Abstract
Due to potential health risks, current recommendations are that individuals who wish to use hormone therapy to treat menopausal symptoms do so for the shortest period of time possible. In our investigation into how short-term use of estrogens in midlife following loss of ovarian function exerts long-term effects on female cognitive aging in rodents, we discovered a link between the ability of previous exposure to estradiol to enhance memory long-term and its ability to increase estrogen receptor (ER) α levels in the hippocampus, a brain area important for memory. Follow-up studies in model systems implicate a role for ERα in enhanced cognitive function independent of ovarian or exogenously administered estrogens. Results are consistent with clinical studies in which brain ERα levels in older women and men are related to cognitive functioning and risk of cognitive decline is associated with polymorphisms in the gene that transcribes ERα. Research in preclinical models reveals mechanisms through which ERα can be activated and affect cognition in the absence of ovarian estrogens including ligand-independent activation via insulin-like growth factor-1 signaling and activation by brain-derived neuroestrogens. This report reviews preclinical and clinical data that collectively point to the importance of ERα in cognition and highlights the need to differentiate the role of estrogen receptors from their classical ligands as we seek approaches to facilitate successful cognitive aging.
Keywords: Estrogen receptor α, estrogens, hippocampus, brain, cognition, aging, IGF-1, neuroestrogens, menopause, Alzheimer’s
The loss of ovarian hormones during menopause coincides with cognitive decline and increased risk of age-related dementias including Alzheimer’s Disease [1, 2]. Several decades of research provides convincing evidence that estrogens play a neuroprotective role in the brain [3, 4]. Thus, expectations were that menopausal hormone therapy would provide benefits to the brain and cognition. In fact, early reports indicated that estrogens used during or near menopause reduced the risk and severity of Alzheimer’s disease (AD) [5, 6]. However, results of the large Women’s Health Initiative Memory Study (WHIMS) conducted by the National Institutes of Health indicated that conjugated equine estrogens (CEE) therapy resulted in small long-term deficits in cognitive function, smaller brain volumes and increased risk of dementia [7, 8, 9]. Following these unexpected results, attention was focused on various factors related to experimental design including type of estrogen administered and tests used to assess cognition. Attention also focused on the importance of the timing of initiation of hormone therapy. In the WHIMS, hormone therapy was administered to women aged 65 years and older (mean age of 73) and well after ovarian hormone levels have declined at menopause. The critical period hypothesis, which proposes that cognitive benefits of estrogens may only be apparent if administered near the time of menopause, is viewed as a possible explanation for discrepant results across studies [10]. Although existing clinical data provide some support for the critical period hypothesis of estrogen effects in women [11], recent results of the Kronos Early Estrogen Prevention Study (KEEPS) indicate no harm, but also no benefit, of either oral CEE or transdermal 17β-estradiol (estradiol) in healthy recently postmenopausal women [12]. Ongoing clinical trials such as KEEPS should provide more insight as women enrolled in the study reach ages at which cognitive decline is more prevalent.
Following publication of the Women’s Health Initiative study results, long-term use of menopausal hormone therapy dropped significantly [13]. Furthermore, because of concerns regarding putative health risks [14, but see 15], the Food and Drug Administration (FDA) now recommends that women who wish to use hormone therapy to treat menopausal symptoms should limit its use for the shortest time period possible [16]. Because hormone therapy is now recommended to be used for only a few years near menopause, understanding if and how short-term exposure to estrogens exert long-term effects on the brain and cognitive aging trajectory has become critical.
In our own work investigating the long-term impacts of short-term use of estrogens on the brain and memory in a preclinical rodent model, we have identified an intriguing role for brain estrogen receptors, in the absence of ovarian or exogenous estrogens, in successful cognitive and brain aging. Consistent with experimental evidence are clinical studies that point to a potential relationship between brain estrogen receptor and cognitive aging. The current report provides an overview of the research from across the translational science spectrum that points to estrogen receptor (ER) α as a vital mediator of cognitive function during aging and highlights the importance of the translational link between preclinical and clinical research, in which results from each can inform questions asked by the other.
Lasting impact for cognition of short-term estrogen use in midlife: A role for ERα
Based on current recommendations that women use hormone therapy, if needed, for a short time, we employed a rodent model to empirically test whether short-term previous exposure to estrogens in middle-age had lasting impact on cognition. We found that in rodents, short-term treatment with estrogens in midlife produce similar benefits for memory to that of continuous estradiol treatment without the prolonged exposure to hormones (See Fig 1A). Rats that received 40 days of estradiol treatment (roughly comparable to 3.5 years in women) immediately following ovariectomy in midlife displayed enhanced performance on a hippocampal dependent radial-maze memory task up to seven months after hormone treatment had been terminated [17]. These rats previously treated with estradiol showed comparable performance on the radial-arm maze to animals continuously receiving estradiol treatment throughout the experiment, demonstrating that short-term exposure to estradiol near the start of loss of ovarian function can have similar cognitive benefits to ongoing estrogen use. More recently, similar results have been found in nonhuman primates. Ovariectomized rhesus monkeys that received 11 months of cyclic estradiol injections displayed enhanced memory when tested one year after hormone treatments had ended [18]. These results provide evidence across species that midlife estradiol use can impact the brain and cognition via mechanisms that persist long after hormone exposure is terminated. Identifying the long-term changes in the brain resulting from midlife estradiol use can therefore provide new avenues for combating age related cognitive decline that avoid traditional long-term hormone treatment.
Fig 1. Evidence that ERα acts in the brain to enhance female cognitive aging in the absence of ovarian or exogenously administered estrogens.
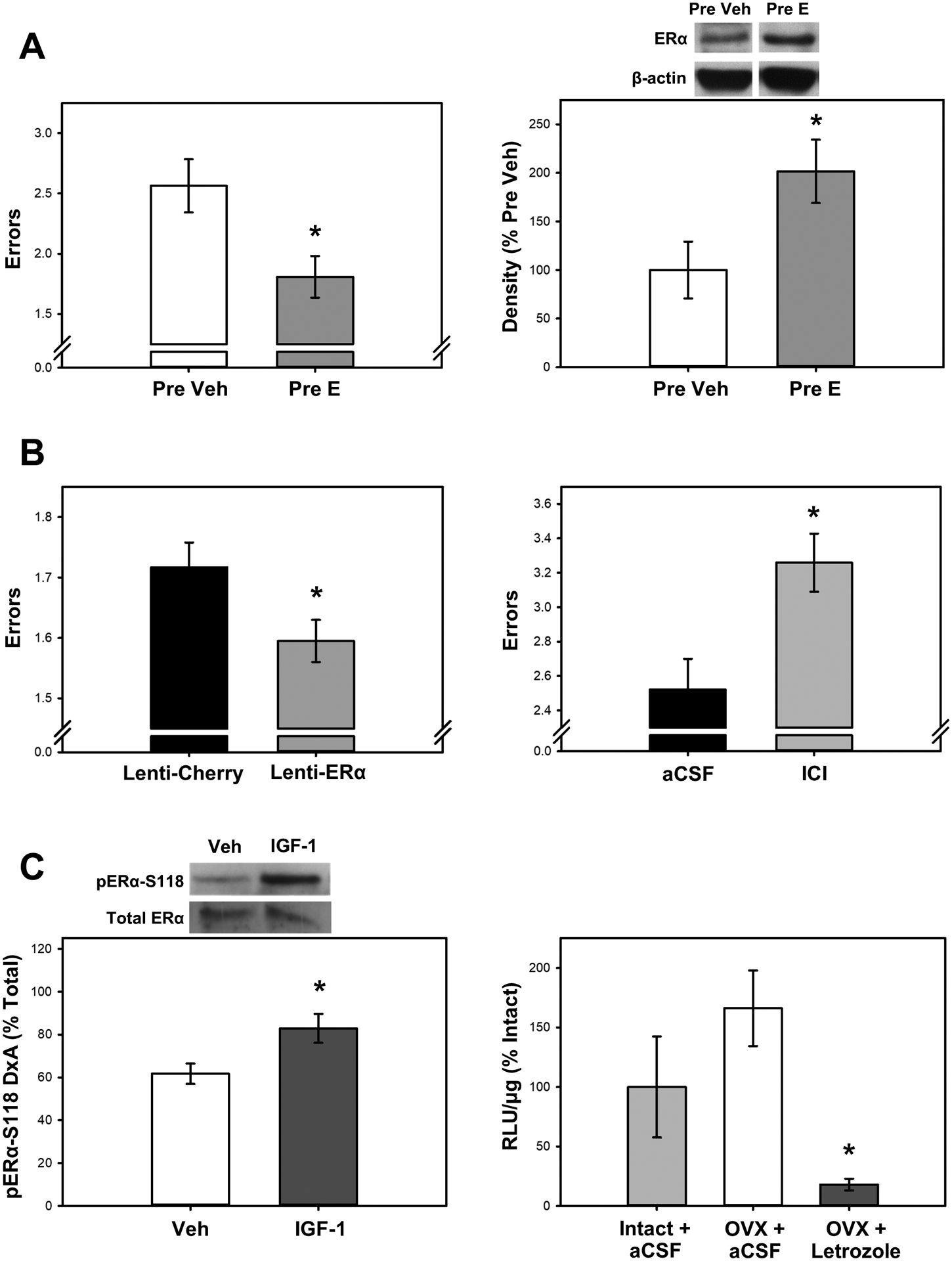
(A) Increased levels of brain ERα are associated with improved memory in the absence of ovarian or exogenous estrogens. Previous midlife estradiol treatment (Pre E) as compared to vehicle treatment (Pre Veh) in aging ovariectomized rats enhances memory (decreased errors) on a radial-arm maze task (left) and increases protein levels of ERα in the hippocampus as measured by western blotting (right) seven months after termination of estradiol treatment. (B) A causal relationship exists between increased levels or availability of brain ERα and enhanced memory in the absence of ovarian or exogenous estrogens. Overexpressing ERα in the hippocampus of aging ovariectomized rats via lenit-viral delivery (Lenti-ERα; left) enhances memory (decreased errors) on a radial-maze task as compared to a control virus (Lenti-Cherry). Antagonizing brain ER via intraventricular (icv) infusion of the ER antagonist ICI 182,780 (ICI; right) in aging ovariectomized rats impairs memory (increased errors) on the radial maze task as compared to infusion of aCSF vehicle. (C) In the absence of ovarian or exogenous estrogens, ERα can be activated by at least two mechanisms. Ligand-independent activation occurs by growth factor signaling (left) in which an icv infusion of insulin-like growth factor-1 (IGF-1) increases levels of phosphorylated ERα at S118 in the hippocampus of ovariectomized rats as compared to infusion of aCSF vehicle. Brain-derived neuroestrogens can activate the receptor (right) in which mice that were ovariectomized (OVX) 10 days prior had similar levels of hippocampal ER-dependent transcription (i.e. luciferase activity indicated by RLU/μg) as gonadally intact females. Chronic icv infusion of the aromatase inhibitor letrozole, which blocks brain estradiol synthesis, blocks ER-dependent transcription in the hippocampus. *p < .05; Adapted from [17, 20, 37, 45, 58].
In our exploration into how short-term exposure to estrogens can exert long-term impacts on the brain, we found that in parallel to effects on memory, previous exposure to estradiol in midlife results in increased levels of ERα, but not ERβ, in the hippocampus up to eight months after hormone treatment had ended [17]. Importantly, the effects of previous exposure to estradiol on ERα were identical to those of animals that received ongoing estradiol treatment. This lasting increase in ERα resulting from previous treatment with estradiol following loss of ovarian function has been replicated in several studies [19, 20]. In addition to the impact on hippocampal levels of ERα, previous estradiol treatment results in lasting increases in choline acetyltransferase (ChAT) expression [17, 19], phosphorylation of p42-MAPK [19], and estrogen receptor mediated transcriptional activity [21] in the hippocampus. The long-lasting elevation of ERα protein levels and downstream mediators of cognitive function in the hippocampus following previous exposure to estradiol in midlife provide mechanistic evidence for a role for ERα in enhancing cognition in the absence of ovarian hormones.
Impacts of ERα on cognitive and brain aging in the absence of ovarian or exogenously administered estrogens
Our findings in a preclinical rodent model of female cognitive aging of elevated brain ERα levels and lasting memory enhancements that persist long after termination of midlife estradiol treatment led us to examine the clinical literature for evidence of a role of ERα in cognitive aging in humans. An overview of that literature is provided in Table 1.
Table 1.
Overview of results of clinical studies investigating the relationship between ERα and pathological and non-pathological age-related cognitive decline
| Relationship | Authors | Reported Population | Measures | Summary of findings | PMID/doi |
|---|---|---|---|---|---|
| Studies supporting an association between ERα/Esr1 and cognitive outcomes | Bojar et al. 2016 | Middle-aged women without dementia | Cognitive tests; E2 levels; Esr1 polymorphisms | Esr1 polymorphism modulated the effect of E2 on cognitive function | PMID: 27680398 |
| Cheng et al. 2014 | Meta-analysis of other studies | Esr1 SNPs Pvull and Xbal; AD risk | Esr1 Pvull SNP significantly increases risk of AD in Caucasian, but not Asian women | PMID: 25061285 | |
| Corbo et al. 2006 | AD patients and age-matched controls (men and women) | Esr1 SNPs Pvull and Xbal; APOE plasma levels | Esr1 SNPs Pvull and Xball increased risk of AD in men only; in women those SNPs increased rate of cognitive decline | PMID: 16699281 | |
| Hu et al. 2003 | AD patients and age-matched controls (women) | ERα localization in hippocampus | Decreased nuclear ERα staining in CA1 and CA2 of AD brains | PMID: 12819990 | |
| Ishunina et al. 2007 | AD patients and age matched controls (women) | Esr1 mRNA and aromatase activity in hippocampus | Decreased Esr1 mRNA (normal and splice variant) and decreased aromatase expression in AD hippocampi | PMID: 17010478 | |
| Kelly et al. 2008 | AD patients (men and women) | Cognitive tests; ERα protein levels | Increased nuclear ERα in frontal cortex associated with better cognitive performance | PMID: 18288931 | |
| Ma et al. 2009 | AD patients and age-matched controls (men and women) | Esr1 SNPs; AD risk | Several Esr1 SNPs associated with AD risk and age of onset | PMID: 19586561 | |
| Ma et al. 2014 | Elderly men and women | Esr1 SNPs; Cognitive tests | Several Esr1 polymorphisms associated with cognitive decline | PMID: 23567436 | |
| Pinkas et al. 2018 | Post-menopausal women | Esr1 and APOE polymorphisms; cognitive tests | Interactive effects with certain Esr1 and APOE polymorphisms on memory | doi.org/10.5114/aoms.2018.72972 | |
| Ryan et al. 2014 | Elderly men and women | Esr1 polymorphisms; Risk of AD and dementia | Esr1 polymorphism slightly assoc. with increased AD risk in women, but Esr1 polymorphism + APOE ε4 allele increased risk of AD | PMID: 23491264 | |
| Yaffe et al 2002 | Elderly women | Cognitive tests; Esr1 SNPs | Esr1 SNPs increase risk of cognitive impairment, independent of estrogen use | PMID: 11955468 | |
| Yaffe et al. 2009 | Elderly men and women without dementia | Cognitive tests; Esr1 and Esr2 SNPs | Esr1 and Esr2 SNPs increased risk of cognitive impairment | PMID: 17889406 | |
| Studies not supporting an association between ERα/Esr1 and cognitive outcomes | Fehsel et al. 2016 | Elderly women without dementia | Cognitive tests; Esr1, Esr2, APOE SNPs | No association of Esr1 SNPs with cognitive effects but did see effects for Esr2 and some interaction of Esr2 with APOE SNPs | PMID: 27629499 |
| Goumidi et al. 2011 | AD patients and age-matched controls (men and women) | Esr1 and Esr2 SNPs; late onset AD risk | No association of Esr1 or Esr2 SNPs with late onset AD risk | PMID: 21673408 | |
| Ryan et al. 2013 | Elderly women without dementia | Cognitive tests; Esr1 and Esr2 polymorphisms | Five common Esr1 polymorphisms not associated with cognitive impairment, but some effect of Esr2 polymorphisms | PMID: 23932494 |
In brief summary, similar to what we found in our preclinical model, levels of ERα in brain areas important for cognition are associated with cognitive and/or brain function in humans. For example, in both men and women with Alzheimer’s disease (AD), increased levels of the full-length 66-kD isoform of ERα in the frontal cortex is associated with better performance on cognitive tests [22], suggesting that levels of ERα in the brain may modulate cognition in AD patients. Additionally, nuclear expression of ERα protein levels in the CA1 and CA2 regions of the hippocampus is decreased in brains of women with AD as compared to those of age-matched controls [23]. Similarly, mRNA expression of Esr1, the gene that encodes for ERα, is decreased in the brains of women with AD as compared to those of age-matched controls [24]. Further supporting a role for ERα in cognitive aging are results that implicate polymorphisms in Esr1 in both pathological and non-pathological cognitive decline. For example, there are several reports of an increased risk of AD in men and women with certain Esr1 polymorphisms [25, 26, 27], although not all studies agree on which specific Esr1 polymorphisms are relevant. Additionally, Esr1 polymorphisms may act as effect modifiers for risk of AD in carriers of the APOE ε4 allele [28] and interact with APOE polymorphisms to impact cognition [29]. However, in contrast to these results are data that show no association between estrogen receptor genotype and susceptibility to late-onset AD [30]. In addition to potential effects on risk of AD, Esr1 polymorphisms have been implicated in non-pathological cognitive decline [31, 32]. Interestingly, the increased risk of cognitive decline associated with Esr1 polymorphisms in women was found to be independent of menopausal estrogen use [33]. However, certain polymorphisms can modulate effect of estrogens on cognitive function [34], suggesting that variations in ERα expression might affect the efficacy of menopausal hormone treatments. Not all reports support the association between Esr1 polymorphisms and cognitive decline. For example, in elderly women without dementia associations were found between cognition and polymorphisms in Esr2, the gene that encodes for ERβ, but for not Esr1 polymorphisms [35, 36].
Although a more thorough and critical review of the clinical data related to the association of estrogen receptor and cognitive aging is warranted, collectively current data suggest a potential role for brain ERα in successful brain and cognitive aging in humans. To test for a causal relationship of this association, we turned to our preclinical model.
Experimental evidence implicating brain ERα as a mediator of cognitive function in the absence of ovarian estrogens
To determine if ERα can directly impact cognitive function in aged females following the loss of ovarian function, we used two approaches – increasing and decreasing availability of brain ERα in aging ovariectomized rats and assessing effects on memory (See Fig 1B). We first overexpressed ERα in the hippocampus of aged ovariectomized females using lentiviral delivery of the gene for the protein (lenti-ERα) [37]. Aged females that received lenti-ERα outperformed rats that received a control virus on a hippocampus-dependent spatial memory task. In a second experiment, we blocked brain ERs in ovariectomized aging females that had previously undergone midlife estradiol treatment [20]. Rats received chronic intracerebroventricular infusion via osmotic minipumps of the ER antagonist, ICI 182,780 (ICI) or aCSF vehicle beginning after estradiol treatment was terminated. Rats treated with ICI had significantly worse memory on a radial-maze task than controls. Results reveal that increased levels or availability of ERs in the hippocampus lead to enhanced memory in the absence of ovarian or ongoing administration of estrogens. They provide support for the hypothesis that treatments that increase or maintain levels of ERα in aging females help to improve or maintain memory processes even in the absence of ovarian or exogenously administered estrogens.
In addition to its direct effects on memory, ERα plays a neuroprotective role in the brain. For instance, increased hippocampal ERα expression protects female neonatal mice following hypoxic ischemia [38], a time point before ovarian estrogen production begins but at which ERα can have a lasting impact on cognitive function through adulthood [39]. This finding is particularly interesting considering the several studies that have shown the neuroprotective impacts of estrogens acting specifically through ERα following stroke in the aging brain [40, 41]. The protective functions of ERα have also been demonstrated in other brain regions and as well as in males. In the nucleus accumbens, stress results in decreased levels of ERα and overexpression of ERα promotes resilience against chronic social defeat stress through transcriptional changes in both male and female mice [42].
Together, data from preclinical models indicates an important role for estrogen receptors—independent of ovarian estrogens—in maintaining cognitive health across the lifespan.
Mechanisms by which brain ERα can be activated in the absence of circulating estrogens
In order for ERα to continue to impact cognition after loss of ovarian function, it must be activated by mechanisms independent of ovarian estrogens. Based on work from our lab and others, we propose two mechanisms for sustained activation of ERα in the absence of ovarian estrogens: ligand-independent activation of ERα by growth factors including insulin-like growth factor-1 (IGF-1) and activation of the receptor by locally synthesized neuroestrogens (See Fig 1C).
Ligand-independent activation by IGF-1
Activation of estrogen receptor-dependent transcriptional activity in the absence of estrogens by growth factor was shown years ago in vitro [43]. Specifically, insulin-like growth factor-1 (IGF-1) is able to activate ERα through activation of the MAPK pathway resulting in phosphorylation of ERα at S118 [44]. More recently, work from our lab demonstrated a similar effect in vivo [45]. Acute infusion of IGF-1 to the lateral ventricle of ovariectomized rats results in increased levels of pS118-ERα in the hippocampus one hour after treatment, and increased levels of total ERα 24 hours after treatment Therefore, ligand-independent activation of ERα by IGF-1 provides a potential mechanism through which increased expression of ERα can enhance cognition in the absence of ovarian estrogens.
IGF-1 is a peptide hormone that acts as the major effector of the pituitary hormone growth hormone (GH). It is structurally very similar to insulin and can bind to insulin receptors in addition to its own IGF-1 receptor [46]. IGF-1 is necessary for neuronal development and regulates plasticity in the developing brain [47]. Because of the critical role it plays in development, levels of IGF-1 change over the lifespan and ultimately decline in the brain with aging [48]. Similar to the decline in estrogens following the loss of ovarian function, this decrease in IGF-1 coincides with increased risk of cognitive decline.
Studies in humans implicate IGF-1 signaling dysfunction in the pathology of age-related dementias including AD, although much debate remains about whether increased IGF-1 levels would be beneficial in combatting these disorders. For instance, IGF-1R expression levels were found to be decreased in the brains of patients with AD as compared to those of controls [49]. On the other hand, work in animal models suggests that increased IGF-1R signaling contributes to the pathological formation of proteins associated with AD [50]. Dysfunction in IGF-1 and insulin signaling likely plays a role in the pathology of Alzheimer’s, although the specific mechanisms remain unclear.
Studies in model systems have demonstrated that the effects of IGF-1 signaling in the brain overlap greatly with the impacts of estrogen receptor signaling and indicate that these two systems closely interact. Most neurons and some astrocytes in the rat brain that express IGF-1R also express either ERα or ERβ, with particularly prevalent colocalization of IGF-1R and ERα in hippocampal neurons [51]. The interaction between these receptors goes beyond simply colocalization, however. ERα and IGF-1R have been shown to form estradiol dependent protein complexes in the brains of ovariectomized rats [52].
These interactions between ERα and IGF-1R are important for maintaining cognition in the aging brain. In ovariectomized rats treated with continuous exposure to estradiol, antagonizing IGF-1R with JB1 directed to the lateral ventricle blocked the ability of estradiol to enhance performance on the radial-arm maze and increase hippocampal expression of synaptic proteins PSD-95 and spinophilin [53]. Furthermore, JB1 blocks the memory enhancements and increased hippocampal expression of ERα, ChAT, and phosphorylated p42-MAPK in ovariectomized rats previously treated to estradiol during midlife [19]. These findings demonstrate that IGF-1R modulates the ability of ERα to impact memory and hippocampal function in both the presence and absence of circulating estrogens.
Neuroestrogen activation of ERα
In addition to ligand-independent activation, following loss of ovarian function, estrogen receptors may be activated by locally synthesized estradiol. These brain-derived neuroestrogens are capable of being synthesized throughout the human brain—including in the hippocampus, cerebral cortex, hypothalamus, and amygdala, among others—as indicated by widespread expression of aromatase, the enzyme that synthesizes estradiol from testosterone [54]. Because aromatase inhibitors are used in breast cancer treatments postmenopause, there are clinical insights into the role of neuroestrogens in cognition—presumably due to activation of estrogen receptors—in the absence of ovarian estrogens. Many, but not all, studies demonstrate that the use of aromatase inhibitors can negatively impact cognitive function in humans [for meta-analysis, see 55]. Interestingly, the use of aromatase inhibitors is associated with decreased hippocampal activity and impaired memory function in postmenopausal women [56]. However, the lack of randomized clinical trials investigating the role of aromatase inhibitors in cognitive function limits the ability to draw conclusions as to the role of neuroestrogens in estrogen receptor function in the absence of ovarian estrogens.
Preclinical model systems have been used to empirically test the effects of aromatase inhibitors on hippocampal memory and estrogen receptor function. Chronic systemic administration of the aromatase inhibitor letrozole, which blocks estradiol synthesis including neuroestrogens, was recently shown to impair spatial memory performance in male and female gonadectomized marmosets [57]. Additionally, we recently showed in a mouse model that neuroestrogens are able to impact estrogen receptor-dependent transcription in the brain for a short-term, but not a long-term following ovariectomy [58]. Further work is necessary to fully elucidate the long-term role of neuroestrogens in mediating estrogen receptor-dependent activity in the brain after loss of ovarian function. Interestingly, recent findings from our lab a potential interaction between neuroestrogens and IGF-1 in sustaining ERα activity in the absence of ovarian estrogens [59].
Conclusion
Across multiple species and paradigms, an increasing body of literature suggests an important role for ERα in maintaining cognitive function in aging females in the absence of ovarian or exogenously administered estrogens. Clinical studies have revealed that variability in expression of ERα in older women (and men) is associated with variability in risk of cognitive impairment. Ongoing work in preclinical model systems is providing valuable insight into the mechanisms through which ERα enhances memory in aging females. Moving forward, identification of factors that contribute to variability in levels of brain ERα during aging, likely including previous use of menopausal estrogen therapy in midlife and perhaps environmental factors such as stress [42], may lead to novel approaches to cognitive aging that capitalize on the potential of ERα to positively impact the aging brain.
Source of funding
Work described in this review that was conducted in our laboratory was supported by the National Institute on Aging under Grant RF1AG041374.
Footnotes
Conflict of interest - The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Pike CJ. Sex and the development of Alzheimer’s disease. Journal of neuroscience research. 2017. January 2;95(1–2):671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheyer O, Rahman A, Hristov H, et al. Female Sex and Alzheimer’s Risk: The Menopause Connection. The journal of prevention of Alzheimer’s disease. 2018;5(4):225–230. doi: 10.14283/jpad.2018.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler-Chiurazzi EB, Singh M, Simpkins JW. From the 90’s to now: A brief historical perspective on more than two decades of estrogen neuroprotection. Brain Res. 2016. February 15;1633:96–100. doi: 10.1016/j.brainres.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luine V, Frankfurt M. Estrogenic regulation of memory: The first 50 years. Horm Behav. 2020. May;121:104711. doi: 10.1016/j.yhbeh.2020.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. [DOI] [PubMed] [Google Scholar]
- 6.Tang M, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. [DOI] [PubMed] [Google Scholar]
- 7.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004. June 23;291(24):2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 8.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004. June 23;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 9.Coker LH, Espeland MA, Hogan PE, et al. Change in brain and lesion volumes after CEE therapies: the WHIMS-MRI studies. Neurology. 2014. February 4;82(5):427–34. doi: 10.1212/wnl.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. The Lancet Neurology. 2005. March;4(3):190–4. doi: 10.1016/s1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- 11.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013. June;20(6):695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller VM, Naftolin F, Asthana S, et al. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause. 2019. September;26(9):1071–1084. doi: 10.1097/gme.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo RA. Where are we 10 years after the Women’s Health Initiative? The Journal of clinical endocrinology and metabolism. 2013. May;98(5):1771–80. doi: 10.1210/jc.2012-4070. [DOI] [PubMed] [Google Scholar]
- 14.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med. 2006. May 8;166(9):1027–32. doi: 10.1001/archinte.166.9.1027. [DOI] [PubMed] [Google Scholar]
- 15.Harman SM, Vittinghoff E, Brinton EA, et al. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. The American journal of medicine. 2011. March;124(3):199–205. doi: 10.1016/j.amjmed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIoA. Hot Flashes: What Can I Do? : National Institute of Health; 2017. [Google Scholar]
- 17.Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010. March;151(3):1194–203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- 18.Baxter M, Santistevan A, Bliss-Moreau E, et al. Timing of cyclic estradiol treatment differentially affects cognition in aged female rhesus monkeys. Beh Neurosci. 2018;132(4):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witty CF, Gardella LP, Perez MC, et al. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013. February;154(2):842–52. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- 20.Black KL, Witty CF, Daniel JM. Previous midlife oestradiol treatment results in long-term maintenance of hippocampal oestrogen receptor alpha levels in ovariectomised rats: mechanisms and implications for memory. Journal of neuroendocrinology. 2016. October;28: doi: 10.1111/jne.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollard KJ, Wartman HD, Daniel JM. Previous estradiol treatment in ovariectomized mice provides lasting enhancement of memory and brain estrogen receptor activity. Horm Behav. 2018. June;102:76–84. doi: 10.1016/j.yhbeh.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly JF, Bienias JL, Shah A, et al. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer’s disease: relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008. February;5(1):45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Qin S, Lu Y, et al. Decreased estrogen receptor-alpha expression in hippocampal neurons in relation to hyperphosphorylated tau in Alzheimer patients. Acta Neuropathol. 2003;106(3):213–20. [DOI] [PubMed] [Google Scholar]
- 24.Ishunina T, Fischer D, Swaab D. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiology of aging. 2007;28:11. [DOI] [PubMed] [Google Scholar]
- 25.Ma S, Tang N, Tam C, et al. Polymorphisms of the estrogen receptor alpha (ESR1) gene and the risk of Alzheimer’s disease in a southern Chinese community. Int Psychogeriatr. 2009;21(5):977–86. [DOI] [PubMed] [Google Scholar]
- 26.Corbo RM, Gambina G, Ruggeri M, et al. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer’s disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- 27.Cheng D, Liang B, Hao Y, et al. Estrogen receptor α gene polymorphisms and risk of Alzheimer’s disease: evidence from a meta-analysis. Clin Interv Aging. 2014;9:1031–8. doi: 10.2147/cia.S65921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan J, Carriere I, Carcaillon L, et al. Estrogen receptor polymorphisms and incident dementia: the prospective 3C study. Alzheimers Dement. 2014. January;10(1):27–35. doi: 10.1016/j.jalz.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Pinkas J, Bojar I, Gujski M, et al. Effect of interactions between APOE and ESR1 polymorphisms on cognitive functions in postmenopausal women. Archives of Medical Science. 2018. doi: 10.5114/aoms.2018.72972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goumidi L, Dahlman-Wright K, Tapia-Paez I, et al. Study of estrogen receptor-α and receptor-β gene polymorphisms on Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2011;26(3):431–9. [DOI] [PubMed] [Google Scholar]
- 31.Yaffe K, Lindquist K, Sen S, et al. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiology of aging. 2009. April;30(4):607–14. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma SL, Tang NL, Leung GT, et al. Estrogen receptor α polymorphisms and the risk of cognitive decline: A 2-year follow-up study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014. May;22(5):489–98. doi: 10.1016/j.jagp.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Lui LY, Grady D, et al. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002. April 15;51(8):677–82. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- 34.Bojar I, Pinkas J, Wierzbińska-Stępniak A, et al. Cognitive Functions, Concentration of Endogenous Estradiol, Estrogen Receptor α (ERα) Polymorphism in Postmenopausal Women. Med Sci Monit. 2016. September 28;22:3469–3478. doi: 10.12659/msm.901247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fehsel K, Schikowski T, Jänner M, et al. Estrogen receptor beta polymorphisms and cognitive performance in women: associations and modifications by genetic and environmental influences. J Neural Transm (Vienna). 2016;123(12):1369–1379. [DOI] [PubMed] [Google Scholar]
- 36.Ryan J, Carrière I, Amieva H, et al. Prospective analysis of the association between estrogen receptor gene variants and the risk of cognitive decline in elderly women. Eur Neuropsychopharmacol. 2013;23(12):1763–8. [DOI] [PubMed] [Google Scholar]
- 37.Witty CF, Foster TC, Semple-Rowland SL, et al. Increasing hippocampal estrogen receptor alpha levels via viral vectors increases MAP kinase activation and enhances memory in aging rats in the absence of ovarian estrogens. PloS one. 2012;7(12):e51385. doi: 10.1371/journal.pone.0051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cikla U, Chanana V, Kintner DB, et al. ERα Signaling Is Required for TrkB-Mediated Hippocampal Neuroprotection in Female Neonatal Mice after Hypoxic Ischemic Encephalopathy(1,2,3). eNeuro. 2016. Jan-Feb;3(1). doi: 10.1523/eneuro.0025-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafer D, Aycan N, Ozaydin B, et al. Sex differences in Hippocampal Memory and Learning following Neonatal Brain Injury: Is There a Role for Estrogen Receptor-α? Neuroendocrinology. 2019;109(3):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubal DB, Rau SW, Shughrue PJ, et al. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006. June;147(6):3076–84. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 41.Dubal DB, Zhu H, Yu J, et al. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2001. February 13;98(4):1952–7. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorsch Z, Loh Y, Purushothaman I, et al. Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat Commun. 2018;9(1):1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Z, S antagati S, Patrone C, et al. Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human neuroblastoma cell line SK-ER3. Molecular endocrinology (Baltimore, Md). 1994;8(7):910–8. [DOI] [PubMed] [Google Scholar]
- 44.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science (New York, NY). 1995. December 1;270(5241):1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 45.Grissom EM, Daniel JM. Evidence for ligand-independent activation of hippocampal estrogen receptor-α by IGF-1 in hippocampus of ovariectomized rats. Endocrinology. 2016;157(8):3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo VC, Gluckman PD, Feldman EL, et al. The insulin-like growth factor system and its pleiotropic functions in brain. Endocrine reviews. 2005. December;26(7):916–43. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- 47.Arsenijevic Y, Weiss S, Schneider B, et al. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(18):7194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonntag W, Lynch C, Bennett S, et al. Alterations in insulin-like growth factor-1 gene and protein expression and type 1 insulin-like growth factor receptors in the brains of ageing rats. Neuroscience. 1999;88(1):269–79. [DOI] [PubMed] [Google Scholar]
- 49.Moloney A, Griffin R, Timmons S, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 2010;31(2):224–43. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill C, Kiely AP, Coakley MF, et al. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer’s disease. Biochem Soc Trans. 2012. August;40(4):721–7. doi: 10.1042/bst20120080. [DOI] [PubMed] [Google Scholar]
- 51.Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99(4):751–60. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 52.Mendez P, Azcoitia I, Garcia-Segura L. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112(1–2):170–6. [DOI] [PubMed] [Google Scholar]
- 53.Nelson BS, Springer RC, Daniel JM. Antagonism of brain insulin-like growth factor-1 receptors blocks estradiol effects on memory and levels of hippocampal synaptic proteins in ovariectomized rats. Psychopharmacology (Berl). 2014. March;231(5):899–907. doi: 10.1007/s00213-013-3310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azcoitia I, Yague J, Garcia-Segur a L. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–147. [DOI] [PubMed] [Google Scholar]
- 55.Lee PE, Tierney MC, Wu W, et al. Endocrine treatment-associated cognitive impairment in breast cancer survivors: evidence from published studies. Breast Cancer Research and Treatment. 2016;158(3):407–420. [DOI] [PubMed] [Google Scholar]
- 56.Bayer J, Rune G, Schultz H, et al. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology. 2015;56:213–25. doi: 10.1016/j.psyneuen.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Gervais N, Remage-Healey L, Starrett J, et al. Adverse Effects of Aromatase Inhibition on the Brain and Behavior in a Nonhuman Primate. J Neurosci. 2019;39(5):918–928. doi: 10.1523/jneurosci.0353-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgartner NE, Grissom EM, Pollard KJ, et al. Neuroestrogen-Dependent Transcriptional Activity in the Brains of ERE-Luciferase Reporter Mice following Short- and Long-Term Ovariectomy. eNeuro. 2019. Sep-Oct;6(5). doi: 10.1523/eneuro.0275-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollard KJ, Daniel JM. Nuclear estrogen receptor activation by insulin-like growth factor-1 in Neuro-2A neuroblastoma cells requires endogenous estrogen synthesis and is mediated by mutually repressive MAPK and PI3K cascades. Mol Cell Endocrinol. 2019. June 15;490:68–79. doi: 10.1016/j.mce.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


