Abstract
During ~5 days of Drosophila neurogenesis (late embryogenesis to the beginning of pupation), a limited number of neural stem cells produce ~200,000 neurons comprising hundreds of cell types. To build a functional nervous system, neuronal types need to be produced in the proper place, appropriate number, and correct time. We discuss how neural stem cells (neuroblasts) obtain “area codes” for their positions in the nervous system (spatial patterning) and how they keep time to sequentially produce neurons with unique fates (temporal patterning). We focus on specific examples that demonstrate how a relatively simple patterning system (Notch) can be used reiteratively to generate different neuronal types. We also speculate how different modes of temporal patterning operating over short time periods versus long time periods might be linked. We end by discussing how specification programs are integrated and lead to the terminal features of different neuronal types.
Keywords: Drosophila, neurogenesis, spatial patterning, temporal patterning, Notch, sensory organ precursor
Introduction
The nervous system is comprised of a staggering number of cells belonging to many cell types (Allen et al. 2020; Konstantinides et al. 2018; Davie et al. 2018; Croset, Treiber, and Waddell 2018). These neuronal types are built following sets of developmental rules that begin to be executed in the early embryo (before any neurons exist) and unfold through space and time. Although how we define neuronal types has changed as our tools to assay different aspects of cells have emerged, neuronal morphology, connectivity, physiology, and molecular profile all contribute to identify neuronal classes. The advancement of single-cell mRNA sequencing has further revealed many more molecularly defined neuronal types that exist in the developing and adult nervous system. This knowledge has led to the next main challenge: linking developmental specification programs to specific neuronal types in the adult and attempting to understand how terminal features of neurons (e.g., their morphology, neurotransmitter expression, connectivity etc.) are regulated. Studying developmental mechanisms such as spatial and temporal patterning provides a framework for understanding how neuronal diversity is generated (Chris Q Doe 2017; Holguera and Desplan 2018). Nevertheless, we are just beginning to link these general mechanisms to specific neuronal fates and the terminal features of neurons. In this review we provide a general overview of some key developmental rules in Drosophila. We begin by reviewing how spatial patterning helps determine where neuronal types will be produced in the central nervous system (comprised of the central brain, optic lobes, and ventral nerve cord (VNC)). We then discuss how temporal patterning (i.e., sequential birth of neuronal types) contributes to diversifying neurons. We also highlight the roles of Notch signaling in fate specification. We emphasize that in most cases we have very little knowledge about how patterning programs are integrated to control neuronal fate. We end by focusing on studies that help us understand how the early patterning events that specify neuronal types control and trigger the following steps of differentiation and the terminal features of neurons.
Spatial patterning of ventral nerve cord and central brain neuroblasts
Drosophila Ventral Nerve Cord (VNC) neuroblasts are perhaps the best studied model of spatial patterning (Bhat 1999; James B. Skeath and Thor 2003; Technau, Berger, and Urbach 2006). The VNC is comprised of a sequence of 14 repeated segments, each divided into two hemisegments by the midline. Within each hemisegment in the embryo, 30 neuroblasts are produced from a sheet of neuroectodermal cells and become organized in an array of rows and columns (Hartenstein and Campos-Ortega 1984). The array is built during embryogenesis as neuroblasts delaminate from the neuroepithelium in five successive waves over the course of ~3 hours (C Q Doe 1992). The identities of neuroblasts within an array are highly stereotypical, thus allowing for detailed analyses of how spatial identities arise. Neuroblasts in different segments are serial homologs and acquire similar but not identical fates based on gene expression profiles since they are essentially located in the same position within the array (C Q Doe 1992). For example, a neuroblast in row 4 and column 2 (4–2) in a thoracic hemisegment is similar to neuroblast 4–2 in an abdominal hemisegment based on the genes they express and the neurons they produce.
How do neuroblasts obtain specific spatial identities? The genes that specify body segmental patterning also function in neurogenesis: Gap, pair-rule, and segment polarity genes act in a sequential manner to regulate the segmentation of the developing embryo along the anterior-posterior (A-P) axis (Bhat 1999; Technau, Berger, and Urbach 2006). The segment polarity genes are expressed in repeating stripes in the neuroectoderm prior to neuroblast delamination (Figure 1A). Neuroblasts in each segment are partially specified by these factors, which include the spatial transcription factors (sTFs) Gooseberry (Gsb), Engrailed (En) and Invected (Inv), the secreted proteins Wingless (Wg) and Hedgehog (Hh) and its receptor Patched (Ptc) (Figure 1A) (McDonald and Doe 1997; J B Skeath et al. 1995; Deshpande et al. 2001; Chu-LaGraff and Doe 1993; Matsuzaki and Saigo 1996). The Cartesian coordinate system that provides neuroblasts with unique spatial identities is completed along the dorsal-ventral (D-V) axis by the expression of the sTFs Ventral nervous system defective (Vnd, medial column), Intermediate neuroblast defective (Ind, intermediate column), and Drop (Dr, lateral column; also called Msh) in three distinct columns (Figure 1A) (Von Ohlen and Doe 2000; McDonald et al. 1998; T Isshiki, Takeichi, and Nose 1997). The strict D-V boundaries of expression are maintained by cross-repressive interactions between these sTFs (Figure 1A) (Von Ohlen and Doe 2000).
Figure 1. Spatial patterning programs that operate in the embryonic VNC and developing optic lobes.
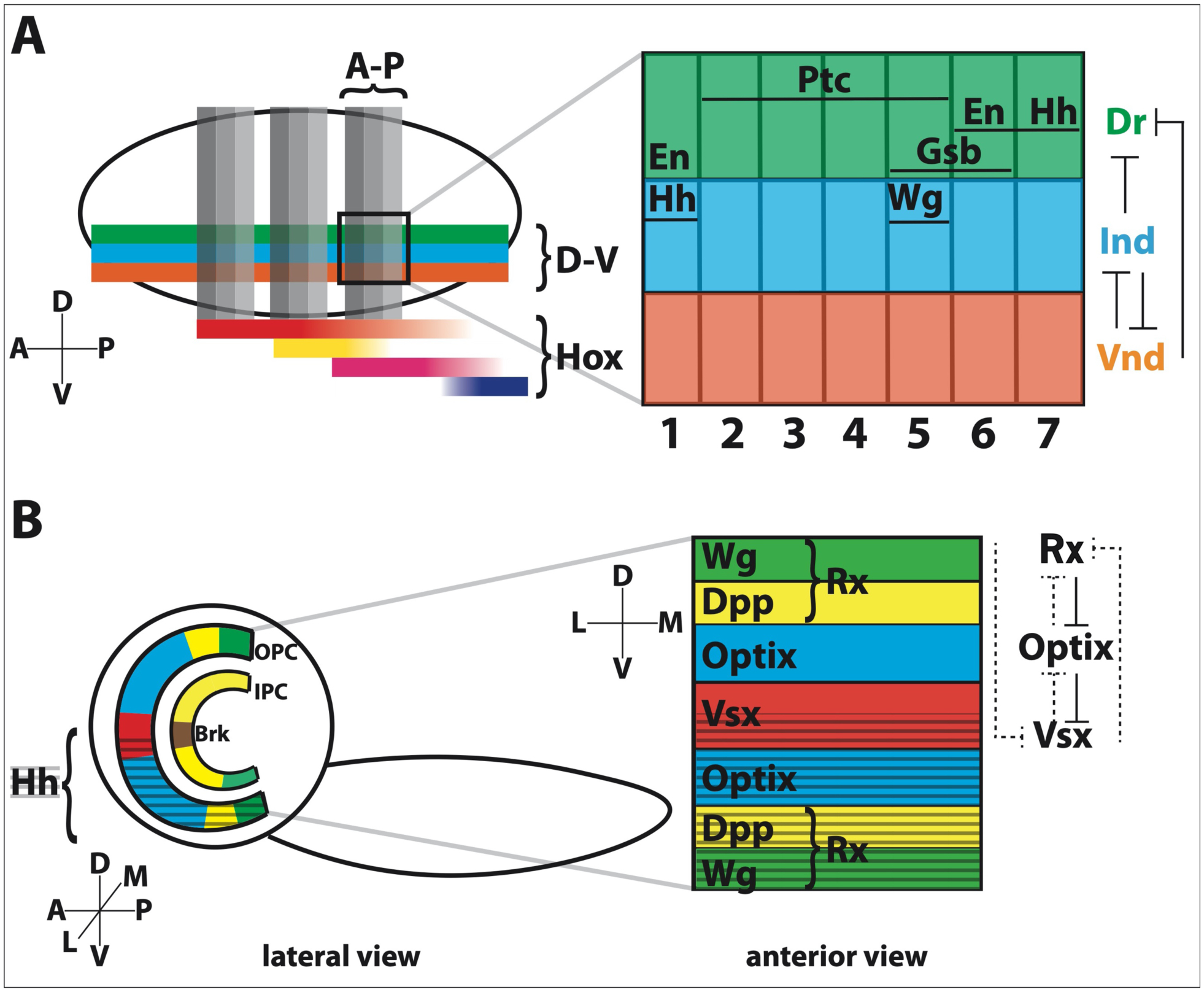
A. A simplified schematic showing how anterior-posterior (A-P) and dorsal-ventral (D-V) patterning leads to neuroblasts with unique spatial identities. Along the A-P axis, the VNC is subdivided into 14 repeated segments, represented here by vertical gray bars. Within each segment, the segment-polarity genes define different rows (numbered 1–7). The D-V axis is subdivided into three columns, represented here by green, blue, and orange bars. Each segment is further defined by the expression of different Hox genes. One segment is magnified to show the expression domains of the different sTFs in each array. Only the interactions between the D-V sTFs are shown for simplicity. En: Engrailed; Hh: Hedgehog; Ptc: Patched; Wg: Wingless; Gsb: Gooseberry; Dr: Drop (also called Msh); Ind: Intermediate neuroblasts defective; Vnd: Ventral nervous system defective.
B. A simplified larval brain is viewed from the side (lateral) to show the crescent shape of the OPC and IPC, which give rise to optic lobe neuroblasts. The OPC can be subdivided into 8 compartments and the IPC into 4 compartments along the D-V axis. The ventral hedgehog (hh) domain (marked by gray hashed lines) is a remnant of earlier developmental expression. Unlike the VNC, there is no indication that the OPC or IPC are spatially patterned along a second axis (i.e., the medial-lateral axis representing the posterior-anterior retina), which is illustrated by rotating the view of the OPC by 90 degrees (clockwise). The boundaries defining D-V compartments are maintained by interactions between the sTFs themselves.
Although serially homologous neuroblasts have nearly identical expression patterns, they can produce different types of neurons or glia depending on their position along the body A-P axis (e.g., in abdominal or thoracic segments), which is controlled by the expression of Hox genes (Figure 1A) (G Udolph et al. 1993). For example, in the abdominal segments, expression of the Hox gene abdominal-A (abd-A) triggers early apoptosis of abdominal neuroblasts while serially homologous thoracic neuroblasts specified by the Hox gene antennapedia (antp) continue dividing and produce neuronal types not present in the abdomen (Berger et al. 2005; Bello, Hirth, and Gould 2003; Karlsson, Baumgardt, and Thor 2010).
Central brain neurons are generated by ~100 neuroblasts (Younossi-Hartenstein et al. 1996). Although their spatial organization into a grid-like array is less apparent, the central brain neuroectoderm also expresses the D-V genes Vnd, Ind, and Dr and well as the A-P segment polarity genes that define VNC neuroblasts (Urbach and Technau 2003). However, unlike in the VNC, central brain neuroectoderm expresses additional A-P and D-V genes such as Empty spiracles (Ems) and Nkx6, respectively (Urbach and Technau 2003, 2004; Seibert, Volland, and Urbach 2009; Seibert and Urbach 2010). Cross repression between Vnd, Ind, and Dr, combined with activation or repression of these sTFs by Ems helps subdivide the neuroectoderm into discrete domains that specify the unique spatial identities of central brain neuroblasts that sequentially produce stereotyped sets of neurons (H. H. Yu et al. 2013; Y.-J. Lee et al. 2020; Ito et al. 2013).
Spatial patterning of optic lobe neuroblasts
Unlike central brain and VNC neuroblasts, which are produced during embryogenesis, optic lobe neuroblasts are born during late larval stages from two crescent-shaped neuroepithelia called the outer and inner proliferation centers (OPC and IPC) (Ngo, Andrade, and Hartenstein 2017). The adult optic lobes are comprised of the lamina, medulla, lobula and lobula plate neuropils, which receive and sequentially process visual information from photoreceptors in the retina. The OPC mostly gives rise to neurons of the lamina and medulla while the smaller IPC produces neuroblasts that give rise to neurons in the lobula and lobula plate (Hadjieconomou, Timofeev, and Salecker 2011; Hofbauer and Campos-Ortega 1990).
Beginning at late larval stages, neuroepithelial cells at the medial edge of the OPC crescent are transformed into neuroblasts that will generate medulla neurons that become innervated by photoreceptors from the posterior part of the retina. A “proneural wave” of neuroblast formation then extends towards the lateral edge of the crescent, progressively giving rise to neurons that process information from more anterior parts of the retina (X. Li et al. 2013; Sato, Suzuki, and Nakai 2013; Suzuki et al. 2013). At the same time, starting at the lateral most edge of the OPC, the epithelium is converted into progenitors called lamina precursor cells (LPCs) that give rise to lamina neurons in response to signals from photoreceptors that are also born sequentially in the eye disc (Huang and Kunes 1996; Huang, Shilo, and Kunes 1998; Fernandes et al. 2017). Precursors born at the dorsal and ventral tips of the lateral OPC give rise to lamina wide field neurons, which send projections to both the medulla and lamina (Chen et al. 2016).
The medulla neuropil is the largest optic neuropil and is composed of ~40,000 neurons divided into ~800 columns, i.e. 800 similar parallel circuits, each of which processes information from the ~800 pixels/ommatidia of the retina. These neurons can be split into two main types: those that innervate a single column (uni-columnar) and thus exist as 800 copies, and those that innervate and integrate information from multiple columns (multi-columnar); these are thus present in less than ~800 times (Fischbach and Dittrich 1989). The current model posits that each medulla neuroblast produces the entire repertoire of uni-columnar neurons, while multi-columnar neurons are only generated by subsets of neuroblasts. Uni-columnar neurons thus ignore OPC spatial patterning while the identity (and number) of multi-columnar neuronal types depends on the region where they were born (Erclik et al. 2017; Courgeon and Desplan 2019b, 2019a). Thus far, there is no evidence to suggest that neuroblasts born at different times during larval or pupal life (i.e. receiving photoreceptor input along the A-P axis) are differentially patterned. However, along the D-V axis, the OPC is spatially patterned into at least 8 domains (or compartments) by sTFs and signaling molecules (Figure 1B) (Erclik et al. 2017; Gold and Brand 2014; Erclik et al. 2008). First, the OPC can be split into dorsal and ventral domains by the immortalized expression of hedgehog (a remnant of its embryonic expression pattern since hedgehog is not expressed by the larval OPC) (Chang et al. 2001; Erclik et al. 2017). The dorsal and ventral tips of the OPC crescent are labeled by the expression of Retinal Homeobox (Rx), which can be further subdivided into two regions that either express wingless (wg) or decapentaplegic (dpp) (Gold and Brand 2014; Dearborn and Kunes 2004; Perez and Steller 1996). The main parts of each dorsal and ventral arm of the crescent are marked by the expression of Optix while the central region is marked by Visual system homeobox 1 (Vsx1). These spatial domains are established early during embryogenesis as lineage restricted compartments, but the boundaries between them are later regulated by cross-regulation among the sTFs themselves, similar to how VNC sTFs cross-regulate one another (Figure 1B) (Erclik et al. 2008, 2017; Gold and Brand 2014; Hakes, Otsuki, and Brand 2018).
The IPC similarly can be divided into four unique spatial domains, which ultimately contribute to diversifying neurons (Figure 1B) (Apitz and Salecker 2015; Pinto-Teixeira et al. 2018). The central region expresses Brinker (Brk) and is surrounded by two Dpp expressing domains (Figure 1B). These three regions give rise to migrating progenitors that mature into neuroblasts in a region called the distal-IPC, which will produce C and T neurons before producing T4 and T5 neurons. The ventral tip of the IPC (also called the surface-IPC) expresses Wingless (Wg) and produces three lobula neuron clusters (Apitz and Salecker 2018). Similar to the OPC, it is known that Wg induces the expression of Dpp in the adjacent epithelial domains (Kaphingst and Kunes 1994; Apitz and Salecker 2018). In addition, cross-repressive interactions between Dpp and Brk helps to maintain strict spatial boundaries once established (Pinto-Teixeira et al. 2018; Apitz and Salecker 2018).
Temporal patterning of neuroblasts
Since temporal patterning of Drosophila neuroblasts has been reviewed in great detail (Chris Q Doe 2017; Rossi, Fernandes, and Desplan 2017; Holguera and Desplan 2018; Maurange 2020), we will only highlight key points of the classic literature and instead focus on more recent contributions. Temporal patterning refers to the observations that different neuronal types are produced sequentially during development. This could be achieved by continuously specifying new neuroblasts through time that will produce distinct neuronal types, or by specifying a limited pool of neuroblasts early that can then produce different lineage-related neurons as they age. As discussed above, only optic lobe neuroblasts are continuously generated during development. However, this does not serve as a means to diversify neuronal identities as these neuroblasts are identical and rather build sequentially the ~800 repetitive columnar units in the optic lobe.
Each Drosophila neuroblast divides multiple times to produce sequentially different neurons depending on their time of birth. At every cell division, neuroblasts self-renew and maintain a neuroblast identity while budding off a progeny cell that will differentiate into neurons. For most Drosophila neuroblasts (Type I), this progeny is an intermediate precursor (ganglion mother cell; GMC) that divides only once to produce two cells that will usually adopt distinct fates based on Notch signaling (one NotchON cell and the other a NotchOFF cell; see below). In some instances, however, neuroblasts produce neurons directly without first producing a GMC (Type 0 mode) (Bertet et al. 2014; Baumgardt et al. 2014). Finally, only 8 Type II neuroblasts located in the central brain produce semi-proliferative cells called intermediate neural progenitors (INPs), which themselves asymmetrically divide 4–6 times to self-renew and produce 4–6 GMCs (Bello et al. 2008; Bowman et al. 2008; Boone and Doe 2008). Neuroblast lifespan is a critical component of how many neurons a given neuroblast will produce. During embryogenesis, VNC and central brain neuroblasts divide between 10–20 times (Monedero Cobeta, Salmani, and Thor 2017). The same neuroblasts post-embryonically divide many more times (~50) and produce many neuronal types before exiting proliferation (H.-H. Yu et al. 2010; J. S. Yang et al. 2013; Homem et al. 2014; Homem, Repic, and Knoblich 2015). Optic lobe neuroblasts are also shorter-lived and divide ~15–20 times. These widely different lineage lengths likely require different modes of temporal patterning.
Temporal patterning of VNC and central brain neuroblasts
The first hint of temporal patterning in Drosophila came from observing the laminar organization of different neuronal types in the embryonic VNC (Kambadur et al. 1998). Ventral nerve cord neuroblasts are born at precise times during embryogenesis and each produces different neuronal types in a stereotyped pattern as they age. The sequential birth of neurons with different identities is controlled by a cell-intrinsic clock or temporal series, with most neuroblasts expressing the temporal transcription factors (tTFs) Hunchback (Hb), Krüppel (Kr), Pdm, Castor, and then Grainyhead (Grh) as they age (Figure 2A) (Brody and Odenwald 2000; Takako Isshiki et al. 2001; Chris Q Doe 2017). Importantly, not all temporal factors need be transcription factors. Although the tTFs of the VNC have been described in great detail, it is not clear whether the same VNC tTFs are expressed and functional in the ~100 embryonic central brain neuroblasts (Kao et al. 2012). However, the eight Type II central brain neuroblasts that are born in the late embryo start by expressing Pdm and Cas (i.e. the last few VNC tTFs) when they are first born, indicating that they may undergo a similar sequential expression of tTFs (Walsh and Doe 2017).
Figure 2. Temporal patterning programs that operate in embryonic VNC, post-embryonic optic lobe, and post-embryonic central brain/VNC neuroblasts.
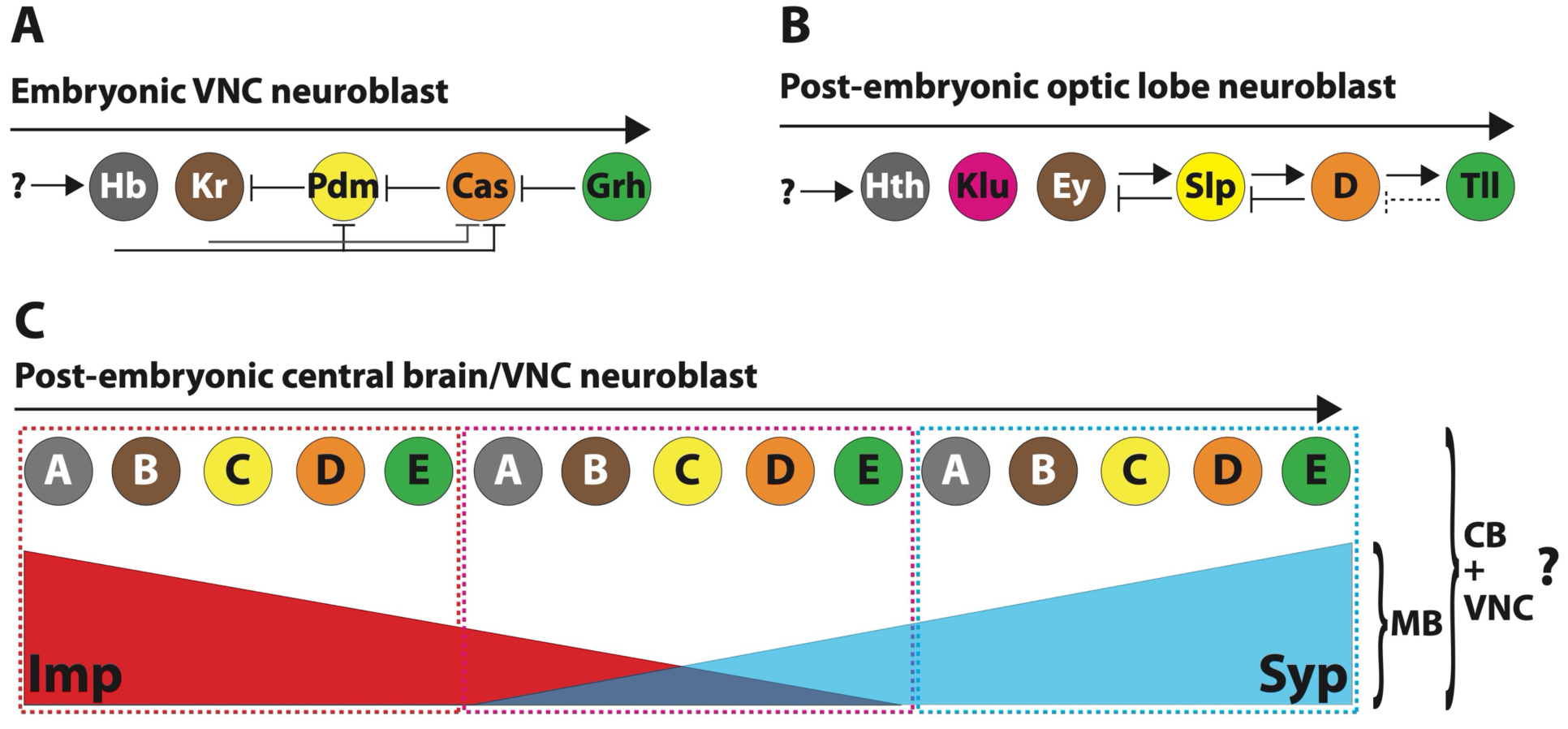
A. Most embryonic VNC neuroblasts progress through a series of five tTFs over the course of 10–20 divisions. Repressive interactions between the tTFs are partially responsible for allowing the intrinsic “clock” to “tick”. It is unknown what initiates the temporal series. Hb: Hunchback; Kr: Kruppel; Pdm: POU domain protein 2; Cas: Castor; Grh: Grainy head.
B. A similar series but with different tTFs operates in medulla optic lobe neuroblasts, which also divide an estimated 15–20 times. Here, some tTFs activate the next factor in the series and repress the previous factor. It is unknown what activates Hth to initiate the series. Hth: Homothorax; Klu: Klumpfuss; Ey: Eyeless; Slp: Sloppy Paired 1 and 2; D: Dichaete; Tll: Tailless.
C. Post-embryonically, central brain and VNC neuroblasts express the RNA-binding proteins Imp and Syp in opposing temporal gradients through time. At this stage, most neuroblasts divide ~50 times, except for mushroom body neuroblasts that divide ~250 times. Imp and Syp gradients alone are necessary for specifying the three main neuronal types produced by mushroom body neuroblasts. In all other central brain and VNC neuroblasts that produce many more neuronal types, it is possible that Imp and Syp act at the top of the temporal patterning hierarchy and that neuroblasts progress through tTF series, that could repeat, as they age. Imp: IGF-II mRNA binding protein; Syp: Syncrip; CB: central brain; VNC: ventral nerve cord.
Similar to the interactions between sTFs, VNC tTFs also regulate each other’s expression to help control the progression of the “clock”, although deletion of a single tTF does not delay the progression of the temporal cascade, indicating that other factors are involved (T Isshiki, Takeichi, and Nose 1997). The transcription factor Seven-up (Svp) promotes the Hb to Kr transition that also requires cytokinesis (Grosskortenhaus et al. 2005; Kanai, Okabe, and Hiromi 2005). Nonetheless, the role of switching factors and cell cycle regulation in temporal transitions is still an area of intense investigation. For example, the relay between tTFs is generally thought to be an activator model, in which the preceding factor activates transcription of the following one. However, mathematical modeling suggests that the timing mechanism may be better described by a repressor-decay model (Averbukh et al. 2018). That is, activation of late factors in the series may be inhibited by early factors and are only expressed once the early factors have decayed. This model helps explain why deleting early tTFs from the series does not inhibit late factors from being expressed.
When comparing VNC/central brain neuroblasts between embryonic and larval stages it is important to keep in mind that they divide over the course of widely different time scales and therefore have much larger lineages post-embryonically. Embryonic neuroblasts divide 10–20 times to produce many different neurons that will function during larval life (Monedero Cobeta, Salmani, and Thor 2017). During this period they rapidly transit through a temporal series with tTFs being expressed for 1–2 divisions, producing a different neuronal type after every division (Chris Q Doe 2017). At the end of embryogenesis, nearly all neuroblasts (in both the VNC and central brain) enter quiescence (Maurange and Gould 2005). Once neuroblasts are reactivated in the early larva, they produce many different neurons over the course of several days that will function in the adult brain/VNC (Birkholz et al. 2015). Therefore, each post-embryonic lineage is much longer with many more neuronal types born sequentially and might therefore utilize different temporal patterning mechanisms. Currently, we know that temporal progression of post-embryonic central brain neuroblasts is at least partially controlled by the expression of two mutually inhibiting RNA-binding proteins: IGF-II mRNA binding protein (Imp) and Syncrip (Syp) (Figure 2C) (Z. Liu et al. 2015). Imp is expressed in early stage neuroblasts in a temporal gradient: its expression progressively decreases through time. Conversely, Syp expression increases in late stage neuroblasts until it promotes the end of the lineage (C.-P. P. Yang et al. 2017; Samuels et al. 2020; Pahl, Doyle, and Siegrist 2019). These temporal factors are inherited by newborn neurons (similar to many of the tTFs in embryonic VNC neuroblasts and optic lobe neuroblasts) and control the temporal identities of neurons through a transcription factor called Chronological inappropriate morphogenesis (Chinmo) (Zhu et al. 2006). Just as Imp and Syp act as gradients, Chinmo also acts in a high-to-low gradient in neurons, since its translation is promoted by Imp and inhibited by Syp. The contribution of these factors in regulating temporal identity appears to be lineage specific and thus dependent on spatial identity. For example, mushroom body neuroblasts, which generate Kenyon cells that have been well-studied for their roles in learning and memory, produce only three main neuronal types sequentially, with each neuron type produced many times over many cell divisions: Each neuroblast divides 250 times to produce γ, then α’/β’ and finally α/β neurons (T. Lee, Lee, and Luo 1999). In this lineage, Imp is required for properly patterning both γ and α’/β’ neurons while Syp is required for patterning α’/β’ and α/β neurons (Z. Liu et al. 2015). Imp and Syp are also expressed in antennal lobe neuroblasts (that produce ~50 types of projection neurons) and in Type II neuroblasts (that produce neurons of the central complex) but in steeper gradients since the lifespans of these neuroblasts are much shorter (~50 divisions) than the lifespan of mushroom body neuroblasts (Z. Liu et al. 2015; C.-P. Yang et al. 2016; Syed, Mark, and Doe 2017; Ren et al. 2017). However, in the antennal lobe anterodorsal 1 (ALad1) neuroblast that generates 22 projection neuronal types, only one neuronal type (the VA3 projection neuron) is missing in imp mutants (Z. Liu et al. 2015). Nonetheless, the ratio of early-to-late neuronal types is severely affected in imp or syp mutants, since neuroblasts stop proliferating early (and Syp is expressed earlier) in imp mutants while neuroblasts are unable to end life (and Imp is maintained for longer) in syp mutants (Z. Liu et al. 2015; C.-P. P. Yang et al. 2017; Samuels et al. 2020).
Temporal patterning by analog gradients of RNA-binding proteins rather than digital counting by tTFs is an important concept to explain how time is kept in long-lived lineages; however, it has yet to explain how opposing protein gradients, beyond contributing to broad temporal windows, produce a vast diversity of neurons. Previous studies suggest that other factors help narrow these broad developmental windows. Central brain and VNC neuroblasts express the tTF Cas and then Seven-up (Svp) in a temporal fashion to promote the progression from Imp to Syp expression, although how they regulate this transition is not understood (Maurange, Cheng, and Gould 2008; Syed, Mark, and Doe 2017; Ren et al. 2017). During the Imp temporal window, Chinmo positive neurons are produced while during the Syp temporal window, Broad positive neurons are generated (Maurange, Cheng, and Gould 2008). In addition, neurons with complex morphologies generally appear earlier in some central brain lineages, while those with simpler morphologies appear later, indicating that Imp and Syp could be used to define morphological complexity (Y.-J. Lee et al. 2020). It is likely that the ratio of Imp to Syp expression is used to delineate smaller temporal windows (e.g., Imp++/Syp−, Imp+/Syp+, Imp−/Syp++). It is possible that within each of these broad windows, temporal expression of a series of tTFs might specify different neural types (Figure 2C). It is even possible that the same series of tTFs is used recurrently (i.e., temporal windows that exist/repeat themselves more than once), with each tTF giving rise to different neurons depending on the status of the overlying Imp/Syp gradients (Figure 2C). In support of this, neurons with similar morphology have the potential to appear multiple times over the course of a lineage (Y.-J. Lee et al. 2020). Thus, it can be speculated that two levels of temporal patterning exist in post-embryonic central brain and VNC neuroblasts: First, broad temporal windows based on Imp/Syp levels act at the top of the temporal patterning hierarchy; downstream, these larger windows may be subdivided into smaller temporal windows, perhaps by a repeating tTF series (Figure 2C). In combination with input from extrinsic cues (e.g., ecdysone and Activin, see below), this would represent a comprehensive model for how the vast diversity of neuronal types could be specified by long-lived neuroblasts with large lineages.
Thus far, only a few factors have been identified to be expressed temporally in the central brain and they are all expressed over long time periods that span many cell divisions (Syed, Mark, and Doe 2017; Genovese et al. 2019). Since there is only one of each neuroblast in each hemisphere, and the neuroblasts are not synchronized, identifying temporally expressed factors is a challenging technical endeavor that has not yet been solved. We note that, in the semi-proliferative INPs born from the Type II neuroblasts, a typical tTF series comprised of Dichaete (D), Grainy head (Grh), and Eyeless (Ey) has been identified (Bayraktar and Doe 2013). These tTFs are expressed in all INPs born throughout the lifespans of Type II neuroblasts and cross-regulate each other, similar to how tTFs in embryonic VNC and optic lobe neuroblasts interact. Importantly, INPs are very short-lived and divide about five times before ending their lineage, perhaps arguing that tTF series define short temporal programs.
As the expression gradients of Imp and Syp are very extended, what determines when neuroblasts transition from one broad temporal window to the next? Since the environment is changing throughout development, incorporating extrinsic signals is one way to both time transitions between the production of different neuronal types as well as to specify neuronal types directly. In Type II neuroblasts, ecdysone signaling (in addition to Svp) times the transition from Imp to Syp expression, which directly regulates the number of early versus late-born cell types (Syed, Mark, and Doe 2017). Activin signaling is also an important extrinsic signal used by mushroom body neuroblasts; this signal times the transition from young to old temporal states, but more importantly, also specifies a second, extended temporal window in which the second-born α’/β’ neuronal type is produced (Rossi and Desplan 2020; Marchetti and Tavosanis 2019). In the absence of the Activin signaling receptor Baboon, the second temporal window (α′/β′) is lost and only the first (γ) and last (α/β) neuronal types are produced. In both the Type II and mushroom body examples, extrinsic signals regulate Imp and Syp levels, linking intrinsic and extrinsic temporal programs (Rossi and Desplan 2020; Syed, Mark, and Doe 2017). It will be important to explore whether extrinsic cues like Activin and Ecdysone (and others) help diversify neuronal types in other neuroblast lineages.
Temporal patterning of optic lobe neuroblasts
Individual optic lobe neuroblasts are relatively short-lived and produce a vast array of neuronal types. Similar to embryonic VNC neuroblasts, medulla neuroblasts quickly progress through a temporal series, although the tTFs differ compared to those in the VNC. The medulla temporal series is currently comprised of 6 tTFs: Homothorax (Hth), Klumpfuss (Klu), Ey, Sloppy paired1/2 (Slp), D, and Tailless (Tll) (Figure 2B) (X. Li et al. 2013; Suzuki et al. 2013). For the most part, progression through the series is controlled by an activation-repression mechanism. For example, Ey activates Slp while Slp represses Ey. It is important to note that each tTF is expressed for varying numbers of divisions, that their expression levels differ through time, and that tTF windows overlap. With this in mind, our understanding of the medulla temporal series (combined with spatial patterning and Notch signaling, see below) can account for many (~20) but not all of the neuronal types produced by each neuroblast. Thus, additional temporal windows likely exist. The unique structure of the optic lobes (i.e. circuits repeated in parallel) allows for the concurrent visualization of multiple temporal windows at the same point in developmental time and allowed for the identification of medulla tTFs (Suzuki et al. 2013; X. Li et al. 2013). In the future, single-cell mRNA sequencing methods will help identify additional, if not all, temporally expressed genes in optic lobe neuroblasts. This is also true for embryonic VNC neuroblasts, since like optic lobe neuroblasts they are reiterated in each of the 14 body segments.
Apart from medulla neuroblasts, other regions go through a temporal series, including in neuroblasts born at the tips of the OPC (Wg-expressing region) (Bertet et al. 2014) where neuroblasts progress through a slightly modified series and first express Distall-less (Dll) instead of Hth before progressing through the same set of tTFs (i.e., Ey then Slp followed by D but ending before Tll). These spatially distinct neuroblasts produce neurons not only for the medulla but also for the lobula and lobula plate. Therefore, temporal series represent a general mechanism but the tTFs can differ widely between neuroblasts from different regions, or can also be slightly modified to produce different neurons.
The roles of Notch signaling in neuronal specification
The Notch pathway is one of the best studied pathways and has been the topic of many reviews (Bray 2016; Gerald Udolph 2012). We therefore only focus on how Notch signaling is recurrently used to diversify the fates of neurons.
When cells divide asymmetrically, Notch signaling is often used to make a binary decision between two alternative fates. For example, when Type I neuroblasts divide, neuroblasts maintain a NotchON status while GMCs are NotchOFF. Importantly, Notch signaling is used again when GMCs divide, as one of the two sibling cells is NotchON and the other NotchOFF. In combination with spatial and temporal inputs, Notch status dictates the different fates of the two sister cells: For instance, Notch can specify whether one sibling lives or dies, where a neuron will project, or which neurotransmitter a neuron will express (Bertet et al. 2014; Truman et al. 2010; Lin et al. 2010; Guo, Jan, and Jan 1996; Eric P. Spana and Doe 1996; E P Spana et al. 1995; J B Skeath and Doe 1998). In the optic lobes, NotchON status is linked to cholinergic identity (Konstantinides et al. 2018), and in the VNC, whole hemilineages (e.g., all NotchON siblings born from a single neuroblast) use the same neurotransmitter, or are all specified to die (while their NotchOFF siblings survive, or vice versa) (Lacin et al. 2019; Lin et al. 2010; Truman et al. 2010). Thus, Notch signaling is context dependent and can play various roles depending on the lineage in which it acts, often acting multiple times (and in multiple manners) within one lineage.
Perhaps the best example of how Notch signaling is integrated with spatiotemporal patterning programs to help generate the proper numbers and types of neurons in the brain was recently described for the optic lobe T4 and T5 neurons, the lobula plate neurons that respond to local motion cues (Figure 3A) (Pinto-Teixeira et al. 2018). Each of the ~800 lobula plate columns contains four T4 and four T5 neurons that respond to moving light (T4) or dark (T5) edges in one of the four cardinal directions (up, down, front-to-back or back-to-front). T4 and T5 neurons are born from neuroblasts that originate from different domains of the IPC (see above). Spatial patterning acts as the first step towards specifying neuronal identity, as neuroblasts born from the Brk domain produce horizontal (front-to-back or back-to-front) motion-sensing T4/T5s while those born from the Dpp domain give rise to vertical (up or down) motion-detecting neurons. Temporal patterning is also used to specify T4/T5 fate. In the IPC, C/T neurons are produced from the first temporal window of thee neuroblasts (D+), but in the later window (Dac/Ato+), T4/T5 neurons are generated. Finally, Notch signaling is utilized twice to subdivide T4/T5s into their eight different subtypes. First, the neuroblasts that generate T4/T5s undergo a terminal asymmetric, Notch-dependent division to produce two GMCs: The NotchON GMC will produce neurons that detect up (or front-to-back) motion while the NotchOFF GMC will produce down (or back-to-front) neurons. Then, the GMC divides once more asymmetrically to produce a T4 NotchOFF and a T5 NotchON neuron. In this way, a single neuroblast from the Dpp or Brk domain produces the full complement of T4 and T5 neurons needed to detect vertical (Dpp) or horizontal (Brk) motion. In this case, Notch signaling, but not temporal patterning, diversifies T4 and T5 fates, which is reminiscent of how SOPs only use spatial patterning and Notch signaling to generate a limited number of cells with different identities (see below).
Figure 3. Notch signaling is used reiteratively to specify a limited number of neuronal types.
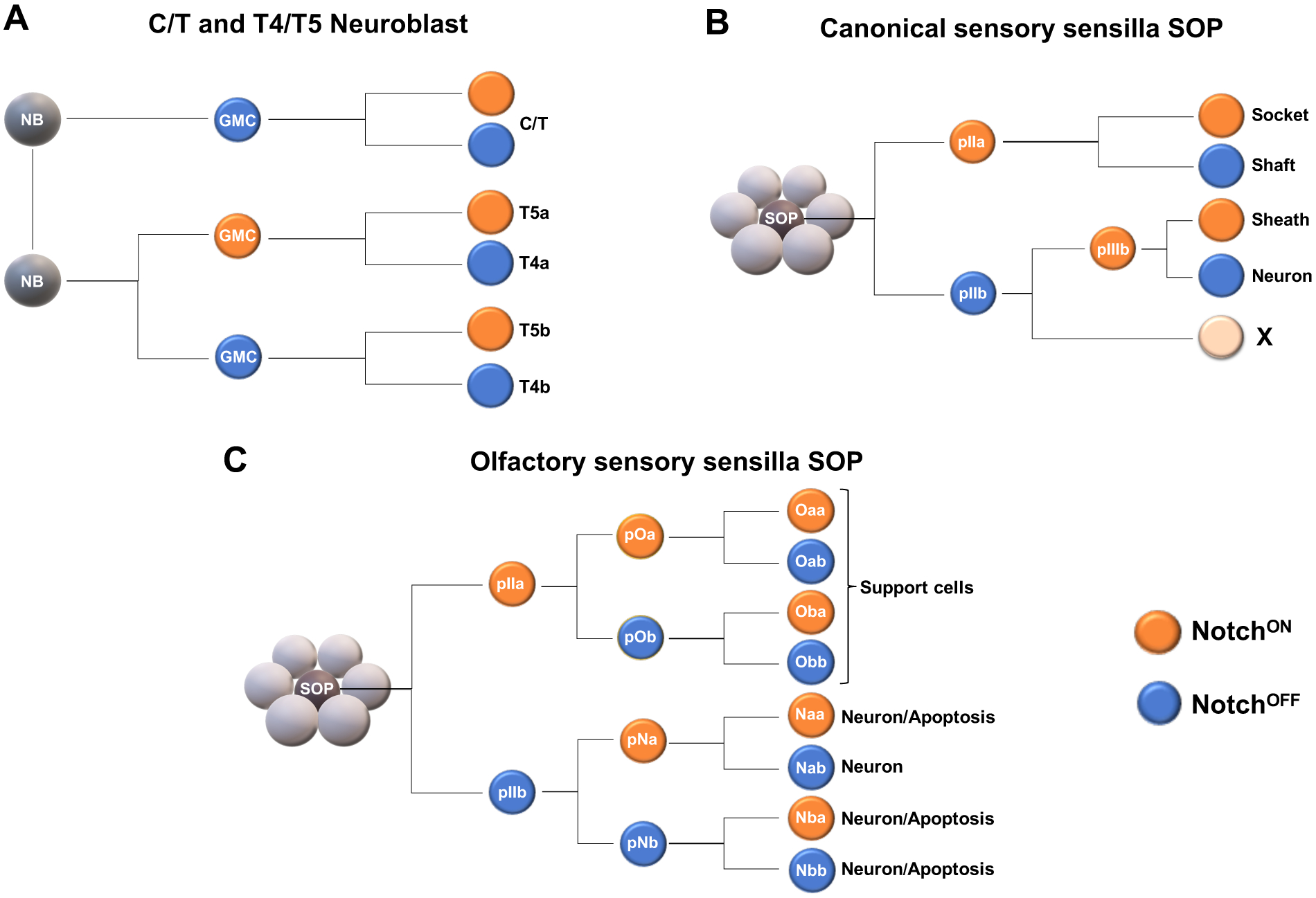
A. Neuroblasts that produce the motion sensitive T4/T5 neurons of the lobula plate are born from the IPC. During the first temporal window, these neuroblasts produce C/T neurons. At the final temporal window and last division, these neuroblasts divide to produce two GMCs: one GMC is NotchON and the other is NotchOFF. This Notch-dependent division specifies GMCs as either back-to-front or front to back (or up versus down depending on the origin of the neuroblast; see text). Each GMC then terminally divides, producing a NotchOFF T4 and a NotchON T5 neuron. For simplicity, only T4/T5 neurons from the horizontal system (a and b subtypes) are illustrated.
B. SOPs in the periphery are selected from a group of epithelial cells. Every division from SOP to the final progeny is Notch-dependent and leads to four different cell types: 1 neuron and 3 support cells.
C. A slight modification to the basic SOP ground plan is followed by SOPs that produce olfactory sensory neurons. Nonetheless, every division is Notch dependent but up to 4 different neuronal types can be produced. Starting from the pIIb cell, the number of divisions and the final outcome is highly similar to the final divisions leading to the four different T4/T5 neuronal types from a single neuroblast.
Limited neuronal diversity generated by spatial patterning and Notch signaling of sensory organ precursors
Although neurons of the central nervous system and optic lobe are generated by neuroblasts that act as stem cells dividing multiple times in a linear manner, non-visual sensory systems are produced by sensory organ precursors (SOPs) that typically divide two or three times and generate very limited neuronal diversity that is exclusively the result of spatial patterning and Notch signaling. For example, more than fifty olfactory receptor neuron (ORN) types—each defined by the expression of different types of olfactory receptor genes—are produced in a highly predetermined manner during development (Couto, Alenius, and Dickson 2005). These neurons are clustered into sensilla, each of which houses a specific subset of 1 to 4 ORNs. The antennal sensilla can be defined into three distinct subtypes: basiconic, coeloconic, and trichoid (Shanbhag, Müller, and Steinbrecht 1999). Initially, the antennal imaginal disc is spatially patterned by the expression of En, Wg, Dpp and Hh, which leads to the division of the disc into zones (Yan et al. 2020; Ruiz-Losada et al. 2018). Zone A3 gives rise to the olfactory SOPs and can be subdivided into eight concentric arcs that dictate the differentiation potential of SOPs (i.e., which sensilla types and ORNs they will produce) (Chai et al. 2019). Thus, spatial patterning determines the types of neurons antenna SOPs produce, similar to how spatial patterning of the OPC delimits the types of multi-columnar neurons a medulla neuroblast will generate.
Like VNC neuroblasts, SOPs that generate sensory organs delaminate from the neuroepithelium, but SOPs typically only generate one neuron whose identity depends on spatial identity and Notch status (Figure 3B). Generally, each SOP divides once asymmetrically to generate a NotchON pIIa and a NotchOFF pIIb precursor cell (Rebeiz, Miller, and Posakony 2011; Reddy and Rodrigues 1999). These two cells divide again in a Notch dependent manner to produce three bristle support cells as well as a single sensory neuron (Figure 3B) (Rebeiz, Miller, and Posakony 2011). A modification of the same system is used to generate a greater diversity of neurons in the olfactory system: Antennal disc SOPs can generate up to four distinct olfactory neurons by relying solely on Notch signaling (and spatial patterning to limit their potential). Just like a canonical SOP, antennal SOPs divide to produce a pIIa (NotchON) precursor that will divide twice more in a Notch dependent manner to generate support cells that form the olfactory sensillum, and a pIIb (NotchOFF) precursor that also divides twice in a Notch-dependent manner to produce up to four neurons that will occupy this sensillum (Figure 3C) (Endo et al. 2007; Singhania and Grueber 2014; Endo et al. 2011). The olfactory pIIb precursor that emerges from an SOP divides with a very similar pattern and a similar outcome (i.e., four different neuron types) as the GMCs that generate T4 and T5 neurons during the last IPC neuroblast division (see above). However, in some olfactory sensilla, up to 3 of the neurons are programed to die (A. Sen, Reddy, and Rodrigues 2003; Reddy and Rodrigues 1999; Chai et al. 2019). These examples illustrate how the same pathways and mechanisms are used repeatedly during neurogenesis to generate (in this case, limited) neuronal diversity.
How specification programs interact and their links to the terminal features of neurons
The spatial, temporal and Notch-dependent mechanisms described above act at the very top of a hierarchy used to pattern neurons into specific fates. In fact, nearly all the mechanisms we have described occur in neuroepithelial cells or neuroblasts, which are steps away from mature neurons that might reach full differentiation several days later. How then do temporal and spatial programs control the final fates of neurons? Are tTFs and sTFs sufficient in each neuroblast to completely determine the final features of the different neuronal types (i.e., are they “terminal-selector” genes?)? Which genes are downstream of sTFs and tTFs in newborn neurons to promote their proper differentiation? We discuss some of the recent advances made in understanding these questions.
Before the differentiation process begins, how do spatial and temporal inputs combine at the very top of the hierarchy to regulate neuronal identity? It should be noted that sTFs are only expressed in the neuroepithelium and not in neuroblasts. Since the same sets of tTFs are used by nearly all neuroblasts in a given brain region (e.g., all medulla neuroblasts use the same tTFs regardless of where they were born from the OPC), sTFs in the neuroepithelium might modify the chromatin landscape in which tTFs act in neuroblasts, or activate downstream effectors that work directly with tTFs. Recent work identified Hb binding sites in two different VNC neuroblasts (NB5–6 and NB7–4) and showed that Hb was bound to different loci in these two neuroblasts, coinciding with the earlier binding sites for the sTF Gsb, which is expressed in NB5-6 but not NB7-4 (S. Q. Sen et al. 2019). This suggests that sTFs limit the genomic space (i.e. chromatin accessibility) in which tTFs can act, allowing for the same tTFs to be used by all neuroblasts, but with different outcomes. It will be important to expand this type of analysis to other tTF/sTF combinations not only in the VNC but also in the central brain and optic lobes.
Once spatial identity is established, are tTFs sufficient to control neuronal fate and their terminal features? For example, Hb is the first tTF in the VNC temporal series and appears to transform late-born neurons into an early fate when overexpressed. That is, overexpressing Hb in neuroblast 7–1 (which normally sequentially produces the 5 distinct U1-U5 motor neuronal types) causes all U neurons to express markers associated with the first U1 fate. However, recent work indicates that this transformation is not complete and that the additional “U1” motor neurons are not identical to their first-born counterpart (Meng et al. 2019; A. Q. Seroka and Doe 2019). This is likely due to the progressive restriction in competence of neuroblasts when they age, which is mediated by the closing of chromatin accessible by tTFs (Kohwi et al. 2013). Thus, it seems that additional criteria must be met in order to completely drive a change in neuronal identity, i.e. individual tTFs are not completely sufficient. As our catalog of neuronal types and their transcriptional profiles during development and in the adult grows, we will be better able to characterize and predict how developmental factors may regulate identity when mutated or mis-expressed.
Moving from specification programs to mature neurons
How are early specification events translated into specific neuronal types with distinct terminal features? In other words, how does the expression of an sTF in a neuroepithelial cell and a tTF in a neuroblast give rise to a neuron that knows where in the brain to connect and which neurotransmitter to use? A classic example that provides some answers to these questions can be found with embryonic VNC neuroblast 5-6T. During the late Cas and Grh temporal windows, four neuronal types that express Apterous (Ap) and Eyes absent (Eya) are produced sequentially: the first expresses the neuropeptide Nplp1 and the last expresses the neuropeptide FMRFa, while the middle-born are considered generic Ap+ neurons. The “general” Ap/Eya identity of these four neurons is controlled by the terminal selector Collier (Col), whose expression is initiated by temporal (Cas) and spatial (Hth, Antennapedia, and Ladybird early) inputs (Baumgardt et al. 2009; Gabilondo et al. 2016; Karlsson, Baumgardt, and Thor 2010). The differential activation of feedforward networks in a precise temporal sequence leads to the three different terminal fates (Stratmann et al. 2016). For example, the level of Grh peaks when the last Ap/Eya+ neuron is born, activating a different feedforward network that leads to FMRFa expression and this terminal cell fate (Baumgardt et al. 2009). Here, we note that Notch signaling is not involved in specifying these neuronal types since during this time window NB5-6T divides in a Type 0 manner.
How spatiotemporal factors control the terminal features of neurons are also beginning to be explored in other parts of the brain and VNC. For example, the tTFs Kr and Pdm appear sufficient to pattern a distinct motor neuron type produced from the embryonic VNC neuroblast 7–1, indicating the presence of a new, Kr/Pdm temporal window. These two tTFs control the expression of Nkx6 in the post-mitotic neuron, where it is necessary and sufficient to control axonal pathfinding (A. Seroka et al. 2020). In the medulla, the morphology of the T1 neuronal type is controlled by three transcription factors (Otd, Sox102F and Ets65A) expressed in differentiating neurons that are each regulated by a tTF, but are maintained when the neuroblast switches to a later tTF (Ey activates Otd, Slp activates Sox102F and D activates Etz65A). These genes become specifically co-expressed in the D temporal window and their co-expression regulates T1 character (Naidu et al. 2020). Some of these transcription factors may be the previously described “concentric genes”, which are expressed in medulla neurons born from the same temporal window and thus appear as rings. These genes are regulated by the medulla tTFs and are likely involved in controlling terminal features of medulla neurons (Hasegawa et al. 2013). These examples only begin to build a bridge from tTF series to the terminal features of neurons.
How extended temporal programs, such as Imp and Syp gradients, translate into specific neuronal types is even less understood. In the post-embryonic mushroom body lineage, Imp and Syp are sufficient to define the three neuronal types and control the translation of Chinmo in neurons, which acts in a high-to-low gradient downstream of Imp and Syp levels. Chinmo can then activate “terminal selectors,” such as Mamo, which is required for establishing the second-born α′/β’ mushroom body neuronal fate (L. Liu et al. 2019). Abrupt is another transiently expressed neuronal factor that likely acts downstream of Imp/Syp (and possibly Chinmo) to inhibit the cell adhesion molecule Fasciclin 2 in α′/β’ cells to allow them to form connections distinct from those of first and last-born mushroom body neuron types (Kucherenko et al. 2012).
Another class of six genes called morphology transcription factors (mTFs) were described to act in different combinations post-mitotically to specifically regulate the morphologies of seven thoracic motor neurons targeting muscles in the leg (Enriquez et al. 2015). These motor neuron types are born sequentially from a specific VNC neuroblast and express different combinations of mTFs, but it remains unknown what programs (e.g., Imp/Syp/Chinmo?) control the mTF codes. These examples provide the first attempts towards defining the large and complicated genetic networks required to progress from specification to mature neuronal types.
Concluding remarks
In order to gain a complete understanding of the mechanisms driving neuronal specification, diversification, and differentiation going forward, we will need access to datasets (e.g. from single-cell mRNA-seq and single-cell ATAC-seq) containing information from every cell as it progresses through development. For example, some datasets are already available for projection neurons in the olfactory network although we have very little understanding of how different projection neuronal types are specified (H. Li et al. 2017). In other cases, we have a deep understanding of general specification programs but very little information about when different neuronal types are produced, or the steps utilized to generate their intermediate and final neuronal states. Thus, the major goal for developmental neurobiologists going forward will be to gather datasets for all cells in the nervous system from the moment of specification to their final differentiated states and annotate all of these cells. Computational advances will certainly make this endeavor easier as any information for a cell (even from a single time point) could be used to help annotate a cluster and then transfer annotations from one time point to the next. This was recently achieved for optic lobe neurons, for which we now have a nearly complete trajectory from when each of the nearly 200 neurons are first specified and into the adult.
Although current single-cell approaches cannot reveal how cells are related to one another by lineage (e.g., whether they are sister cells or born from overlapping temporal windows), combining single cell approaches with advanced lineage tracing techniques promises to provide this information (Espinosa-Medina et al. 2019). With these types of datasets in hand we are ready to understand how specification programs are transformed into lasting neuronal terminal features. Once characterized, the long-term aim will be to predict which factors act as master regulators and terminal selectors, and then decipher how they help specify and maintain unique neuronal types.
References
- Allen Aaron M., Neville Megan C., Birtles Sebastian, Croset Vincent, Treiber Christoph Daniel, Waddell Scott, and Goodwin Stephen F.. 2020. “A Single-Cell Transcriptomic Atlas of the Adult Drosophila Ventral Nerve Cord.” ELife 9 (April). 10.7554/eLife.54074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apitz Holger, and Salecker Iris. 2015. “A Region-Specific Neurogenesis Mode Requires Migratory Progenitors in the Drosophila Visual System.” Nature Neuroscience 18 (1): 46–55. 10.1038/nn.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2018. “Spatio-Temporal Relays Control Layer Identity of Direction-Selective Neuron Subtypes in Drosophila.” Nature Communications 9 (1): 2295. 10.1038/s41467-018-04592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbukh Inna, Lai Sen-Lin, Doe Chris Q, and Barkai Naama. 2018. “A Repressor-Decay Timer for Robust Temporal Patterning in Embryonic Drosophila Neuroblast Lineages.” ELife 7 (December): 354969. 10.7554/eLife.38631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgardt Magnus, Karlsson Daniel, Salmani Behzad Y., Bivik Caroline, MacDonald Ryan B., Gunnar Erika, and Thor Stefan. 2014. “Global Programmed Switch in Neural Daughter Cell Proliferation Mode Triggered by a Temporal Gene Cascade.” Developmental Cell 30 (2): 192–208. 10.1016/j.devcel.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Baumgardt Magnus, Karlsson Daniel, Terriente Javier, Díaz-Benjumea Fernando J., and Thor Stefan. 2009. “Neuronal Subtype Specification within a Lineage by Opposing Temporal Feed-Forward Loops.” Cell 139 (5): 969–82. 10.1016/j.cell.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Bayraktar Omer Ali, and Doe Chris Q. 2013. “Combinatorial Temporal Patterning in Progenitors Expands Neural Diversity.” Nature 498 (7455): 449–55. 10.1038/nature12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello Bruno C, Hirth Frank, and Gould Alex P. 2003. “A Pulse of the Drosophila Hox Protein Abdominal-A Schedules the End of Neural Proliferation via Neuroblast Apoptosis.” Neuron 37 (2): 209–19. 10.1016/S0896-6273(02)01181-9. [DOI] [PubMed] [Google Scholar]
- Bello Bruno C, Izergina Natalya, Caussinus Emmanuel, and Reichert Heinrich. 2008. “Amplification of Neural Stem Cell Proliferation by Intermediate Progenitor Cells in Drosophila Brain Development.” Neural Development 3 (February): 5. 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Christian, Pallavi SK, Prasad Mohit, Shashidhara LS, and Technau Gerhard M.. 2005. “A Critical Role for Cyclin E in Cell Fate Determination in the Central Nervous System of Drosophila Melanogaster.” Nature Cell Biology 7 (1): 56–62. 10.1038/ncb1203. [DOI] [PubMed] [Google Scholar]
- Bertet Claire, Li Xin, Erclik Ted, Cavey Matthieu, Wells Brent, and Desplan Claude. 2014. “Temporal Patterning of Neuroblasts Controls Notch-Mediated Cell Survival through Regulation of Hid or Reaper.” Cell 158 (5): 1173–86. 10.1016/j.cell.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat Krishna Moorthi. 1999. “Segment Polarity Genes in Neuroblast Formation and Identity Specification during Drosophila Neurogenesis.” BioEssays 21 (6): 472–85. . [DOI] [PubMed] [Google Scholar]
- Birkholz Oliver, Rickert Christof, Nowak Julia, Coban Ivo C., and Technau Gerhard M.. 2015. “Bridging the Gap between Postembryonic Cell Lineages and Identified Embryonic Neuroblasts in the Ventral Nerve Cord of Drosophila Melanogaster.” Biology Open 4 (4): 420–34. 10.1242/bio.201411072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone Jason Q., and Doe Chris Q.. 2008. “Identification of Drosophila Type II Neuroblast Lineages Containing Transit Amplifying Ganglion Mother Cells.” Developmental Neurobiology 68 (9): 1185–95. 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman Sarah K., Rolland Vivien, Betschinger Joerg, Kinsey Kaolin A., Emery Gregory, and Knoblich Juergen A.. 2008. “The Tumor Suppressors Brat and Numb Regulate Transit-Amplifying Neuroblast Lineages in Drosophila.” Developmental Cell 14 (4): 535–46. 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray Sarah J. 2016. “Notch Signalling in Context.” Nature Reviews Molecular Cell Biology 9 (August). 10.1038/nrm.2016.94. [DOI] [PubMed] [Google Scholar]
- Brody Thomas, and Odenwald Ward F. 2000. “Programmed Transformations in Neuroblast Gene Expression during Drosophila CNS Lineage Development.” Developmental Biology 226 (1): 34–44. 10.1006/dbio.2000.9829. [DOI] [PubMed] [Google Scholar]
- Chai Phing Chian, Cruchet Steeve, Wigger Leonore, and Benton Richard. 2019. “Sensory Neuron Lineage Mapping and Manipulation in the Drosophila Olfactory System.” Nature Communications 10 (1). 10.1038/s41467-019-08345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T, Mazotta J, Dumstrei K, Dumitrescu A, and Hartenstein V. 2001. “Dpp and Hh Signaling in the Drosophila Embryonic Eye Field.” Development (Cambridge, England) 128 (23): 4691–4704. http://www.ncbi.nlm.nih.gov/pubmed/11731450. [DOI] [PubMed] [Google Scholar]
- Chen Zhenqing, Del Valle Rodriguez Alberto, Li Xin, Erclik Ted, Fernandes Vilaiwan M., and Desplan Claude. 2016. “A Unique Class of Neural Progenitors in the Drosophila Optic Lobe Generates Both Migrating Neurons and Glia.” Cell Reports, April, 1–13. 10.1016/j.celrep.2016.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu-LaGraff Q, and Doe CQ. 1993. “Neuroblast Specification and Formation Regulated by Wingless in the Drosophila CNS.” Science (New York, N.Y.) 261 (5128): 1594–97. 10.1126/science.8372355. [DOI] [PubMed] [Google Scholar]
- Courgeon Maximilien, and Desplan Claude. 2019a. “Coordination of Neural Patterning in the Drosophila Visual System.” Current Opinion in Neurobiology 56: 153–59. 10.1016/j.conb.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2019b. “Coordination between Stochastic and Deterministic Specification in the Drosophila Visual System.” Science 6727 (October): eaay6727. 10.1126/science.aay6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto Africa, Alenius Mattias, and Dickson Barry J.. 2005. “Molecular, Anatomical, and Functional Organization of the Drosophila Olfactory System.” Current Biology 15 (17): 1535–47. 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Croset Vincent, Treiber Christoph Daniel, and Waddell Scott. 2018. “Cellular Diversity in the Drosophila Midbrain Revealed by Single-Cell Transcriptomics.” ELife 7: e34550. 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie Kristofer, Janssens Jasper, Koldere Duygu, Maxime De Waegeneer Uli Pech, Kreft Łukasz, Aibar Sara, et al. 2018. “A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain.” Cell 174 (4): 982–998.e20. 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn Richard, and Kunes Sam. 2004. “An Axon Scaffold Induced by Retinal Axons Directs Glia to Destinations in the Drosophila Optic Lobe.” Development (Cambridge, England) 131 (10): 2291–2303. 10.1242/dev.01111. [DOI] [PubMed] [Google Scholar]
- Deshpande N, Dittrich R, Technau GM, and Urban J. 2001. “Successive Specification of Drosophila Neuroblasts NB 6–4 and NB 7–3 Depends on Interaction of the Segment Polarity Genes Wingless, Gooseberry and Naked Cuticle.” Development (Cambridge, England) 128 (17): 3253–61. http://www.ncbi.nlm.nih.gov/pubmed/11546742. [DOI] [PubMed] [Google Scholar]
- Doe CQ 1992. “Molecular Markers for Identified Neuroblasts and Ganglion Mother Cells in the Drosophila Central Nervous System.” Development 116 (4): 855–63. 10.1146/annurev.ge.24.120190.002131. [DOI] [PubMed] [Google Scholar]
- Doe Chris Q. 2017. “Temporal Patterning in the Drosophila CNS.” Annual Review of Cell and Developmental Biology 33 (1): 219–40. 10.1146/annurev-cellbio-111315-125210. [DOI] [PubMed] [Google Scholar]
- Endo Keita, Aoki Tomoko, Yoda Yuka, Kimura Ken Ichi, and Hama Chihiro. 2007. “Notch Signal Organizes the Drosophila Olfactory Circuitry by Diversifying the Sensory Neuronal Lineages.” Nature Neuroscience 10 (2): 153–60. 10.1038/nn1832. [DOI] [PubMed] [Google Scholar]
- Endo Keita, Karim M. Rezaul, Taniguchi Hiroaki, Krejci Alena, Kinameri Emi, Siebert Matthias, Ito Kei, Bray Sarah J., and Moore Adrian W.. 2011. “Chromatin Modification of Notch Targets in Olfactory Receptor Neuron Diversification.” Nature Neuroscience 15 (2): 224–33. 10.1038/nn.2998. [DOI] [PubMed] [Google Scholar]
- Enriquez Jonathan, Venkatasubramanian Lalanti, Baek Myungin, Peterson Meredith, Aghayeva Ulkar, and Mann Richard S.. 2015. “Specification of Individual Adult Motor Neuron Morphologies by Combinatorial Transcription Factor Codes.” Neuron 86 (4): 955–70. 10.1016/j.neuron.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erclik Ted, Hartenstein Volker, Lipshitz Howard D., and McInnes Roderick R.. 2008. “Conserved Role of the Vsx Genes Supports a Monophyletic Origin for Bilaterian Visual Systems.” Current Biology 18 (17): 1278–87. 10.1016/j.cub.2008.07.076. [DOI] [PubMed] [Google Scholar]
- Erclik Ted, Li Xin, Courgeon Maximilien, Bertet Claire, Chen Zhenqing, Baumert Ryan, Ng June, et al. 2017. “Integration of Temporal and Spatial Patterning Generates Neural Diversity.” Nature 541 (7637): 365–70. 10.1038/nature20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Medina Isabel, Garcia-Marques Jorge, Cepko Connie, and Lee Tzumin. 2019. “High-Throughput Dense Reconstruction of Cell Lineages.” Open Biology. Royal Society Publishing. 10.1098/rsob.190229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Vilaiwan M, Chen Zhenqing, Rossi Anthony M, Zipfel Jaqueline, and Desplan Claude. 2017. “Glia Relay Differentiation Cues to Coordinate Neuronal Development in Drosophila.” Science (New York, N.Y.) 357 (6354): 886–91. 10.1126/science.aan3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach KF, and Dittrich APM. 1989. “The Optic Lobe of Drosophila Melanogaster. I. A Golgi Analysis of Wild-Type Structure.” Cell and Tissue Research 258 (3): 441–75. 10.1007/BF00218858. [DOI] [Google Scholar]
- Gabilondo Hugo, Stratmann Johannes, Rubio-Ferrera Irene, Millán-Crespo Irene, Contero-García Patricia, Bahrampour Shahrzad, Thor Stefan, and Benito-Sipos Jonathan. 2016. “Neuronal Cell Fate Specification by the Convergence of Different Spatiotemporal Cues on a Common Terminal Selector Cascade.” PLoS Biology 14 (5): 1–21. 10.1371/journal.pbio.1002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese Sara, Clément Raphaël, Gaultier Cassandra, Besse Florence, Narbonne-Reveau Karine, Daian Fabrice, Foppolo Sophie, Luis Nuno Miguel, and Maurange Cédric. 2019. “Coopted Temporal Patterning Governs Cellular Hierarchy, Heterogeneity and Metabolism in Drosophila Neuroblast Tumors.” ELife 8 (September): 1–36. 10.7554/eLife.50375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold Katrina S., and Brand Andrea H.. 2014. “Optix Defines a Neuroepithelial Compartment in the Optic Lobe of the Drosophila Brain.” Neural Development 9 (1). 10.1186/1749-8104-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskortenhaus Ruth, Pearson Bret J., Marusich Amanda, and Doe Chris Q.. 2005. “Regulation of Temporal Identity Transitions in Drosophila Neuroblasts.” Developmental Cell 8 (2): 193–202. 10.1016/j.devcel.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Guo Ming, Jan LY, and Jan YN. 1996. “Control of Daughter Cell Fates during Asymmetric Division: Interaction of Numb and Notch.” Neuron 17 (1): 27–41. 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Hadjieconomou Dafni, Timofeev Katarina, and Salecker Iris. 2011. “A Step-by-Step Guide to Visual Circuit Assembly in Drosophila.” Current Opinion in Neurobiology 21 (1): 76–84. 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Hakes Anna E., Otsuki Leo, and Brand Andrea H.. 2018. “A Newly Discovered Neural Stem Cell Population Is Generated by the Optic Lobe Neuroepithelium during Embryogenesis in Drosophila Melanogaster.” Development (Cambridge) 145 (18). 10.1242/dev.166207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein Volker, and Campos-Ortega Jose A.. 1984. “Early Neurogenesis in Wild-TypeDrosophila Melanogaster.” Wilhelm Roux’s Archives of Developmental Biology 193 (5): 308–25. 10.1007/BF00848159. [DOI] [PubMed] [Google Scholar]
- Hasegawa Eri, Kaido Masako, Takayama Rie, and Sato Makoto. 2013. “Brain-Specific-Homeobox Is Required for the Specification of Neuronal Types in the Drosophila Optic Lobe.” Developmental Biology 377 (1): 90–99. 10.1016/j.ydbio.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Hofbauer Alois, and Campos-Ortega José A. 1990. “Proliferation Pattern and Early Differentiation of the Optic Lobes in Drosophila Melanogaster.” Roux’s Archives of Developmental Biology : The Official Organ of the EDBO 198 (5): 264–74. 10.1007/BF00377393. [DOI] [PubMed] [Google Scholar]
- Holguera Isabel, and Desplan Claude. 2018. “Neuronal Specification in Space and Time.” Science 362 (6411): 176–80. 10.1126/science.aas9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem Catarina C. F., Repic Marko, and Knoblich Jürgen A.. 2015. “Proliferation Control in Neural Stem and Progenitor Cells.” Nature Reviews Neuroscience 16 (11): 647–59. 10.1038/nrn4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem Catarina C.F, Steinmann Victoria, Burkard Thomas R., Jais Alexander, Esterbauer Harald, and Knoblich Juergen A.. 2014. “Ecdysone and Mediator Change Energy Metabolism to Terminate Proliferation in Drosophila Neural Stem Cells.” Cell 158 (4): 874–88. 10.1016/j.cell.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Huang Zhen, and Kunes Samuel. 1996. “Hedgehog, Transmitted along Retinal Axons, Triggers Neurogenesis in the Developing Visual Centers of the Drosophila Brain.” Cell 86 (3): 411–22. 10.1016/S0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Huang Zhen, Shilo Ben Zion, and Kunes Sam. 1998. “A Retinal Axon Fascicle Uses Spitz, an EGF Receptor Ligand, to Construct a Synaptic Cartridge in the Brain of Drosophila.” Cell 95 (5): 693–703. 10.1016/S0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- Isshiki T, Takeichi M, and Nose A. 1997. “The Role of the Msh Homeobox Gene during Drosophila Neurogenesis: Implication for the Dorsoventral Specification of the Neuroectoderm.” Development (Cambridge, England) 124 (16): 3099–3109. http://www.ncbi.nlm.nih.gov/pubmed/9272951. [DOI] [PubMed] [Google Scholar]
- Isshiki Takako, Pearson Bret, Holbrook Scott, and Doe Chris Q.. 2001. “Drosophila Neuroblasts Sequentially Express Transcription Factors Which Specify the Temporal Identity of Their Neuronal Progeny.” Cell 106 (4): 511–21. 10.1016/S0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Ito Masayoshi, Masuda Naoki, Shinomiya Kazunori, Endo Keita, and Ito Kei. 2013. “Systematic Analysis of Neural Projections Reveals Clonal Composition of the Drosophila Brain.” Current Biology 23 (8): 644–55. 10.1016/j.cub.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Kambadur Ravi, Koizumi Keita, Stivers Chad, Nagle James, Poole Stephen J., and Odenwald Ward F.. 1998. “Regulation of POU Genes by Castor and Hunchback Establishes Layered Compartments in the Drosophila CNS.” Genes and Development 12 (2): 246–60. 10.1101/gad.12.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Makoto I., Okabe Masataka, and Hiromi Yasushi. 2005. “Seven-up Controls Switching of Transcription Factors That Specify Temporal Identities of Drosophila Neuroblasts.” Developmental Cell 8 (2): 203–13. 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kao Chih-Fei, Yu Hung-Hsiang, He Yisheng, Kao Jui-Chun, and Lee Tzumin. 2012. “Hierarchical Deployment of Factors Regulating Temporal Fate in a Diverse Neuronal Lineage of the Drosophila Central Brain.” Neuron 73 (4): 677–84. 10.1016/j.neuron.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst Kimberly, and Kunes Samuel. 1994. “Pattern Formation in the Visual Centers of the Drosophila Brain: Wingless Acts via Decapentaplegic to Specify the Dorsoventral Axis.” Cell 78 (3): 437–48. 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Karlsson Daniel, Baumgardt Magnus, and Thor Stefan. 2010. “Segment-Specific Neuronal Subtype Specification by the Integration of Anteroposterior and Temporal Cues.” PLoS Biology 8 (5). 10.1371/journal.pbio.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Minoree, Lupton Joshua R., Lai Sen-Lin, Miller Michael R., and Doe Chris Q.. 2013. “Developmentally Regulated Subnuclear Genome Reorganization Restricts Neural Progenitor Competence in Drosophila.” Cell 152 (1–2): 97–108. 10.1016/j.cell.2012.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides Nikolaos, Kapuralin Katarina, Fadil Chaimaa, Barboza Luendreo, Satija Rahul, and Desplan Claude. 2018. “Phenotypic Convergence: Distinct Transcription Factors Regulate Common Terminal Features.” Cell, 1–14. 10.1016/j.cell.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherenko Mariya M, Barth Jonas, Fiala André, and Shcherbata Halyna R. 2012. “Steroid-Induced MicroRNA Let-7 Acts as a Spatio-Temporal Code for Neuronal Cell Fate in the Developing Drosophila Brain.” The EMBO Journal 31 (24): 4511–23. 10.1038/emboj.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacin Haluk, Chen Hui-Min, Long Xi, Singer Robert H, Lee Tzumin, and Truman James W. 2019. “Neurotransmitter Identity Is Acquired in a Lineage-Restricted Manner in the Drosophila CNS.” ELife 8: 1–26. 10.7554/elife.43701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Lee A, and Luo L. 1999. “Development of the Drosophila Mushroom Bodies: Sequential Generation of Three Distinct Types of Neurons from a Neuroblast.” Development (Cambridge, England) 126 (18): 4065–76. [DOI] [PubMed] [Google Scholar]
- Lee Ying-Jou, Yang Ching-Po, Miyares Rosa Linda Huang Yu-Fen, He Yisheng, Ren Qingzhong, Chen Hui-Min, et al. 2020. “Conservation and Divergence of Related Neuronal Lineages in the Drosophila Central Brain.” ELife 9 (April). 10.7554/eLife.53518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Hongjie, Horns Felix, Wu Bing, Xie Qijing, Li Jiefu, Li Tongchao, Luginbuhl David J., et al. 2017. “Classifying Drosophila Olfactory Projection Neuron Subtypes by Single-Cell RNA Sequencing.” Cell 171 (5): 1206–1220.e22. 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Xin, Erclik Ted, Bertet Claire, Chen Zhenqing, Voutev Roumen, Venkatesh Srinidhi, Morante Javier, Celik Arzu, and Desplan Claude. 2013. “Temporal Patterning of Drosophila Medulla Neuroblasts Controls Neural Fates.” Nature 498 (7455): 456–62. 10.1038/nature12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Suewei, Lai S-L, Yu H-H, Chihara Takahiro, Luo Liqun, and Lee Tzumin. 2010. “Lineage-Specific Effects of Notch/Numb Signaling in Post-Embryonic Development of the Drosophila Brain.” Development 137 (1): 43–51. 10.1242/dev.041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Ling-yu, Long Xi, Yang Ching-po, Miyares Rosa L, Sugino Ken, Singer Robert H, and Lee Tzumin. 2019. “Mamo Decodes Hierarchical Temporal Gradients into Terminal Neuronal Fate.” ELife 8 (September): 1–28. 10.7554/eLife.48056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang C-P, Sugino Ken, Fu C-C, Liu L-Y, Yao X, Lee LP, and Lee T. 2015. “Opposing Intrinsic Temporal Gradients Guide Neural Stem Cell Production of Varied Neuronal Fates.” Science 350 (6258): 317–20. 10.1126/science.aad1886. [DOI] [PubMed] [Google Scholar]
- Marchetti Giovanni, and Tavosanis Gaia. 2019. “Modulators of Hormonal Response Regulate Temporal Fate Specification in the Drosophila Brain.” Edited by Wang Hongyan. PLOS Genetics 15 (12): e1008491. 10.1371/journal.pgen.1008491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, and Saigo K. 1996. “Hedgehog Signaling Independent of Engrailed and Wingless Required for Post-S1 Neuroblast Formation in Drosophila CNS.” Development (Cambridge, England) 122 (11): 3567–75. http://www.ncbi.nlm.nih.gov/pubmed/8951072. [DOI] [PubMed] [Google Scholar]
- Maurange Cédric. 2020. “Temporal Patterning in Neural Progenitors: From Drosophila Development to Childhood Cancers.” Disease Models & Mechanisms 13 (7): dmm044883. 10.1242/dmm.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurange Cédric, Cheng Louise, and Gould Alex P. 2008. “Temporal Transcription Factors and Their Targets Schedule the End of Neural Proliferation in Drosophila.” Cell 133 (5): 891–902. 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Maurange Cédric, and Gould Alex P.. 2005. “Brainy but Not Too Brainy: Starting and Stopping Neuroblast Divisions in Drosophila.” Trends in Neurosciences. 10.1016/j.tins.2004.10.009. [DOI] [PubMed] [Google Scholar]
- McDonald JA, and Doe CQ. 1997. “Establishing Neuroblast-Specific Gene Expression in the Drosophila CNS: Huckebein Is Activated by Wingless and Hedgehog and Repressed by Engrailed and Gooseberry.” Development (Cambridge, England) 124 (5): 1079–87. http://www.ncbi.nlm.nih.gov/pubmed/9056782. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Holbrook Scott, Isshiki Takako, Weiss Joseph, Doe Chris Q, and Mellerick Dervla M. 1998. “Dorsoventral Patterning in the Drosophila Central Nervous System: The Vnd Homeobox Gene Specifies Ventral Column Identity.” Genes & Development 12 (22): 3603–12. 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Julia L, Marshall Zarion D, Lobb-Rabe Meike, and Heckscher Ellie S. 2019. “How Prolonged Expression of Hunchback, a Temporal Transcription Factor, Re-Wires Locomotor Circuits.” ELife 8 (September): 1–30. 10.7554/eLife.46089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monedero Cobeta, Behzad Yaghmaeian Salmani Ignacio, and Thor Stefan. 2017. “Anterior-Posterior Gradient in Neural Stem and Daughter Cell Proliferation Governed by Spatial and Temporal Hox Control.” Current Biology : CB 27 (8): 1161–72. 10.1016/j.cub.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Naidu Vamsikrishna G., Zhang Yu, Lowe Scott, Ray Alokananda, Zhu Hailun, and Li Xin. 2020. “Temporal Progression of Drosophila Medulla Neuroblasts Generates the Transcription Factor Combination to Control T1 Neuron Morphogenesis.” Developmental Biology 464 (1): 35–44. 10.1016/j.ydbio.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo Kathy T., Andrade Ingrid, and Hartenstein Volker. 2017. “Spatio-Temporal Pattern of Neuronal Differentiation in the Drosophila Visual System: A User’s Guide to the Dynamic Morphology of the Developing Optic Lobe.” Developmental Biology 428 (1): 1–24. 10.1016/j.ydbio.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlen Tonia Von, and Doe Chris Q.. 2000. “Convergence of Dorsal, Dpp, and Egfr Signaling Pathways Subdivides the Drosophila Neuroectoderm into Three Dorsal-Ventral Columns.” Developmental Biology 224 (2): 362–72. 10.1006/dbio.2000.9789. [DOI] [PubMed] [Google Scholar]
- Pahl Matthew C., Doyle Susan E., and Siegrist Sarah E.. 2019. “E93 Integrates Neuroblast Intrinsic State with Developmental Time to Terminate MB Neurogenesis via Autophagy.” Current Biology, 1–13. 10.1016/j.cub.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Sharon E., and Steller Hermann. 1996. “Migration of Glial Cells into Retinal Axon Target Field in Drosophila Melanogaster.” Journal of Neurobiology 30 (3): 359–73. . [DOI] [PubMed] [Google Scholar]
- Pinto-Teixeira Filipe, Koo Clara, Rossi Anthony Michael Neriec Nathalie, Bertet Claire, Li Xin, Del-Valle-Rodriguez Alberto, and Desplan Claude. 2018. “Development of Concurrent Retinotopic Maps in the Fly Motion Detection Circuit.” Cell 173 (2): 485–498.e11. 10.1016/j.cell.2018.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz Mark, Miller Steven W., and Posakony James W.. 2011. “Notch Regulates Numb: Integration of Conditional and Autonomous Cell Fate Specification.” Development 138 (2): 215–25. 10.1242/dev.050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GV, and Rodrigues V. 1999. “A Glial Cell Arises from an Additional Division within the Mechanosensory Lineage during Development of the Microchaete on the Drosophila Notum.” Development (Cambridge, England) 126 (20): 4617–22. http://www.ncbi.nlm.nih.gov/pubmed/10498695. [DOI] [PubMed] [Google Scholar]
- Ren Qingzhong, Yang Ching-Po, Liu Zhiyong, Sugino Ken, Mok Kent, He Yisheng, Ito Masayoshi, Nern Aljoscha, Otsuna Hideo, and Lee Tzumin. 2017. “Stem Cell-Intrinsic, Seven-up-Triggered Temporal Factor Gradients Diversify Intermediate Neural Progenitors.” Current Biology, April, 1–11. 10.1016/j.cub.2017.03.047. [DOI] [PubMed] [Google Scholar]
- Rossi Anthony M, and Desplan Claude. 2020. “Extrinsic Activin Signaling Cooperates with an Intrinsic Temporal Program to Increase Mushroom Body Neuronal Diversity.” ELife 9 (July). 10.7554/eLife.58880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi Anthony M, Fernandes Vilaiwan M, and Desplan Claude. 2017. “Timing Temporal Transitions during Brain Development.” Current Opinion in Neurobiology 42 (February): 84–92. 10.1016/j.conb.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Losada Mireya, Blom-Dahl David, Córdoba Sergio, and Estella Carlos. 2018. “Specification and Patterning of Drosophila Appendages.” Journal of Developmental Biology 6 (3). 10.3390/jdb6030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels Tamsin J., Järvelin Aino I., Ish-Horowicz David, and Davis Ilan. 2020. “Imp/IGF2BP Levels Modulate Individual Neural Stem Cell Growth and Division through Myc MRNA Stability.” ELife 9 (January): 1–27. 10.7554/eLife.51529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Makoto, Suzuki Takumi, and Nakai Yasuhiro. 2013. “Waves of Differentiation in the Fly Visual System.” Developmental Biology 380 (1): 1–11. 10.1016/j.ydbio.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Seibert Janina, and Urbach Rolf. 2010. “Role of En and Novel Interactions between Msh, Ind, and Vnd in Dorsoventral Patterning of the Drosophila Brain and Ventral Nerve Cord.” Developmental Biology 346 (2): 332–45. 10.1016/j.ydbio.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Seibert Janina, Volland Dagmar, and Urbach Rolf. 2009. “Ems and Nkx6 Are Central Regulators in Dorsoventral Patterning of the Drosophila Brain.” Development 136 (23): 3937–47. 10.1242/dev.041921. [DOI] [PubMed] [Google Scholar]
- Sen Anindya, Reddy G. Venugopala, and Rodrigues Veronica. 2003. “Combinatorial Expression of Prospero, Seven-up, and Elav Identifies Progenitor Cell Types during Sense-Organ Differentiation in the Drosophila Antenna.” Developmental Biology 254 (1): 79–92. 10.1016/S0012-1606(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Sen Sonia Q, Chanchani Sachin, Southall Tony D, and Doe Chris Q. 2019. “Neuroblast-Specific Open Chromatin Allows the Temporal Transcription Factor, Hunchback, to Bind Neuroblast-Specific Loci.” ELife 8: 1–26. 10.7554/eLife.44036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroka Austin Q., and Doe Chris Q.. 2019. The Hunchback Temporal Transcription Factor Determines Motor Neuron Axon and Dendrite Targeting in Drosophila. Development. Vol. 8029. 10.1242/dev.175570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroka Austin, Yazejian Rita M., Lai Sen-Lin, and Doe Chris Q.. 2020. “A Novel Temporal Identity Window Generates Alternating Eve+/Nkx6+ Motor Neuron Subtypes in a Single Progenitor Lineage.” Neural Development 15 (1): 9. 10.1186/s13064-020-00146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag SR, Müller B, and Steinbrecht RA. 1999. “Atlas of Olfactory Organs of Drosophila Melanogaster.” International Journal of Insect Morphology and Embryology 28 (4): 377–97. 10.1016/S0020-7322(99)00039-2. [DOI] [Google Scholar]
- Singhania Aditi, and Grueber Wesley B.. 2014. “Development of the Embryonic and Larval Peripheral Nervous System of Drosophila.” Wiley Interdisciplinary Reviews: Developmental Biology 3 (3): 193–210. 10.1002/wdev.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB, and Doe CQ. 1998. “Sanpodo and Notch Act in Opposition to Numb to Distinguish Sibling Neuron Fates in the Drosophila CNS.” Development (Cambridge, England) 125 (10): 1857–65. http://www.ncbi.nlm.nih.gov/pubmed/9550718. [DOI] [PubMed] [Google Scholar]
- Skeath JB, Zhang Y, Holmgren R, Carroll SB, and Doe CQ. 1995. “Specification of Neuroblast Identity in the Drosophila Embryonic Central Nervous System by Gooseberry-Distal.” Nature 376 (6539): 427–30. 10.1038/376427a0. [DOI] [PubMed] [Google Scholar]
- Skeath James B., and Thor Stefan. 2003. “Genetic Control of Drosophila Nerve Cord Development.” Current Opinion in Neurobiology 13 (1): 8–15. 10.1016/S0959-4388(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Spana EP, Kopczynski C, Goodman CS, and Doe CQ. 1995. “Asymmetric Localization of Numb Autonomously Determines Sibling Neuron Identity in the Drosophila CNS.” Development (Cambridge, England) 121 (11): 3489–94. http://www.ncbi.nlm.nih.gov/pubmed/8582263. [DOI] [PubMed] [Google Scholar]
- Spana Eric P., and Doe Chris Q.. 1996. “Numb Antagonizes Notch Signaling to Specify Sibling Neuron Cell Fates.” Neuron 17 (1): 21–26. 10.1016/S0896-6273(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Stratmann Johannes, Gabilondo Hugo, Benito-Sipos Jonathan, and Thor Stefan. 2016. “Neuronal Cell Fate Diversification Controlled by Sub-Temporal Action of Kruppel.” ELife 5 (October): 1–17. 10.7554/eLife.19311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Takumi, Kaido Masako, Takayama Rie, and Sato Makoto. 2013. “A Temporal Mechanism That Produces Neuronal Diversity in the Drosophila Visual Center.” Developmental Biology 380 (1): 12–24. 10.1016/j.ydbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Syed Mubarak Hussain, Mark Brandon, and Doe Chris Q. 2017. “Steroid Hormone Induction of Temporal Gene Expression in Drosophila Brain Neuroblasts Generates Neuronal and Glial Diversity.” ELife 6 (April): 1–23. 10.7554/eLife.26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau Gerhard M., Berger Christian, and Urbach Rolf. 2006. “Generation of Cell Diversity and Segmental Pattern in the Embryonic Central Nervous System of Drosophila.” Developmental Dynamics. 10.1002/dvdy.20566. [DOI] [PubMed] [Google Scholar]
- Truman James W, Moats Wanda, Altman Janet, Marin Elizabeth C, and Williams Darren W. 2010. “Role of Notch Signaling in Establishing the Hemilineages of Secondary Neurons in Drosophila Melanogaster.” Development 137 (1): 53–61. 10.1242/dev.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udolph G, Prokop A, Bossing T, and Technau GM. 1993. “A Common Precursor for Glia and Neurons in the Embryonic CNS of Drosophila Gives Rise to Segment-Specific Lineage Variants.” Development (Cambridge, England) 118 (3): 765–75. http://www.ncbi.nlm.nih.gov/pubmed/8076516. [DOI] [PubMed] [Google Scholar]
- Udolph Gerald. 2012. “Notch Signaling and the Generation of Cell Diversity in Drosophila Neuroblast Lineages.” Advances in Experimental Medicine and Biology 727: 47–60. 10.1007/978-1-4614-0899-4_4. [DOI] [PubMed] [Google Scholar]
- Urbach Rolf, and Technau Gerhard M.. 2003. “Segment Polarity and DV Patterning Gene Expression Reveals Segmental Organization of the Drosophila Brain.” Development (Cambridge, England) 130 (16): 3607–20. 10.1242/dev.00532. [DOI] [PubMed] [Google Scholar]
- ———. 2004. “Neuroblast Formation and Patterning during Early Brain Development in Drosophila.” BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology 26 (7): 739–51. 10.1002/bies.20062. [DOI] [PubMed] [Google Scholar]
- Walsh Kathleen T., and Doe Chris Q.. 2017. “Drosophila Embryonic Type II Neuroblasts: Origin, Temporal Patterning, and Contribution to the Adult Central Complex.” Development, dev.157826. 10.1242/dev.157826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Hua, Jafari Shadi, Pask Gregory, Zhou Xiaofan, Reinberg Danny, and Desplan Claude. 2020. “Evolution, Developmental Expression and Function of Odorant Receptors in Insects.” The Journal of Experimental Biology 223 (Pt Suppl 1). 10.1242/jeb.208215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Ching-Po, Fu Chi-Cheng, Sugino Ken, Liu Zhiyong, Ren Qingzhong, Liu Ling-Yu, Yao Xiaohao, Lee Luke P, and Lee Tzumin. 2016. “Transcriptomes of Lineage-Specific Drosophila Neuroblasts Profiled by Genetic Targeting and Robotic Sorting.” Development (Cambridge, England) 143 (3): 411–21. 10.1242/dev.129163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Ching-Po Po, Samuels Tamsin J., Huang Yaling, Yang Lu, Ish-Horowicz David, Davis Ilan, and Lee Tzumin. 2017. “Imp and Syp RNA-Binding Proteins Govern Decommissioning of Drosophila Neural Stem Cells.” Development 144 (19): 3454–64. 10.1242/dev.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Jacob S., Awasaki Takeshi, Yu Hung-Hsiang, He Yisheng, Ding Peng, Kao Jui-Chun, and Lee Tzumin. 2013. “Diverse Neuronal Lineages Make Stereotyped Contributions to the Drosophila Locomotor Control Center, the Central Complex.” Journal of Comparative Neurology 521 (12): 2645–62. 10.1002/cne.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi-Hartenstein Amelia, Nassif Claude, Green Patricia, and Hartenstein Volker. 1996. “Early Neurogenesis of TheDrosophila Brain.” The Journal of Comparative Neurology 370 (3): 313–29. . [DOI] [PubMed] [Google Scholar]
- Yu Hung-Hsiang, Kao Chih-Fei, He Yisheng, Ding Peng, Kao Jui-Chun, and Lee Tzumin. 2010. “A Complete Developmental Sequence of a Drosophila Neuronal Lineage as Revealed by Twin-Spot MARCM.” Edited by Bellen Hugo J.. PLoS Biology 8 (8): e1000461. 10.1371/journal.pbio.1000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Hung Hsiang, Awasaki Takeshi, Schroeder Mark David, Long Fuhui, Yang Jacob S., He Yisheng, Ding Peng, et al. 2013. “Clonal Development and Organization of the Adult Drosophila Central Brain.” Current Biology 23 (8): 633–43. 10.1016/j.cub.2013.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Sijun, Lin Suewei, Kao Chih-Fei, Awasaki Takeshi, Chiang Ann-Shyn, and Lee Tzumin. 2006. “Gradients of the Drosophila Chinmo BTB-Zinc Finger Protein Govern Neuronal Temporal Identity.” Cell 127 (2): 409–22. 10.1016/j.cell.2006.08.045. [DOI] [PubMed] [Google Scholar]


