Abstract
Objectives:
This study sought to better characterize the quality of life and economic impact in patients with symptoms of ischemia and no obstructive coronary disease (INOCA) and to identify the influence of coronary microvascular dysfunction (CMD).
Background:
Patients with INOCA have a high symptom burden and an increased incidence of major adverse cardiac events. CMD is a frequent cause of INOCA. The morbidity associated with INOCA and CMD has not been well-characterized.
Methods:
Sixty-six patients with INOCA underwent stress cardiac magnetic resonance (CMR) imaging with calculation of myocardial perfusion reserve (MPR); MPR 2.0 – 2.4 was considered borderline-reduced (possible CMD) and MPR < 2.0 was defined as reduced (definite CMD). Subjects completed quality of life questionnaires to assess the morbidity and economic impact of INOCA. Questionnaire results were compared between INOCA patients with and without CMD. In addition, logistic regression was used to determine the predictors of CMD within the INOCA population.
Results:
The prevalence of definite CMD was 24%. Definite or borderline CMD was present in 59% (MPR ≤ 2.4). INOCA patients reported greater physical limitation, angina frequency, and reduced quality of life compared to referent stable CAD and acute myocardial infarction populations. In addition, INOCA patients reported frequent time missed from work and work limitations, suggesting a substantial economic impact. No difference was observed in reported symptoms between INOCA patients with and without CMD. Glomerular filtration rate and body-mass index were significant predictors of CMD in multivariable regression analysis.
Conclusion:
INOCA is associated with high morbidity similar to other high-risk cardiac populations, and work limitations reported by INOCA patients suggest a substantial economic impact. CMD is a common cause of INOCA but is not associated with increased morbidity. These results suggest that there is significant symptom burden in the INOCA population regardless of etiology.
Keywords: Coronary Microvascular Dysfunction, Exercise, INOCA, Quality of Life
INTRODUCTION
While epicardial coronary artery disease (CAD) has traditionally been considered the cause of myocardial ischemia, more than 50% of patients with stable angina undergoing elective invasive coronary angiography are found to have no obstructive coronary artery disease (CAD) (1). This group of patients with symptoms suggestive of ischemia but no obstructive CAD (INOCA) (2) has a high morbidity (3) and an increased risk of major adverse cardiac events (MACE) (4). While the INOCA population is heterogeneous with multiple potential etiologies, as many as 50–65% of these patients are found to have coronary microvascular dysfunction (CMD) (5). CMD is thought to occur due to multiple processes including impaired arteriolar vasodilation, inflammation, and thrombosis which lead to ischemia from an insufficient increase in coronary blood flow from rest to stress (5).
This impaired increase in coronary blood flow can be assessed invasively through calculation of coronary flow reserve (CFR), the ratio of coronary blood flow under maximal vasodilation to blood flow at rest as measured through intracoronary Doppler ultrasonography or thermodilution techniques. In the absence of obstructive epicardial CAD, a reduced CFR identifies CMD (6). However, CMD can now also be evaluated noninvasively through measurement of the myocardial perfusion reserve (MPR), calculated as the ratio of stress to rest absolute myocardial blood flow (MBF) derived from myocardial perfusion. MPR can be determined using stress positron-emission tomography (PET) or cardiovascular magnetic resonance imaging (CMR). In animal models, quantification of myocardial blood flow with CMR has shown excellent correlation to microsphere analysis, considered the gold standard (7,8). Similarly, in human studies, CMR has shown good correlation with PET (9). The evaluation of CMD patients with stress CMR has shown perfusion abnormalities on qualitative analysis (10) and a significant difference in MPR from control patients on quantitative analysis (11).
While there have been significant advances in the understanding of INOCA and CMD, knowledge gaps still exist. The morbidity of INOCA and CMD regarding symptom burden and economic impact, has not been well-described or compared to other cardiac populations. Accordingly, the goal of this study was to characterize the quality of life and economic impact in patients with INOCA and evaluate the influence of CMD as the cause of INOCA on these parameters.
METHODS
Study population
Consecutive patients age 18–85 who presented with chest pain or exertional dyspnea concerning for ischemia with no obstructive epicardial CAD on invasive or computed tomographic coronary angiography within the past year were enrolled between July 2014 and May 2018. Obstructive epicardial CAD was defined as ≥50% stenosis or fractional flow reserve <0.8. Exclusion criteria included history of coronary artery bypass grafting, prior myocardial infarction (MI), hypertrophic or restrictive cardiomyopathy, coronary vasospasm during angiography, severe obstructive valvular disease, pregnancy, severe liver disease, contraindications to CMR (metallic implants, glomerular filtration rate (GFR) <45 mL/min/1.73m2, acute kidney injury, or cardiac arrhythmias that would interfere with electrocardiographic (ECG) gating), contraindications to regadenoson (severe asthma/chronic obstructive pulmonary disease, bradyarrhythmias, or systolic blood pressure <90 mmHg), inability to provide informed consent, and life expectancy < 2 years. This study was approved by the University of Virginia Health System Institutional Review Board, and informed written consent was provided by all subjects.
Stress Testing
A proportion of subjects underwent stress testing as part of their work-up for CAD prior to inclusion in this study. Patients able to exercise underwent an exercise treadmill stress using the Bruce protocol. Exercise workload was assessed as the total number of metabolic equivalents (METs) achieved. For patients unable to exercise, regadenoson was administered. Following stress, subjects underwent either single photon-emission computed tomography (SPECT) or echocardiography to evaluate for evidence of ischemia. A stress test was considered positive if there were ECG findings suggestive of ischemia or a stress perfusion abnormality on SPECT/wall motion abnormality on echocardiography.
Calcium scoring
Subjects underwent coronary artery calcium scoring on a Siemens FLASH computed tomography (CT) scanner (Siemens Healthineers, Munich, Germany). High-pitch spiral acquisition was used. CT acquisition parameters included 120 kV, 80 reference mAs, and 3mm reconstructed slice thickness.
CMR
All subjects underwent regadenoson stress perfusion CMR imaging on a SIEMENS 1.5T AERA scanner according to a previously described protocol (11). Quantitative first-pass myocardial perfusion imaging (MPI) was performed on 3 short-axis slices at the left ventricle (LV) base, mid, and apical levels. Rest perfusion was assessed, followed by stress perfusion 15 minutes after regadenoson stress to allow for contrast washout and recovery of vasodilation. Gadolinium contrast was administered via intravenous bolus of 0.075 mmol/kg at 4 cm3/s. An accelerated saturation recovery variable-density spiral perfusion pulse sequence with integrated proton density and arterial input function acquisition was then acquired to quantify MBF.
First-pass perfusion was quantified using the Fermi function deconvolution method in MATLAB (MathWorks, Natick, Massachusetts) (12). For each subject, global stress and rest MBF were determined and MPR was calculated as the ratio of stress to rest MBF. Subjects were classified into one of three categories based on their MPR: reduced (<2.0), consistent with definite CMD; borderline (2.0–2.4), consistent with possible CMD; and normal (>2.4). These cutoffs were chosen based on literature review of diagnostic cutoffs for CMD (13) and were validated in our previous study, in which 20 healthy controls were compared with 46 patients with risk factors for CMD (11). The mean MPR in the 20 healthy controls was 2.93±0.44. Our cutoff for borderline CMD was greater than 1 standard deviation below the mean of this group, while our cutoff for definite CMD was greater than 2 standard deviations below the mean.
Quality of Life/Economic Burden Questionnaires
Seattle Angina Questionnaire
Subjects completed the Seattle Angina Questionnaire (SAQ), which assesses the impact of angina on 5 domains: physical limitation; angina stability; angina frequency; treatment satisfaction; and quality of life (14). A validated summary score averages the subscores for physical limitation, angina frequency and quality of life (15). The mean SAQ subdomain scores and summary score were compared between our study population and previously-published referent SAQ scores for patients with stable angina, acute myocardial infarction (MI), and presentation for elective percutaneous coronary intervention of obstructive CAD (15).
Cardiac Anxiety Questionnaire
Subjects completed the Cardiac Anxiety Questionnaire (CAQ), which evaluates heart-focused anxiety. The CAQ consists of 18 items, each rated on a 5-point scale from 0 (never) to 4 (always). The CAQ produces an overall anxiety score and three subscores (cardiac fear, heart-focused attention, and avoidance) (16). The mean CAQ scores in our study population were calculated and compared with scores from other studies evaluating cardiac anxiety following acute MI (17) and in subjects with hypertrophic cardiomyopathy and sudden cardiac death (18).
Work Limitations Questionnaire
To determine the economic impact of INOCA and CMD, subjects completed the Work Limitations Questionnaire (WLQ). The WLQ measures the effects of chronic health conditions on worker productivity (19). It was developed as a 25-item questionnaire that captures work limitations across four domains: physical demands; time demands; mental-interpersonal demands; and output demands. An 8-item WLQ score was subsequently developed based on the 8 questions shown to be most predictive of productivity loss; this abbreviated score has demonstrated good reliability and validity (20). We utilized the 8-item WLQ score in this study and derived the WLQ At-Work Productivity Loss Score, a measure of overall productivity loss (21). In addition to the WLQ questionnaire, patients completed a time loss questionnaire detailing their time missed from work due to their symptoms in the previous 2 weeks.
Economic Analysis
To convert the WLQ/time loss questionnaires to an estimate of the economic impact of INOCA, we utilized the “lost wages” method (22,23). Productivity loss was assessed through two components, absenteeism (time missed from work) and presenteeism (productivity loss at work due to health impairment). Productivity loss from absenteeism was calculated as the total workdays missed per year multiplied by the average daily compensation for American workers ($36.32 per hour × 8 hour days) (24). This was then adjusted by a wage multiplier of 1.28, the median across 35 jobs, to capture the cost to the employer of the worker’s absence (25). To estimate the cost of presenteeism, total productivity loss was estimated by converting WLQ scores into an overall At-Work Productivity Loss Score (21). This percentage was then applied to total annual compensation (assuming 240 workdays/year and subtracting out days missed due to absenteeism). The sum of the annual cost for presenteeism and absenteeism was taken to be the total annual productivity loss per INOCA patient; this was then multiplied by the total number of INOCA patients in the United States workforce to determine total annual productivity costs related to INOCA. The number of INOCA patients in the workforce was estimated by taking the total number of patients with INOCA (estimated at 3 million (26)) and multiplying by the percentage of INOCA patients in our study that were currently working (50%).
Statistical analysis
Baseline characteristics, exercise capacity, and stress CMR results were compared between MPR groups (reduced, borderline, and normal). Continuous variables are given as medians with interquartile range. Categorical variables are reported as percentages. Differences by MPR group were compared using Kruskal-Wallis testing for continuous variables and Chi-square testing or Fisher’s exact testing where appropriate for categorical variables. Quality of life questionnaire data are expressed as means ± standard deviation. Subjects were grouped by MPR levels as above, and 1- way ANOVA was used to compare for survey data differences by MPR level. For both SAQ and CAQ, scores were compared between INOCA patients with and without CMD using Student t test. Simple linear regression was used to assess the association of CMD and SAQ and CAQ summary and subscores. For all comparisons, a p-value <0.05 was considered significant. Univariable logistic regression analysis was used to identify predictors of definite CMD (MPR <2.0). Variables with p<0.10 on univariable testing were included in a stepwise multivariable logistic regression model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Patient characteristics
The study population was comprised of 66 subjects with INOCA. The median age of the entire population was 60 years old (IQR 50–66); 56% of subjects were female. The prevalence of borderline MPR 2.0–2.4, consistent with possible CMD, was 35% (23/66 subjects) and 24% (16/66 subjects) had MPR <2.0, consistent with definite CMD. Together, these groups comprised 59% of the total study cohort (39/66 subjects).
Baseline demographics, cardiac risk factors, and relevant medications are compared by MPR level in Table 1. The prevalence of hypertension was significantly higher in the reduced MPR group compared with the borderline and normal MPR groups (p=0.013). Likewise, the calculated GFR was significantly lower in the reduced MPR group (p=0.010). There was a stepwise increase in total cholesterol with decreasing MPR that was borderline significant (p=0.06). All other baseline clinical variables were similar between MPR groups.
Table 1.
Study cohort baseline characteristics in total and subdivided by myocardial perfusion reserve.
| Clinical Characteristic | Reduced <2.0 (n (%)) |
Normal >2.4 (n (%)) |
|||
|---|---|---|---|---|---|
| Total Patients | 66 | 16 (24.2) | 23 (34.9) | 27 (40.9) | - |
| MPR† | 2.26 (2.01, 2.85) | 1.84 (1.70, 1.94) | 2.23 (2.06, 2.27) | 2.88 (2.69, 3.02) | - |
| Age | 60 (50,66) | 61 (54.5,68) | 63 (53,69) | 55 (48,63) | 0.1870 |
| Female | 37 (56.1) | 11 (68.8) | 12 (52.2) | 14 (51.9) | 0.5167 |
| Caucasian | 57 (86.4) | 14 (87.5) | 20 (87.0) | 23 (85.2) | 1.000 |
| Diabetes | 13 (19.7) | 5 (31.3) | 4 (17.4) | 4 (14.8) | 0.4470 |
| Hemoglobin A1c (n=43) | 5.7 (5.4,6.5) | 5.8 (5.6, 6.7) | 5.7 (5.4, 6.5) | 5.5 (5.4, 6.1) | 0.4894 |
| Hypertension | 44 (66.7) | 15 (93.8) | 14 (60.9) | 15 (55.6) | 0.0199 |
| Dyslipidemia | 42 (63.6) | 12 (75) | 16 (69.6) | 14 (51.9) | 0.2680 |
| LDL (n=60) | 105.5 (83.5, 131.5) | 114 (99,139) | 115 (88,131) | 100.5 (82.5, 124) | 0.3710 |
| HDL (n=63) | 49 (39,58) | 49 (39,60) | 51 (41,60) | 48 (37,52) | 0.6162 |
| Triglycerides (n=63) | 118 (74, 175) | 141 (90, 249) | 101 (72, 148) | 120 (78, 153) | 0.5179 |
| Total cholesterol (n=63) | 178 (158, 207) | 197 (161,228) | 181 (162, 207) | 167 (137,193) | 0.0603 |
| Tobacco use | 33 (50.0) | 8 (50.0) | 14 (60.9) | 11 (40.7) | 0.3655 |
| Tobacco pack years (n=65) | 0 (0,13) | 2.5 (0,20.5) | 4.5 (0,15) | 0 (0,11) | 0.4800 |
| Family history CAD | 52 (78.8) | 11 (68.8) | 19 (82.6) | 22 (81.5) | 0.5523 |
| Body-mass index | 28.5 (25.8, 34.2) | 34.0 (30.1, 36.0) | 28.1 (25.8, 33.4) | 27.5 (25.1, 33.6) | 0.1094 |
| Glomerular filtration rate | 86.5 (73, 95) | 73 (64, 87.5) | 89 (75, 94) | 89 (80, 99) | 0.0347 |
| Statin | 43 (65.2) | 13 (81.3) | 15 (65.2) | 15 (55.6) | 0.2344 |
| Beta-blocker | 26 (39.4) | 9 (56.3) | 9 (39.1) | 8 (29.6) | 0.2250 |
| ACE or ARB | 29 (43.9) | 9 (56.3) | 11 (47.8) | 9 (33.3) | 0.3075 |
| Aspirin | 49 (74.2) | 12 (75.0) | 20 (87.0) | 17 (63.0) | 0.1438 |
| Amlodipine | 15 (22.7) | 5 (31.3) | 4 (17.4) | 6 (22.2) | 0.6549 |
P-value from Kruskal-Wallis test for continuous variables or Chi-square/Fisher’s exact test for categorical variables. P-values <0.05 were considered statistically significant.
Continuous variables given as median (25th to 75th percentiles).
ACE indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; BMI, body-mass index; GFR, glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MPR, myocardial perfusion reserve.
Noninvasive Testing
Stress testing, CMR, and coronary CT calcium results are compared by MPR level in Table 2. In total, 54 patients received a stress test prior to inclusion in the study; 42 underwent exercise stress while 12 were unable to exercise. All 66 patients underwent a stress CMR. LVEF and LV volumes were similar between MPR groups. In the 55 subjects who underwent calcium scoring, neither calcium score nor presence of nonobstructive coronary disease on invasive or CT angiography differed significantly by MPR level.
Table 2.
Noninvasive testing results in total and subdivided by myocardial perfusion reserve.
| Clinical Characteristic | Reduced <2.0 (n (%)) |
Normal >2.4 (n (%)) |
|||||
|---|---|---|---|---|---|---|---|
| Nuclear Stress Test | |||||||
| Total Patients | 54 | 12 (22.2) | 19 (35.2) | 23 (42.6) | - | ||
| Exercise Stress | 42 | 7 (16.7) | 15 (35.7) | 20 (47.6) | - | ||
| METs achieved (n=42)† | 8.0 (7.0, 10.1) | 7.0 (5.4, 7.5) | 9.1 (7.0, 11.0) | 10.1 (7.0, 10.2) | 0.0815 | ||
| ≥10 METs (n=42) | 16 (38.1) | 0 (0) | 6 (40.0) | 10 (50.0) | 0.0585 | ||
| Ischemic stress ECG/imaging (n=54) | 26 (48.2) | 7 (58.3) | 10 (52.6) | 9 (39.1) | 0.5372 | ||
| Coronary CT Calcium Scan | |||||||
| Total Patients | 55 | 12 (21.8) | 18 (32.7) | 25 (45.5) | - | ||
| Agatston score | 1.1 (0,102.4) | 0.30 (0, 7.55) | 3.34 (0,182.9) | 8.0 (0, 63) | 0.5969 | ||
| Nonobstructive CAD | 23 (34.9) | 6 (37.5) | 6 (26.1) | 11 (40.7) | 0.5379 | ||
| Stress CMR | |||||||
| Total Patients | 66 | 16 (24.2) | 23 (34.9) | 27 (40.9) | - | ||
| LVEF | 58 (54,63) | 57.5 (52, 63.5) | 59 (54, 64) | 58 (52, 60) | 0.4802 | ||
| LV mass index | 42.9 (36.9, 49.1) | 42.9 (36.5, 58.7) | 40.2 (34.4, 47.1) | 43.5 (38.7, 51.7) | 0.2530 | ||
| LVEDV index | 67.3 (59.2, 73.8) | 69.9 (66.2, 76.5) | 66.6 (57.8, 74.2) | 67.2 (59.2, 73.8) | 0.4161 | ||
| LVESV index | 28.8 (24.1, 30.8) | 29.5 (24.4, 36.2) | 26.5 (23.7, 30.2) | 28.7 (25.3, 30.8) | 0.5351 | ||
| SV index | 40.2 (34.8, 43.6) | 40.8 (39.2, 46.6) | 40.2 (32.9, 45.5) | 38.9 (32.7, 42.8) | 0.3369 | ||
| Cardiac index | 2.5 (2.1, 2.8) | 2.5 (2.3, 3.2) | 2.3 (2.0, 2.8) | 2.4 (1.9, 2.8) | 0.3359 | ||
| RVEF | 57 (52, 62) | 58 (51, 63) | 56 (49, 64) | 58 (53, 62) | 0.7195 | ||
| Rest absolute MBF (mL/min/g) | 1.07 (0.98,1.36) | 1.31 (1.05, 1.59) | 1.07 (0.98, 1.37) | 1.07 (0.93, 1.20) | 0.1285 | ||
| Stress absolute MBF (mL/min/g) | 2.65 (2.24, 3.18) | 2.31 (1.95, 2.80) | 2.30 (1.97,3.07) | 3.11 (2.61, 3.80) | 0.0003 | ||
P-value from Kruskal-Wallis test for continuous variables or Chi-square/Fisher’s exact test for categorical variables. P-values <0.05 were considered statistically significant.
Continuous variables given as median (25th to 75th percentiles).
CAD indicates coronary artery disease; ECG, electrocardiogram; LV, left ventricle; LVEDV, left ventricle end diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricle end systolic volume; MBF, myocardial blood flow; METs, metabolic equivalents; RVEF, right ventricle ejection fraction; SV, stroke volume.
Morbidity
Seattle Angina Questionnaire, Cardiac Anxiety Questionnaire, and Work-Limitations Questionnaire scores did not differ by MPR level (Table 3). However, scores were notable for a significant amount of angina (average SAQ summary score 64 ± 20) and anxiety (average overall CAQ score 1.5 ± 0.6) in this total study population with INOCA. A comparison of SAQ scores from our study to scores from patients with stable CAD, post-PCI for refractory angina, and post-acute MI, as compiled from multiple registries (15), is shown in the Central Illustration. INOCA patients had lower SAQ scores, indicating worse symptoms, than patients with stable CAD and post MI, and were comparable to patients undergoing PCI for refractory angina. A comparison of CAQ scores in our study to previously published scores for patients with hypertrophic cardiomyopathy and sudden cardiac death (18) is shown in the Central Illustration; INOCA patients reported higher levels of cardiac anxiety than patients with a history of sudden cardiac death and were comparable to patients with hypertrophic cardiomyopathy. A comparison of CAQ/SAQ scores between INOCA patients with and without CMD showed no difference (Central Illustration). On linear regression analysis, CMD was not a significant predictor of SAQ summary score (β coefficient 1.02 ± 6.58, p=0.8774), nor of any of the SAQ subscores. Similarly, CMD was not a significant predictor of CAQ total score (β coefficient 0.14 ± 0.17, p=0.4148), nor of any of the CAQ subscores.
Table 3.
Quality of Life Questionnaire results in total and subdivided by myocardial perfusion reserve
| Clinical Characteristic | Reduced <2.0 (n (%)) |
Normal >2.4 (n (%)) |
|||
|---|---|---|---|---|---|
| Total Patients | 66 | 16 (24.2) | 23 (34.9) | 27 (40.9) | - |
| SAQ Summary Score (n=51)† | 64.0 ± 20.3 | 64.8 ± 19.5 | 67.5 ± 18.8 | 60.4 ± 22.3 | 0.5561 |
| SAQ: Physical Limitations (n=51) | 66.3± 24.8 | 58.1± 27.8 | 69.7± 23.9 | 68.4± 23.5 | 0.3930 |
| SAQ: Angina Stability (n=49) | 53.1± 28.7 | 56.3± 32.2 | 56.9± 26.9 | 47.4± 28.7 | 0.5518 |
| SAQ: Angina Frequency (n=51) | 73.9± 26.3 | 74.4± 25.8 | 75.9± 26.5 | 71.7± 27.6 | 0.8844 |
| SAQ: Treatment Satisfaction (n=49) | 76.8± 24.9 | 80.9± 27.0 | 78.2± 24.1 | 72.5± 25.0 | 0.6267 |
| SAQ: Disease Perception (n=48) | 49.6 ± 23.5 | 59.7± 21.6 | 52.6± 22.1 | 41.0± 23.7 | 0.074 |
| CAQ: Heart-Focused Attention (n=51) | 1.45± 0.77 | 1.51± 0.86 | 1.23± 0.57 | 1.60± 0.85 | 0.3348 |
| CAQ: Avoidance (n=51) | 1.45± 0.77 | 1.63± 0.78 | 1.29± 0.57 | 1.47± 0.92 | 0.4717 |
| CAQ: Fear (n=51) | 1.65± 0.60 | 1.73± 0.67 | 1.42± 0.58 | 1.82± 0.54 | 0.1211 |
| CAQ: Overall Score (n=51) | 1.54± 0.55 | 1.64± 0.55 | 1.33± 0.42 | 1.66± 0.63 | 0.1445 |
| WLQ-8: Time Demands (n=31) | 31.0± 30.1 | 35.9± 19.4 | 43.1± 38.1 | 20.5± 27.6 | 0.1901 |
| WLQ-8: Physical Demands (n=36) | 21.9± 24.9 | 20.3± 14.8 | 22.9± 31.0 | 21.9± 25.2 | 0.9755 |
| WLQ-8: Mental-interpersonal Demands (n=37) | 20.6± 22.3 | 16.7± 15.3 | 24.0± 32.6 | 20.3± 16.4 | 0.7677 |
| WLQ-8: Output Demands (n=31) | 25.0 ± 26.8 | 31.3± 20.0 | 32.5± 35.0 | 15.4± 21.7 | 0.2422 |
| WLQ-8: Overall score (n=37) | 23.3± 21.8 | 23.5± 15.1 | 28.6± 30.1 | 19.2± 17.7 | 0.5399 |
| Full workdays missed in last 2 weeks (n=31) | 1.10 ± 2.17 | 0.71 ± 1.50 | 1.38 ± 1.41 | 1.13 ± 2.73 | 0.8473 |
| Days where part of workday missed in last 2 weeks (n=31) | 1.59 ± 2.83 | 1.43 ± 1.51 | 1.38 ± 1.69 | 1.13 ± 2.75 | 0.9450 |
P-value from 1-way ANOVA. P-values <0.05 were considered statistically significant.
Continuous variables given as mean ± standard deviation.
CAQ indicates Cardiac Anxiety Questionnaire; SAQ, Seattle Angina Questionnaire; WLQ, Work Limitations Questionnaire.
Central Illustration: Morbidity in INOCA and CMD.
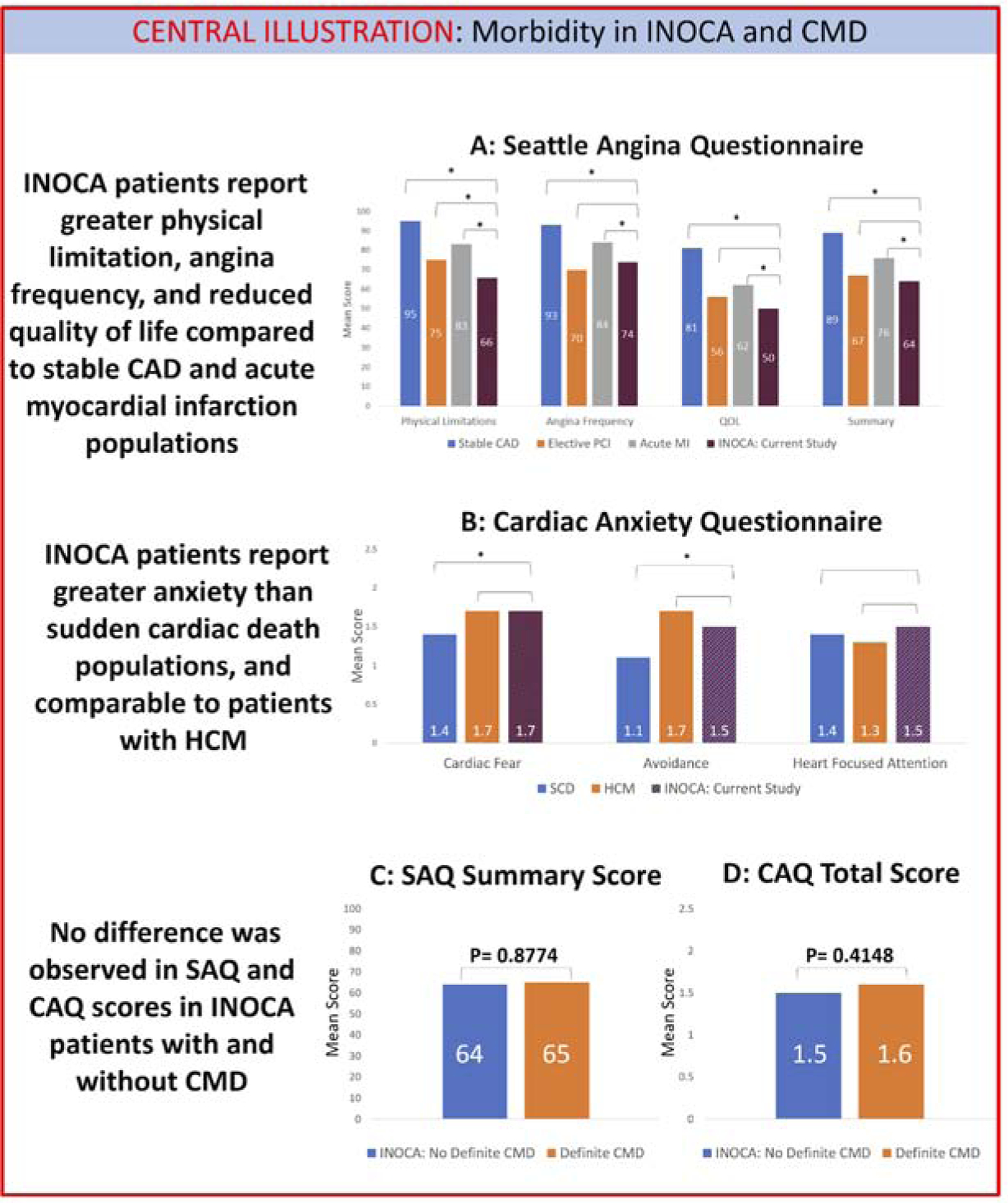
A: INOCA patients report significant angina, with lower SAQ scores than patients with stable CAD or presenting with acute MI, and comparable to patients undergoing elective PCI for refractory angina. B: INOCA patients also report significant cardiac anxiety, with CAQ scores higher than patients with sudden cardiac death and comparable to patients with hypertrophic cardiomyopathy. C: There is no difference in SAQ summary score between INOCA patients with and without CMD. D: There is no difference in CAQ total score between INOCA patients with and without CMD. * indicates P-value <0.05 on Student t test. CAD=coronary artery disease; CAQ=Cardiac Anxiety Questionnaire; INOCA=ischemia and no obstructive coronary disease; HCM=hypertrophic cardiomyopathy; MI=myocardial infarction; PCI=percutaneous coronary intervention; SAQ=Seattle Angina Questionnaire; SCD=sudden cardiac death.
WLQ scores showed significant work limitations (average score 23 ± 22) and time missed from work, with an average of 1.1 full workdays missed per 2 weeks in the overall study population. Economic analysis of the WLQ/time loss questionnaires indicated an estimated annual cost per INOCA patient due to absenteeism of $9,819 and cost due to presenteeism of $4,158, for a total per patient annual cost of $13,977. Applying this economic impact to an estimated 1.5 million INOCA patients in the workforce in the United States, the total estimated annual cost due to productivity loss from INOCA could be as high as $21 billion (Figure 1).
Figure 1: Potential economic impact from productivity loss in the INOCA population.
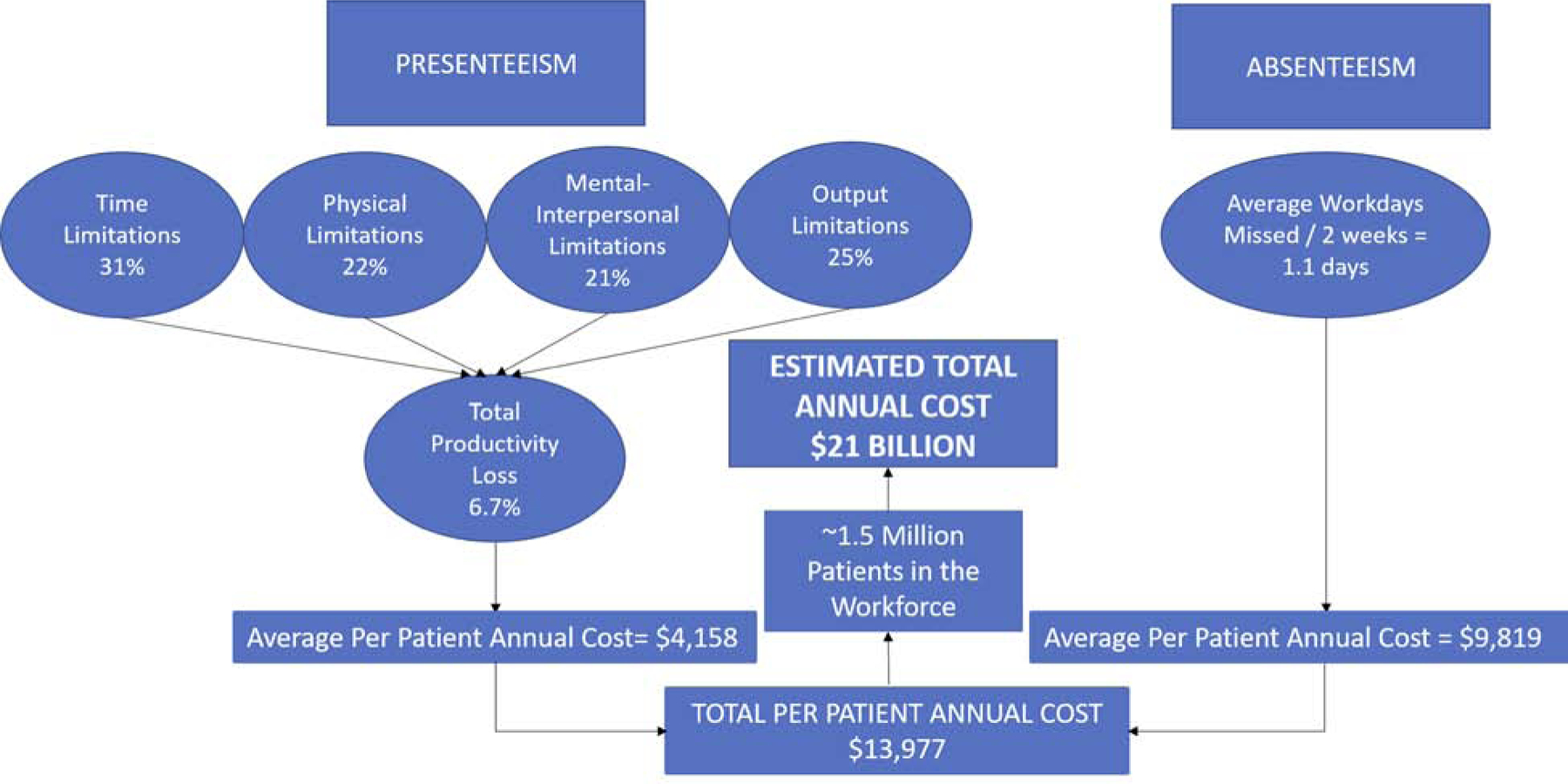
Results from Work Limitations Questionnaire and time loss questionnaire indicate the potential for significant economic impact from INOCA, with estimated total annual cost in the United States of $21 billion. INOCA=ischemia and no obstructive coronary disease.
Predictors of CMD
The significant predictors of definite CMD on univariable logistic regression analysis were glomerular filtration rate (GFR), history of hypertension, and exercise workload on exercise stress testing. There was a significant difference in METs performed between the reduced MPR patients with definite CMD (6.4 ± 1.7) and those with borderline or normal MPR without definite CMD (9.3 ± 3.2), p=0.027 as shown in Figure 2. Body-mass index (BMI) and total cholesterol were borderline significant (p<0.10) on univariable regression and were included in the multivariable analysis. Given that 16 patients had definite CMD in this study, the multivariable logistic regression model was limited to 2 variables. Using ROC curve analysis, the two variables that produced the model with the highest area under the curve (AUC) were BMI and GFR (Table 4). The AUC for this model was 0.79 (95% CI 0.65–0.93).
Figure 2: Exercise capacity in CMD patients.
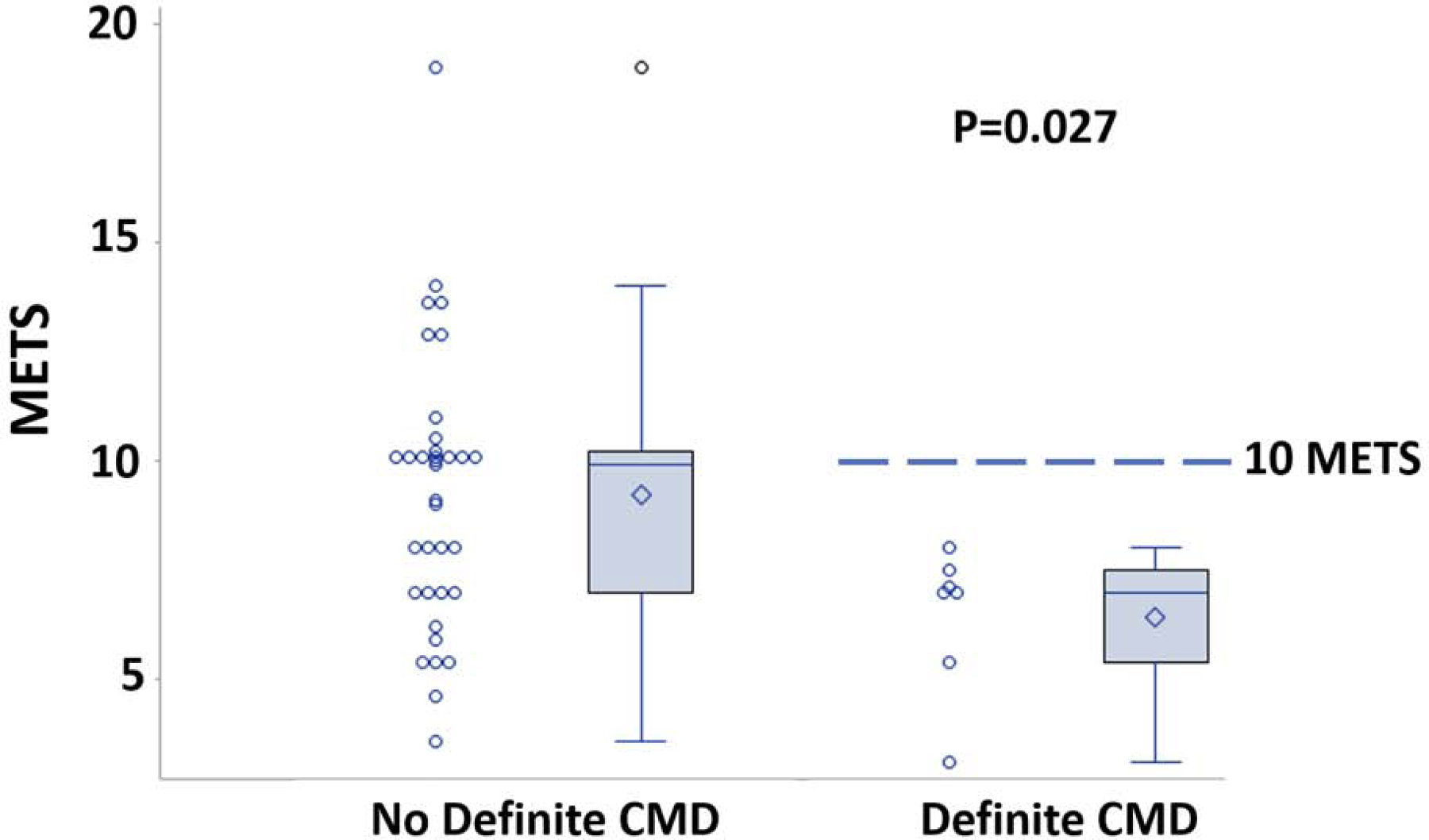
Significant difference in exercise capacity was observed between patients with and without definite CMD. No patients with CMD achieved ≥10 METS. CMD=coronary microvascular dysfunction; METS=metabolic equivalents.
Table 4:
Univariable/Multivariable Logistic Regression Models for Predictors of CMD.
| Univariable Logistic Analysis | Multivariable Logistic Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | χ2 | Odds Ratio (95% CI) | P-value | χ2 | Odds Ratio (95% CI*) | P-value* |
| GFR (per mL/min/1.73m2) | 5.67 | 0.951 (0.909 – 0.989) | 0.017 | 6.28 | 0.95 (0.90 – 0.99) | 0.012 |
| BMI (per kg/m2) | 3.43 | 1.107 (0.998 – 1.242) | 0.064 | 4.07 | 1.13 (1.0 – 1.28) | 0.044 |
| HTN | 4.95 | 10.9 (1.96 – 240) | 0.026 | |||
| Total cholesterol (per mg/dL) | 3.66 | 1.017 (1.0 – 1.035) | 0.056 | |||
| METs on stress (per MET) | 4.46 | 0.634(0.384 – 0.915) | 0.035 | |||
P-values <0.05 were considered statistically significant.
BMI indicates body-mass index; GFR, glomerular filtration rate; HTN, hypertension; METs, metabolic equivalents.
DISCUSSION
The primary findings of this study include: 1) there is a high prevalence of CMD in the INOCA population; 2) the INOCA population has significant morbidity and INOCA creates a high economic burden as assessed through quality of life metrics; and 3) no difference was observed in morbidity between INOCA patients with and without CMD.
Patient Characteristics/Prevalence
While there remains no universally accepted flow/perfusion reserve cutpoint to define CMD, and various studies have used flow/perfusion reserve values ranging from 1.5–2.6 (13), an MPR greater than 2.4 is generally considered normal. In our prior study evaluating stress CMR to measure MPR, we found a mean MPR of 2.93 ± 0.44 in 20 health controls (11). Defining abnormal MPR as less than 2.4, we observed a high prevalence of borderline or reduced MPR in the INOCA population (59%). This high prevalence is similar to other studies in the INOCA population, in which approximately 50–65% of patients presenting with INOCA have reduced flow/perfusion reserve (5). It is noteworthy that the INOCA patients in our study, regardless of MPR, had a significant risk factor burden (with high rates of hypertension, hyperlipidemia, smoking, and family history of CAD). This finding is consistent with high clinical risk in INOCA patients irrespective of CMD.
Whereas there is a focus on CMD specifically in women, and many of the larger studies in the CMD literature have included only women (27), 29/66 (43.9%) of our study subjects were men. In one of the largest studies including both men and women, Murthy et al. examined the prevalence of CMD among patients presenting with INOCA in 405 men and 813 women (28). Defining CMD as CFR <2.0 using PET, they found no difference in the prevalence of CMD in men and women (51% vs. 54%, p=0.39). Similarly, there was no difference in our study in the prevalence of men vs. women with possible or definite CMD (55% vs. 62%, p=0.57) These findings suggest a high prevalence of CMD in the INOCA population regardless of gender, and CMD should be considered in both men and women presenting with INOCA.
Morbidity
Our study illustrates the substantial effects of INOCA on quality of life. Our patients with INOCA reported lower SAQ scores, indicating worse symptoms, than those with stable CAD or patients tested after an acute MI; their scores were comparable to patients undergoing elective PCI for refractory angina. This comparison illustrates the significant symptom burden in the INOCA patient population, which represents an unmet clinical need.
INOCA patients in our study demonstrated high CAQ scores indicating a significant burden of cardiac anxiety. These scores were higher than those in sudden cardiac death survivors and patients post-acute MI (18). These results are consistent with a study that showed an inverse correlation between degree of cardiac injury (as measured with ST-elevation and troponin rise) and cardiac anxiety (as measured using CAQ) in patients presenting with an acute coronary syndrome (29). The authors hypothesized that patients without known significant cardiac injuries can have anxiety driven by diagnostic uncertainty of the cause of their symptoms. This hypothesis is plausible in the INOCA population, as the lack of a diagnosis post-angiography has the potential to cause a significant amount of anxiety with no symptom etiology identified. Testing for microvascular dysfunction may assuage anxiety by making a definitive diagnosis or ruling-out additional cardiac dysfunction.
We did not observe a difference in SAQ or CAQ scores by MPR, suggesting that patients with CMD do not experience more severe symptoms compared to INOCA patients without CMD. This similarity in scores is comparable to the ImProve diagnOsis and treatment of Women with angina pEctoris and micRovessel disease (iPOWER) study (27), in which there were no differences in SAQ Anginal Stability, Anginal Frequency, and Disease Perception subscores by flow reserve. However, iPOWER did note a significant worsening of scores for Physical Limitation and Disease Perception in patients with lower flow reserve. Our small sample size (n=48) may have been insufficient to detect such a difference in these subscores.
The WLQ-8 and time loss questionnaire data illustrate the significant economic impact of INOCA, with an estimated annual cost from presenteeism/absenteeism in the United States of $21 billion (Figure 1). For comparison, the annual productivity loss due to coronary artery disease has been estimated at $55 billion (30,31). Notably, productivity loss from CAD was estimated to be primarily due to presenteeism ($43 billion) rather than absenteeism ($12 billion), while the economic impact from INOCA in our study was primarily driven by absenteeism ($15 billion) rather than presenteeism ($6 billion).
Clinical Predictors
The two clinical variables that were most predictive of definite CMD in our multivariable analysis were BMI and GFR. Obesity has been shown to be associated with a reduction in flow reserve (32). In a large study using PET to evaluate CFR, Bajaj et al. observed a J-shaped relationship between CFR and BMI, with CFR decreasing linearly with increasing BMI in obese patients (33). GFR has also been shown to be a significant predictor of flow reserve (34). Chade et al. examined 605 patients with normal or mild nonobstructive CAD, and evaluated CFR using intracoronary adenosine, finding significantly lower CFR values in patients with GFR <60 mL/min/1.73m2. They hypothesized that microvascular dysfunction occurs in both coronary and renal vascular beds prior to development of significant obstructive disease and represents an early marker of disease (35).
While we did observe a significant reduction in exercise capacity between INOCA patients with CMD and those without (Figure 2), and exercise capacity was a significant predictor of MPR on univariable regression analysis, it was not significant on multivariable analysis. Inclusion of exercise capacity into our multivariable model was limited by only a subset of subjects (n=42) in the study underwent exercise testing. In the Women’s Ischemia Syndrome Evaluation (WISE) study, the Duke Activity Status Index score, a self-reported estimate of functional capacity, had a significant correlation to flow reserve on univariate analysis, but was not significant when controlling for age (36). Bechsgaard et al found that CMD patients had no difference in self-reported physical activity levels to asymptomatic controls, but were found to have diminished exercise capacity on cardiopulmonary exercise testing (37). In addition, there have been small studies investigating exercise as treatment for CMD, in which regular exercise has led to improvement in patient symptoms, exercise capacity, and measures of ischemia (38,39). These results suggest that further evaluation is needed to ascertain the relationship between exercise and CMD, both as a predictor and potential treatment.
Study Limitations
This was a single-center study with a relatively limited sample size and associated limitation in the number of predictors that could be included in multivariable regression analysis. A small study population limits comparison of study cohort characteristics. Moreover, the sample size limits the complexity of the statistical models that can be fit. These limitations suggest that further exploration and investigation with larger populations is warranted. Additionally, the lack of exercise data in all patients reduced our ability to fully test this variable. Another limitation was that not all patients in the study completed quality of life questionnaires. The work-life questionnaire results may be impacted by report completion near the time of medical procedures. Furthermore, there is limited data on the MPR cut-off to diagnose CMD using CMR; we utilized 20 healthy controls to establish normal values, but the optimal CMD cut-point will need to be validated in larger populations. In addition, the diagnostic criteria for CMD includes not only measurement of flow reserve but also testing for vasospasm.(40) By not assessing for this cause of INOCA, we may not have captured all the CMD patients within our study population.
CONCLUSION
In this prospective cohort study, we have characterized the quality of life and economic burden of INOCA and examined prevalence and predictors of CMD. Patients with INOCA were found to have a significant burden of angina symptoms and cardiac anxiety exceeding morbid populations such as those post-MI and post-sudden cardiac death. INOCA led to significant rates of both absenteeism and presenteeism, resulting in a substantial economic impact. Reduced or borderline MPR indicative of definite or possible CMD was found to be highly prevalent within the INOCA population. However, there was no difference in reported symptoms between INOCA patients with and without CMD, suggesting there is significant morbidity in the INOCA population regardless of etiology.
PERSPECTIVES.
Competency in Medical Knowledge:
Patients with symptoms of ischemia and no obstructive coronary disease are found to have symptom burden exceeding other morbid cardiac populations. There is no difference in symptoms in INOCA patients with and without CMD.
Translational Outlook:
This analysis highlights the substantial symptom burden and reduced quality of life in patients with symptoms of ischemia and no obstructive coronary disease. Further research is needed in larger populations to confirm this finding and to definitively determine the impact of coronary microvascular dysfunction.
Acknowledgements:
The authors would like to acknowledge our study coordinators, Robyn McKenzie RN and Isabelle Giordano RN, as well as our research CMR technologists Jose Reyes RT(R)MR and Joseph Hylton RT(R)MR.
Funding:
This study was funded by NIH K23 HL119620, T32 EB003841, a University of Virginia clinical research grant, and Astellas Pharma.
Disclosures:
Dr Bourque owns equity in Locus Health and is a consultant for Pfizer and General Electric. Dr Salerno receives research support from Siemens. The other authors report no conflicts.
ABBREVIATIONS:
- BMI
body-mass index
- CAQ
Cardiac Anxiety Questionnaire
- CMD
coronary microvascular dysfunction
- CMR
cardiac magnetic resonance
- INOCA
ischemia and no obstructive coronary artery disease
- GFR
glomerular filtration rate
- MBF
myocardial blood flow
- METS
metabolic equivalents
- MPI
myocardial perfusion imaging
- MPR
myocardial perfusion reserve
- PET
positron emission tomography
- SAQ
Seattle Angina Questionnaire
- SPECT
single-photon emission computed tomography
- WLQ
Work Limitations Questionnaire
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Patel MR, Peterson ED, Dai D, et al. Low Diagnostic Yield of Elective Coronary Angiography. N Engl J Med 2010;362(10):886–95. Doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL Ischemia and No Obstructive Coronary Artery Disease (INOCA). Circulation 2017;135(11):1075–92. Doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of Hospital Admission and Repeat Angiography in Angina Pectoris Patients with and without Coronary Artery Disease: A Registry-Based Cohort Study. PLoS One 2014;9(4):e93170. Doi: 10.1371/journal.pone.0093170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33(6):734–44. Doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 5.Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC Cardiovasc Imaging 2015;8(2):210–20. Doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camici PG, d’Amati G, Rimoldi O Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12(1):48–62. Doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 7.Christian TF, Rettmann DW, Aletras AH, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology 2004;232(3):677–84. Doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 8.Schuster A, Zarinabad N, Ishida M, et al. Quantitative assessment of magnetic resonance derived myocardial perfusion measurements using advanced techniques: Microsphere validation in an explanted pig heart system. J Cardiovasc Magn Reson 2014;16(1). Doi: 10.1186/s12968-014-0082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engblom H, Xue H, Akil S, et al. Fully quantitative cardiovascular magnetic resonance myocardial perfusion ready for clinical use: A comparison between cardiovascular magnetic resonance imaging and positron emission tomography. J Cardiovasc Magn Reson 2017;19(1). Doi: 10.1186/s12968-017-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalnapurkar S, Zarrini P, Mehta PK, et al. Role of Stress Cardiac Magnetic Resonance Imaging in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease. J Radiol Nurs 2017;36(3):180–3. Doi: 10.1016/j.jradnu.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zorach B, Shaw PW, Bourque J, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson 2018;20(1):14. Doi: 10.1186/s12968-018-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerosch-Herold M Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12(1). Doi: 10.1186/1532-429X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löffler AI, Bourque JM Coronary Microvascular Dysfunction, Microvascular Angina, and Management. Curr Cardiol Rep 2016;18(1):1. Doi: 10.1007/s11886-015-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25(2):333–41. [DOI] [PubMed] [Google Scholar]
- 15.Chan PS, Jones PG, Arnold SA, Spertus JA Development and Validation of a Short Version of the Seattle Angina Questionnaire. Circ Cardiovasc Qual Outcomes 2014;7(5):640–7. Doi: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eifert GH, Thompson RN, Zvolensky MJ, et al. The Cardiac Anxiety Questionnaire: development and preliminary validity. Behav Res Ther 2000;38(10):1039–53. Doi: 10.1016/S0005-7967(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 17.van Beek MHCT, Mingels M, Voshaar RCO, et al. One-year follow up of cardiac anxiety after a myocardial infarction: A latent class analysis. J Psychosom Res 2012;73(5):362–8. Doi: 10.1016/j.jpsychores.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Rosman L, Whited A, Lampert R, Mosesso VN, Lawless C, Sears SF Cardiac anxiety after sudden cardiac arrest: Severity, predictors and clinical implications. Int J Cardiol 2015;181:73–6. Doi: 10.1016/j.ijcard.2014.11.115. [DOI] [PubMed] [Google Scholar]
- 19.Lerner D, Amick BC, Rogers WH, Malspeis S, Bungay K, Cynn D The Work Limitations Questionnaire. Med Care 2001;39(1):72–85. [DOI] [PubMed] [Google Scholar]
- 20.Walker TJ, Tullar JM, Diamond PM, Kohl HW, Amick BC Validity and Reliability of the 8-Item Work Limitations Questionnaire. J Occup Rehabil 2017;27(4):576–83. Doi: 10.1007/s10926-016-9687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner D, Rogers WH CH. Technical Report: Scoring the Short Form of the Work Limitations Questionnaire (WLQ-SF) 2009.
- 22.Berger ML, Murray JF, Xu J, Pauly M Alternative valuations of work loss and productivity. J Occup Environ Med 2001;43(1):18–24. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RJ, Bates P Measuring Health-Related Productivity Loss. Popul Health Manag 2011;14(2):93–8. Doi: 10.1089/pop.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Department of Labor. Employer costs for Employee Compensation: December 2018. Washington, DC; 2018. [Google Scholar]
- 25.Nicholson S, Pauly MV, Polsky D, Sharda C, Szrek H, Berger ML Measuring the effects of work loss on productivity with team production. Health Econ 2006;15(2):111–23. Doi: 10.1002/hec.1052. [DOI] [PubMed] [Google Scholar]
- 26.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN Ischemia and No Obstructive Coronary Artery Disease (INOCA): What Is the Risk? J Am Heart Assoc 2018;7(17). Doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mygind ND, Michelsen MM, Pena A, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J Am Heart Assoc 2016;5(3):e003064. Doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy VL, Naya M, Taqueti VR, et al. Effects of Sex on Coronary Microvascular Dysfunction and Cardiac Outcomes. Circulation 2014;129(24):2518–27. Doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Beek MHCT, Oude Voshaar RC, van Deelen FM, van Balkom AJLM, Pop G, Speckens AEM Inverse Correlation Between Cardiac Injury and Cardiac Anxiety. J Cardiovasc Nurs 2014;29(5):448–53. Doi: 10.1097/JCN.0b013e3182982550. [DOI] [PubMed] [Google Scholar]
- 30.Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin 2006;22(6):1203–10. Doi: 10.1185/030079906X112552. [DOI] [PubMed] [Google Scholar]
- 31.Gordois AL, Toth PP, Quek RG, Proudfoot EM, Paoli CJ, Gandra SR Productivity losses associated with cardiovascular disease: a systematic review. Expert Rev Pharmacoecon Outcomes Res 2016;16(6):759–69. Doi: 10.1080/14737167.2016.1259571. [DOI] [PubMed] [Google Scholar]
- 32.Ahmari SAL, Bunch TJ, Modesto K, et al. Impact of individual and cumulative coronary risk factors on coronary flow reserve assessed by dobutamine stress echocardiography. Am J Cardiol 2008;101(12):1694–9. Doi: 10.1016/j.amjcard.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj NS, Osborne MT, Gupta A, et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol 2018;72(7):707–17. Doi: 10.1016/j.jacc.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohandas R, Segal MS, Huo T, et al. Renal Function and Coronary Microvascular Dysfunction in Women with Symptoms/Signs of Ischemia. PLoS One 2015;10(5):e0125374. Doi: 10.1371/journal.pone.0125374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006;69(2):266–71. Doi: 10.1038/SJ.KI.5000031. [DOI] [PubMed] [Google Scholar]
- 36.Wessel TR., Arant CB, McGorray SP, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin Cardiol 2007;30(2):69–74. Doi: 10.1002/clc.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bechsgaard DF, Hove JD, Suhrs HE, et al. Women with coronary microvascular dysfunction and no obstructive coronary artery disease have reduced exercise capacity. Int J Cardiol 2019;293:1–9. Doi: 10.1016/j.ijcard.2019.07.048. [DOI] [PubMed] [Google Scholar]
- 38.Szot W, Zajaç J, Kubinyi A, Kostkiewicz M The effects of cardiac rehabilitation on overall physical capacity and myocardial perfusion in women with microvascular angina. Kardiol Pol 2016;74(5):431–8. Doi: 10.5603/KP.a2015.0198. [DOI] [PubMed] [Google Scholar]
- 39.de Carvalho EEV, Santi GL, Crescêncio JC, et al. Pilot study testing the effect of physical training over the myocardial perfusion and quality of life in patients with primary microvascular angina. J Nucl Cardiol 2015;22(1):130–7. Doi: 10.1007/s12350-014-9949-6. [DOI] [PubMed] [Google Scholar]
- 40.Ong P, Camici PG, Beltrame JF, et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. Doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]


