population (123,939).
Prevalence of COVID-19, Temporal Trends, and Characteristics of COVID-19 Cases
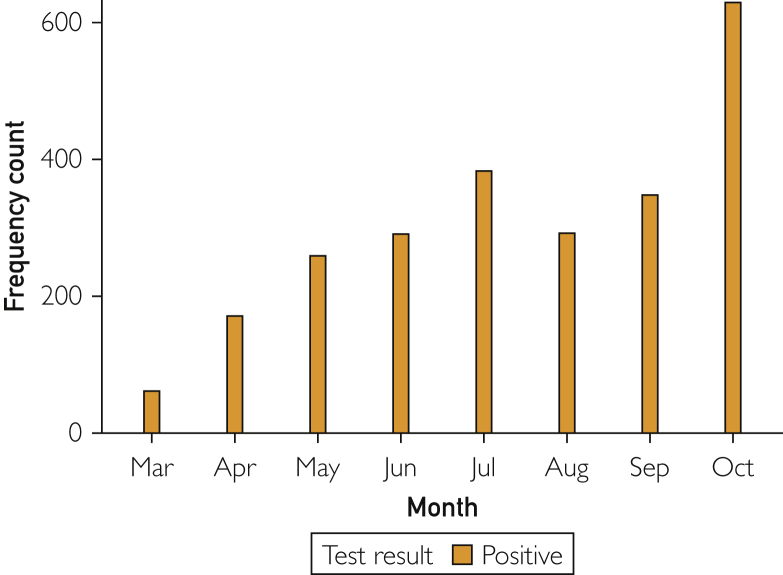
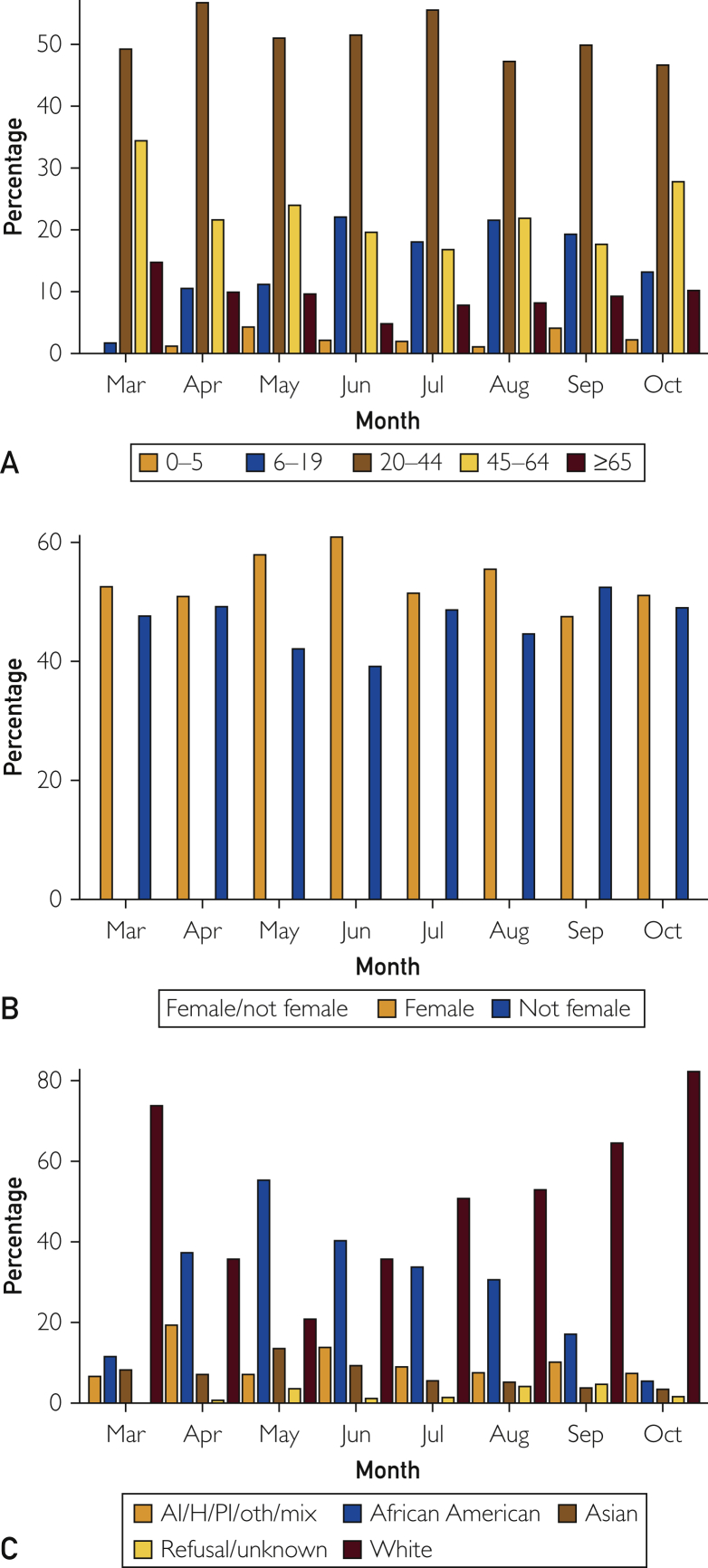
As of October 31, 2020, a total of 37,141 individuals (30% of urban residents) were tested at least once, of whom 2433 (2%) tested positive, accounting for 86% of the total number of COVID-19 cases in Olmsted County, MN, during the study period. Since the first COVID-19 case was confirmed on March 11, the total number of new cases per month initially increased until July and decreased during summer. Subsequently, the total number of cases markedly increased since September and reached a peak in October (Figure 1). Figure 2 depicts temporal (semimonthly) trends of COVID-19 cases in relation to demographic characteristics for age (Figure 2A), sex (Figure 2B), race/ethnicity (Figure 2C), and socioeconomic status (SES) (Figure 2D). The proportion of African Americans (8% [10,383/123,939] of the Olmsted County urban population) among COVID-19 cases were substantially higher between April and June (45% [324/721]) than that of whites (77% [94,940/123,939] of population, 30% [219/721] of cases) and dramatically decreased since then (34% [129/383] of urban cases in July and 5% [34/629] in October). A majority of positive cases since July was driven by whites, especially from September (77% [596/774] of urban cases). Despite disparities in COVID-19 cases, no differences in testing rates were found: 29% [2984/10,343] (black), 30% [2632/8910] (Hispanic), 25% [2030/8058] (Asian), and 31% [29,082/94,940] (white). Populations from the lowest socioeconomic background (quartile 1) consistently had a higher burden of COVID-19 up to July 2020, and since then, populations from quartile 3 and quartile 4 (highest SES) had a higher burden of COVID-19.
Figure 1.
Temporal trends of coronavirus disease 2019 in urban areas of Olmsted County, Minnesota.
Figure 2.
Temporal (semimonthly) trends of coronavirus disease 2019 cases in relation to demographic characteristics for (A) age, (B) sex, (C) race, (D) ethnicity, and (E) socioeconomic status. AI/H/PI/Oth/Mix, American Indian, Hawaiian, Pacific Islander, Other, and Two or More Races; Q1, Q2, Q3, Q4, quartiles 1, 2, 3, 4.
Geospatial Trends of COVID-19 in the Community
The results of the longitudinal (semimonthly) geospatial analysis for COVID-19 are presented in the Supplemental Figure (available online at http://www.mcpiqojournal.org). The combined geospatial analysis results based on the entire study period are presented in Figure 3.
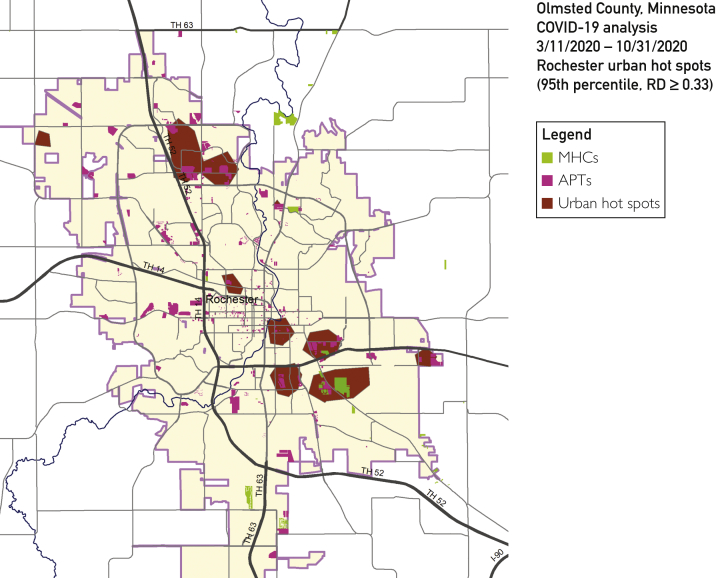
Figure 3.
Geospatial analysis and hot spots for coronavirus disease 2019 (COVID-19) in the city of Rochester, Minnesota, from March 11, 2020, through October 31, 2021. Overall, urban hot spots were concentrated in 3 types of areas with low-income family apartment (APT) complexes, mobile home communities (MHCs), and nearby moderate-income single family house residential areas. RD, relative density.
During the early phase (March) of COVID-19, hot spots were concentrated in all housing types including APTs, MHCs, and SFHs in most regions of Rochester. However, from mid-April, hot spots exhibited clear new trends of being concentrated in APTs and MHCs, primarily in northwest and southeast regions, with less affecting population residing in SFHs and southwest areas, which were relatively persistent until September. Since September, hot spots exhibited in almost all regions in Rochester, and in October, populations were affected regardless of housing types, which coincided with the peak of COVID-19 cases in the community, as shown in Figure 1. According to the geospatial analysis for the entire study period as shown in Figure 3, overall, urban hot spots were concentrated in 3 types of areas: (1) low-income family APT complexes, (2) MHCs, and (3) nearby moderate-income SFH residential areas.
Comparison of Population Characteristics Between Those Residing in Hot Spots and Those Outside Hot Spots
Table 2 summarizes characteristics between those residing in hot spots and those outside hot spots. Ten urban hot spots meeting the 95th percentile and relative difference of 0.33 or higher thresholds were limited to only the city of Rochester. Urban cases affected 1512 addresses with 2433 persons tested positive. Ten urban hot spots accounted for 590 cases at 220 addresses (2.68 cases per address) as compared with 1843 cases at 1292 addresses in areas outside hot spots (1.43 cases per address). Overall, 12% (14,651/123,939) of the population residing in hot spots accounted for 24% (590/2433) of all urban COVID-19 cases (n=590). People living in hot spots tend to be minorities (eg, African American and Hispanic) and from a lower socioeconomic background.
Discussion
To our knowledge, this is the first longitudinal geospatial analysis for COVID-19 epidemiology at a county level in the United States. Our geospatial trend analysis revealed that hot spots for COVID-19 are a major unrecognized geographic risk factor for COVID-19 and appears to be concentrated in neighborhoods with lower-income APTs and MHCs. Minorities and socioeconomically underresourced populations with COVID-19 disproportionately resided in hot spots. These significant disparities occurred despite the reported community factors mitigating health disparities such as low dissimilarity index and higher mean family income than the national average. Our temporal trend analysis revealed distinctive patterns of COVID-19 epidemiology at a community level.
Overall, 12% population residing in hot spots for COVID-19 accounted for 24% of urban COVID-19 cases and such hot spots appear to be concentrated in areas of urban lower-income APTs and MHCs. These results are novel and suggest that hot spots for COVID-19 and their associated housing types could be a major unrecognized geographic risk factor for COVID-19, which might account for widely recognized racial/ethnic and socioeconomic disparities in COVID-19 cases4,43, 44, 45 as individuals residing in hot spots were more likely to be minorities (eg, African American and Hispanic) and those from a lower socioeconomic background. These results might imply that combinations of both crowded residential units and minorities and those with lower SES might pose substantially increased risks of transmission of COVID-19 in populations residing in APTs or MHC settings in the community. These observations may provide important implications for policy prescription and public health interventions. For example, although testing and tracing is a major mitigation strategy for the pandemic apart from the recommended public health measures (we did not observe differences in access to COVID-19 testing among different race/ethnic groups in our study),46,47 families living in a crowded housing unit who had a COVID-19–affected family member might not have much choice other than all members being exposed and developing COVID-19.4,48, 49, 50 This finding highlights the importance of the role of SDHs, such as housing or even arranging a temporary place for isolation or quarantine of the affected family members for those living in crowded residential units (eg, APTs) beyond testing and tracing to mitigate the risk of COVID-19 transmissibility in the community.45,51,52
Another important implication of our geospatial analysis results is to provide geospatial guidance to reach out to underresourced populations with a higher burden of COVID-19, which is useful for prioritizing COVID-19 vaccines to populations at risk instead of controversial race/ethnicity-based prioritization.53 For example, a community outreach team of clinical practice used our geospatial analysis, which identified high-priority neighborhoods for influenza vaccination (“Pop-Up Flu Immunization Clinic”) and its potential locations to reach out to underresourced populations for influenza vaccination on the basis of geospatial data on SES, burden of COVID-19, and high-risk conditions for influenza (unpublished data). These underresourced populations would not have otherwise had access to influenza vaccination. A recent study identified racial/ethnic, sex, age, language, and socioeconomic differences in accessing telemedicine (an important communication measure) for primary care during the pandemic, which may exacerbate existing inequities in care among vulnerable populations.54 Thus, this geographically targeted approach guided by geospatial analysis can be valuable for prioritizing COVID-19 vaccine delivery for populations at risk in the community and public health education, given the reported low likelihood of getting a COVID-19 vaccine in African American individuals and those with lower educational backgrounds despite their disproportionately higher burden of COVID-19.55
Apart from our geospatial analysis, our temporal geospatial trend analysis of COVID-19 epidemiology reveals a few noteworthy findings. First, it appears that there are 3 phases of COVID-19 epidemiology during the study period. During the early phase (March) of COVID-19 in our community, the pandemic started with a geographically widespread outbreak in the community relatively without regard to housing types and geographic locations. We postulate that this phase might reflect a relatively uninformed phase of the community to COVID-19 since the first case reported on March 10, 2020. During the second phase from April through July, the community became cognizant of the pandemic and the state of Minnesota issued a shelter-in-place (lockdown) order from March 27, 2020, to May 13, 2020. During this phase, significant disparities in the burden of COVID-19 cases emerged, disproportionately affecting the minority population and socioeconomically underresourced population. Many minorities and underresourced populations are employed as essential workers, which substantially increased the risk of COVID-19 transmission within their household.4,11,43, 44, 45,56 We postulate that this phase reflects a phase when SDHs operated their effects on the acquisition and transmission of COVID-19 and their outcomes. During the third phase after July (summer), a majority of positive cases was driven by whites and those with relatively higher SES, whereas the total number of COVID-19 cases drastically reduced in minorities and those from a lower socioeconomic background. People from a higher socioeconomic background and white people seem to be associated with the second spike of COVID-19 (first spike during July). Small gatherings have been one of the main sources of the second spike per the Olmsted County Public Health Services according to their epidemiological investigation (M. Sherden, MPH, oral communication, December 2020) and tracing as recognized by the Centers for Disease Control and Prevention.57 This phase might reflect relaxation of restrictions, politicization of some protective matters (eg, masking),58,59 and pandemic fatigue in some subgroups of populations becoming nonadherent to the recommended public health measures in addition to other factors.60 These observations provide an important insight into the effect of changes in distinctive population behaviors and the time-dependent differential effect of SDHs during the pandemic over time on the risk of transmission of COVID-19 at a community level. It may provide an important policy prescription for preparing the community for future inevitable pandemics.
Our study has a few important strengths. First, our study is a population-based study leveraging a self-contained health care environment and REP, electronic data repository for our community population. Second, our study is the first longitudinal temporal geospatial analysis for COVID-19 epidemiology in a Midwest community with low dissimilarity index. Third, the prevalence of COVID-19 was weighted by the number of tests and population size and was characterized by individual level SES for the study population. Also, our study has some limitations. Some COVID-19 tests and cases might be missed in our data surveillance system if they were performed outside our study setting, and 5% of the population did not authorize to use their medical records for research. Our study setting has a unique feature such as a higher proportion (22%) of health care workers, which might affect the interpretation of our study results with caution. Our geospatial analysis results were not tested for statistical significance given the frequent update of the results (semimonthly).
Conclusion
Our longitudinal geospatial analysis reveals novel geographic and residential risk factors that might considerably account for the overall burden of COVID-19 and its associated racial/ethnic and socioeconomic disparities in the community. The results could geospatially guide community outreach efforts (eg, public health education, testing/tracing, and vaccine rollout) for populations at risk for COVID-19.
Acknowledgments
We thank Meaghan Sherden, MPH, at Olmsted County Public Health Services for sharing her comments on the potential source for the transmission of coronavirus disease 2019 in the community during the second spike. We also thank Kelly Okeson, BS, for her administrative assistance in preparing the manuscript.
Footnotes
Grant Support: This study was supported by the Housing-Based Socioeconomic Status Program of Mayo Foundation, the National Institutes of Health (R01 HL126667), and the National Center for Advancing Translational Sciences (UL1 TR02377). This study was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigators: Walter A. Rocca, MD, MPH, and Jennifer L. St Sauver, PhD).
Potential Competing Interests: Dr Juhn is a consultant to AstraZeneca and has received grants from the National Institutes of Health/Genentech/GlaxoSmithKline; he owns US Patent 10,654,923 (all outside the submitted work). The other authors report no competing interests.
Supplemental material can be found online at: http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.COVID data tracker Centers for Disease Control and Prevention website. https://covid.cdc.gov/covid-data-tracker/#datatracker-home Accessed January 2, 2021.
- 2.Baum A., Wisnivesky J., Basu S., Siu A.L., Schwartz M.D. Association of geographic differences in prevalence of uncontrolled chronic conditions with changes in individuals’ likelihood of uncontrolled chronic conditions. JAMA. 2020;324(14):1429–1438. doi: 10.1001/jama.2020.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen S.A., Khoury M.J., Del Rio C. Precision public health as a key tool in the COVID-19 response. JAMA. 2020;324(10):933–934. doi: 10.1001/jama.2020.14992. [DOI] [PubMed] [Google Scholar]
- 4.Emeruwa U.N., Ona S., Shaman J.L. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324(4):390–392. doi: 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew D.A., Nguyen L.H., Steves C.J., COPE Consortium Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science. 2020;368(6497):1362–1367. doi: 10.1126/science.abc0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franch-Pardo I., Napoletano B.M., Rosete-Verges F., Billa L. Spatial analysis and GIS in the study of COVID-19: a review. Sci Total Environ. 2020;739:140033. doi: 10.1016/j.scitotenv.2020.140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjardins M.R., Hohl A., Delmelle E.M. Rapid surveillance of COVID-19 in the United States using a prospective space-time scan statistic: detecting and evaluating emerging clusters. Appl Geogr. 2020;118:102202. doi: 10.1016/j.apgeog.2020.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Response Team Geographic differences in COVID-19 cases, deaths, and incidence—United States, February 12-April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller I.F., Becker A.D., Grenfell B.T., Metcalf C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26(8):1212–1217. doi: 10.1038/s41591-020-0952-y. [DOI] [PubMed] [Google Scholar]
- 10.Karaca-Mandic P., Georgiou A., Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. 2021;181(1):131–134. doi: 10.1001/jamainternmed.2020.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selden T.M., Berdahl T.A. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood) 2020;39(9):1624–1632. doi: 10.1377/hlthaff.2020.00897. [DOI] [PubMed] [Google Scholar]
- 12.Martinez D.A., Hinson J.S., Klein E.Y. SARS-CoV-2 positivity rate for Latinos in the Baltimore-Washington, DC region. JAMA. 2020;324(4):392–395. doi: 10.1001/jama.2020.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 14.2010 Census urban and rural classification and urban area criteria. United States Census Bureau website. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural.html
- 15.Harrison B.D. Psychosocial aspects of asthma in adults. Thorax. 1998;53(6):519–525. doi: 10.1136/thx.53.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Census. 2010. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF. Accessed September 16, 2021.
- 17.St Sauver J.L., Grossardt B.R., Yawn B.P. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minnesota Department of Health. Weekly COVID-19 Report. https://www.health.state.mn.us/diseases/coronavirus/stats/covidweekly44.pdf. Accessed January 9, 2021.
- 19.Wi C.I., Wheeler P.H., Kaur H., Ryu E., Kim D., Juhn Y. Spatio-temporal comparison of pertussis outbreaks in Olmsted County, Minnesota, 2004-2005 and 2012: a population-based study. BMJ Open. 2019;9(5):e025521. doi: 10.1136/bmjopen-2018-025521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A.A., Wheeler P.H., Wi C.-I. Mobile home residence as a risk factor for adverse events among children in a mixed rural-urban community: a case for geospatial analysis. J Clin Transl Sci. 2020;4(5):443–450. doi: 10.1017/cts.2020.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhn Y.J., Beebe T.J., Finnie D.M. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944. doi: 10.1007/s11524-011-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang D.W., Manemann S.M., Gerber Y. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. Int J Environ Res Public Health. 2014;11(11):11597–11615. doi: 10.3390/ijerph111111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghawi H., Crowson C.S., Rand-Weaver J., Krusemark E., Gabriel S.E., Juhn Y.J. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ Open. 2015;5(4):e006469. doi: 10.1136/bmjopen-2014-006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi P.Y., Ryu E., Hathcock M.A. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. doi: 10.1136/jech-2015-205925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu E., Juhn Y.J., Wheeler P.H. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol. 2017;27(7):415–420.e412. doi: 10.1016/j.annepidem.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Wi C.I., Gauger J., Bachman M. Role of individual-housing-based socioeconomic status measure in relation to smoking status among late adolescents with asthma. Ann Epidemiol. 2016;26(7):455–460. doi: 10.1016/j.annepidem.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wi C.I., St Sauver J.L., Jacobson D.J. Ethnicity, socioeconomic status, and health disparities in a mixed rural-urban US community—Olmsted County, Minnesota. Mayo Clin Proc. 2016;91(5):612–622. doi: 10.1016/j.mayocp.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield M.C., Williams A.R., Beebe T. A two-county comparison of the HOUSES index on predicting self-rated health. J Epidemiol Community Health. 2011;65(3):254–259. doi: 10.1136/jech.2008.084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris M.N., Lundien M.C., Finnie D.M. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. doi: 10.1038/npjpcrm.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson M.D., Urm S.H., Jung J.A. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141(4):880–887. doi: 10.1017/S0950268812001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer R., Capili C., Wi C.I., Ryu E., Rand-Weaver J., Juhn Y.J. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC Public Health. 2016;16(1):1000. doi: 10.1186/s12889-016-3673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens M.A., Beebe T.J., Wi C.I., Taler S.J., St Sauver J.L., Juhn Y.J. HOUSES index as an innovative socioeconomic measure predicts graft failure among kidney transplant recipients. Transplantation. 2020;104(11):2383–2392. doi: 10.1097/TP.0000000000003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan C.S., Juhn Y.J., Kaur H. Long-term incidence of glioma in Olmsted County, Minnesota, and disparities in postglioma survival rate: a population-based study. Neurooncol Pract. 2020;7(3):288–298. doi: 10.1093/nop/npz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thacher T.D., Dudenkov D.V., Mara K.C., Maxson J.A., Wi C.I., Juhn Y.J. The relationship of 25-hydroxyvitamin D concentrations and individual-level socioeconomic status. J Steroid Biochem Mol Biol. 2020;197:105545. doi: 10.1016/j.jsbmb.2019.105545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patten C.A., Juhn Y.J., Ryu E. Rural-urban health disparities for mood disorders and obesity in a Midwestern community. J Clin Transl Sci. 2020;4(5):408–415. doi: 10.1017/cts.2020.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barwise A., Wi C.I., Frank R. An innovative individual-level socioeconomic measure predicts critical care outcomes in older adults: a population-based study. J Intensive Care Med. 2021;36(7):828–837. doi: 10.1177/0885066620931020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu E., Olson J.E., Juhn Y.J. Association between an individual housing-based socioeconomic index and inconsistent self-reporting of health conditions: a prospective cohort study in the Mayo Clinic Biobank. BMJ Open. 2018;8(5):e020054. doi: 10.1136/bmjopen-2017-020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barwise A., Juhn Y.J., Wi C.I. An individual housing-based socioeconomic status measure predicts advance care planning and nursing home utilization. Am J Hosp Palliat Care. 2019;36(5):362–369. doi: 10.1177/1049909118812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bjur K.A., Wi C.I., Ryu E., Crow S.S., King K.S., Juhn Y.J. Epidemiology of children with multiple complex chronic conditions in a mixed urban-rural US community. Hosp Pediatr. 2019;9(4):281–290. doi: 10.1542/hpeds.2018-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjur K.A., Wi C.I., Ryu E. Socioeconomic status, race/ethnicity, and health disparities in children and adolescents in a mixed rural-urban community—Olmsted County, Minnesota. Mayo Clin Proc. 2019;94(1):44–53. doi: 10.1016/j.mayocp.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryu E., Wi C.I., Crow S.S. Assessing health disparities in children using a modified housing-related socioeconomic status measure: a cross-sectional study. BMJ Open. 2016;6(7):e011564. doi: 10.1136/bmjopen-2016-011564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynch B.A., Finney Rutten L.J., Jacobson R.M. Health care utilization by body mass index in a pediatric population. Acad Pediatr. 2015;15(6):644–650. doi: 10.1016/j.acap.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muñoz-Price L.S., Nattinger A.B., Rivera F. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open. 2020;3(9):e2021892. doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogedegbe G., Ravenell J., Adhikari S. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chowkwanyun M., Reed A.L., Jr. Racial health disparities and Covid-19—caution and context. N Engl J Med. 2020;383(3):201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 46.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 47.Steinbrook R. Contact tracing, testing, and control of COVID-19—learning from Taiwan. JAMA Intern Med. 2020;180(9):1163–1164. doi: 10.1001/jamainternmed.2020.2072. [DOI] [PubMed] [Google Scholar]
- 48.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo L., Liu D., Liao X-l. Modes of contact and risk of transmission in COVID-19 among close contacts. https://doi.org/10.1101/2020.03.24.20042606 [published online ahead of print March 26, 2020]. medRxiv.
- 50.Selden T.M., Berdahl T.A. Risk of severe COVID-19 among workers and their household members. JAMA Intern Med. 2021;181(1):120–122. doi: 10.1001/jamainternmed.2020.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berkowitz S.A., Cené C.W., Chatterjee A. Covid-19 and health equity—time to think big. N Engl J Med. 2020;383(12):e76. doi: 10.1056/NEJMp2021209. [DOI] [PubMed] [Google Scholar]
- 52.Egede L.E., Walker R.J. Structural racism, social risk factors, and Covid-19—a dangerous convergence for black Americans. N Engl J Med. 2020;383(12):e77. doi: 10.1056/NEJMp2023616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt H., Gostin L.O., Williams M.A. Is it lawful and ethical to prioritize racial minorities for COVID-19 vaccines? JAMA. 2020;324(20):2023–2024. doi: 10.1001/jama.2020.20571. [DOI] [PubMed] [Google Scholar]
- 54.Eberly L.A., Kallan M.J., Julien H.M. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic [published correction appears in JAMA Netw Open. 2021;4(2):e211913] JAMA Netw Open. 2020;3(12):e2031640. doi: 10.1001/jamanetworkopen.2020.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szilagyi P.G., Thomas K., Shah M.D. National trends in the US public’s likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA. 2020;325(4):396–398. doi: 10.1001/jama.2020.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubin R. Household composition may explain COVID-19 racial/ethnic disparities. JAMA. 2020;324(8):732. doi: 10.1001/jama.2020.14375. [DOI] [PubMed] [Google Scholar]
- 57.Holiday celebrations and small gatherings. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/holidays.html
- 58.Leventhal A.M., Dai H., Barrington-Trimis J.L. Association of political party affiliation with physical distancing among young adults during the COVID-19 pandemic. JAMA Intern Med. 2021;181(3):399–403. doi: 10.1001/jamainternmed.2020.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gollwitzer A., Martel C., Brady W.J. Partisan differences in physical distancing are linked to health outcomes during the COVID-19 pandemic. Nat Hum Behav. 2020;4(11):1186–1197. doi: 10.1038/s41562-020-00977-7. [DOI] [PubMed] [Google Scholar]
- 60.World Health Organization . World Health Organization, Regional Office for Europe; Copenhagen, Denmark: 2020. Pandemic Fatigue: Reinvigorating the Public to Prevent COVID-19: Policy Framework for Supporting Pandemic Prevention and Management: revised version November 2020. Licence: CC BY-NC-SA 3.0 IGO2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.