Abstract
Background
Age‐related macular degeneration (AMD) is one of the leading causes of blindness in high‐income countries. The majority of cases of AMD are of the non‐exudative type. Experts have proposed photobiomodulation (PBM) therapy as a non‐invasive procedure to restore mitochondrial function, upregulate cytoprotective factors and prevent apoptotic cell death in retinal tissue affected by AMD.
Objectives
To assess the effectiveness and safety of PBM compared to standard care, no treatment or sham treatment for people with non‐exudative AMD.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (Issue 5, 2020), Ovid MEDLINE, Embase, ISRCTN, ClinicalTrials.gov and the WHO ICTRP to 11 May 2020 with no language restrictions.
Selection criteria
The review included randomised controlled trials (RCTs) on participants receiving any type of PBM therapy for non‐exudative AMD compared to standard care, sham treatment or no treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We considered the following outcome measures at 12 months: best‐corrected visual acuity (BCVA) ; contrast sensitivity; near vision; low luminance density score; reading speed; vision‐related quality of life score; and adverse events such as progression of AMD and conversion to exudative AMD. We graded the certainty of the evidence using GRADE.
Main results
We included two published RCTs from single centres in the UK and Canada, which recruited 60 participants (60 eyes) and 30 participants (46 eyes) respectively. Participants in these trials were people with non‐exudative AMD with Age‐Related Eye Disease Study (AREDS) categories 2 to 4. One study compared single wavelength PBM with no treatment. This study was at risk of performance bias because the study was not masked, and there was attrition bias. One study compared multi‐wavelength PBM with sham treatment and conflicts of interest were reported by study investigators. We also identified three eligible ongoing RCTs from searching the clinical trials database.
When comparing PBM with sham treatment or no treatment for non‐exudative AMD, there was no evidence of any meaningful clinical difference in BCVA at 12 months (mean difference (MD) 0.02 logMAR, 95% confidence interval (CI) ‐0.02 to 0.05; 2 RCTs, 90 eyes; low‐certainty evidence). One study comparing multi‐wavelength PBM with sham treatment showed an improvement in contrast sensitivity at Level E (18 cycles/degree) at 12 months (MD 0.29 LogCS, 95% CI 0.23 to 0.35; 1 RCT, 46 eyes; low‐certainty evidence). Visual function and health‐related quality of life scores were comparable between single wavelength PBM and no treatment groups at 12 months (VFQ‐48 score MD 0.43, 95% CI ‐0.17 to 1.03; P = 0.16; 1 RCT, 47 eyes; low‐certainty evidence).
When comparing PBM with sham treatment or no treatment for non‐exudative AMD, there was no evidence of any meaningful clinical difference in conversion to exudative AMD (risk ratio (RR) 0.97, 95% CI 0.17 to 5.44; 2 RCTs, 96 eyes; very low‐certainty evidence) at 12 months. There was inconclusive evidence that single wavelength PBM prevents the progression of AMD (RR 0.79, 95% CI 0.41 to 1.53; P = 0.48; 1 RCT, 50 eyes; low‐certainty evidence). Disease progression was defined as the development of advanced AMD or significant increase in drusen volume.
No included study reported near vision, low luminance vision or reading speed outcomes.
Authors' conclusions
Currently there remains uncertainty whether PBM treatment is beneficial in slowing progression of non‐exudative macular degeneration. There is a need for further well‐designed controlled trials assessing dosimetry, powered for both effectiveness and safety outcomes. Consideration should be given to the adoption of agreed clinical outcome measures and patient‐based outcome measures for AMD.
Plain language summary
What are the benefits and risks of low level light therapy (photobiomodulation) for treating dry age‐related macular degeneration (a degenerative eye condition)?
Why is this question important? Age‐related macular degeneration (AMD) is a common condition of the eyes. It usually develops in people aged over 50, and leads to progressive loss of central vision. People with AMD may find it difficult to read or recognise faces, and they can become partially sighted.
AMD progresses in stages. To begin with, yellow spots (drusen) develop under the retina (the back of the eye). These are not visible to the naked eye, but can be seen by health professionals during examinations of the eyes. As AMD progresses, cells located in the macula (the central area of the retina) that are needed for vision die. If blood vessels in the eye go on to leak, the condition is classed as ‘wet’ AMD. If there is no leakage, the condition is known as ‘dry’ AMD.
There is no cure for dry AMD. However, it may be possible to use low level light therapy (photobiomodulation) to stop vision from worsening. To find out how effective photobiomodulation is for treating dry AMD and whether it is associated with unwanted effects, we reviewed the evidence from research studies.
How did we identify and evaluate the evidence? First, we searched for randomised controlled studies (clinical studies where people are randomly put into one of two or more treatment groups), because these studies provide the most robust evidence about the effects of a treatment. We then compared the results, and summarised the evidence from the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find? We found two studies that involved a total of 90 people with dry AMD. Both studies received public funding.
In one study, with 60 people, set in the UK, 30 people wore a light‐emitting eye mask for eight hours every night for one year. Their results were compared to those of 30 people who received no treatment.
In the other study, with 30 people, set in Canada, people received either:
→ low level light therapy for five minutes per eye, three times a week for three weeks, which was repeated after a six‐month break; or
→ a sham treatment, three times a week for three weeks, which was repeated after a six‐month break.
Five of the investigators of this study had links to the manufacturer of the light therapy device used (three of these were employed by the manufacturer).
We have little to very little confidence in the evidence we found, because it is based on only two small studies. The way these studies were conducted is likely to have introduced errors in their results.
The evidence suggests that:
→ photobiomodulation may make little or no difference to changes in clarity of vision one year after starting treatment compared to no treatment or a sham treatment;
→ photobiomodulation may make little or no difference to disease progression of AMD after one year;
→ photobiomodulation may improve a person’s ability to distinguish an object against its background;
→ photobiomodulation may make little or no difference to clarity of vision as reported by patients.
We have too little confidence in the evidence to be able to determine whether, after one year of treatment, photobiomodulation affects:
→ progression to wet AMD.
Since neither study reported information about some outcomes, we cannot determine from the evidence whether, after one year of treatment, photobiomodulation affects:
→ near vision;
→ vision in low light; or
→ reading speed.
What does this mean? Compared to no treatment or a sham treatment, photobiomodulation may make little or no difference to clarity of vision or disease progression in people with dry AMD one year after starting treatment.
We do not know if photobiomodulation affects other aspects such as progression to wet AMD or changes in near vision. This is because too few, or no, robust studies have investigated this.
How up‐to‐date is this review? The evidence in this Cochrane Review is current to May 2020.
Summary of findings
Summary of findings 1. Photobiomodulation treatment compared to sham or no treatment for non‐exudative age‐related macular degeneration.
| PBM treatment compared to sham or no treatment for non‐exudative AMD | ||||||
| Patient or population: people with non‐exudative AMD (AREDS 2 to 4) Setting: ophthalmology clinics Intervention: PBM treatment Comparison: no treatment or sham treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with PBM treatment | |||||
|
Best‐corrected visual acuity at 12 months [logMAR] Measured on the logMAR scale, range ‐1.3 to 1.0. Lower scores represent better vision. |
The mean best‐corrected visual acuity ranged across control groups from 0.24 to 0.96 logMAR | Mean logMAR score in the PBM group was on average 0.02 logMAR higher (worse) (‐0.02 lower to 0.05 higher) | ‐ | 90 (2 RCTs) | ⊕⊕⊝⊝a,b LOW | |
|
Contrast sensitivity at Level E [18 cycles/degree] at 12 months Higher scores represent better contrast sensitivity. |
On average there was no change In contrast sensitivity at Level E from baseline | Mean change In contrast sensitivity (Level E, 18 cycles/degree) was approximately 0.3 higher (better) with PBM (0.23 higher to 0.35 higher) | ‐ | 30 (1 RCT) |
⊕⊕⊝⊝a,b LOW | |
| Near vision | No studies reported this outcome | |||||
| Low luminance deficit score | No studies reported this outcome | |||||
| Reading speed | No studies reported this outcome | |||||
|
Self‐reported visual function at 12 months Measured with VFQ‐48 questionnaire. Higher scores represent better reported visual function. |
Average self‐reported visual function score was 5.19 |
Mean visual function score was 0.43 higher (worse)in the PBM group (‐0.17 lower to 1.03 higher) | ‐ | 47 (1 RCT) | ⊕⊕⊝⊝a,b LOW | |
|
Adverse events: AMD progression at 12 months Defined as increased drusen volume or onset of advanced AMD. Conversion to exudative AMD at 12 months Assessed by clinical examination and retinal optical coherence tomography. |
483 per 1,000 study population | 381 per 1,000 (198 to 739) | RR 0.79 (0.41 to 1.53) | 50 (1 RCT) | ⊕⊕⊝⊝a,b LOW | |
| 43 per 1,000 study population | 41 per 1,000 (7 to 231) | RR 0.97 (0.17 to 5.44) | 96 (2 RCTs) | ⊕⊝⊝⊝b,c VERY LOW | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AMD: age‐related macular degeneration; AREDS: age‐related eye disease study classification; CI: confidence interval; OR: odds ratio; PBM: photobiomodulation; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
Reason for downgrading certainty of evidence
a. ‐1 for imprecision as sample size was underpowered b. ‐1 for performance and attrition bias c. ‐2 for very wide confidence intervals including 1 (null effect)
Background
Description of the condition
Age‐related macular degeneration (AMD) is an irreversible, degenerative eye condition involving the central retina. AMD is the leading cause of irreversible blindness in people aged 50 years or older in the UK. It accounts for 50% of blind and partially sighted registrations (Macular Society 2017).
Clinically, AMD occurs in two advanced forms, non‐exudative ('dry') or neovascular ('wet'). AMD is characterised by cellular debris, clinically identifiable as round deposits called drusen which accumulate between the choroid and the retina. This is followed by gradual retinal cell death representing non‐exudative AMD, which can be further complicated by the 'wet' form where blood vessels grow from the choroid underneath the retina. Wet AMD is more rapidly advancing. The exact cause of the underlying disease process is unknown but the major aetiological factors are oxidative stress, abnormalities in autophagy and heterophagy, and innate immune activation exacerbated by genetic predispositions. AMD has early, intermediate and advanced forms.
Description of the intervention
Photobiomodulation (PBM) is the process by which low level light technology affects cellular function. Visible to near‐infrared (NIR) light wavelength can be delivered by low‐level laser therapy (LLLT) or light‐emitting diodes (LED) and has been proposed to have beneficial clinical effects in the AMD process. LLLT is coherent monochromatic light and can be delivered as a continuous emission or pulsed beam. LED light is quasi‐monochromatic and non‐coherent.
How the intervention might work
By exposing people with AMD to light ranging from low‐intensity visible to NIR (500 nm to 1000 nm), PBM therapy allows for high tissue penetration and offers a possible non‐invasive approach for the treatment of AMD. PBM utilising low‐intensity visible green/blue light (500 nm to 560 nm) delivered at night, can act as a visual cycle modulator by reducing the metabolic demand of the retina by preventing full dark adaptation and thereby night‐time retinal outer segment hypoxia (Arden 2010). The pathophysiology of AMD is complex. Hypoxia associated with high metabolic demand of rods has been implicated as a main driver in the pathology of AMD. NIR irradiation is shown to enhance mitochondrial activity, in particular mitochondrial cytochrome C oxidase (COX) activity and production of adenosine triphosphate (ATP) in the retina, leading to a reduction in free radical production and oxidative damage (Sivapathasuntharam 2017). Mitochondrial COX acts as a photo‐acceptor for NIR irradiation. Spectral analysis study showed inhibitory effects of certain NIR wavelengths (750 nm and 950 nm) on COX activity, suggesting different biological effects within the NIR wavelength spectrum (Sanderson 2018).
PBM has also been shown to reduce gene expression of retinal stress and inflammatory mediators in the outer retina (Begum 2013; Kokkinopoulos 2013). Animal studies have shown PBM reduces complement propagation, increases phagocytosis and improves aged retinal function (Fuma 2015; Rutar 2012). A review by Fitzgerald and colleagues concluded that there is a growing body of preclinical evidence to support the hypothesis that PBM has disease‐modifying potential (Fitzgerald 2013). This has prompted investigators to conduct clinical trials to assess the efficacy of PBM therapy in improving visual function and reducing disease progression in people with AMD.
Why it is important to do this review
Consultation with patients and eye care professionals prioritised the need for a treatment to stop non‐exudative AMD progression (James Lind Alliance 2013). In the EU, 400,000 people are diagnosed with advanced AMD, a figure which is expected to rise to 700,000 per year in 2050 (Li 2020). The cumulative cost of detection, treatment and provision of social care for people with AMD from 2010 to 2020 was estimated as GBP 16.4 billion (RNIB 2009). Over 15% of people with AMD develop advanced AMD, with profound central vision loss or blindness (Klein 2007; Macular Society 2017).
AMD limits day‐to‐day activities due to vision‐related impairment (Berdeaux 2005; Owsley 2006; Scilley 2002). There is currently no available treatment. An effective and cost‐effective intervention would be of considerable benefit globally to those with this untreatable and debilitating condition. PBM offers the possibility of a safe and non‐invasive therapy for AMD. Currently, there is wide heterogeneity of PBM treatment regimens and uncertainty exists regarding its efficacy. It is important to do this review to obtain an overall estimate of the effectiveness of PBM treatment in non‐exudative AMD and to assess any harmful effects.
Objectives
To assess the effectiveness and safety of PBM compared to standard care, no treatment or sham treatment for people with non‐exudative AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only in this review. Trials included had analyses based on one or both eyes per participant.
Types of participants
Participants aged 50 years or older with non‐exudative AMD of the early, intermediate or late stage, as defined by the Age‐Related Eye Disease Study (AREDS 2001), were eligible irrespective of their lens status. Late stages feature geographic atrophy: a well‐demarcated area of retinal pigmented epithelium atrophy with or without central foveal involvement.
Types of interventions
We included trials that compared PBM to standard care or no treatment (or sham treatment) and where both groups were monitored equally. We included trials using visible to NIR light (500 nm to 1000 nm) delivered by low‐level lasers or light‐emitting diodes. We included trials that adequately described the PBM therapy parameters in terms of wavelength(s), dose, frequency, duration and coverage. Standard care consists of modifying risk factors; smoking cessation, nutritional advice and supportive measures; and referral to low‐vision services and visual rehabilitation.
Types of outcome measures
Primary outcomes
Mean best‐corrected visual acuity (BCVA) using a logMAR chart at 12 months follow‐up
Secondary outcomes
Mean best‐corrected visual acuity (BCVA) using a logMar chart at three months after treatment (range one to three months)
Mean contrast sensitivity measured using a Pelli Robson chart at short‐ (one to three months) and long‐term (12 months) follow‐up
Mean near vision at short‐ (one to three months) and long‐term (12 months) follow‐up
Mean low luminance deficit (LLD) score (the difference between low luminance visual acuity and BCVA), measured in logMAR chart units, at short‐ (one to three months) and long‐term (12 months) follow‐up
Mean reading speed at short‐ (one to three months) and long‐term (12 months) follow‐up using a calibrated reading chart
Mean vision‐related quality of life score at short‐ (one to three months) and long‐term (12 months) follow‐up, measured using a validated questionnaire
Where studies measured outcomes using alternative techniques ‐ for example, other visual acuity or contrast sensitivity charts ‐ we planned to collect and include data in the analysis by converting scales. We planned to report any cost benefit data reported in included studies.
Adverse events
The main complication of concern for PBM is vision loss, especially due to choroidal neovascularisation or advancing geographic atrophy. Adverse events included but were not limited to:
the proportion of participants with worse vision following PBM therapy. Worse vision was defined by a loss of 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters;
the proportion of participants who developed new geographic atrophy or progression of geographic atrophy; and
the proportion of participants who developed neovascular macular degeneration.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for randomised controlled trials and controlled clinical trials. There were no restrictions to language or year of publication. The electronic databases were last searched on 11 May 2020.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 5) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 11 May 2020) (Appendix 1).
MEDLINE Ovid (1946 to 11 May 2020) (Appendix 2).
Embase Ovid (1980 to 11 May 2020) (Appendix 3).
International Standard Randomised Controlled Trial Number (ISRCTN) registry(www.isrctn.com/editAdvancedSearch; searched 11 May 2020) (Appendix 4).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 11 May 2020) (Appendix 5).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 11 May 2020) (Appendix 6).
Searching other resources
We searched reference lists of included trial reports and related systematic reviews to identify additional potentially relevant trials. We contacted medical device companies conducting studies on PBM for information about any ongoing or completed but not published data. We also searched abstracts from the annual meetings of the:
European VitreoRetinal Society (2006 to 2018);
European Society of Retina Specialists (2009 to 2018);
American Academy of Ophthalmology (2000 to 2018);
American Society of Retinal Surgeons (2017 to 2018); and
Association for Research in Vision and Ophthalmology for ongoing trials (2006 to 2018).
Data collection and analysis
Selection of studies
Both review authors independently screened the titles and abstracts resulting from the searches using web‐based software (Covidence). We assessed full‐text articles of potentially relevant trials and contacted trial investigators for further information if required. We resolved discrepancies as to whether or not studies met inclusion criteria through discussion. We recorded excluded studies and the reasons for exclusion.
For potentially eligible studies identified on trials registers, we sought data using the following methods.
If the study completed in May 2018 or earlier, we searched for publications of this trial and contacted the investigators if necessary to obtain published or unpublished data from the trial. If eligible, we included the study in the review irrespective of whether we could identify a publication.
If the study had a completion date of May 2018, or in the future, we documented the study in the ongoing studies section.
Data extraction and management
Both review authors extracted data independently using an online form developed by Cochrane Eyes and Vision, and Covidence. We resolved discrepancies through discussion. We made two attempts to contact trial investigators for missing data. We imported data directly into Review Manager 5 (RevMan 5) (Review Manager 2014). One author checked the accuracy of the data import.
Study characteristics
We collected the following information on study characteristics (Appendix 7).
Study design: parallel group RCT/within‐person RCT/one or both eyes reported
Participants: country, total number of participants, age, sex, inclusion and exclusion criteria
Intervention and comparator details: including number of people (eyes) randomised to each group
Intervention details in terms of wavelength, dose, fluence, intensity, coverage, treatment time, frequency, intervals, total number of sessions and route of administration
Primary and secondary outcomes as measured and reported in the trials
Adverse events
Length of follow‐up
Date study conducted
Sample size and study power
Funding and conflicts of interest
Trial registration, if available
Outcome data
We extracted, separately, the following data from each included study, for both intervention and comparator groups.
Mean, standard deviation and number of participants on which outcome measured for continuous variables (visual acuity, contrast sensitivity, reading speed, LLD score)
Number of events and number of participants on which outcome data collected for dichotomous variables (adverse events)
When ETDRS letter visual acuity was reported, we converted it to logMAR prior to data entry into the review; more negative logMAR represented better visual function.
Assessment of risk of bias in included studies
Both review authors independently assessed the risk of bias using Cochrane's 'Risk of bias' tool for assessing risk of bias in each included study (Higgins 2011). We resolved disagreements through discussion. We considered the following sources of bias.
Selection bias (random sequence generation, allocation concealment): was the sequence of allocation generated using a random procedure and was the allocation concealed to people recruiting and enrolling participants and to participants?
Performance bias (masking of participants and researchers): were the recipients of care unaware of their assigned intervention? Were persons providing care unaware of the assigned intervention?
Detection bias (masking of outcome assessors): were persons evaluating outcomes unaware of the assigned intervention?
Attrition bias: were the rates of follow‐up and compliance similar in the groups? Was the analysis by intention‐to‐treat and were there any post‐randomisation exclusions?
Selective outcome reporting bias: is there any evidence that the outcomes measured have not been reported?
We graded each domain as low risk of bias, high risk of bias or unclear (lack of information or uncertainty of potential for bias).
Measures of treatment effect
We calculated mean difference for the following continuous outcomes: visual acuity, contrast sensitivity, reading speed and LLD score. It was not possible to check for skewness of continuous data which were reported as change from baseline (Altman 1996). We calculated risk ratio for adverse events.
Unit of analysis issues
PBM can be applied unilaterally or bilaterally. Usually it is applied to the affected eye(s). A pilot interventional study showed bilateral drusen size reduction when an ultra low‐energy nanosecond laser was applied unilaterally in AMD‐affected eyes (Guymer 2014; Jobling 2015). Application to only one eye may have an effect in the fellow eye, although the evidence is unclear and beyond the scope of this review.
Eyes and people
Trials may randomise one or both eyes to the intervention or comparator. The ALIGHT trial analysis was based on one eye per participant and there was no unit of analysis issue. In this case, investigators documented how the eye was selected. However, the LIGHTSITE I trial included more than one eye from some participants. Participants were randomly allocated to treatment or sham treatment but both eyes were included and reported for some participants. It is unclear whether LIGHTSITE I investigators adjusted for within‐person correlation in the tabulated data presented.
Cross‐over trials
Cross‐over trials are feasible; however, the washout period of the PBM therapy is undetermined which would make designing a cross‐over trial difficult. We identified no cross‐over trials.
Dealing with missing data
As intention‐to‐treat (ITT) data were not available, we performed available case analysis which assumed that data are missing at random. We did not impute missing data. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group.
Assessment of heterogeneity
We examined overall characteristics of studies ‐ in particular, the type of participants and types of interventions ‐ to assess the extent to which the studies were similar enough to make pooling study results sensible. We assessed forest plots to check for consistency between studies, in particular, the size and direction of effects. We calculated I², which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2002). There were no outcomes with I² values over 50% which would have indicated substantial inconsistency. We also considered Chi² P value. A P value less than 0.1 indicated statistical significance of the Chi² test.
Assessment of reporting biases
We used the 'Risk of bias' assessment tool (Higgins 2011) to look for selective or incomplete reporting (see Assessment of risk of bias in included studies).
Data synthesis
We pooled results using a fixed‐effect model in RevMan 5 for comparisons containing two studies. If the effects were in different directions and I² greater than 50% and P value less than 0.1, we did not pool data but instead gave a description of the pattern of the individual study results.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis to compare the effect of treatment in early, intermediate and late non‐exudative‐AMD was not possible.
Sensitivity analysis
We examined the impact of excluding: studies at high risk of bias in one or more domains; unpublished data; and industry‐funded data.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table presenting relative and absolute risks for the following outcomes at 12 months.
Mean best‐corrected visual acuity
Mean contrast sensitivity
Mean near vision
Mean LLD score
Mean reading speed
Mean vision‐related quality of life score
Adverse events
Two authors independently graded the overall certainty of the evidence for each outcome using the GRADE classification (GRADEpro GDT).
Results
Description of studies
See Characteristics of included studies.
Results of the search
The electronic searches yielded 445 records (Figure 1). After 56 duplicates were removed, the Cochrane Information Specialist (CIS) screened the remaining 389 records and removed 348 records that were not within the scope of the review. We identified two reports of trials from handsearching conference abstracts and, along with the 41 records identified by the CIS searches, we screened 43 records for potential inclusion in the review. We excluded 30 records based on information in the title and abstract, and obtained full‐text reports of 13 records for further investigation. We included two studies (five reports) ‐ (ALIGHT and LIGHTSITE I ) ‐ and excluded five reports of five studies; see Characteristics of excluded studies for details. Three ongoing studies (NCT03878420 (LIGHTSITE II), NCT04065490 (LIGHTSITE III) and NCT03859245), potentially met the inclusion criteria and will be assessed when data become available, see Characteristics of ongoing studies.
1.
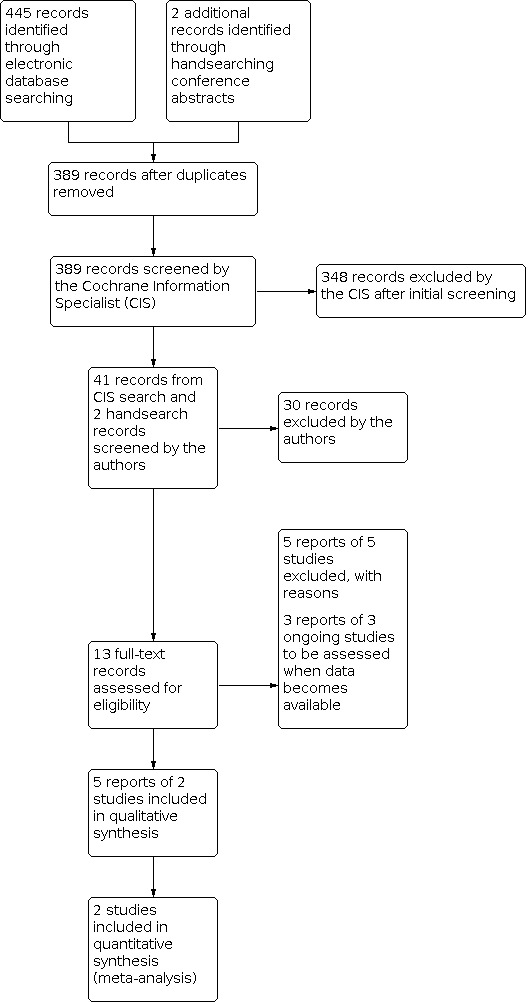
Study flow diagram.
Included studies
We included two published RCTs from single centres in the UK (ALIGHT) and Canada (LIGHTSITE I), which recruited 60 participants and 30 participants (46 eyes), respectively. One study compared single wavelength PBM with no treatment (ALIGHT). This study was at risk of performance bias because the treatment and control were different. One study compared multi‐wavelength PBM with sham treatment and was at risk of bias due to conflict of interests held by study investigators (LIGHTSITE I).
The average age of participants ranged between 76 years (LIGHTSITE I) and approximately 78 years (ALIGHT). The percentage of women enrolled ranged from 57% in ALIGHT to 60% in LIGHTSITE I. Participants in these trials were people with non‐exudative AMD with Age‐Related Eye Disease Study (AREDS) categories 2 (early) to 4 (advanced non‐exudative) in the treatment eye. In the ALIGHT study, approximately 33.3% of participants had AREDS category 4 (advanced non‐exudative) i.e. foveal involving geographic atrophy, and in the LIGHTSITE I study, approximately 43.5% of eyes enrolled were AREDS category 4.
ALIGHT was a single centre RCT conducted at Bristol Eye Hospital, Bristol, UK. It enrolled 60 participants with AREDS grades 2 to 4 and ETDRS BCVA score of 0.3 logMAR or better in the study eye. Participants were randomly assigned to receive LED‐PBD (505 nm) or no treatment in the study eye. PBM was delivered for 8 hours every night for 12 months via Noctura 500 mask. All participants were receiving ranibizumab injections for neovascular AMD in the fellow eye. The structural and functional primary outcome was disease progression. Disease progression was defined as the proportion of participants developing advanced AMD or an increase in drusen volume beyond test‐retest 95% confidence intervals at 12 months in the study eye. Investigators assessed participants on a monthly basis with optical coherence tomography (OCT) imaging to allow measurement of drusen volume and assess for the development of neovascular AMD. A co‐primary outcome measure was the change in cone τ time to recover sensitivity to 63% pre‐bleach value at 12 months. Secondary outcome measures included: change in ETDRS BCVA, drusen volume, chromatic and flicker thresholds, self‐reported visual function (VFQ‐48), health‐related quality of life (EQ‐5D) and the ranibizumab re‐treatment rate in the fellow eye. Safety and acceptability outcomes included 12 month compliance data, change in self‐reported sleep disturbance and adverse events.
The LIGHTSITE I pilot study was a double‐masked RCT comparing multi‐wavelength PBM with sham treatment in 30 people (46 eyes). Participants with AREDS classification 2 to 4 (non‐exudative) were included. Participants with visual significant cataracts or other ocular diseases were excluded. PBM treatment was administered with a multi‐wavelength system, the Valeda™ light delivery system emitting yellow (590 nm 5 mW/cm2), red (660 nm, 65 mW/cm2),and near‐infra red (850 nm, 8 mW/cm2) wavelengths via light‐emitting diodes (LED). The total treatment time was 4 minutes 10 seconds and consisted of four phases. First, pulsed yellow and NIR wavelengths were delivered through the participants' open eyes for 35 seconds, followed by continuous red wavelength for 90 seconds through the participants' closed eyes. This was then repeated. Sham treatment emitted lower 660 nm wavelength dose by approximately a factor of 100, producing a slightly duller light. The 850 nm (non‐visible light) was not provided in the sham treatment. Participants received sham or PBM for 4 to 5 minutes/eye three times a week for three weeks which was then repeated 6 months from baseline. LIGHTSITE I demonstrated an improvement in BCVA, contrast sensitivity and reduction in central drusen volume and thickness at 3 months. Initial results demonstrated that PBM therapy was more beneficial in people with early stage non‐exudative AMD with better vision (more than 74 letters ETDRS BCVA). The majority of a subset of high PBM responders, with more than 5 letter ETDRS improvement, did not have foveal involving geographical atrophy (AREDS 2 or 3). The Valeda™ light delivery system obtained CE marking in 2018 but is limited to investigational use by the Food & Drug Administration (FDA). The results of LIGHTSITE I have informed a larger multi‐centre RCT, NCT03878420 (LIGHTSITE II).
Ongoing studies
We also identified three eligible ongoing RCTs from clinical trials database search (NCT03878420 (LIGHTSITE II); NCT04065490 (LIGHTSITE III); NCT03859245).
We identified two RCTs, comparing PBM with sham treatment, from the searches of clinical trial registries (NCT03878420 (LIGHTSITE II), NCT04065490 (LIGHTSITE III)). NCT03878420 (LIGHTSITE II) and NCT04065490 (LIGHTSITE III) are double‐masked, sham‐controlled, prospective multi‐site studies for the use of PBM as a treatment for non‐exudative AMD using the Valeda™ Light Delivery System. NCT03878420 (LIGHTSITE II) aims to enrol 144 participants and NCT04065490 (LIGHTSITE III) aims to enrol 96 participants with early to intermediate AMD (AREDS 2 to 3), excluding centre involving geographic atrophy and an ETDRS BCVA letter score of between 50 and 75. NCT03878420 (LIGHTSITE II) is expected to reach completion by December 2020 and NCT04065490 (LIGHTSITE III) by June 2022. Wavelengths 590 nm and 850 nm are delivered together through the open eyelid and the 660 nm wavelength delivered through the closed eyelid. Sham 660 nm wavelength dose is lower than the treatment dose by approximately a factor of 100, producing a slightly duller light. BCVA, contrast sensitivity, and central drusen volume and thickness are some of the outcome measures set.
The third RCT (NCT03859245) compares PBM and a ketogenic diet with PBM alone for the treatment of retinal disorders. NCT03859245 is an ongoing open label RCT due to complete in September 2020. PBM therapy is delivered via Joovv red light/infrared LED device, three times per week, 20 minutes per session. The primary outcome for the non‐exudative AMD group is reduction in size and number of drusen in early and intermediate stages of non‐exudative AMD.
Excluded studies
We excluded five full‐text articles: one investigated subthreshold nanosecond laser (Guymer 2018); two were prospective case series (TORPA I 2012; TORPA II 2016); and two were retrospective case series (Ivandic 2008; Koev 2018). None met the inclusion criteria. We have provided the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias assessments are detailed in the Characteristics of included studies table and are summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for included study.
Allocation
ALIGHT generated an unpredictable sequence, combined with an adequate allocation sequence concealment method to prevent selection bias. Random permuted blocks were computer generated with varying block sizes, stratified for AMD AREDS grading. LIGHTSITE I did not report the method of randomisation or allocation concealment.
Blinding
In ALIGHT, two independent graders assessed fundus images according to AREDS simplified severity scale. There was no masking of the participant and trial investigator to the intervention. However, treating clinicians at the AMD clinic were masked to the intervention. Automated drusen volume assessment was made at baseline and at monthly follow‐up visits using software available for the Cirrus OCT imaging system. Masked treating ophthalmologists determined the development of advanced AMD at monthly follow‐up appointments in the AMD clinic.
In LIGHTSITE I, participants and investigators were masked to the treatment. Masked independent graders assessed baseline and follow‐up fundus images and OCT scans. Masking of LIGHTSITE I was achieved by sham wavelengths and identical graphical user interfaces and outputs from the Valeda instrumentation.
Incomplete outcome data
Nine of 30 (30%) participants from the intervention arm withdrew primarily due to mask discomfort, compared to three of 30 (10%) participants who withdrew from the control group (ALIGHT). It was unclear how investigators handled missing data of drusen volume for participants who withdrew. It appears that participants who withdrew for reasons other than conversion to exudative AMD were excluded from treatment effect analysis. ALIGHT reported six protocol violations where four control and two intervention participants were assessed outside the schedule window of 12 months ± 4 weeks. Investigators collected drusen volume data for 24 participants on average 7.2 ± 2.89 weeks after the final study visit. Compliance with wearing the treatment mask was 78%.
Investigators of LIGHTSITE I reported complete primary outcome data at one month for both arms of the study. However, at 12 months there was missing BCVA data for two eyes in the sham group and one eye in the PBM group. It is unclear if missing data from the sham group at 12 months was due to loss of follow‐up. Investigators performed intention‐to‐treat analysis.
Selective reporting
Although ALIGHT authors commented on all outcomes listed in the protocol (see McKeague 2014), LIGHTSITE I authors reported on all prespecified outcomes, did not report adequately on non‐significant results. The ALIGHT investigators provided unpublished summary data of non‐significant results upon request to avoid bias in future meta‐analysis.
In the ALIGHT study, investigators did not report adverse events according to CONSORT guidelines (Ioannidis 2004), as overall absolute risk per arm, per adverse event type data were not provided. Investigators did not fully report the seriousness of adverse events, and it was unclear if any adverse events were recurrent events in the same participant.
It remains unclear whether PBM application to only one eye may have an effect in the fellow eye. None of the other studies identified investigated the effect of PBM to the fellow eye.
Other potential sources of bias
There was an insufficient number of studies included in this review to assess the likelihood of potential publication bias. Two authors of ALIGHT declared a personal financial interest. Five LIGHTSITE I study authors declared some type of fees from LumiThera Inc., which manufactures Valeda Light Delivery System and three authors are employees at LumiThera Inc.
Effects of interventions
See: Table 1
See Table 1 for PBM versus control treatment in non‐exudative AMD.
Primary outcome
Best‐corrected visual acuity at 12 months
When comparing PBM with sham treatment or no treatment control for non‐exudative AMD, we found no meaningful clinical difference in BCVA (mean difference (MD) 0.02 logMAR, 95% confidence interval (CI) ‐0.02 to 0.05; 2 RCTs, 90 eyes; low‐certainty evidence) at 12 months. There was no heterogeneity of effect (I 2= 0%; P value = 0.34). There was uncertainty and the confidence intervals included the line of no effect (Figure 3).
3.

Forest plot of comparison: 1 PBM treatment versus no treatment for non‐exudative AMD, outcome: 1.1 BCVA at 12 months [LogMar].
The LIGHTSITE I study was underpowered for its primary outcome BCVA at 12 months. Participants received two series of multiple‐wavelength PBM treatments six months apart. Each series consisted of nine treatment sessions (three sessions per week). By 12 months follow‐up, participants would have received two series of multiple‐wavelength PBM treatments or sham treatment. There was no difference in BCVA between the two groups at 12 months. Observed improvements in BCVA after PBM treatment diminished with time to baseline BCVA levels, suggesting that a treatment interval shorter than six months is needed to maintain therapeutic effect. Similarly, ALIGHT showed no difference in BCVA between participants receiving single‐wavelength PBM and no treatment at one year (MD 0.02 logMAR, 95% CI ‐0.02 to 0.06).
Secondary outcomes
Best‐corrected visual acuity at 3 months
Both studies reported this outcome at three months (Analysis 1.2). We found a statistical difference in BCVA (MD ‐0.07 logMAR, 95% CI ‐0.13 to 0.00; P = 0.03; 2 RCTs, 78 eyes) at three months, although not clinically meaningful (less than 1 line gain on the logMAR chart). There was no heterogeneity of effect (I2 = 0%; P value = 0.46). Both effect estimates were in the same direction (‐0.11 and ‐0.05).
1.2. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 2: BCVA at 3 months [LogMar]
At three months after receiving the first series of multiple‐wavelength PBM, treated participants showed an average increase in BCVA of 4.1 (±5.4) letters compared to 1.4 (±6) ETDRS letters in the sham group (LIGHTSITE I). Both groups had similar baseline BCVA. ALIGHT showed no difference in BCVA between participants receiving single‐wavelength PBM and no treatment at three months (MD ‐0.11 logMAR, 95% CI ‐0.24 to 0.02; P = 0.10).
Contrast sensitivity
LIGHTSITE I showed an improvement in contrast sensitivity at Level E (18 cycles/degree) that was maintained at 12 months (MD 0.29 LogCS, 95% CI 0.23 to 0.35; 1 RCT, 46 eyes; low‐certainty evidence) (Figure 4). This trend was not observed for contrast sensitivity at Levels A, B, C and D. ALIGHT did not measure contrast sensitivity.
4.

Forest plot of comparison: 1 PBM treatment versus control for non‐exudative AMD, outcome: 1.3 Contrast sensitivity at 12 months Level E [18cycles/degree].
Vision‐related quality of life score
In ALIGHT, quality of life scores for: visual function (VFQ‐48 score) (MD 0.43, 95% CI ‐0.17 to 1.03; 1 RCT, 47 eyes; P = 0.16; Figure 5); health‐related quality of life (EQ‐5D score) (MD ‐0.03, 95% CI ‐0.14 to 0.08; P = 0.58; Analysis 1.5); and sleep quality (PSQI) were comparable between the PBM and control groups. Although there was a high attrition rate in the PBM group due to mask discomfort, in LIGHTSITE I, investigators assessed quality of life (QOL) using Visual Function Questionnaire‐25 (VFQ‐25), which showed an overall improvement in QOL composite score and a perceived improvement with PBM in relation to difficulties in daily activities at three months (P = 0.003), seven months (P = 0.015) and nine months (P = 0.003).
5.

Forest plot of comparison: 1 PBM treatment versus control for non‐exudative AMD, outcome: 1.4 Self‐reported visual function [VFQ‐48].
1.5. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 5: Health‐related quality of life EQ‐5D
Adverse events
Conversion to exudative AMD
Both studies reported conversion to exudative AMD at 12 months (Figure 6). There was no heterogeneity (I2 = 0%; P value = 0.39). In ALIGHT, fewer eyes receiving PBM converted to exudative AMD. However, in LIGHTSITE I, one eye converted to exudative AMD. There remains uncertainty as the confidence intervals included 1 (risk ratio (RR) 0.97, 95% CI 0.17 to 5.44; 2 RCTs, 96 eyes), and it is an infrequent event within this follow‐up period.
6.

Forest plot of comparison: 1 PBM treatment versus control for non‐exudative AMD, outcome: 1.6 Conversion to exudative AMD.
ALIGHT reported 33 adverse events (eight were ocular) and nine serious adverse events (of which three related to conversion of exudative AMD). Of the eight ocular adverse events, four were in the PBM group. None of the adverse or serious adverse events were considered by the investigators to be associated with the PBM. There was no significant difference found between the re‐treatment rate with anti‐vascular endothelial growth factor (VEGF) injections for exudative AMD in the fellow eye.
In LIGHTSITE I, there was a total of 21 adverse events: four participants in the sham group reported five adverse events whereas seven participants in the PBM group reported 16 adverse events. None of these adverse events were considered to be related to the PBM treatment by investigators. One eye converted to neovascular AMD in the PBM group within one month of receiving treatment. There was no significant difference in growth of geographic atrophy lesion between PBM and sham treatment groups at 12 months.
Disease progression
ALIGHT reported disease progression and defined it as the onset of advanced AMD or significant increase in drusen volume beyond test‐retest 95% confidence intervals (95% CIs) within 12 months (Figure 7). One participant in the PBM group and two participants in the control group developed exudative AMD during the 12 months follow‐up period. There was no difference in drusen volume at 12 months between PBM and no treatment (MD ‐0.02 mm3,95% CI ‐0.04 to 0.0; P = 0.06). When number of participants who developed advanced AMD and significant increase of drusen volume were combined, there remained no difference in the overall disease progression between PBM and no treatment (RR 0.79, 95% CI 0.41 to 1.53; P = 0.48). The PBM group exhibited greater delay in cone dark adaptation at 12 months compared to the control group (MD 1 minute, 95% CI 0.68 to 1.32; P < 0.001).
7.

Forest plot of comparison: 1 PBM treatment versus no treatment for non‐exudative AMD, outcome: 1.5 AMD progression at 12 months.
We downgraded the quality of the evidence due to performance and attrition biases, and insufficient sample size. The included studies did not measure these outcomes: near vision, LLD score, loss of 15 or more EDTRS letters, and reading speed.
Discussion
Summary of main results
Two published RCTs met our inclusion criteria investigating single‐wavelength PBM (ALIGHT) and multi‐wavelength PBM (LIGHTSITE I) for non‐exudative AMD. We found three ongoing RCTs: NCT03878420 (LIGHTSITE II) and NCT04065490 (LIGHTSITE III) investigating multi‐wavelength PBM compared to sham treatment for non‐exudative AMD; and NCT03859245 comparing PBM and a ketogenic diet with PBM alone for the treatment of retinal disorders.
ALIGHT investigated single wavelength 505 nm organic light‐emitting diode (OLED) in participants with non‐exudative AMD in the study eye. Evidence from this study suggests PBM 505 nm OLED may disrupt retinal physiology by prolonging dark adaptation. It would be interesting to elucidate if this change is temporary and for how long these effects are sustained. Despite the apparent delay in dark adaptation amongst the PBM group on testing cone adaptation time, participants were not symptomatic. There was no difference in disease progression between participants receiving PBM and no treatment, although the follow‐up period was limited to only 12 months. Just over a quarter (26%) of participants withdrew within three months of treatment as a result of mask‐related issues such as discomfort.
LIGHTSITE I demonstrated some improvement in BCVA and contrast sensitivity (Level E 18 cycles/degree) following multi‐wavelength PBM in non‐exudative AMD. However, the therapeutic effects of multi‐wavelength PBM diminished by six months after treatment, suggesting a shorter time interval between treatments is needed to maintain benefit. Post‐hoc analysis identified high PBM responders as participants with earlier stage disease without foveal involving geographic atrophy. The findings of LIGHTSITE I have informed the inclusion criteria and treatment regime for NCT03878420 (LIGHTSITE II) and NCT04065490 (LIGHTSITE III) studies, which will exclude patients with central involving geographic atrophy and will schedule PBM treatment series at four‐monthly intervals with follow‐up periods of nine months and 21 months, respectively.
We have also described other relevant studies identified in the searches in order to comment on the current evidence base for clinical practice. There was the TORPA I 2012 pilot study and TORPA II 2016, both interventional case series, which investigated multi‐wavelength LED‐PBM (590 nm, 4 mW; 670 nm, 50 mW; 790 nm, 0.6 mW; pulsed at 2.5 Hz; 0.1 J/cm2/treatment) through open or closed eyes in non‐exudative AMD. LED‐PBM was delivered by two off‐label instruments (Warp10 and Gentlewaves) sequentially three times per week for six weeks. The investigators for LIGHTSITE I conducted these studies. TORPA I 2012 investigated functional outcomes of LED‐PBM in non‐exudative AMD. Although improvement in visual acuity and contrast sensitivity was maintained at 12 months, there was some decline after four months, suggesting a repeat dosing to maintain therapeutic effect. TORPA II 2016 expanded clinical outcomes to include functional and morphological endpoints. It evaluated 24 participants (42 eyes), of which nine had foveal involving geographic atrophy. Compared to LIGHTSITE I, TORPA II 2016 recruited fewer participants with advanced non‐exudative AMD (AREDS 4). Participants with a baseline BCVA between 70‐89 EDTRS letters were more likely to gain more than 5 letters with LED‐PBM. TORPA II 2016 reported an improvement in mean BCVA of 5.14 EDTRS letters, contrast sensitivity (0.16 three cycles per degree), drusen volume (decrease by 0.029 mm3) and thickness (decrease by 0.34 µm) at three months. Evaluation of optical coherence tomography (OCT) and colour fundoscopy showed that drusen regression did not contribute to new geographic atrophy formation.
Two interventional case control studies in a mixed AMD population showed visual improvement in eyes treated with LLLT‐PBM. Ivandic 2008 conducted the largest retrospective case series to date in a heterogenous group of AMD patients, non‐exudative and exudative forms with or without cataracts. In this study, treatment consisted of transscleral delivery of low level laser therapy (LLLT), as a semiconductor laser diode 780 nm (7.5 mW, 292 Hz, spot diameter 3 mm) with continuous emission, to irradiate the macula. Investigators delivered treatment for 40 seconds (0.3 J/cm2) resulting in a total dose of 1.2 J/cm2. The treatment schedule consisted of twice weekly sessions for two weeks. Investigators treated 193 participants (328 eyes) with PBM and 10 participants (20 eyes) received sham treatment. A greater proportion of eyes without cataracts gained 3 or more Snellen lines compared to eyes with cataracts (40.4% versus 28.6%). It was not clear how long improved visual function was maintained as follow‐up ranged from three to 36 months. Neither local nor systemic adverse effects were observed. Over a quarter of the eyes receiving PBM were exudative AMD and showed a reduction in oedema and bleeding but no sub‐group analysis was performed. It was not clear if participants with exudative AMD received treatment with anti‐VEGF therapy. Koev 2018 conducted the longest case control study to date in participants with all stages of AMD. In this study, treatment consisted of transpupillary delivery of LLLT, a helium‐neon (He‐NE) laser with continuous emission at 633 nm (0.1 mW/cm2), to the macula for three minutes on alternative days for 12 days. Eight of 66 eyes that received PBM were exudative AMD. The study did not report the proportion of eyes with foveal involving geographical atrophy. At five year follow‐up, Koev and colleagues reported visual improvement from baseline in 93.9% of eyes (62/66) receiving PBM, of these 19% (12/62) gained 3 or more lines. Surprisingly, there was no change in vision in the control group at five years.
Ivandic 2008 and Koev 2018 reported no local or systemic adverse effects. However, these studies did not define harms particularly well. We would suggest proactive reporting of the proportion of participants who progress to advanced AMD (exudative or non‐exudative) or time to progression to advanced AMD; loss of 3 lines ETDRS BCVA; deterioration in low luminance BCVA; in addition to the incidence and severity of other ocular conditions. Where possible, studies should report clinical characteristics as described in the International Consortium for Health Outcomes Measurement Macular Degeneration Standard Set (Rodrigues 2016), and more specifically for trials assessing geographic atrophy (Krezel 2019), so that data can be more easily compared across studies. Data from available case series suggest some benefit from PBM in improving vision, contrast sensitivity and drusen volume in early AMD, but whether it meets minimally clinically important differences is unclear. Longer follow‐up durations is warranted to assess prevention of progression to advanced AMD.
Equivalence between LED‐PBM and LLLT‐PBM, as well as continuous emission versus pulsed laser, in the treatment of retinal injury has not been elucidated from pre‐clinical studies and will remain controversial until future studies compare them. PBM dosimetry can be very complicated as altering any one of the parameters may impact the biological effect. There is no international consensus on PBM dosimetry as this would need to be specifically tailored for the tissue type and disease. The first PBM therapy to be approved by National Institute for Health and Care Excellence (NICE) in 2018 was for preventing or treating oral mucositis caused by radiotherapy or chemotherapy (NICE 2018). PBM in this field continues to have a wide range of dosing schedules. However, the evidence base lacks clarity on PBM route of administration, spectral analysis and dosimetry for retinal disease. The World Association of Laser Therapy has published a consensus agreement on key dosimetric parameters of PBM as part of item 4 of the CONSORT guidelines (WALT 2004). It recommends reporting laser type, wavelength, delivery mode, peak power, beam irradiation area, power density, irradiation duration, fluence, number of treatments, dose per treatment and cumulative dose.
We excluded subthresold micropulse laser therapy from this review. Although this treatment is designed to minimise thermal retinal tissue damage, it causes RPE cell damage visible on histology (Yu 2013), and has a different mechanism of action to PBM. Subthreshold nanosecond laser treatment with a 532 nm Q‐switched neodymium‐dopedyttrium‐aluminum‐garnet laser with 3 ns pulse duration for intermediate AMD did not slow progression to advanced AMD (Guymer 2018). However, post hoc analysis showed a change in effect direction (hazard ratio 0.23) in participants without reticular pseudodrusen, advocating caution when considering treatment for this clinical phenotype. Future PBM studies should carefully characterise the clinical phenotypes included and adequately power the study to allow for subgroup analysis, potentially identifying non‐responders.
Overall completeness and applicability of evidence
Our search strategy is likely to have returned all relevant articles in this area. Handsearching returned two conference proceedings and one report. We identified an exploratory study in Russian which compared different PBM wavelengths in non‐exudative AMD (Abramov 1996), but we could not retrieve the full‐text. The updated evidence remains incomplete for three main reasons. Firstly, the applicability of this review is limited by the lack of studies of sufficient quality. Short‐term benefits observed with PBM in the observational studies could be neither confirmed nor refuted with enough confidence in this current review. Secondly, the long‐term beneficial effects, if any, in terms of favourable structural and functional outcomes are not yet known. Thirdly, uncertainty remains regarding the safety profile of PBM therapy in treated and fellow eyes.
Quality of the evidence
We have assessed the quality of evidence for single and multiple wavelength PBM therapies with the reasons for downgrading shown in Table 1. Two published studies met the inclusion criteria for this review, and we graded them as high risk in at least one or more domain. We intended to include all primary and secondary outcomes of the review in the 'Summary of findings' table. However, many of the outcomes were not reported in the included studies. We graded the overall quality of evidence as low and very low for all reported outcomes. As such, further research is very likely to have an important impact on the effect estimate and is likely to change the estimate.
Potential biases in the review process
We followed standard Cochrane methods to minimise potential biases in the review process. Most outcomes of the review were not reported in the included studies. We are contacting the authors of the studies to collect additional information on these outcomes. Also, we could not perform a subgroup analysis according to type of PBM due to paucity of studies.
Agreements and disagreements with other studies or reviews
Compared to a recent review, we identified additional studies (Waugh 2018).
Authors' conclusions
Implications for practice.
Currently there remains uncertainty whether PBM treatment is beneficial in slowing progression of non‐exudative macular degeneration. Low‐certainty evidence comparing PBM with sham treatment or no treatment showed no clinically meaningful difference in BCVA and contrast sensitivity at 12 months. Further research is likely to have an important impact on the confidence in the estimate of effect and may change the estimate. There is no evidence comparing low level laser therapy with LED‐PBM, or transpupillary delivery with a transscleral route of administration. There is a need for further well‐designed controlled trials assessing dosimetry, powered for both effectiveness and safety outcomes. Consideration should be given to the adoption of core clinical outcomes measures that include patient‐based outcome measures.
Implications for research.
This review raises several issues for further trials in PBM. Future trials should be powered appropriately and include definitions of success that reflect more clinically relevant gains in visual acuity than the lower levels reported to date. Several important outcomes that we pre‐specified in the review protocol (Henein 2018), but are under‐investigated, deserve more attention in future trials. These include near vision, low luminance vision and reading speed. Methodologically, there are several modifications that future trials could make to minimise bias. Specifically, trials would benefit from standardised methods for allocation concealment and clear reporting of these methods. Additionally, studies could decrease bias by following an intention‐to‐treat analysis and by including all randomised participants in all analyses from all follow‐up time points, and by using multiple imputation methods for missing data when necessary. It would also be beneficial to increase the sample size in trials to make subgroup analyses possible or power the studies appropriately to assess specific disease subtypes.
In conclusion, the role of PBM in the management of non‐exudative macular degeneration remains unclear. With growing use of PBM therapy in the treatment of many retinal diseases, further adequately powered trials that compare PBM with sham treatment or carefully designed outcome masking, and other PBM treatment parameters, are needed for improved patient care.
History
Protocol first published: Issue 5, 2018 Review first published: Issue 4, 2021
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Anupa Shah (Managing Editor for CEV) and the CEV UK editorial base for their assistance with this review and Tim Jackson and Noemi Lois for peer review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees #2 MeSH descriptor: [Retinal Degeneration] explode all trees #3 MeSH descriptor: [Retinal Neovascularization] explode all trees #4 MeSH descriptor: [Choroidal Neovascularization] explode all trees #5 MeSH descriptor: [Macula Lutea] explode all trees #6 macula* near lutea* #7 (macula* or retina* or choroid*) near/4 degenerat* #8 (macula* or retina* or choroid*) near/4 neovascul* #9 AMD or ARMD or maculopath* #10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 #11 MeSH descriptor: [Low‐Level Light Therapy] this term only #12 (low near/2 level near/2 light) #13 (low near/2 level near/2 laser*) #14 (low near/2 power near/2 laser*) #15 (low near/2 energy near/2 laser*) #16 (low near/2 intensity near/2 laser*) #17 (light near/2 based near/2 technolog*) #18 light emitting diode* #19 (LLLT or LLL or LED‐T) #20 (photobiomodulat* or PBM or photomodulat*) #21 (Gentlewaves or LT‐300) #22 laser* near/2 (irradiat* or phototherap* or biostimulat*) #23 (phototherap* near/2 infrared) #24#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25#10 and #24
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp retinal degeneration/ 14. retinal neovascularization/ 15. choroidal neovascularization/ 16. exp macula lutea/ 17. (macula$ adj2 lutea).tw. 18. ((macul$ or retina$ or choroid$) adj4 degener$).tw. 19. ((macul$ or retina$ or choroid$) adj4 neovasc$).tw. 20. (AMD or ARMD or CNV).tw. 21. maculopath$.tw. 22. or/13‐21 23. Low‐Level Light Therapy/ 24. (low adj2 level adj2 light).tw. 25. (low adj2 level adj2 laser$).tw. 26. (low adj2 power adj2 laser$).tw. 27. (low adj2 energy adj2 laser$).tw. 28. (low adj2 intensity adj2 laser$).tw. 29. (light adj2 based adj2 technolog$).tw. 30. light emitting diode$.tw. 31. (LLLT or LLL or LED‐T).tw. 32. (photobiomodulat$ or PBM or photomodulat$).tw. 33. (Gentlewaves or LT‐300).tw. 34. (laser$ adj2 (irradiat$ or phototherap$ or biostimulat$)).tw. 35. (phototherap$ adj2 infrared).tw. 36. or/23‐35 37. 22 and 36 38. 12 and 37
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula degeneration/ 34. exp retina degeneration/ 35. exp retina neovascularization/ 36. exp subretinal neovascularization/ 37. (AMD or ARMD or CNV).tw. 38. ((macul$ or retina$ or choroid$) adj4 degener$).tw. 39. ((macul$ or retina$ or choroid$) adj4 neovasc$).tw. 40. exp retina macula lutea/ 41. (macula$ adj2 lutea$).tw. 42. maculopath$.tw. 43. or/33‐42 44. low level laser therapy/ 45. light emitting diode/ 46. (low adj2 level adj2 light).tw. 47. (low adj2 level adj2 laser$).tw. 48. (low adj2 power adj2 laser$).tw. 49. (low adj2 energy adj2 laser$).tw. 50. (low adj2 intensity adj2 laser$).tw. 51. (light adj2 based adj2 technolog$).tw. 52. light emitting diode$.tw. 53. (LLLT or LLL or LED‐T).tw. 54. (photobiomodulat$ or PBM or photomodulat$).tw. 55. (Gentlewaves or LT‐300).tw. 56. (laser$ adj2 (irradiat$ or phototherap$ or biostimulat$)).tw. 57. (phototherap$ adj2 infrared).tw. 58. or/44‐57 59. 43 and 58 60. 32 and 59
Appendix 4. ISRCTN search strategy
(Condition: Macular Degeneration OR AMD OR ARMD OR CNV AND Interventions: photobiomodulation OR low level light OR low level laser)
Appendix 5. ClinicalTrials.gov search strategy
Interventional Studies | Macular Degeneration OR AMD OR ARMD OR CNV | photobiomodulation OR low light OR low laser
Appendix 6. WHO ICTRP search strategy
Condition = Macular Degeneration OR AMD OR ARMD OR CNV AND Interventions = photobiomodulation OR low level light OR low level laser
Appendix 7. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design | · Parallel group RCTi.e. people randomised to treatment · Within‐person RCTi.e. eyes randomised to treatment · Cluster RCTi.e. communities randomised to treatment · Cross‐over RCT · Other, specify |
Exclusions after randomisation Losses to follow up Number randomised/analysed How were missing data handled? e.g., available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomisation/ unit of analysis |
· One eye included in study, specify how eye selected · Two eyes included in study, both eyes received same treatment, briefly specify how analysed (best/worst/average/both and adjusted for within person correlation/both and not adjusted for within person correlation) and specify if mixture one eye and two eye · Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done |
|
| Participants | ||
| Country | Setting Ethnic group Participation rate Equivalence of baseline characteristics (Y/N) Diagnostic criteria |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) |
· Number of people randomised to each group · Wavelength of photobiomodulation therapy · Dose, fluence, intensity, coverage, treatment time · Frequency, intervals and total number of sessions · Route of administration · Specify whether another intervention was performed at same time as intervention |
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports | · Best‐corrected visual acuity at 12 months · Contrast sensitivity · Near visual acuity · Reading speed · Low luminance deficit score · Quality of life score · Cost benefit data (if available) · The proportion of participants with worse vision following photobiomodulation therapy. Worse vision is defined by a loss of 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters. · The proportion of participants who developed new geographic atrophy or progression of geographic atrophy. · The proportion of participants who developed neovascular macular degeneration. |
Planned/actual length of follow up · Length of follow up · Loss to follow up · Intervals at which outcomes assessed |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name: (if applicable) Reported subgroup analyses (Y/N) Were trial investigators contacted? Trial Registration Number: (if applicable) |
| Sources of funding | ||
| Declaration of interest | ||
Data and analyses
Comparison 1. PBM treatment versus control for non‐exudative AMD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 BCVA at 12 months [LogMar] | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.05] |
| 1.2 BCVA at 3 months [LogMar] | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 1.3 Contrast sensitivity at 12 months Level E [18cycles/degree] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Self‐reported visual function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Health‐related quality of life EQ‐5D | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Conversion to exudative AMD | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.17, 5.44] |
| 1.7 AMD progression at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 1: BCVA at 12 months [LogMar]
1.3. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 3: Contrast sensitivity at 12 months Level E [18cycles/degree]
1.4. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 4: Self‐reported visual function
1.6. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 6: Conversion to exudative AMD
1.7. Analysis.

Comparison 1: PBM treatment versus control for non‐exudative AMD, Outcome 7: AMD progression at 12 months
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ALIGHT.
| Study characteristics | ||
| Methods | Phase I/IIa, prospective, parallel group, single‐centre, unmasked, randomised controlled trial One eye selected, non‐exudative age‐related macular degeneration |
|
| Participants | Country: United Kingdom
Number of people randomised: 60
Age: range 55 to 88 years
Sex: 57% female
Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics |
|
| Interventions | Intervention: Noctura 500 eye mask (LED 505 nm 23 scotopic Trolands) worn eight hours every night for 12 months n = 30 Comparator: no treatment n = 30 |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Contrast sensitivity, near vision, low luminance deficit score, loss of 15 or more EDTRS letters and reading speed were not reported. |
|
| Notes | Study name: Alight Dates of recruitment of participants: 04/2014 to 02/2015 Sources of funding: research grant from the College of Optometrists, London, UK Declaration of interest: two authors declared a personal financial interest, indicating they are "inventor/developer designated on a patent, patent application, copyright, or trade secret, whether or not the patent, copyright, etc. is presently licensed or otherwise commercialized, which is the subject matter of your publication or could be in competition with the technology described". Trial investigators were contacted. Trial registration: ISCTRN 82148651 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence using permutated blocks stratified according to AREDs scores 2, 3 and 4. |
| Allocation concealment (selection bias) | Low risk | Randomisation allocation number provided in envelopes to study investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unmasked to treatment. Clinicians treating fellow eye with neovascular age‐related macular degeneration masked to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of treating clinicians; however, communication from participants could remove masking. Assessors of primary outcome were masked but not for secondary outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Compliance with wearing treatment mask was 78%. Nine participants withdrew from study due to mask discomfort. Drusen volume data was missing for 24 participants. Final visits for six participants were conducted outside study protocol. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were reported. |
LIGHTSITE I.
| Study characteristics | ||
| Methods | Double‐masked randomised sham‐controlled single‐centre trial One eye or both eyes selected, non‐exudative age‐related macular degeneration |
|
| Participants | Country: Canada Number of participants randomised: 30 (46 eyes) Age: 76 years Sex: 60% female Inclusion criteria:
Exclusion criteria:
Equivalence of baseline characteristics 43.5% of eyes included were AREDS 4 with foveal involving geographic atrophy. |
|
| Interventions | Intervention: LT‐300 multi‐wavelength treatment 590 nm, 670 nm, and 850 nm delivered 3 times a week for 3 weeks then repeated after 6 months. Treatment lasted total of 5 minutes per eye. Comparator: Sham treatment using LT‐300 system delivered 3 times a week for 3 weeks then repeated after 6 months. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Study Name: LIGHTSITE I Dates of recruitment of participants: not reported Sources of funding: National Institutes of Health, National Eye Institute #3R43EY025508‐01S1 Declarations of interest: five authors declared a conflict of interest with manufacturer of photobiomodulation device, LumiThera. Three authors are employees of LumiThera. Trial investigators were contacted. Trial registration: NCT02725762 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how randomisation was conducted. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were masked to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The Valeda instrumentation was designed in such a way that user interface and outputs were identical for treatment and sham modalities. The operator used the machine under a cloth shield to prevent any accidental viewing of incidental light during treatment. A masked independent expert reviewed optical coherence tomography and fundus autofluorescence images. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants withdrew from the sham group: one after month 1 due to long commute and the other did not attend final follow up at month 12. An intention‐to‐treat analysis was performed for the primary outcome measure. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the clinical trials register and methods section were reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Guymer 2018 | Subthreshold nanosecond laser |
| Ivandic 2008 | Retrospective case series |
| Koev 2018 | Retrospective case series in mixed type age‐related macular degeneration |
| TORPA I 2012 | Non‐peer‐reviewed prospective case series |
| TORPA II 2016 | Prospective case series in non‐exudative age‐related macular degeneration |
Characteristics of ongoing studies [ordered by study ID]
NCT03859245.
| Study name | Photobiomodulation & Ketogenic Diet for Treatment of Mid‐periphery Retinal Disorders (Diabetic Retinopathy, Dry AMD, Hard Drusen Formation) for Alzheimer's Disease Prevention |
| Methods | Open label, randomised controlled trial, parallel group |
| Participants | Diabetic retinopathy, age‐related macular degeneration, mid‐peripheral drusen formation, diabetic macular oedema |
| Interventions | Experimental: photobiomodulation therapy, via Joovv red light/infrared LED device, three days/week, 20 minutes per session and a clinically prescribed ketogenic diet. Comparator: photobiomodulation therapy, via Joovv red light/infrared LED device, three days/week, 20 minutes per session. |
| Outcomes | Primary:
Secondary:
|
| Starting date | February 2019 |
| Contact information | Kelly Gibas kgibas@bristleconemedical.com Julie Gomer jgomer@bristleconemedical.com |
| Notes |
NCT03878420 (LIGHTSITE II).
| Study name | Study of Photobiomodulation to Treat Dry Age‐Related Macular Degeneration (LIGHTSITE II) |
| Methods | Double‐masked randomised sham‐controlled |
| Participants | Non‐exudative age‐related macular degeneration |
| Interventions | The Valeda™ Light Delivery System delivering 590, 660 and 850nm wavelengths together or the Valeda™ Light Delivery System delivering non‐effective treatment of the 590 and 660nm wavelengths together |
| Outcomes | Primary:
Secondary:
|
| Starting date | February 2019 |
| Contact information | Clark Tedford ctedford@lumithera.com |
| Notes |
NCT04065490 (LIGHTSITE III).
| Study name | Study to Assess the Safety and Efficacy of Photobiomodulation (PBM) in Subjects With Dry Age‐Related Macular Degeneration (AMD) (LIGHTSITE III) |
| Methods | Double‐masked, randomised, sham‐controlled, parallel group, multi‐centre |
| Participants | Non‐exudative age‐related macular degeneration |
| Interventions | Experimental: the Valeda™ Light Delivery System Comparator: the sham mode of the Valeda™ Light Delivery System |
| Outcomes | Primary:
Secondary:
Other:
|
| Starting date | September 2019 |
| Contact information | Clark Tedford ctedford@lumithera.com Cindy Croissant ccroissant@lumithera.com |
| Notes |
Differences between protocol and review
We could not conduct an intention‐to‐treat (ITT) analysis as ITT data was not available in the included trials. As there were fewer than 10 trials included in a meta‐analysis, assessment of publication bias was not possible (Chapter 8 of the Cochrane Handbook for Systematic Reviews of InterventionsHiggins 2011).
Contributions of authors
CH prepared the review draft and DS reviewed and edited the draft. CH prepared the protocol draft and DS reviewed and edited the draft.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledged financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
CH: holds a National Institute for Health Research (NIHR) Academic Clinical Fellowship Award. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. DS: has acted as a consultant to a number of companies unrelated to the subject matter of this review.
New
References
References to studies included in this review
ALIGHT {published data only}
- Robinson DG, Margrain TH, Dunn MJ, Bailey C, Binns AM. Low-level nighttime light therapy for age-related macular degeneration: a randomized clinical trial. Investigative Ophthalmology and Visual Science 2018;59(11):4531-41. [DOI] [PubMed] [Google Scholar]
LIGHTSITE I {published data only (unpublished sought but not used)}
- Markowitz SN, Devenyi RG, Munk MR, Croissant CL, Tedford SE, Rückert R, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 2020 Aug;40(8):1471-1482. [DOI: 10.1097/IAE.0000000000002632] [DOI] [PMC free article] [PubMed]
- Markowitz SN. LIGHTSITE1: a double-masked, randomized, sham-control study with photobiomodulation in dry AMD subjects. secure.aao.org/aao/meeting-archive 2018.
- Munk MR, Markowitz SN, Devenyi R, Croissant C, Tedford S, Ruckert R, et al. Final analysis of LIGHTSITE I: a double-masked, randomized, sham-controlled study with photobiomodulation in dry age-related macular degeneration subjects. Investigative Ophthalmology and Visual Science 2018;59(11):ARVO E-abstract 2415.
References to studies excluded from this review
Guymer 2018 {published data only}
- Guymer RH, Wu Z, Hodgson LA, Caruso E, Brassington KH, Tindill N, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration. The LEAD randomised controlled clinical trial. Ophthalmology 2019;126(6):829-38. [DOI] [PubMed] [Google Scholar]
Ivandic 2008 {published data only}
- Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomedicine and Laser Surgery 2008;26(3):241-5. [DOI] [PubMed] [Google Scholar]
Koev 2018 {published data only (unpublished sought but not used)}
- Koev K, Avramov L, Borissova E. Five-year follow-up of low-level laser therapy (LLLT) in patients with age-related macular degeneration (AMD). Journal of Physics: Conference Series 2018;992(1):012061. [DOI: ] [Google Scholar]
TORPA I 2012 {published data only}
- Merry G, Devenyi R, Dotson R, Markowitz SN, Reyes SV. Treatment of dry age-related macular degeneration with photobiomodulation. www.lumithera.com/wp-content/uploads/sites/7/TORPA-Clinical-Study.pdf (accessed 10 December 2018).
TORPA II 2016 {published data only}
- Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmologica 2016;95(4):e270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT03859245 {published data only}
- NCT03859245. Photobiomodulation and ketogenic diet for treatment of mid-periphery retinal disorders for Alzheimer's disease prevention. clinicaltrials.gov/ct2/show/NCT03859245 (first received 1 March 2019).
NCT03878420 (LIGHTSITE II) {published data only}
- NCT03878420. Study of photobiomodulation to treat dry age-related macular degeneration (LIGHTSITE II). clinicaltrials.gov/ct2/show/NCT03878420 (first received 18 March 2019).
NCT04065490 (LIGHTSITE III) {published data only}
- NCT04065490. Study of photobiomodulation to treat dry age-related macular degeneration (LIGHTSITE III). clinicaltrials.gov/ct2/show/NCT04065490 (first received 22 August 2019).
Additional references
Abramov 1996
- Abramov MV, Egorov EA. Dependence of the efficiency of low-intensity laser therapy in involution chorioretinal dystrophy on a used wavelength. Vestnik Oftalmologii 1996;112(2):25-6. [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arden 2010
- Arden GB, Gündüz MK, Kurtenbach A, Völker M, Zrenner E, Gündüz SB, et al. A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye 2010;24(7):1149–55. [DOI] [PubMed] [Google Scholar]
AREDS 2001
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report number 8. Archives of Ophthalmology 2001;119(10):1417-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begum 2013
- Begum R, Powner MB, Hudson N, Hogg C, Jeffery G. Treatment with 670 nm light up regulates cytochrome C oxidase expression and reduces inflammation in an age-related macular degeneration mode. PLoS One 2013;8(2):e57828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berdeaux 2005
- Berdeaux GH, Nordmann JP, Colin E, Arnould B. Vision-related quality of life in patients suffering from age-related macular degeneration. American Journal of Ophthalmology 2005;139(2):271-9. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 21 June 2020. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Fitzgerald 2013
- Fitzgerald M, Hodgetts S, Van Den Heuvel C, Natoli R, Hart NS, Valter K, et al. Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Reviews in Neuroscience 2013;24(2):205-26. [DOI] [PubMed] [Google Scholar]
Fuma 2015
- Fuma S, Murase H, Kuse Y, Tsuruma K, Shimazawa M, Hara H. Photobiomodulation with 670 nm light increased phagocytosis in human retinal pigment epithelial cells. Molecular Vision 2015;21:883-92. [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 22 June 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guymer 2014
- Guymer RH, Brassington KH, Dimitrov P, Makeyeva G, Plunkett M, Xia W, et al. Nanosecond-laser application in intermediate AMD: 12-month results of fundus appearance and macular function. Clinical and Experimental Ophthalmology 2014;42(5):466-79. [DOI] [PubMed] [Google Scholar]
Henein 2018
- Henein C, Steel DH. Photobiomodulation for non-exudative age-related macular degeneration. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD013029. [DOI: 10.1002/14651858.CD013029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editors. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781-8. [DOI] [PubMed] [Google Scholar]
James Lind Alliance 2013
- James Lind Alliance Sight Loss and Vision Priority Setting Partnership. Setting Priorities for Eye Research. www.sightlosspsp.org.uk 2018.
Jobling 2015
- Jobling AI, Guymer RH, Vessey KA, Greferath U, Mills SA, Brassington KH, et al. Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage. FASEB Journal 2015;29(2):696-710. [DOI] [PubMed] [Google Scholar]
Klein 2007
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology 2007;114(2):253-62. [DOI] [PubMed] [Google Scholar]
Kokkinopoulos 2013
- Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiology of Aging 2013;34(2):602-9. [DOI] [PubMed] [Google Scholar]
Krezel 2019
Li 2020
- Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis.. British Journal of Ophthalmology 2020;104(8):1077-84. [DOI] [PubMed] [Google Scholar]
Macular Society 2017
- Macular Society. Your guide to age-related macular degeneration. www.macularsociety.org/sites/default/files/resource/Macular Society Guide to AMD accessible pdf MS002 JUN17.pdf (accessed prior to 31 October 2017).
McKeague 2014
- McKeague C, Margrain TH, Bailey C, Binns AM. Low-level night-time light therapy for age-related macular degeneration (ALight): study protocol for a randomized controlled trial. Trials 2014;15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Low-level laser therapy for preventing or treating oral mucositis caused by radiotherapy or chemotherapy. www.nice.org.uk/guidance/ipg615 (accessed 10 December 2018).
Owsley 2006
- Owsley C, McGwin G Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Investigative Ophthalmology and Visual Science 2006;47(2):528-35. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RNIB 2009
- Minassian D, Reidy A, RNIB. Future sight loss in the decade 2010 to 2020: an epidemiological and economic model. www.rnib.org.uk/sites/default/files/FSUK_Report_2.doc (accessed prior to 31 October 2017).
Rodrigues 2016
- Rodrigues IA, Sprinkhuizen SM, Barthelmes D, Blumenkranz M, Cheung G, Haller J, et al. Defining a minimum set of standardized patient-centered outcome measures for macular degeneration. American Journal of Ophthalmology 2016;168:1-12. [DOI] [PubMed] [Google Scholar]
Rutar 2012
- Rutar M, Natoli R, Albarracin R, Valter K, Provis J. 670-nm light treatment reduces complement propagation following retinal degeneration. Journal of Neuroinflammation 2012;26(9):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanderson 2018
- Sanderson TH, Wider JM, Lee I. Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Scientific Reports 2018;8(1):article number 3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scilley 2002
- Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology 2002;109(7):1235-42. [DOI] [PubMed] [Google Scholar]
Sivapathasuntharam 2017
- Sivapathasuntharama C, Sivaprasad S, Hogg C, Jeffery G. Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiology of Aging 2017;52:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
WALT 2004
- World Association of Laser Therapy. Consensus agreement on the design and conduct of clinical studies with low level laser therapy and light therapy for musculoskeletal pain and disorders. Available at waltza.co.za/wp-content/uploads/2012/08/walt_standard_for_conduct_of_randomized_controlled_trials.pdf. [DOI] [PubMed]
Waugh 2018
- Waugh N, Loveman E, Colquitt J, Royle P, Yeong JL, Hoad G, et al. Treatments for dry age-related macular degeneration and Stargardt disease: a systematic review. Health Technology Assessment 2018;22(27):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2013
- Yu AK, Merrill KD, Truong SN, Forward KM, Morse LS, Telander DG. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Investigative Ophthalmology and Visual Science 2013;54(3):2216-24. [DOI] [PubMed] [Google Scholar]


