Abstract
Background:
There is no widely adopted severity grading system for acute allergic reactions, including anaphylactic and nonanaphylactic reactions, thus limiting the ability to optimize and standardize management practices and advance research.
Objective:
The aim of this study was to develop a severity grading system for acute allergic reactions for use in clinical care and research.
Methods:
From May to September 2020, we convened a 21-member multidisciplinary panel of allergy and emergency care experts; 9 members formed a writing group to critically appraise and assess the strengths and limitations of prior severity grading systems and develop the structure and content for an optimal severity grading system. The entire study panel then revised the grading system and sought consensus by utilizing Delphi methodology.
Results:
The writing group recommended that an optimal grading system encompass the severity of acute allergic reactions on a continuum from mild allergic reactions to anaphylactic shock. Additionally, the severity grading system must be able to discriminate between clinically important differences in reaction severity to be relevant in research while also being intuitive and straightforward to apply in clinical care. Consensus was reached for all elements of the proposed severity grading system.
Conclusion:
We developed a consensus severity grading system for acute allergic reactions, including anaphylactic and nonanaphylactic reactions. Successful international validation, refinement, dissemination, and application of the grading system will improve communication among providers and patients about the severity of allergic reactions and will help advance future research.
Keywords: Allergic reactions, anaphylaxis, Delphi, emergency department, severity
Acute allergic reactions may occur following exposure to a wide variety of allergens (eg, foods, medications, biologic compounds, plants, venoms), in a broad range of settings (eg, home, school, recreational, health care), and during oral food challenges and clinical trials evaluating the efficacy of allergen immunotherapy.1-3 Reactions occur on a severity continuum from mild (requiring no or minimal intervention) to anaphylactic shock (necessitating treatment with resuscitative therapies).1
The societal burden of acute allergic reactions (including anaphylaxis) on patients, families, and the US health care system is considerable. From 2008 to 2016, there were more than 400,000 emergency department visits for anaphylaxis alone.4 Despite research advances to determine the epidemiology of allergic reactions and develop therapies and public health interventions to mitigate disease burden, recent studies have found that in the past decade, emergency department visits for anaphylaxis in the United States doubled across all age groups and tripled among children.4 Direct medical costs from food allergies alone total $4.3 billion dollars annually, including average individual direct medical costs of $2081.5
Although severity grading systems have been developed in the past,6-14 they have not been widely implemented, thus hindering standardization of clinical practice and research efforts. Our group recently published definitions of anaphylaxis outcomes to harmonize clinical care and facilitate research15 and concluded that there is also a need to develop a novel grading system to classify the severity of these outcomes. This conclusion is supported by a position paper by the European Academy of Allergy and Clinical Immunology recommending the “need for an appropriately developed and validated severity scoring system for allergic reactions that works across the range of allergenic triggers and addresses the needs of different stakeholder groups.”9
Thus, the objective of this study was to assemble a multidisciplinary group of allergy and emergency care experts to develop a consensus severity grading system for acute allergic reactions (including both anaphylactic and nonanaphylactic reactions) that is applicable to clinical care and research. This serves as a key first step before validation, dissemination, and implementation of our severity grading system. Successful implementation of this grading system will improve communication among providers and patients, facilitate standardization efforts in anaphylaxis management, and serve as a framework for reporting outcomes and adverse events during clinical trials.
METHODS
Literature search
We performed a literature review of the Embase, PubMed, Cochrane, and Scopus databases for English articles published from 1997 to March 2020; we used the following search terms: anaphylaxis severity, acute allergic reaction grading system, anaphylaxis grading system, acute allergic reaction grading scale, anaphylaxis grading scale, allergic reaction grade, and anaphylaxis grade (see Table E1 in the Online Repository at www.jacionline.org). Articles published before 1997 were also included if they were cited at least 5 times in references from the literature search. Articles were made available to panel members in an online study folder.
Delphi group panel members
We convened a 21-member panel of experts in the field of anaphylaxis, including allergists/immunologists and general and pediatric emergency medicine specialists. Panel members were selected on the basis of expert recommendations, published research, and membership in national research networks and/or anaphylaxis interest groups. From this group, 9 panel members (T.D., D.S., J.S., R.C., M.S., M.N., K.M., P.C., and H.S.) additionally served on a writing group tasked with (1) reviewing the literature search to assess the strengths and limitations of prior grading systems; (2) developing criteria and attributes for an optimal grading system, including how it should be structured and used in clinical care and research; and (3) creating and revising a draft grading system based on discussions during monthly teleconference calls and electronic communications from May to June 2020. The grading system was subsequently refined by the entire study panel during monthly teleconference calls from July to September 2020.
Conference calls
During the writing group conference calls, panel members discussed relevant research, research gaps, and clinical expertise relevant to the study objective. On the first call, panel members reviewed prior severity grading systems from the literature search and summarized their strengths and limitations. The panel then developed a framework for creating an ideal severity grading system that would address the needs of both clinicians and researchers. After each conference call, the draft grading system was circulated to the writing group for revisions and subsequently modified. This process was repeated until all panel members agreed that no further revisions should be made. The grading system, along with a document outlining central themes from the calls, was then disseminated to the entire study panel, and during monthly conference calls, the grading system was revised and subsequently finalized. Audio recordings of the calls were made available to panel members who could not join the live calls.
Delphi surveys
After finalization of the grading system during conference calls, Delphi methodology was used to determine whether there was consensus for the severity grading system and all severity grades, the subgrading system and all subgrades, and the definitions specific to the grading system. We used previously applied Delphi methodology for developing consensus outcomes in other fields of medicine,16 including the definition and clinical criteria for septic shock.17 In each survey round, an electronic Research Electronic Data Capture (REDCap) survey (Nashville, Tenn)18 was sent to panel members, who were asked to rate each statement on level of agreement (with 1 meaning strongly disagree, 2 meaning agree, 3 meaning neutral, 4 meaning agree, and 5 indicating strongly agree), or not applicable if panel members did not think that they had the necessary expertise or background knowledge to rank a particular statement. The responses strongly agree and agree were grouped together, and the responses strongly disagree and disagree were grouped together.16 Additionally, panel members were encouraged to submit free text comments to clarify their response to every question, suggest additional questions, or recommend modifications to the severity grading system. Consensus was defined as agreement equal to or exceeding 65% for statements. Our methodology (including the threshold of 65%) was based on that used by Shankar-Hari et al to develop a new definition and clinical criteria for septic shock; the threshold of 65% was determined a priori before the surveys were sent to panel members.16
If consensus for a question was not reached after the initial survey, subsequent surveys incorporating the aggregate deidentified responses from prior surveys were sent to panel members with the goal of narrowing responses to reach consensus. This process was repeated until consensus was reached for each statement or after 4 survey rounds. If no consensus was achieved by the fourth round, the question was categorized as “no consensus reached.”
The institutional review board at Cincinnati Children’s Hospital Medical Center approved this study.
RESULTS
Literature review
In total, 31 articles were included and reviewed by the writing group and made available to the entire study panel. The most common methodology for creating prior severity grading systems was expert opinion. Of note, no previous grading system had been developed by using Delphi methodology. For a summary of the literature review, please see Table E1.
Limitations of prior grading systems and attributes of an optimal, novel severity grading system
The panel members reviewed previous grading systems and agreed that they have a variety of limitations (detailed later in this article) preventing consistent use across clinical care and research. However, the grading systems provide a framework for developing a broadly applicable grading system. For example, the grading system proposed by Sampson et al (in 2012)7 to standardize double-blind, placebo-controlled food challenges affords researchers the ability to account for a broad range of symptoms within each organ system. Although this approach works well for research, it does not allow clinicians or researchers to assign a summative severity grade based on the ranking and/or combinations of symptoms. In contrast, the grading system developed by Brown in 20046 is easy to use in clinical care because it separates hypersensitivity reactions into 3 clearly defined severity grades. Unfortunately, such an approach was thought to be overly simplistic because it does not allow researchers the ability to account for the wide spectrum of disease severity for acute allergic reactions (eg, mild local reactions to life-threatening anaphylaxis). Beyond these grading systems, other systems have been used in clinical trials; they include the grading system developed by Burks et al (in 2012) to assess acute allergic reactions during oral immunotherapy for treatment of egg allergy.11 Although the grading system is not overly complex and matches reaction severity based on clinical gestalt, it is suboptimal because it incorporates symptoms, nonspecific terminology (eg, extreme limitation in activity), and subjective clinical outcomes (eg, need for hospitalization) to classify reaction severity instead of relying solely on symptom severity.
-
Lack of granularity. Prior grading systems have not been able to discriminate between clinically important phenotypes (eg, cardiovascular involvement with presyncope, hypotension, anaphylactic shock, or cardiac arrest).9
The optimal severity grading system should convey that the severity of acute allergic reactions is on a continuum from mild to fatal. It does not simply categorize reactions as anaphylactic or nonanaphylactic, given that anaphylaxis diagnostic criteria may change with time19,20 and because the number of organ systems affected may vary throughout a patient’s clinical course. This is also important because patients may have fatal allergic reactions with apparently “isolated” organ system involvement, including respiratory failure.21
-
Nonspecific terminology. Prior systems have used nonspecific terms to describe organ system involvement (eg, cardiovascular, neurologic, bronchial) without accounting for severity differences within organ systems (eg, cardiovascular involvement with syncope vs shock).9 Grading systems do not uniformly define terms used to classify reaction severity, including hypotension, shock,10 cardiovascular collapse, confusion,6 tachycardia,10 or respiratory failure.8 This includes using subjective terms such as life-threatening,9 dyspnea (without mention of the use of accessory muscles), or change in mental status (without reference to a quantifiable scoring system such as the Glasgow Coma Scale [GCS]).
Signs (clinical and physical examination findings) and symptoms (hereafter signs and symptoms are referred to as symptoms) included in the optimal severity grading system should be easily recognized in clinical care and be measurable and objective with the intent that they will have high interrater reliability among clinicians in validation studies. The grading system incorporates medical instead of lay terminology, given that it is designed to be used by clinicians who interpret patient-reported symptoms.
-
Nonequivalency within grades. Symptoms that denote different reaction severities (eg, dyspnea, shock) are included in the same severity grade(s).10 Subjective and objective findings are treated as equal when that is unlikely to be the case.
The symptoms included in each severity grade should represent equivalent or near-equivalent reaction severity. However, within each grade there may be a spectrum of disease severities, which supports the need for a subgrading system to account for the totality of symptoms.
-
Number of organ systems and reaction severity. Grading systems have proposed that a greater number of involved organ systems usually equates to increased reaction severity;9 however, this is not always the case, as fatal anaphylaxis can occur with apparently isolated respiratory or cardiovascular involvement.22
The optimal severity grading system should discriminate between severity and number of organ systems involved, because life-threatening reactions may occur with isolated organ system involvement.
-
Therapeutics. Grading systems have incorporated therapies as a means of categorizing reaction severity11,23; however, there may be subjective use of treatments such as intramuscular epinephrine or inhaled β-agonists. Likewise, incorporating objective responses to therapies, such as improvement in pulmonary function tests after treatment with inhaled β-agonists, is not feasible in most clinical settings.23
The optimal severity grading system should not include routine therapies for allergic reactions (intramuscular epinephrine, inhaled β-agonists, or supplemental oxygen) or response to therapies, given that use of these therapies may be subject to medication availability and practice variation. In addition, patients may experience fatal anaphylaxis before they receive medical care.24 Panel members did agree that high-acuity or invasive therapies associated with life-threatening reactions should be included in the novel severity grading system (eg, positive pressure ventilation, intubation, intravenous vasopressors, extracorporeal membrane oxygenation), given that these therapies are generally used only in critically ill patients with respiratory failure and/or shock.
-
Symptom duration. Symptom duration has been used to classify reaction severity.11 However, it is difficult to include the duration of some but not all symptoms, and if time descriptions were included, the grading system would also need to account for the association between therapies and symptom duration (eg, symptoms that persist despite vs without therapies).
The optimal severity grading system should not include the duration of symptoms; instead, it can be used to grade the reaction severity longitudinally at various stages (such as in persistent, refractory, and biphasic reactions).15 For example, a patient may have an initial anaphylactic grade 5 acute allergic reaction and then develop a biphasic, nonanaphylactic grade 2 allergic reaction.
-
Impracticality. Some grading systems include different degrees of severity within each organ system but do not account for the totality of symptoms when used to assess overall reaction severity.20 Although such a grading system may be useful in certain research studies, it is not easily applied in clinical care.
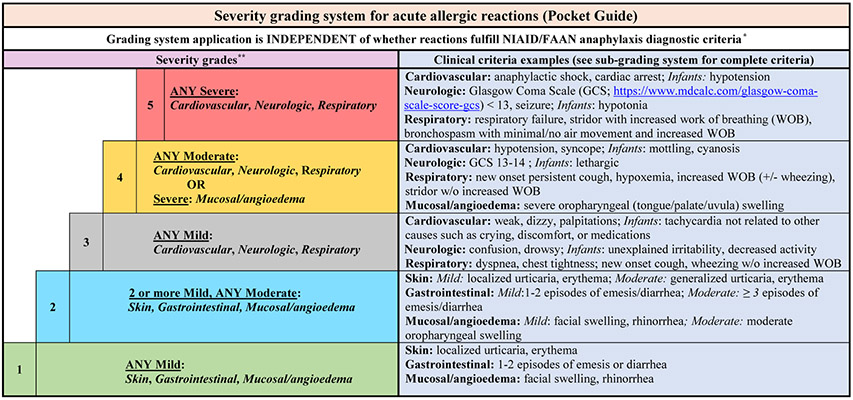
The optimal severity grading system should be intuitive for use in clinical care, given that severity grades (1-5) reflect a logical progression of disease severity. Researchers can use the subgrading system to account for the totality of symptoms within each organ system. We present a cohesive visual representation of the grading and subgrading system (Fig 1) as well as an abridged “pocket guide” version that includes the grading system and important examples of the subgrading system (Fig 2). The pocket guide version is intended to promote easier use in clinical care (of note, Delphi methodology was not used when creating the pocket guide, specifically which elements from the subgrading system should be included in the figure]). Additionally, we have provided a detailed version of the subgrading system (Table I) that could be used as a teaching tool or incorporated into the electronic medical record for easy access and use.
-
Generalizability. Grading systems have been developed for narrow clinical applications, including singular allergens and modes of exposure (eg, oral food challenges vs subcutaneous immunotherapy), care settings, and study designs.7,23 They also do not consistently include symptoms specific to children (particularly, infants or young children who are not able to verbalize symptoms and may have nonspecific symptoms).25
The optimal severity grading system should be widely applicable, including across multiple clinical settings, varied allergens, and all patient ages (including infants, young children, adults, and the elderly).26 Although it would be reasonable to develop a separate severity grading system for infants and young children, the panel thought that it was important to develop a singular grading system applicable to all patient age groups, while highlighting age-dependent specifications. In research, the system can be incorporated into observational or interventional studies to describe outcomes or report adverse events.
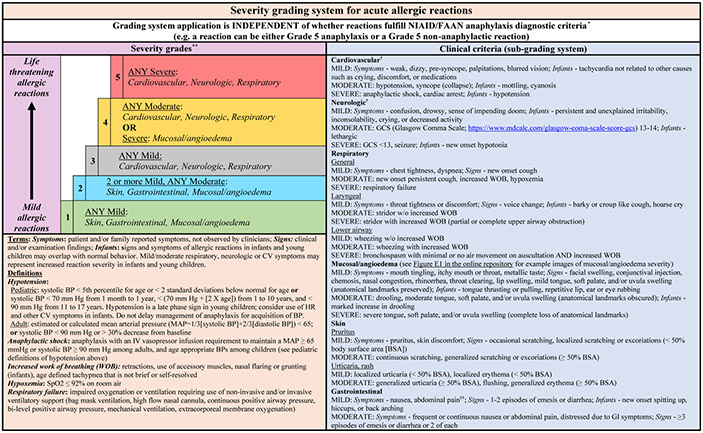
FIG 1.
Severity grading system for acute allergic reactions. *The severity grading system is designed for use across the spectrum of acute allergic reactions, as depicted by the vertical arrow (mild to life-threatening reactions), regardless of whether they fulfill the NIAID/FAAN criteria for anaphylaxis. **For patients with multiple symptoms, reaction severity is based on the most severe symptom; symptoms that constitute more severe grades always supersede symptoms from less severe grades. The grading system can be used to assign reaction severity at any time during the course of reactions; reactions may progress rapidly (within minutes) from one severity grade to another. The grading system does not dictate management decisions; reactions of any severity grade may require treatment with epinephrine. †Patients with severe cardiovascular and/or neurologic involvement may have urinary or stool incontinence. However, the significance of incontinence as an isolated symptom is unclear, and it is therefore not included as a symptom in the subgrading system. ††Abdominal pain may also result from uterine cramping.
FIG 2.
Severity grading system for acute allergic reactions (pocket guide). *The severity grading system is designed for use across the spectrum of acute allergic reactions regardless of whether they fulfill the NIAID/FAAN criteria for anaphylaxis. **For patients with multiple symptoms, reaction severity is based on the most severe symptom; symptoms that constitute more severe grades always supersede symptoms from less severe grades. The grading system can be used to assign reaction severity at any time during the course of reactions; reactions may progress rapidly (within minutes) from one severity grade to another. The grading system does not dictate management decisions; reactions of any severity grade may require treatment with epinephrine.
TABLE I.
Severity subgrading system for acute allergic reactions
| Subgrade | Description |
|---|---|
| Cardiovascular* | |
| Mild | - Symptoms: weak, dizzy, presyncope, palpitations, blurred vision - Infants: tachycardia not related to other causes, such as crying, discomfort, or medications |
| Moderate | - Hypotension, syncope (collapse) - Infants: mottling, cyanosis |
| Severe | - Anaphylactic shock, cardiac arrest - Infants: hypotension |
| Neurologic* | |
| Mild | - Symptoms: confusion, drowsy, sense of impending doom - Infants: persistent and unexplained irritability, inconsolability, crying, or decreased activity |
| Moderate | - GCS score (GCS; https://www.mdcalc.com/glasgow-coma-scale-score-gcs) of 13-14 - Infants: lethargic |
| Severe | - GCS score <13, seizure - Infants: new-onset hypotonia |
| Respiratory | |
| General | |
| Mild | - Symptoms: chest tightness, dyspnea - Signs: new-onset cough |
| Moderate | - New-onset persistent cough, increased WOB, hypoxemia |
| Severe | - Respiratory failure |
| Laryngeal | |
| Mild | - Symptoms: throat tightness or discomfort - Signs: voice change - Infants: barky or croup-like cough, hoarse cry |
| Moderate | - Stridor with no increased WOB |
| Severe | - Stridor with increased WOB (partial or complete upper airway obstruction) |
| Lower airway | |
| Mild | - Wheezing with no increased WOB |
| Moderate | - Wheezing with increased WOB |
| Severe | - Bronchospasm with minimal or no air movement on auscultation AND increased WOB |
| Mucosal/angioedema† | |
| Mild | - Symptoms: mouth tingling, itchy mouth or throat, metallic taste - Signs: facial swelling; conjunctival injection; chemosis; nasal congestion; rhinorrhea; throat clearing; lip swelling; mild tongue, soft palate, and/or uvula swelling (anatomic landmarks preserved) - Infants: tongue thrusting or pulling, repetitive lip, ear or eye rubbing |
| Moderate | - Drooling; moderate tongue, soft palate, and/or uvula swelling (anatomic landmarks obscured) - Infants: marked increase in drooling |
| Severe | - Severe tongue, soft palate, and/or uvula swelling (complete loss of anatomic landmarks) |
| Skin | |
| Pruritus | |
| Mild | - Symptoms: pruritus, skin discomfort - Signs: occasional scratching, localized scratching or excoriations (<50% of BSA) |
| Moderate | - Continuous scratching, generalized scratching or excoriations (≥50% BSA) |
| Urticaria, rash | |
| Mild | - Localized urticaria (<50% BSA), localized erythema (<50% BSA) |
| Moderate | - Generalized urticaria (≥50% BSA), flushing, generalized erythema (≥50% BSA) |
| Gastrointestinal | |
| Mild | - Symptoms: nausea, abdominal pain‡ - Signs: 1-2 episodes of emesis or diarrhea - Infants: new-onset spitting up, hiccups, or back arching |
| Moderate | - Symptoms: frequent or continuous nausea or abdominal pain, distressed as a result of gastrointestinal symptoms - Signs: ≥3 episodes of emesis or diarrhea or 2 of each |
BSA, Body surface area; WOB, work of breathing.
See Fig 1 for lists of terms and definitions.
Patients with severe cardiovascular and/or neurologic involvement may have urinary or stool incontinence. However, the significance of incontinence as an isolated symptom is unclear, and it is therefore not included as a symptom in the subgrading system.
See Fig E1 (in the Online Repository at www.jacionline.org) (eg, images of mucosal/angioedema severity).
Abdominal pain may also result from uterine cramping.
Development of the severity grading system
The grading system is organized into a grading and subgrading system to ensure that it is intuitive and straightforward to apply in clinical care while allowing clinicians and researchers the ability to account for the entirety of symptoms within each organ system and definition. The severity grading system (Figs 1 and 2) is used to assign the final severity grade and is based on the severity (mild, moderate, or severe) of organ system involvement (cardiovascular, neurologic, respiratory, mucosal/angioedema, skin, and gastrointestinal). The subgrading system (Fig 1 and Table I) was developed by modifying the system developed by Sampson et al to score adverse reactions during oral food challenges7 and is used to determine the severity (mild, moderate, or severe) of symptoms, as well as other clinical findings specific to each organ system. As an example, a patient with stridor and retractions would be assigned a respiratory subgrade of severe, and thus the reaction would be categorized as a grade 5 acute allergic reaction. A patient with generalized erythema (≥50% of body surface area) would be assigned a skin subgrade of moderate and the reaction would be categorized as a grade 2 acute allergic reaction.
For patients with multiple symptoms, reaction severity is based on the most severe symptom. Thus, symptoms that constitute more severe grades always supersede symptoms from less severe grades. For example, a patient who has severe mucosal/angioedema involvement (grade 4) as well as severe laryngeal involvement (grade 5) would thus be classified as having a grade 5 acute allergic reaction. The subgrading system also includes clear descriptions of subjective symptoms and symptoms specific to infants.25 It does not include a “severe” subgrade for all organ systems given that this subgrade is intended to denote potentially life-threatening symptoms. As an example, although a patient may have significant isolated dermatologic involvement, the panel agreed there was not usually a degree of dermatologic involvement considered to be life-threatening.
The definitions for pediatric and adult hypotension (Table I and Fig 1) are based on the 2006 National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network (NIAID/FAAN) anaphylaxis diagnostic criteria.20 Low (<65) mean arterial pressure (MAP) was also used to define hypotension in adults, as it is a criterion for hypotension in adult septic shock;17 however, MAP was not used to define pediatric hypotension, given that there is not a consensus threshold for low MAP in pediatric septic shock.27 Additionally, the definition of anaphylactic shock (the need for intravenous vasopressors to maintain targeted blood pressure) is based on the adult definition of septic shock and may not be as applicable to pediatric patients, who can experience compensated shock without hypotension.17 The grading system also includes objective symptoms for different organ systems to minimize ambiguity when using it in clinical care and research. Moderate and severe neurologic involvement are classified by GCS scores (13-14 and <13, respectively).17 Although there are no data about the relationship between GCS scores and anaphylaxis severity, we extrapolated GCS scores from the sequential organ failure assessment score, which is used to predict mortality in adult sepsis.17,28
Delphi results
The panel members voted on the newly developed grading system and each of its individual components, as presented in Table E2 (available in the Online Repository at www.jacionline.org), which also includes free text comments for each question). The grading system achieved 95.2% overall agreement, and the subgrading system achieved 90.5% overall agreement. No individual component had less than 80% agreement. Consensus for each item and for the overall system was reached after 1 Delphi round. As agreement was greater than the Delphi consensus threshold (≥65% agreement),16 there was no need for iterative rounds as outlined in the Methods section.
DISCUSSION
We have developed a consensus-based severity grading system for acute allergic reactions (including anaphylactic and nonanaphylactic allergic reactions) for use in clinical care and research settings. This is the first study to develop a severity grading system for acute allergic reactions by using Delphi methodology, which is a broadly used and preferred methodology to achieve consensus in comparison with relying solely on expert opinion.15-17,29 The severity grading system is generalizable to both domains because it is intuitive while also being granular and specific. Importantly, the grading system is not intended to dictate patient-level management decisions, including when to administer epinephrine, given that it is not reasonable to have prescriptive management recommendations for every patient, type of reaction, or combination of symptoms within each severity grade.
Before it can be accepted for widespread use in clinical care and research, the grading system should be validated and refined with input from an international multidisciplinary group of experts, for which every effort should be made to ensure sex parity among representatives.30,31 This includes validating the grading system by using preexisting prospectively obtained data sets in addition to enrolling prospective cohorts to compare and contrast its applicability and functionality with those of previous developed grading systems.6,32 Prospective studies will also afford researchers the ability to collect biologic specimens to evaluate whether current and novel anaphylaxis biomarkers are correlated with symptom severity and/or are predictive of adverse clinical outcomes (eg, severe, refractory, persistent, or biphasic anaphylaxis).21,33,34 Biomarkers may one day be incorporated into severity grading systems and could be used by clinicians to resolve diagnostic ambiguity (eg, Is this anaphylaxis?) and to inform therapeutic (eg, When should epinephrine be administered?) and management decisions (eg, How long should patients be observed after reaction onset?).
Use of the severity grading system in clinical care and research
The severity grading system is designed to evaluate the severity of acute allergic reactions and not chronic or nonallergic conditions. However, there may be diagnostic uncertainty or inconsistent application of the term acute allergic reactions, especially depending on the clinical setting. To account for potential diagnostic heterogeneity and/or uncertainty, the inclusion criterion for using the grading system for acute allergic reactions is intentionally broad and is predicated on clinical judgment.
Although the severity grading system encompasses anaphylactic and nonanaphylactic reactions, providers and/or researchers can determine whether reactions meet the criteria for anaphylaxis on the basis of whether reactions fulfill the 2006 NIAID/FAAN anaphylaxis diagnostic criteria.20 For example, a patient with urticaria, stridor, and respiratory distress after peanut exposure would be categorized as having an anaphylactic grade 5 acute allergic reaction, whereas a patient with isolated upper airway obstruction following Hymenoptera envenomation would be categorized as having a nonanaphylactic grade 5 allergic reaction (based on the 2006 NIAID/FAAN criteria).20 However, it is important to note that the definition of anaphylaxis itself may evolve, as evidenced by the 2019 revised definition by the Anaphylaxis Committee of the World Allergy Organization, which defines anaphylaxis as “a serious systemic hypersensitivity reaction that is usually rapid in onset and may cause death. Severe anaphylaxis is characterized by potentially life-threatening compromise in breathing and/or the circulation and may occur without typical skin features or circulatory shock being present.”19
Although there is clinical heterogeneity within each severity grade, the subgrading system can be used to account for differences in reaction severity (eg, 2 grade 4 reactions, 1 with isolated hypotension and the other with hypotension and vomiting). However, we recognize that the sub grading system and definitions specific to it do not include every possible symptom; for example, the definition of increased work of breathing includes retractions but does not account for every type of retraction, nor does it include other potential symptoms of increased work of breathing, such as inability to speak in full sentences. The subgrading system can also be used to account for changes in reaction severity. For example, a patient with isolated, moderate lower airway involvement (wheezing with increased work of breathing) would be classified as having a grade 4 reaction; however, if the patient continued to have increased work of breathing and developed worsening aeration on examination (minimal or no air movement), the reaction would be classified as a grade 5 reaction, given that these symptoms may be a sign of impending respiratory failure.
Providers can use the severity grading system to improve communication with patients, who often struggle with the burden and uncertainty of having severe or biphasic reactions. Unfortunately, to date, there has been no standard terminology or shared language to describe reaction severity. Clinicians can use the grading system to educate patients about the continuum of symptoms of allergic and anaphylactic reactions. Patients may be relieved to know that although their symptoms may be distressing or uncomfortable, they are not life-threatening and are mild on the spectrum of disease severity. Furthermore, the grading system will improve communication among providers about the severity of reactions by standardizing the terminology used in documentation and during handoffs.
Importantly, the grading system can be used in clinical research to describe the severity of acute allergic reactions in observational and interventional trials. It could also be used to report adverse events in drug trials, including reaction severity immediately following exposure to the trial drug, the highest severity grade during the course of the reaction, or the totality of all symptoms.
Limitations
As is typical of grading systems and scales developed on the basis of expert opinion, the main limitation of our study is that it has not yet been validated with patient data. However, this is the first allergic reaction severity grading system achieved by using rigorous Delphi methodology. Although we incorporated the best available evidence, including evidence regarding symptoms that have been shown to be associated with life-threatening/fatal reactions,24,35-37 we had to infer many grading system elements from other clinical conditions, including extrapolating the GCS scores and blood pressure thresholds used in septic shock. However, extrapolating these criteria seemed reasonable, given that both conditions are forms of distributive shock.17 Although patient perspectives were not included in development of the grading system, patient-centered studies are needed to determine the interrater reliability of key symptoms and their association with patient-related outcomes.
Although we believe that the grading system is clinically intuitive and balances the need to be broad but sufficiently specific, the 24-element subgrading system may require paper-based or electronic memory aids, standardized documentation elements, or decision support tools to be fully functional. This approach has been used to incorporate other more complex grading systems into clinical care (eg, the National Institutes of Health Stroke Scale).38 Finally, the grading system does not account for fatal allergic reactions owing to the challenge of cause of death adjudication; however, all fatal cases would presumably escalate along the severity continuum and could be categorized as grade 6 reactions for adverse event reporting in research.
Conclusions
We have developed a consensus-based severity grading system for acute allergic reactions (including anaphylactic and nonanaphylactic reactions) for use in clinical care and research. Successful international validation, refinement, dissemination, and application of the grading system will help optimize clinical care practice and facilitate research to advance understanding of the epidemiology and pathophysiology of acute allergic reactions, as well as the efficacy of current and novel therapeutics.
Supplementary Material
Clinical implications: The acute allergic reaction severity grading system proposed in this study has the potential to improve and standardize communication among providers and the care of patients with acute allergic reactions, including anaphylaxis.
Acknowledgments
Supported by the Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center.
Disclosure of potential conflict of interest: D. Schnadower receives funding from the National Institutes of Health (NIH). J. M. Spergel has grant support from DBV Technology, AImmune, and the NIH; in addition, he has consulted for Kaleo. R. L. Campbell has been a peer reviewer for EB Medicine, a consultant for Bryn Pharma, and an author for UpToDate. M. Shaker has a brother who is chief executive officer of Altrix Medical; in addition, he is a member of the Joint Task Force on Practice Parameters, and he serves as an editorial board member for the Journal of Allergy and Clinical Immunology: In Practice; Annals of Allergy, Asthma, and Immunology, and the Journal of Food Allergy. K. A. Michelson receives funding from AHRQ. C. A. Camargo has consulted for Bryn Pharma and Kaleo. D. C. Brousseau receives funding from the NIH and the Maternal and Child Health Bureau. A. H. Assa’ad has research grants from the NIH, Aimmune, DBV technologies, Astellas, AbbVie, and Sanofi. M. Castells is the Brigham and Women’s Hospital principal investigator for the PIONEER BluPrint Clinical Trial for Indolent Systemic Mastocytosis. L. C. Schneider has received research support from Regeneron Pharmaceuticals, DBV Technologies, Pfizer, and Genentech; has consulted for Aimmune Therapeutics; and is on the medical advisory board of FARE (Food Allergy Research and Education). J. Wang receives research support from the National Institute of Allergy and Infectious Disease (NIAID), Aimmune, DBV Technologies, and Regeneron, as well as consultancy fees from, ALK-Abelló, DBV Technologies, and Genentech. R. Mistry receives funding from the NIAID. M. Pistiner has served on advisory boards of DBV Technologies, Kaleo, and Novartis; in addition, he has received research funding from Kaleo and programming funding from DBV Technologies, and he is cofounder of AllergyHome and Allergy Certified Training. H. A. Sampson receives funding to his institution for grants from the NIH/NIAID; in addition, he is employed part-time by and has received stock options from DBV Technologies. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- GCS
Glasgow Coma Scale
- MAP
Mean arterial pressure
- NIAID/FAAN
National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network
REFERENCES
- 1.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020;145:1082–123. [DOI] [PubMed] [Google Scholar]
- 2.The PALISADE Group of Clinical Investigators. AR101 oral immunotherapy for peanut allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 3.Leung DYM, Sampson HA, Yunginger JW, Burks AW, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003;348:986–93. [DOI] [PubMed] [Google Scholar]
- 4.Michelson KA, Dribin TE, Vyles D, Neuman MI. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract 2020;8:767–8.e2. [DOI] [PubMed] [Google Scholar]
- 5.Bilaver LA, Chadha AS, Doshi P, O’Dweyer L, Gupta RS. Economic burden of food allergy- a systematic review. Ann Allergy Asthma Immunol 2019;122:373–80. [DOI] [PubMed] [Google Scholar]
- 6.Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004;114:371–6. [DOI] [PubMed] [Google Scholar]
- 7.Sampson HA, van Wijk RG, Carsten Bindslev-Jensen SS, Teuber SS, Burks AW, Dubois AEJ, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology - European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol 2012;130:1260–74. [DOI] [PubMed] [Google Scholar]
- 8.Cox LS, Sanchez-Borges M, Lockey RF. World Allergy Organization Systemic Allergic Reaction Grading System: is a modification needed? J Allergy Clin Immunol Pract 2017;5:58–62.e5. [DOI] [PubMed] [Google Scholar]
- 9.Muraro A, Fernandez-Rivas M, Beyer K, Cardona V, Clark A, Eller E, et al. The urgent need for a harmonized severity scoring system for acute allergic reactions. Allergy 2018;73:1792–800. [DOI] [PubMed] [Google Scholar]
- 10.Niggemann B, Beyer K. Time for a new grading system for allergic reactions? Allergy 2016;71:135–6. [DOI] [PubMed] [Google Scholar]
- 11.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 2012;367:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol 2018;3:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics 2003;111(suppl 3):1601 LP-1608. [PubMed] [Google Scholar]
- 14.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dribin TE, Sampson HA, Camargo CA Jr, Brousseau DC, Spergel JM, Neuman MI, et al. Persistent, refractory, and biphasic anaphylaxis: a multidisciplinary Delphi study. J Allergy Clin Immunol 2020;146:1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar-Hari M, Phillips GS, Levy ML, Seymour C, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner PJ, Worm M, Ansotegui IJ, El-gamal Y, Rivas MF, Fineman S, et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J 2019;12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second Symposium on the Definition and Management of Anaphlaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117:391–7. [DOI] [PubMed] [Google Scholar]
- 21.Brown SGA, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol 2013;132:1141–9.e5. [DOI] [PubMed] [Google Scholar]
- 22.Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Ann Allergy Asthma Immunol 2007;98:252–7. [DOI] [PubMed] [Google Scholar]
- 23.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 2010. March 1;125:569–74.e7. [DOI] [PubMed] [Google Scholar]
- 24.Bock S, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol 2007;119:1016–8. [DOI] [PubMed] [Google Scholar]
- 25.Greenhawt M, Gupta RS, Meadows JA, Pistiner M, Spergel JM, Camargo CA, et al. Guiding principles for the recognition, diagnosis, and management of infants with anaphylaxis: an expert panel consensus. J Allergy Clin Immunol Pract 2019;7:1148–56.e5. [DOI] [PubMed] [Google Scholar]
- 26.Pistiner M, Mendez-Reyes JE, Eftekhari S, Carver M, Lieberman J, Wang J, et al. Caregiver reported presentation of severe food-induced allergic reactions in infants and toddlers. J Allergy Clin Immunol Pract 2021;311–20.e2. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein B, Giroir B, Randolph A. The M of, Sepsis ICC on P. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 28.Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 29.Fitch K, Bernstein SJ, Mcdonnell J, Kahan JP. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: Rand, 2001. [Google Scholar]
- 30.Mehta S, Burns KEA, Machado FR, Fox-Robichaud AE, Cook DJ, Calfee CS, et al. Gender parity in critical care medicine. Am J Respir Crit Care Med 2017;196:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front Public Health 2018;6:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Y-M, Kim MK, Kang H-R, Kim T-B, Sohn S-W, Koh Y-I, et al. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res 2015;7:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SGA. Elevated serum cytokines during human anaphylaxis: identification of potential mediators of acute allergic reactions. J Allergy Clin Immunol 2009;124:786–92.e4. [DOI] [PubMed] [Google Scholar]
- 34.Korosec P, Turner PJ, Silar M, Kopac P, Kosnik M, Gibbs BF, et al. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J Allergy Clin Immunol 2017;140:750–8.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishoo E, Shah UK, Grillone GA, Stram JR, Fuleihan NS. Predicting airway risk in angioedema: staging system based on presentation. Otolaryngol Neck Surg 1999;121:263–8. [DOI] [PubMed] [Google Scholar]
- 36.Sampson HA, Mendelson L, Rosen J. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 1992;327:380–4. [DOI] [PubMed] [Google Scholar]
- 37.Summers CW, Pumphrey RS, Woods CN, Mcdowell G, Pemberton PW, Arkwright PD, et al. Factors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist center. JACI 2008;121:632–8.e2. [DOI] [PubMed] [Google Scholar]
- 38.Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.