Abstract
Intolerance of uncertainty and worry about future events are cardinal features of anxiety. However, the neurobiological and physiological mechanisms underlying these characteristics of anxiety remain to be fully elucidated. Individuals with diagnosed anxiety disorders (n=29, 22 female) and age-matched comparison subjects (n=28, 17 female) completed a task in which pictures (aversive or neutral content) were preceded by cues indicating certainty or uncertainty about the emotional valence of the subsequent pictures. We assessed fMRI and heart rate activity with respect to: (1) the cue period, (2) the emotional valence of the pictures, and (3) the modulatory effect of uncertainty on responses to subsequent pictures. Individuals with anxiety disorders and comparison subjects exhibited similar fMRI and cardiac activity during the cue period and for the aversive vs. neutral picture contrast. However, individuals with anxiety disorders exhibited greater modulatory effects of uncertainty on their responses to subsequent pictures. Specifically, they displayed greater fMRI activity in a number of cortical regions (visual cortex, anterior cingulate cortex, superior temporal gyrus, and anterior insula), as well as significantly reduced cardiac deceleration, to pictures preceded by the uncertainty cue. These findings suggest that heightened neural and autonomic reactivity to stimuli during conditions of uncertainty may be a key psychobiological mechanism of anxiety.
Keywords: Anxiety, fMRI, heart rate, uncertainty, affective neuroscience
Introduction
Anxiety disorders are the most common form of psychopathology, with a lifetime prevalence of approximately 30% in the United States (1). The total annual cost of anxiety disorders has been estimated to be around $40 billion (2, 3). These costs are mostly attributed to morbidity, mortality, lost productivity, and other indirect costs (4). Most individuals with anxiety disorders do not present with a single disorder. There is high comorbidity between anxiety disorders and other anxiety disorders, depressive disorders, substance use disorders, and/or personality disorders (5). Individuals with anxiety disorders have suboptimal rates of recovery (~58% over a 12-year period for individuals receiving treatment) and in cases where patients do recover, there are significant recurrence rates (45%) (6, 7). Both cognitive-behavioral psychotherapy (CBT) and psychopharmacological treatments have been found to be effective in the treatment of anxiety (8), but symptom reduction and remission remains a problem.
One avenue of research that may enhance efficacy of anxiety diagnosis and treatment is elucidating the neurobiological and psychological mechanisms underlying anxiety. With a deeper understanding of these psychobiological mechanisms, we can hopefully increase our ability to assess and treat anxiety disorders effectively and efficiently. For example, the identification of reliable neuroimaging or physiological markers could be used to develop more objective diagnoses including psychobiological subtypes; predict course of illness or the efficacy of different treatment options; and identify potential anatomical targets for interventions such as neurostimulation techniques. In this study, we use a combination of functional neuroimaging and peripheral physiology measures to probe three putative neuropsychological mechanisms underlying anxiety disorders.
One psychological feature that is thought to play a central role in anxiety is intolerance of uncertainty (IU)—the distress from the possibility that negative events may occur unpredictably (9–11). It has been suggested that a common feature across anxiety disorders is aberrant and excessive anticipatory responding under conditions of threat uncertainty (9). Prior work has also suggested that IU is a broad specifier for worry, which is a cognitive strategy that individuals use in an attempt to control the unknown (12, 13). Moreover, the network of brain regions that becomes active during the anticipation of uncertain outcomes (e.g., anterior insula, amygdala, ventromedial prefrontal cortex (vmPFC), and anterior cingulate cortex (ACC) (14–18) has also been widely implicated in the pathogenesis of anxiety disorders (9, 19–23).
A second feature that may play a role in anxiety is the intensity of emotional responses to negative events. According to the emotion dysregulation model of anxiety (24), individuals with anxiety experience heightened intensity of emotions, poor understanding of their emotions, and deficient emotion regulation. It has been suggested that individuals with anxiety have emotional responses that occur more easily, quickly, and intensely than those without anxiety (25). Additionally, previous studies have shown that individuals with anxiety display maladaptive peripheral physiological responses during emotional contexts (i.e., reduced heart rate variability (26, 27) and reduced cardiac deceleration, indicating a shift to cardiac acceleration and potentiated, defensive responding (28)). Despite these theories suggesting emotional hyper-reactivity in anxiety disorders, evidence from prior neuroimaging studies examining the neurobiological correlates of emotional hyper-reactivity in anxiety disorders remains mixed (29–31).
A third feature that may play a role in anxiety disorders is the modulation of emotional responses by uncertainty. Clinically, individuals with anxiety often worry about the possibility of an aversive stimulus, which can lead to an exaggerated emotional response regardless of whether the aversive stimulus is present or not. Previous research has shown that, in healthy subjects, aversive events elicit heightened subjective and physiological responses when preceded by uncertainty (14, 32–34). Previous research has also shown that exposure to unpredictable neutral tones elicits greater amygdala activity and anxious behavior than predictably timed tones, indicating that uncertainty can modulate subsequent stimulus-evoked responses (35). However, to date, no research has examined the effect of uncertainty on subsequent stimulus response in a population of individuals with anxiety disorders.
In the current study, we addressed this empirical gap through an application of fMRI and heart rate responses in individuals with anxiety disorders. Our aim was to examine neural and cardiac activity during uncertainty and in response to emotional stimuli separately, as well as the effect of uncertainty on subsequent responses to visual stimuli. To address these aims, we used a paradigm previously shown to elicit neural response to aversive pictures and uncertain cues in healthy subjects (14). Specifically, we hypothesized that, for individuals with anxiety disorders as compared to comparison subjects: (1) uncertainty during the anticipation of aversive or neutral stimuli would be associated with greater activity in anterior insula and amygdala, as well as potentiated cardiac response (i.e., reduced heart rate deceleration); (2) viewing aversive relative to neutral images would be associated with greater activity in amygdala, less activity in vmPFC, and potentiated cardiac response; and (3) the modulatory effect of uncertainty on responses to subsequent stimuli would be associated with greater activity in anterior insula and amygdala and potentiated cardiac response.
Materials and Methods
Participants
This study was approved by the University of Wisconsin-Madison Institutional Review Board; all subjects provided written informed consent. Psychiatric diagnoses were based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), through the structured clinical interview for DSM-5 (SCID) (36). Subjects were eligible to be included in the individuals with anxiety group (n=34) if they were 18 years or older, had a primary diagnosis of GAD (based on DSM-5 criteria), and were free of psychotropic medications for six weeks prior to study enrollment. Exclusion criteria were history of significant brain injury, neurological disorder, psychosis, bipolar disorder, post-traumatic stress disorder, or moderate or severe substance use disorder. Additionally, n=34 healthy comparison (HC) subjects with no history of brain injury, neurologic or psychiatric illness, current use of psychoactive medication, or history of psychotherapy treatment were recruited. n=6 participants from the HC group and n=5 from the anxiety group were removed from the analysis due to excessive motion during the experiment (greater than 10% of volumes censored), resulting in a final sample of n=57 (n=28 HC and n=29 anxiety). Due to the high rate of comorbidity among anxiety disorders (1), comorbid mood and anxiety disorders were permitted. In the individuals with anxiety disorders group, n=6 participants had comorbid major depression, n=14 had social anxiety disorder, n=5 had obsessive-compulsive disorder, n=9 had phobic disorder, and n=17 had panic disorder. Demographic and neuropsychological data for the anxiety and HC groups are summarized in Supplementary Table 1 and 2.
Experimental Design
Before scanning, subjects were informed of all cue-picture contingencies and complete a practice task consisting of 16 unique trials (4 per cue-picture pair) to ensure task comprehension. During the fMRI task, which was adapted from previous studies (14, 37–39), subjects viewed 64 unique images drawn from the International Affective Picture System (40), divided evenly among pictures with aversive or neutral content. Aversive stimuli consisted of 32 aversive/unpleasant and arousing images, based on published norms (40, 41). Neutral stimuli consisted of 32 images with neutral valence and low arousal ratings. Further information about the stimuli used can be found in Supplementary Table 3. All images were preceded by one of three visual cues (“X,” “O,” or “?”). The “X” and “O” cues indicated that the subsequent image would be aversive or neutral, respectively, whereas the “?” cue provided no information regarding the emotional content of the image (equal likelihood of aversive or neutral content). Thus, aversive (“X”) and neutral (“O”) cues predicted certain outcomes, whereas ambiguous (“?”) cues indicated uncertainty about the impending stimulus. Each experimental trial consisted of a cue presented for 2 sec, followed, after a jittered interstimulus interval (ISI; range: 2–8 sec), by a 1 sec picture presentation. After a second jittered ISI (range: 5–9 sec), subjects have 4 sec to rate their emotional response to the image using a 4-item scale ranging from 1 (“very positive”) to 4 (“very negative”). See Supplementary Figure 1 for a schematic diagram of the paradigm.
MRI data acquisition
The MRI data acquisition details are described in the supplement.
Statistical Analyses
Data analysis was conducted using AFNI (42). Individual task runs were slice time corrected, motion corrected, smoothed with a 6-mm full-width half-maximum (FWHM) Gaussian kernel, and scaled to percentage signal change (PSC). Preprocessed task data were concatenated and analyzed as previously described to separately model phasic and sustained components of anticipatory activity (39). Phasic activity was modeled using stick regressors at the onset of each cue and sustained activity is modeled using a duration-modulated boxcar regressor, beginning at cue offset and spanning the 2–8 sec anticipatory ISI. All six cue regressors (3 phasic and 3 sustained) were included in a general linear model (GLM) with additional regressors for each picture type, a single regressor for the rating period, and several regressors of no interest, including six motion covariates from rigid-body alignment (43) and a fourth-order polynomial to model baseline and slow signal drift. Blood oxygen level-dependent (BOLD) signal was modeled by convolving each regressor with AFNI’s default canonical hemodynamic response function (HRF; gamma function). To avoid potential confounds introduced by subject motion, volumes in which 10% of voxels were time series outliers were censored before conducting the general linear model; there are no group differences in the average proportion of censored volumes (p=0.41), or in mean framewise displacement (p=0.20). Resulting whole-brain maps of voxelwise-values for phasic and sustained responses were aligned to MNI space and resampled to 3 mm3 isotropic resolution for second-level analyses.
To identify brain regions in which cue-related activity, picture-response activity, and the modulatory effect of uncertainty on responses to subsequent stimuli differed between groups, we performed three between-group t-tests. The picture response model includes valence (aversive vs neutral), group (HC vs anxiety), as well as covariates for age and gender. The cue-related model included cue (?, X, O), group (HC vs anxiety), as well as covariates for age and gender. Finally, the modulation model included pictures preceded by uncertain cues vs pictures preceded by certain cues, group (HC vs anxiety), as well as covariates for age and gender. Resulting statistical maps were familywise error (FWE) corrected for multiple comparisons across the whole brain at the cluster level (pFWE<.05), using a height threshold of p<.002 (44, 45). A corrected pFWE<.05 was achieved using a cluster extent threshold of 15 contiguous voxels, calculated using Monte Carlo simulations with 3dClustSim in AFNI. In addition to whole-brain analyses, we also performed ROI-based analyses for each between-group t-test using anatomically derived ROIs for a group mask including vmPFC, bilateral amygdala, and bilateral anterior insula (Supplementary Figure 2). For ROI-based analyses, a corrected pFWE<.05 was achieved using a cluster extent threshold of 6 contiguous voxels within the ROI, calculated using Monte Carlo simulations with 3dClustSim in AFNI.
To assess cardiac responses, we analyzed cardiac plethysmography data to compute trial-wise estimates of heart rate change for each subject, as previously described (28). Cardiac R-spikes were identified using interactive beat detection software. Trials with ectopic beats, missed beats, or periods of noisy signal (where beat detection failed), were excluded from further analysis (HC group, n=4 with one excluded trial; anxiety group, n=3 with one excluded trial). R-R intervals were transformed into heart rate in beats per minute, averaged in 500-msec bins. Changes in heart rate were determined by subtracting the mean heart rate for 1 sec preceding each stimulus (picture or cue) from the heart rate over 7 sec after stimulus onset (in 500 msec bins). As in previous studies, the maximum cardiac deceleration (i.e., heart rate decrease) during the first 3 sec of stimulus onset was used as an index of the physiologic response to each picture (28).
Results
Behavioral data
During the fMRI task, both groups rated aversive pictures as significantly more aversive than neutral pictures, with no significant differences between groups in ratings (p>0.43) or reaction times (p>0.17). There were no significant differences (within or between groups) for pictures that are preceded by uncertain or certain cues in ratings (p>0.38) or reaction times (p>0.31). See Supplementary Results and Supplementary Table 3 for full behavioral data.
fMRI data: Response to certain/uncertain cues
To test the hypothesis that individuals with anxiety disorders would have elevated activity in anterior insula and amygdala during the uncertain anticipation period (relative to HC subjects), we conducted whole-brain and ROI-based between-group t-tests on PSC in response to uncertain versus certain cues. Contrary to our hypothesis, there were no significant group differences (whole-brain or ROI-based) in neural response to uncertain versus certain cues.
fMRI data: Response to aversive/neutral pictures
Consistent with previous studies, aversive relative to neutral pictures (regardless of preceding cue type), elicited robust subcortical and visual cortex activation in HC subjects and individuals with anxiety disorders (46–48) (Supplementary Figure 3). To test the hypothesis that individuals with anxiety disorders would have elevated amygdala responses and decreased vmPFC responses to aversive pictures (relative to HC subjects), we conducted whole-brain and ROI-based between-group t-tests on PSC in response to aversive versus neutral pictures. Again, contrary to our hypothesis, there were no significant group differences (whole-brain or ROI-based) in neural response to aversive versus neutral pictures.
fMRI data: Modulatory effect of cues on response to pictures
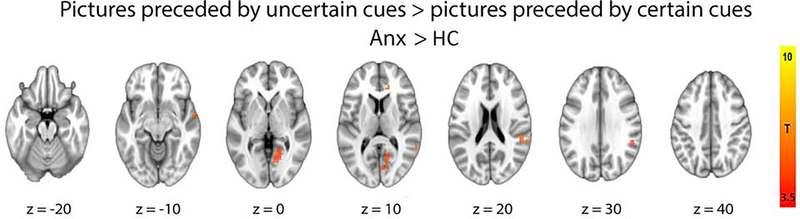
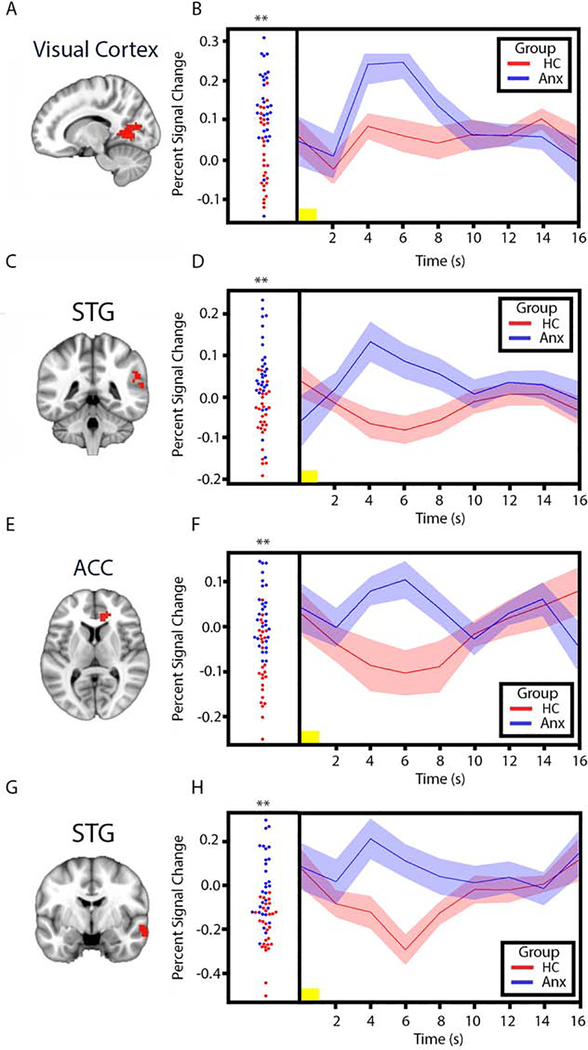
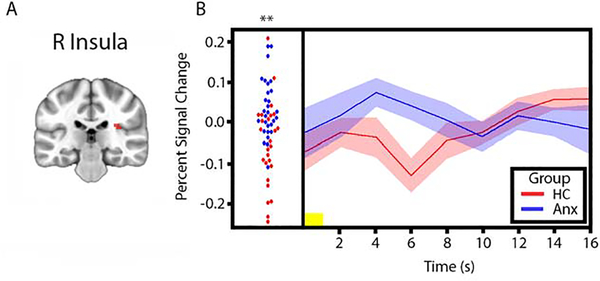
To test the hypothesis that the modulatory effect of uncertainty on responses to stimuli would be associated with greater activity in anterior insula and amygdala in individuals with anxiety disorders (relative to comparison subjects), we conducted whole-brain and ROI-based between-group t-tests on PSC in response to pictures preceded by uncertain cues versus pictures preceded by certain cues. The whole-brain analysis revealed significantly greater activity in the individuals with anxiety group in a number of cortical regions, including right visual cortex, right ACC, and superior temporal gyrus (STG) (Table 2, Figures 1 and 2). In addition, to confirm that this effect was driven by heightened response to pictures preceded by uncertain cues, we examined both trial types separately (Supplementary Figure 4). The ROI-based analysis revealed significantly greater activity for individuals with anxiety disorders in right anterior insula, however only when examining response to aversive images (Figure 3). The whole-brain analysis revealed trending bilateral anterior insula activation, but did not meet statistical significance (i.e., these clusters survive at a threshold of p=.0025, but not p=.002).
Table 2.
Cluster Maxima for Regions with Statistically Significant Increased BOLD Signal for ROI-based Group Difference between Aversive Pictures Proceeded by Uncertain Cues Relative to Aversive Pictures Preceded to Certain Cues.
| Cluster | Peak voxel | |||||
|---|---|---|---|---|---|---|
| Brain Region | BA | Size | t | x | y | z |
| R Ant Insula | 13 | 7 | 4.22 | 33.2 | 22 | 13.5 |
Note. Clusters ordered by t score, for the group difference of pictures preceded by uncertain cues > pictures preceded. by certain cues contrast. Corrected p thresholds indicate minimum FWE-corrected p value for each cluster. BA, Broadman area; BOLD, blood oxygen level-dependent; FWE, familywise error; L, left; R, Right.
Figure 1.
Whole-brain group difference in neural responses to pictures preceded by uncertain cue > pictures preceded by certain cue (anxiety > HC; pFWE < .05). Table 2 contains full cluster list. Anx: Individuals with Anxiety Disorders; FWE: familywise error; HC: Healthy Comparison subjects.
Figure 2.
Visual cortex, superior temporal gyrus, anterior cingulate cortex, and superior temporal gyrus responses to pictures preceded by uncertain cues > pictures preceded by certain cues. A. Task-derived visual cortex region of interest (red) used to extract mean percent signal change (PSC) estimates for group comparison. B. Left, plots of visual cortex PSC for individual HC subjects (red circles) and individuals with anxiety disorders (blue circles) in response to pictures preceded by uncertain cues > pictures preceded by certain cues. Right, mean time series of visual cortex PSC in response to pictures preceded by uncertain cues > pictures preceded by certain cues for HC subjects (red line) and individuals with anxiety disorders (blue line) (width of shaded area corresponds to ±1 SEM). C. Task-derived superior temporal gyrus region of interest (red) used to extract mean PSC estimates for group comparison. D. Plot and mean time series of PSC extracted from the superior temporal gyrus region of interest. E. Task-derived right anterior cingulate cortex region of interest (red) used to extract mean PSC estimates for group comparison. F. Plot and mean time series of PSC extracted from the anterior cingulate cortex of interest. G. Task-derived superior temporal gyrus region of interest (red) used to extract mean PSC estimates for group comparison. H. Plot and mean time series of PSC extracted from the superior temporal gyrus region of interest. Yellow horizontal bars on the time series plots indicate picture duration (1 sec). **p < .01. ACC: Anterior cingulate cortex; Anx: Individuals with Anxiety Disorders; HC: Healthy Comparison subjects; s: seconds; STG: Superior temporal gyrus.
Figure 3.
Right anterior insula responses to pictures preceded by uncertain cues > pictures preceded by certain cues from ROI-based analysis. A. Task-derived visual cortex region of interest (red) used to extract mean percent signal change (PSC) estimates for group comparison. B. Left, plots of right anterior insula PSC for individual HC subjects (red circles) and individuals with anxiety disorders (blue circles) in response to pictures preceded by uncertain cues > pictures preceded by certain cues. Right, mean time series of right anterior insula PSC in response to pictures preceded by uncertain cues > pictures preceded by certain cues for HC subjects (red line) and individuals with anxiety disorders (blue line) (width of shaded area corresponds to ±1 SEM). Yellow horizontal bars on the time series plots indicate picture duration (1 sec). **p < .01. Anx: Individuals with Anxiety Disorders; HC: Healthy Comparison subjects; s: seconds; R: Right.
HR data: Response to certain/uncertain cues
To test the hypothesis that individuals with anxiety disorders would have potentiated cardiac responses (i.e., less HR deceleration indicating a shift towards HR acceleration) during the uncertain anticipation period (relative to HC subjects), we conducted between-group t-tests on cardiac deceleration in response to uncertain versus certain cues. Contrary to our hypothesis, there were no significant group differences in cardiac deceleration to uncertain versus certain cues.
HR data: Response to aversive/neutral pictures
To test the hypothesis that individuals with anxiety disorders would have potentiated cardiac responses to aversive pictures (relative to HC subjects), we conducted between-group t-tests on cardiac deceleration in response to aversive versus neutral pictures. Again, contrary to our hypothesis, there were no significant group differences in cardiac deceleration to aversive versus neutral pictures.
HR data: Modulatory effect of cues on response to pictures
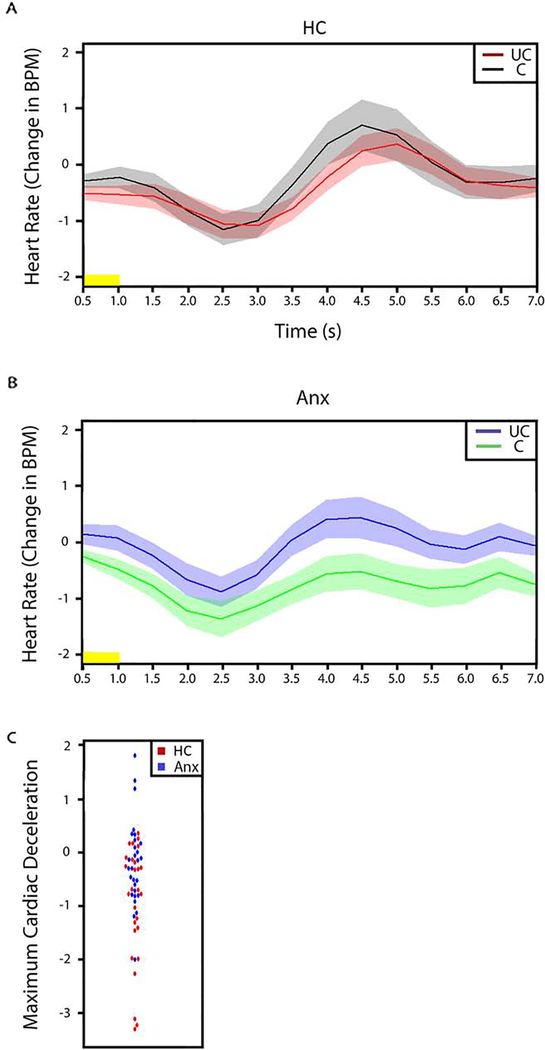
To test the hypothesis that the modulatory effect of uncertainty on responses to stimuli would be associated with potentiated cardiac responses in individuals with anxiety disorders (relative to comparison subjects), we conducted between-group t-tests on cardiac deceleration in response to pictures preceded by uncertain cues versus pictures preceded by certain cues. The magnitude of stimulus-evoked cardiac deceleration was significantly lower in individuals with anxiety disorders than in the comparison subjects for pictures preceded by the uncertain cue, relative to pictures preceded by a certain cue (HC, −1.03 (1.09); 95% CI: −1.46 - −0.58; anxiety disorder group, −0.26 (0.82); 95% CI: − 0.58 – 0.5; t=−2.94, p<0.01, effect size = 0.8) (Figure 4).
Figure 4.
Stimulus-evoked reductions in heart rate (cardiac deceleration) in response to pictures preceded by uncertain cue (UC) and pictures preceded by certain cue (C). A. Comparison group. B. Individuals with anxiety group. C. Plot of the maximum cardiac deceleration in response to pictures preceded by uncertain cue > pictures preceded by certain cue. Our analysis revealed significant group differences in cardiac deceleration in response to pictures preceded by uncertain cue > pictures preceded by certain cue. Yellow horizontal bars on the time series plots indicate picture duration (1 sec). Width of shaded area corresponds to ±1 SEM. Anx: Individuals with Anxiety Disorders; BPM: Beats per minute; C: Certain; HC: Healthy Comparison subjects; S: seconds; UC: Uncertain.
Follow-up analyses
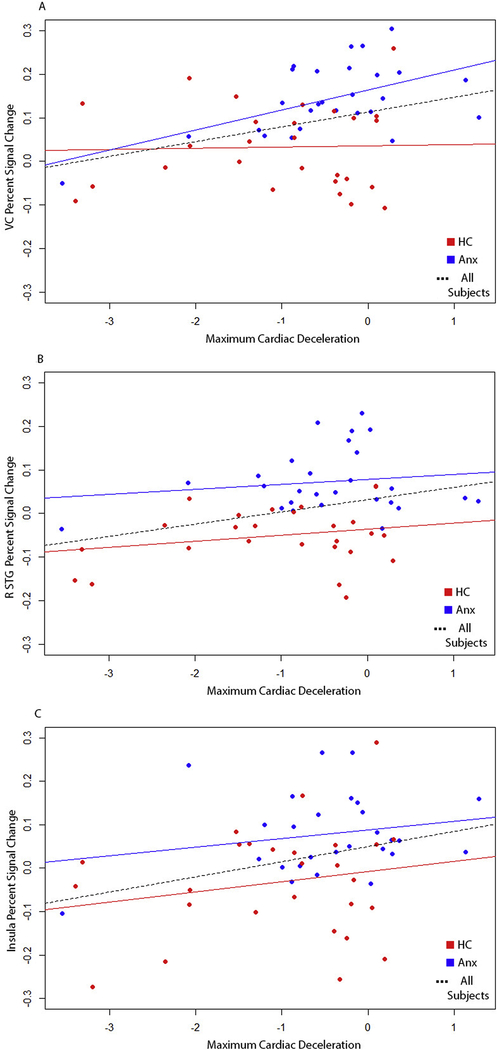
To determine whether the significant group differences in the modulatory effect of cues on response to pictures varied with respect to the valence of the picture (neutral vs aversive), we conducted whole-brain between-group t-tests on PSC in response to both aversive and neutral pictures (i.e., preceded by uncertain cues versus aversive pictures preceded by certain cues) separately. These separate whole-brain analyses for each valence revealed significant differences in a number of the same cortical regions as the main analysis, including right visual cortex and right ACC (Supplementary Figure 5), thus indicating that the modulation effect was present regardless of picture valence. To determine whether the observed group differences in fMRI activity were correlated with the observed group differences in HR deceleration for the modulatory effect of cues on response to pictures, we computed correlations across all subjects and within each group (individuals with anxiety disorders and HC subjects) separately for each cluster. For visual cortex, right STG, and right anterior insula clusters, larger fMRI modulation effects were associated with larger HR modulation effects (across the entire sample, and within the individuals with anxiety disorders group for visual cortex), indicating that, overall, heightened neural responses were accompanied by reduced cardiac deceleration in response to pictures during periods of uncertainty (Figure 5; visual cortex: rNC-Only=0.03, p=0.88, rGAD-Only = 0.54, p<0.01, rFULL=0.34, p=0.01 (rFULL 95% CI 0.09–0.55); right STG: rNC-Only=0.23, p=0.24, rGAD-Only = 0.14, p=0.46, rFULL=0.32,p=0.01 (rFULL 95% CI 0.07–0.53)). No such relationship is present for the other significant fMRI clusters, although the relationship for ACC is trending towards significance in the entire sample (rFULL=0.24, p=0.07).
Figure 5.
Scatter plots depicting the relationship between heart rate (cardiac deceleration) and percent signal change estimates for visual cortex (A), right superior temporal gyrus (B), and right anterior insula (C) in response to pictures preceded by uncertain cues > pictures preceded by certain cues. Blue dots represent individuals with anxiety disorders and red dots represent HC subjects. Solid blue lines indicate the regression line for the n=29 individuals with anxiety disorders, solid red lines indicate the regression line for the n=29 HC subjects, and dotted black lines indicate the regression line across all HC and individuals with anxiety disorders.
In sum, individuals with anxiety disorders had significantly greater neural activation in a number of regions (right visual cortex, right ACC, STG, and anterior insula) as well as significantly lower stimulus-evoked cardiac deceleration in response to the modulatory effect of cues on the response to pictures, but not to cues or pictures alone.
Discussion
In this study, neural activity (fMRI) and peripheral physiological (heart rate) data converge to demonstrate a novel psychobiological correlate of anxiety: potentiated stimulus-evoked responses in the context of uncertainty. Individuals with anxiety disorders had significantly heightened neural responses in a host of cortical brain regions—including visual cortex, ACC, STG, and within an anterior insula ROI—in response to pictures preceded by uncertainty. Similarly, individuals with anxiety disorders had significantly reduced cardiac deceleration in response to pictures preceded by uncertainty. Here we discuss each of these main findings in turn.
Two of the brain regions identified in this analysis—ACC and anterior insula—are known to be highly functionally interconnected, as the major hubs of a “salience network” that mediates attentional control (49, 50). Moreover, both regions have been linked to anxiety (51–55) and the processing of uncertainty in comparison subjects (14–16, 56, 57). The present results connect the previous findings on attention, anxiety, and uncertainty by demonstrating enhanced activation in these brain regions in individuals with anxiety disorders for stimuli preceded by uncertainty. This analysis also revealed greater activity in STG and right visual cortex among the individuals with anxiety disorders. Previous fMRI studies have found abnormal activity and functional connectivity (58) and structural differences in the STG (59–61). The visual cortex, along with the amygdala and pulvinar nucleus of the thalamus, has been hypothesized to play an important role in the feedback loops that support allocation of attention to salient or significant stimuli (62). Collectively, the observed pattern of activation thus suggests enhanced neural mechanisms of attentional processing to stimuli preceded by uncertainty in individuals with anxiety disorders.
Complementing the fMRI findings, we also found that individuals with anxiety disorders had significantly reduced cardiac deceleration in response to pictures preceded by uncertainty. Reduced cardiac deceleration in response to emotional stimuli is a well-established metric of physiological defensive motivation (28). A shift to cardiac acceleration during emotional picture viewing has been suggested to indicate a high level of defense activation (28). For example, previous research has shown this shifting effect when patients with phobic disorder view pictures of the phobic object (63). Consistent with this line of work, individuals with anxiety disorders in our study had significantly reduced cardiac deceleration in response to pictures preceded by uncertainty, which may indicate a shift to cardiac acceleration. This finding suggests high levels of defense activation in response to stimuli presented in the context of uncertainty. Taken together, our neural activity (fMRI) and peripheral physiological (heart rate) data provide complementary evidence of group differences in the modulatory effect of uncertainty on response to pictures. These findings appear in line with other recent literature that has suggested an association between higher IU and greater threat generalization (9, 64, 65). Therefore, based on the previous literature and the findings we present here, we propose that heightened cardiac and neural reactivity following threat uncertainty may be a key psychobiological mechanism of anxiety.
Contrary to our hypotheses, we found inconclusive results strictly in response to the cues (certain vs uncertain) or pictures (neutral vs aversive). To test whether our null findings were due to insufficient power, we conducted a power analysis based on effect sizes extracted from a priori ROIs. The effect sizes (range: 0.25–0.34) extracted from these ROIs would require a sample size of more than 200 participants per group to yield a statistically significant result. Additionally, a recent meta-analysis (66) indicates that, among neuroimaging studies of individuals with anxiety disorders, the median number of individuals with anxiety disorders sample size has been 17. In fact, out of 30 such studies, only 3 (10%) have had sample sizes larger than the current sample size in this study (n=29 individuals with anxiety disorders). Thus, our sample size exceeds the standard in this area of research.
Neurobiological models of anxiety disorders have suggested a key role of uncertainty in the pathogenesis of anxiety disorders (9–11). For example, intolerance of uncertainty scores are elevated in GAD (67), social anxiety disorder (68), and obsessive-compulsive disorder (68). However, in this study and other previous studies, no group differences were found in response to uncertainty alone (see 69 for a review of mixed results). These results suggest a more complex role for uncertainty processing in the pathogenesis of anxiety disorders.
Other models of anxiety have proposed hyperactivity to threat within the amygdala and deficient vmPFC function leading to inadequate regulation of fear response (70, 71). For example, previous studies of emotional anticipation (22) and emotion regulation (30) in GAD have demonstrated amygdala hyperactivity. Nevertheless, in the current study and other previous studies (29–31), no group differences in response to emotional pictures were found between individuals with anxiety disorders and comparison subjects, thus challenging neuropsychological models that emphasize a primary role for amygdala/vmPFC and threat hyper-reactivity in anxiety disorders.
In conclusion, the study results suggest novel neural and physiological correlates of anxiety disorders in the modulatory effect of uncertainty on responses to stimuli. Individuals with anxiety disorders had significantly heightened neural responses in multiple brain regions involved in attentional control and significantly reduced cardiac deceleration in response to pictures following periods of uncertainty. By contrast, neural and cardiac responses were similar between groups for responses to the cues or pictures alone. Our findings thus provide unique evidence regarding the psychobiological mechanisms underlying anxiety disorders.
Limitations
While this study examined the response of individuals with anxiety disorder to uncertainty, negatively-valenced images, and the modulatory effect of uncertainty on responses to subsequent pictures, there remain several limitations. Future studies could expand the scope of the present findings by using more diverse or individual specifically-tailored stimuli and/or task paradigms. Additionally, the use of further psychophysiology measures (i.e., skin conductance or eye tracking), could be helpful to validate the current results. Recent studies have used eye-tracking to monitor avoidance behaviors during experimental paradigms (72). Similarly, as we know clinically, individuals with anxiety disorders have varying levels of learned coping skills to deal with anxiety. In this study, and many others, researchers typically expect participants to attend to the stimuli, but it should be examined whether or not individuals are using coping skills to reduce anxiety during the task (i.e., emotion regulation, distraction, reassurance). As described earlier, anxiety disorder symptoms are often unique to the individual fears and by simply using negatively-valenced images, we may not capture a true anxiety response if this set of pictures does not fall within the participants’ core fears. Ideally, we would be able to increase the sample size to allow us to examine how specific symptoms and/or diagnoses play an integral role in the response to uncertainty. Also, this paradigm elicits one part of an extremely complex presentation of symptoms that combine to form psychopathology. Additionally, we did not measure IU in our sample, which varies within anxiety disorder patients (68). A measure of IU could have been used to examine neural/cardiac responding in a continuous way instead of relying on grouping.
Future Directions and Clinical Implications
We believe it will be important to replicate and extend the current findings by addressing the specific limitations of the current study that we mentioned previously. This could be achieved by taking several steps. First, it would be important to expand the sample size to examine how specific symptom clusters of anxiety disorders may alter response during periods of uncertainty. It would also be important to individualize stimuli to ensure they are eliciting a consistent anxious response across participants. To this end, it is also important to attempt to measure (through methods like eye-tracking) participants’ attempts to regulate anxiety during the task. Often, patients report several modalities of anxiety (i.e., cognitive, emotional, bodily sensations, etc.) but there tend to be unique differences to how each individual experience these responses. A deeper examination of these differences could help further our understanding of how participants experience uncertainty and anticipation of negative events. We plan to continue to further this line of research with more complex and nuanced paradigms in a hope to increase our field’s ability to assess and treat anxiety disorders effectively and efficiently. The neural and physiological correlates of uncertainty on responses to stimuli in anxiety disorders could be explored as biomarkers for treatment planning. Finally, the findings of the current study could inform future anxiety treatment development and refinement efforts, such as targeting these regions using neurostimulation and/or neurofeedback.
Supplementary Material
Table 1.
Cluster Maxima for Regions with Statistically Significant Increased BOLD Signal for Whole-Brain Group Difference between Pictures Proceeded by Uncertain Cues Relative to Pictures Preceded to Certain Cues.
| Cluster | Peak voxel | |||||
|---|---|---|---|---|---|---|
| Brain Region | BA | Size | t | x | y | z |
| R Visual Cortex | 30 | 86 | 4.63 | 15.8 | −57 | 3 |
| R Superior Temporal Gyrus | 22 | 59 | 4.36 | −61.2 | 39.5 | 17 |
| R Anterior Cingulate Cortex | 32 | 22 | 4.00 | −15.8 | −34 | 6.5 |
| R Superior Temporal Gyrus | 21 | 21 | 4.34 | −61.2 | 1 | −7.5 |
Note. Clusters ordered by t score, for the group difference of pictures preceded by uncertain cues > pictures preceded. by certain cues contrast. Corrected p thresholds indicate minimum FWE-corrected p value for each cluster. BA, Broadman area; BOLD, blood oxygen level-dependent; FWE, familywise error; L, left; R, Right.
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH101162).
Footnotes
Conflict of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund MBA, Demler O (2005): Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62:593–602. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. (1999): The Economic Burden of Anxiety Disorders in the 1990s. J Clin Psychiatry. 60:427–435. [DOI] [PubMed] [Google Scholar]
- 3.DuPont RL, Rice DP, Miller LS, Shiraki SS, Rowland CR, Harwood HJ (1996): Economic costs of anxiety disorders. Anxiety. 2:167–172. [DOI] [PubMed] [Google Scholar]
- 4.Devane CL, Chiao E, Franklin M, Kruep EJ (2005): Anxiety Disorders in the 21st Century: Status, Challenges, Opportunities, and Comorbidity With Depression. Am J Manag Care. 11:344–353. [PubMed] [Google Scholar]
- 5.Phillips KA, Friedman MJ, Stein DJ, Craske MG (2010): Special DSM-V Issues on Anxiety, Obsessive-Compulsive Spectrum, Posttraumatic, And Dissociative Disorders. Depression and Anxiety. 27:91–92. [DOI] [PubMed] [Google Scholar]
- 6.Yonkers KA, Dyck IR, Warshaw M, Keller MB (2000): Factors predicting the clinical course of generalised anxiety disorder. British Journal of Psychiatry. 176:544–549. [DOI] [PubMed] [Google Scholar]
- 7.Yonkers KA, Warshaw M, Massion AO, Keller MB (1996): Phenomenology and Course of Generalised Anxiety Disorder. British Journal of Psychiatry. 168:308–313. [DOI] [PubMed] [Google Scholar]
- 8.Crits-Christoph P, Newman MG, Rickels K, Gallop R, Gibbons MB, Hamilton JL, et al. (2011): Combined medication and cognitive therapy for generalized anxiety disorder. J Anxiety Disord. 25:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupe DW, Nitschke JB (2013): Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEvoy PM, Mahoney AE (2012): To be sure, to be sure: intolerance of uncertainty mediates symptoms of various anxiety disorders and depression. Behav Ther. 43:533–545. [DOI] [PubMed] [Google Scholar]
- 11.Garfinkle EJ, Behar E (2012): Advances in psychotherapy for generalized anxiety disorder. Curr Psychiatry Rep. 14:203–210. [DOI] [PubMed] [Google Scholar]
- 12.Dugas MJ, Buhr K, Ladouceur R (2004): The Role of Intolerance of Uncertainty in Etiology and Maintenance. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized anxiety disorder: Advances in research and practice. New York, NY: Guilford Press. [Google Scholar]
- 13.Ladouceur R, Dugas MJ, Freeston MH, Léger E, Gagnon F, Thibodeau N (2000): Efficacy of a cognitive-behavioral treatment for generalized anxiety disorder: Evaluation in a controlled clinical trial. Journal of Consulting and Clinical Psychology. 68:957–964. [PubMed] [Google Scholar]
- 14.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, et al. (2010): Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 20:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM (2013): Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 23:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunsmoor JE, Bandettini PA, Knight DC (2007): Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 121:635–642. [DOI] [PubMed] [Google Scholar]
- 17.Critchley HD, Mathias CJ, Dolan RJ (2001): Neural Activity in the Human Brain relating to Uncertainty and Arousal during Anticipation. Neuron. 29:537–545. [DOI] [PubMed] [Google Scholar]
- 18.Morriss J, Gell M, van Reekum CM (2019): The uncertain brain: A co-ordinate based meta-analysis of the neural signatures supporting uncertainty during different contexts. Neurosci Biobehav Rev. 96:241–249. [DOI] [PubMed] [Google Scholar]
- 19.Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, et al. (2004): Neural correlates of speech anticipatory anxiety in generalized social phobia. Brain Imaging. 15:2701–2705. [PubMed] [Google Scholar]
- 21.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB (2006): Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 60:402–409. [DOI] [PubMed] [Google Scholar]
- 22.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. (2009): Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 166:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauch SL, Shin LM, Phelps EA (2006): Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 60:376–382. [DOI] [PubMed] [Google Scholar]
- 24.Mennin D, Turk C, Heimberg R (2004): Clinical presentation and descriptive psychopathology of generalized anxiety disorder. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford Press, pp 3–28. [Google Scholar]
- 25.Mennin DS, Heimberg RG, Turk CL, Fresco DM (2005): Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther. 43:1281–1310. [DOI] [PubMed] [Google Scholar]
- 26.Chalmers JA, Quintana DS, Abbott MJ, Kemp AH (2014): Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front Psychiatry. 5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers JA, Heathers JA, Abbott MJ, Kemp AH, Quintana DS (2016): Worry is associated with robust reductions in heart rate variability: a transdiagnostic study of anxiety psychopathology. BMC Psychol. 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley MM, Codispoti M, Cuthbert BN, Lang PJ (2001): Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 1:276–298. [PubMed] [Google Scholar]
- 29.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. (2008): Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 165:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF (2010): Failure of Anterior Cingulate Activation and Connectivity With the Amygdala During Implicit Regulation of Emotional Processing in Generalized Anxiety Disorder. Am J Psychiatry. 167:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, et al. (2008): A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 63:858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grillon C, Baas JP, Lissek S, Smith K, Milstein J (2004): Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 118:916–924. [DOI] [PubMed] [Google Scholar]
- 33.Shankman SA, Robison-Andrew EJ, Nelson BD, Altman SE, Campbell ML (2011): Effects of predictability of shock timing and intensity on aversive responses. Int J Psychophysiol. 80:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunsmoor JE, Bandettini PA, Knight DC (2008): Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 40:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. (2007): Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 27:5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.First MB, Williams JBW, Karg RS, Spitzer RL (2015): Structured Clinical Interview for DSM-5--Research Version. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 37.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2014): Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. J Neurosci. 34:10430–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2015): Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grupe DW, Oathes DJ, Nitschke JB (2013): Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 23:1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lang PJ, Bradley MM, Cuthbert BN (2008): International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. [Google Scholar]
- 41.Ewbank MP, Barnard PJ, Croucher CJ, Ramponi C, Calder AJ (2009): The amygdala response to images with impact. Soc Cogn Affect Neurosci. 4:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox RW (1996): AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 29:162–173. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, et al. (2006): Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum Brain Mapp. 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carp J (2012): The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage. 63:289–300. [DOI] [PubMed] [Google Scholar]
- 45.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance In Medicine. 33:636–647. [DOI] [PubMed] [Google Scholar]
- 46.Adolphs R, Tranel D, Damasio H, Damasio A (1994): Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 371. [DOI] [PubMed] [Google Scholar]
- 47.McHugh SB, Marques-Smith A, Li J, Rawlins JN, Lowry J, Conway M, et al. (2013): Hemodynamic responses in amygdala and hippocampus distinguish between aversive and neutral cues during Pavlovian fear conditioning in behaving rats. Eur J Neurosci. 37:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zald DH, Pardo JV (1997): Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci. 94:411904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menon V (2015): Salience Network.597–611. [Google Scholar]
- 51.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C (2012): The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 60:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, et al. (2015): Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry. 5:e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duval ER, Javanbakht A, Liberzon I (2015): Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 11:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin LM, Liberzon I (2010): The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 13:334–340. [DOI] [PubMed] [Google Scholar]
- 57.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ (2006): Functional neuroanatomy of aversion and its anticipation. Neuroimage. 29:106–116. [DOI] [PubMed] [Google Scholar]
- 58.Zhao X, Xi Q, Wang P, Li C, He H (2014): Altered activity and functional connectivity of superior temporal gyri in anxiety disorders: a functional magnetic resonance imaging study. Korean J Radiol. 15:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strawn JR, Wehry AM, Chu WJ, Adler CM, Eliassen JC, Cerullo MA, et al. (2013): Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress Anxiety. 30:842–848. [DOI] [PubMed] [Google Scholar]
- 60.Yun JY, Kim JC, Ku J, Shin JE, Kim JJ, Choi SH (2017): The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J Affect Disord. 214:53–59. [DOI] [PubMed] [Google Scholar]
- 61.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. (2002): Superior Temporal Gyrus Volumes in Pediatric Generalized Anxiety Disorder. Society of Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- 62.Pessoa L, Adolphs R (2010): Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 11:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamm AO, Cuthbert BN, Globisch J, Vaitl D (1997): Fear and the startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Pscyhophysiology. 34:97–107. [DOI] [PubMed] [Google Scholar]
- 64.Bauer EA, MacNamara A, Sandre A, Lonsdorf TB, Weinberg A, Morriss J, et al. (2020): Intolerance of uncertainty and threat generalization: A replication and extension. Psychophysiology. 57:e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morriss J, Biagi N, Dodd H (2020): Your guess is as good as mine: A registered report assessing physiological markers of fear and anxiety to the unknown in individuals with varying levels of intolerance of uncertainty. Int J Psychophysiol. 156:93–104. [DOI] [PubMed] [Google Scholar]
- 66.Fonzo GA, Etkin A (2017): Affective neuroimaging in generalized anxiety disorder: an integrated review. Dialogues Clin Neurosci. 18:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dugas MJ, Marchand A, Ladouceur R (2005): Further validation of a cognitive-behavioral model of generalized anxiety disorder: diagnostic and symptom specificity. J Anxiety Disord. 19:329–343. [DOI] [PubMed] [Google Scholar]
- 68.Carleton RN, Mulvogue MK, Thibodeau MA, McCabe RE, Antony MM, Asmundson GJ (2012): Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 26:468–479. [DOI] [PubMed] [Google Scholar]
- 69.Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE (2014): A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J Affect Disord. 167:336–342. [DOI] [PubMed] [Google Scholar]
- 70.Rauch SL, Shin LM, Wright CI (2003): Neuroimgaing Studies of Amygdala Function in Anxiety Disorders. Ann N Y Acad Sci. 985:389–410. [DOI] [PubMed] [Google Scholar]
- 71.Kent JM, Rauch SL (2003): Neurocircuitry of Anxiety Disorders. Current Psychiatry Reports. 5:266–273. [DOI] [PubMed] [Google Scholar]
- 72.Weeks JW, Howell AN, Srivastav A, Goldin PR (2019): “Fear guides the eyes of the beholder”: Assessing gaze avoidance in social anxiety disorder via covert eye tracking of dynamic social stimuli. J Anxiety Disord. 65:56–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.