Summary
Uniquely human cognitive faculties arise from flexible interplay between specific local neural modules, with hemispheric asymmetries in functional specialization. Here, we discuss how these computational design principles provide a scaffold that enables some of the most advanced cognitive operations, such as semantic understanding of world structure, logical reasoning, and communication via language. We draw parallels to dual processing theories of cognition by placing a focus on Kahneman’s System 1 and System 2. We propose integration of these ideas with the global workspace theory to explain dynamic relay of information products between both systems. Deepening the current understanding of how neurocognitive asymmetry makes humans special can ignite the next wave of neuroscience-inspired artificial intelligence.
Keywords: human intelligence, artificial general intelligence, computational design principles, deep learning, language, global workspace theory
Computational design principles behind the right and left hemisphere probably provide a scaffold that serves several human-defining cognitive feats. Hartwigsen and colleagues reason that better understanding this biological asymmetry for dual processing could ignite new forms of neuroscience-inspired artificial intelligence.
Introduction
The human body plan appears symmetrical from the outside only. We consistently lead with one foot when we climb stairs to enter a building. We fixate with our dominant eye when we span the bow and aim at the target in archery. We also naturally turn our right ear to a colleague who is whispering a secret message at a party. Understanding the meaning of the message works better through the right ear, given its privileged access to the language-dominant left hemisphere in the brain (the so-called right-ear advantage, Westerhausen, 2019).
Most biological systems show some degree of asymmetry (Geschwind and Galaburda, 1985). Functional asymmetries shape the human brain and behavioral functions in profound ways. Prototypical classes of human-defining cognition feature hemispheric differentiation in human brain organization. In particular, the left hemisphere plays a key role in communicative language functions and logical reasoning. Instead, the right hemisphere is relatively more specialized for spatial reasoning and emotional processing (Sun and Walsh, 2006; see below for details). Yet, neuroscientists are only beginning to understand hemispheric brain asymmetries. The precise adaptive advantages reaped from hemispheric asymmetries for cognition and behavior are still far from clear (Esteves et al., 2020).
Here we adopt a neurocognitive perspective on the computational design principles that may be a precondition for the emergence of human-defining cognition. The aim of our review is to point out salient parallels between asymmetries in i) biological neural networks, ii) special human cognitive functions, and iii) artificial neural networks. We recontextualize asymmetries in human neurocognitive functions and artificial neural network algorithms by integrating the System 1 – System 2 perspective. We argue that hemispheric asymmetries may provide a scaffold for information processing that evolved to enable an increasing degree of cognitive sophistication. Hemispheric specialization allows for parallel processing of several complex mental operations, like language and social cognition, that are uniquely powerful in the human species. We will mainly focus on language here because it is the key faculty for human communication. We will argue that insights into functional brain asymmetries could turn into a well of fresh ideas for designing the next breed of deep learning algorithms.
Hemispheric asymmetry as a core design principle of the brain
As a rule of thumb, we typically have one dominant hemisphere for certain types of cognitive operations. Such specialized responsibilities become especially apparent with regards to emotional responsiveness, visuo-spatial attention, as well as conscious problem solving and language capacities (cf. below). These functional asymmetries beg the question of the special purpose and neurocomputational infrastructure of how humans have evolved to perceive, understand, and navigate the world.
The emergence of the symmetrical body plan in animals likely co-occurred with improved mobility, more astute resource-seeking behavior and more effective predator defense (see Corballis, 2019). Why have human brains evolved to be organized into two specialized hemispheres? In the symmetrical brain, modular organization of specialized subsystems probably allows for a more efficient brain architecture, that is, denser packing of neurons in the same space (Kaas, 2000; Kaas and Herculano-Houzel, 2017; Van Essen, 1997). Brain organization into two hemispheres expands the amount of cortical surface area. This brain tissue growth was argued to afford advantages in terms of cooling and energy consumption (Kaas and Herculano-Houzel, 2017). Functional cerebral asymmetry expanded to override a purely symmetrical design of brain and body, and likely confers important evolutionary benefits.
The continuous refinement of specialized neural circuits may cohere with selection pressure for lateralization to enable more efficient functional organization and less redundancy between brain regions (Corballis, 2019; Vallortigara and Rogers, 2005). Moreover, hemispherically divergent processing is assumed to increase the ability to perform multiple tasks at the same time (Vallortigara et al., 2011), and prevent response competition between both hemispheres (Bisazza et al., 1998; Cantalupo et al., 1995). Having two exactly equivalent hemispheres would require numerous long-range connections in large-brained mammals such as human and non-human primates (Kaas, 2000). Long-range wiring comes at the cost of metabolic energy requirements for maintenance and conduction (Laughlin and Sejnowski, 2003; Lennie, 2003; Levy and Baxter, 1996; Ringo et al., 1994).
The emergence of unilateral functional networks might also have been favored by time constraints in information transfer via the corpus callosum between both hemispheres (Toga and Thompson, 2003). The necessary number of long-range axonal connections can be reduced by departing from the basic plan of symmetry in both hemispheres wherever possible, as well as grouping functions in the same hemisphere (Gazzaniga, 1995; Kaas, 2000). This need for local functional specialization (Sporns et al., 2004; Sporns et al., 2007) within each hemisphere may have compounded across primate evolution. This is because our monkey ancestors had to adapt to solve increasingly complex problems, such as coping with life in social groups (Kaas, 2000; see also Dunbar and Shultz, 2007; Tomasello et al., 2005). Of relevance for the following considerations, unilateral aggregation of function manifests in hemispheric asymmetry, thereby increasing the total neural capacity (Vallortigara and Rogers, 2005). Thus, asymmetrical features of functional brain organization enable efficient specialized and parallel processing of neurocognitive operations.
Functional specialization within each hemisphere is not meant to imply that each module works in complete isolation. Efficient processing in the asymmetrically specialized brain requires effective information transfer between both hemispheres (Davis and Cabeza, 2015; Genc et al., 2011; Westerhausen et al., 2011). Some authors argue that the need for flexible coordination of interhemispheric interaction is a direct consequence of brain lateralization (Hinkley et al., 2016; Karolis et al., 2019; Westerhausen et al., 2006). The development of the biggest white-matter pathway that channels information between both hemispheres, the corpus callosum (Schmahmann and Pandya, 2006), shortens the connection between both brain sides and the signal transmission times (Kaas and Herculano-Houzel, 2017; Paul et al., 2007).
As noted, long-range white-matter tracts come at the cost of increased metabolic energy demands for maintenance and conduction (Laughlin and Sejnowski, 2003; Lennie, 2003; Levy and Baxter, 1996; Ringo et al., 1994). The strength of connection through the corpus callosum is dependent on the extent of lateralization of functionally specialized regions. Homologous brain regions that are functionally distinct, that is, lateralized, tend to be less strongly linked to one another through the corpus callosum on a neural level compared to homologous brain regions that perform the same task (Karolis et al., 2019). This bottleneck constraint illustrates a potential evolutionary driver towards lateralization as a mechanism of increasing metabolic efficiency.
It is a repeatedly proposed idea that hemispheric asymmetries themselves may be a unique feature that characterizes the human brain. However, more recent accounts emphasize the presence of asymmetrical brain specialization in several distinct animal taxa (e.g., Corballis, 2009; Gunturkun and Ocklenburg, 2017; Vallortigara et al., 2011). Rodents, songbirds, and non-human primates show certain indicators of hemispheric asymmetry of brain function. For example, several avian species show prominent lateralization of visual processing (Gunturkun and Ocklenburg, 2017). The left hemisphere excels at categorization, discrimination, and memorization of visual patterns in different kinds of birds (Rogers, 2014; Valenti et al., 2003; Yamazaki et al., 2007). In contrast, the right hemisphere tends to assist more in visually guided interactions with emotional stimuli (Vallortigara et al., 2011; Ventolini et al., 2005), attentional shifts (Diekamp et al., 2005), social interactions (Vallortigara and Andrew, 1994), or relational analysis of pictorial and spatial information (Vallortigara et al., 2004; Yamazaki et al., 2007).
Some of these functional asymmetries appear to match those reported in human brains (see next section). Hemispheric asymmetries hence suggest themselves as a conserved design principle according to which localized functional specialization in the brain may bring a computational advantage. In fact, forms of hemispheric specialization have been identified in many species that evolved and live in different ecosystems. Hence, brain asymmetry probably emerged repeatedly and independently to boost cerebral processing capacity in a way that ultimately enhances survival (Gunturkun et al., 2020; Vallortigara and Rogers, 2005). Hemispheric specialization allows to improve execution of several differentiated processing operations in parallel. For example, when searching for food, it is crucial for survival to simultaneously screen the environment for predators and other threats (Gunturkun and Ocklenburg, 2017).
Given current knowledge, asymmetries in homologous brain regions in monkeys bear some degree of resemblance to left-hemispheric language-related brain regions in humans (e.g., Gannon et al., 1998; Hopkins et al., 1998; Sherwood et al., 2003). This observation suggests that brain regions linked to serving communicative abilities may take root before the inception of human language. Nevertheless, the functional relevance of these asymmetries in non-human primates remains unresolved (Vallortigara and Rogers, 2005). Based on the above-described parallels in hemispheric asymmetries between various animals, the more pertinent question is probably about the extent to which human brain lateralization is unique. That is, brain lateralization may be more appropriately viewed of degree, not of kind.
How does human brain organization then depart from that of non-human primates? A step-up in the cognitive sophistication and computational processing budget in the human species is closely tied to the considerable expansion of the higher association cortex in general as well as specific parts of the prefrontal, temporal and parietal cortex in particular (e.g., Glasser et al., 2014; Hill et al., 2010; Schleicher et al., 1999; Van Essen and Dierker, 2007). Asymmetries observed in comprehensive comparative assessments provide strong evidence that favor phylogenetic origins of brain lateralization (Toga and Thompson, 2003). Such clues show that differences between species are apparent especially in the inferior parietal lobule, aside from homologies between non-human primates and humans in the organization of certain brain areas (see Orban, 2016 for a review). Of relevance to our present considerations, there are probably 1.3 times more specialized cortical areas in humans than in monkeys.
Additionally, putatively unique human areas in the inferior parietal lobule are major contenders to make essential contributions to complex human-defining abilities, such as tool use or communicative language functions, problem solving, and future planning (Orban, 2016; Dohmatob et al., 2020; see also Seghier, 2013). There is also accumulating evidence for organizational elaboration of the prefrontal cortex between humans and other primates (Semendeferi et al., 2011; Teffer and Semendeferi, 2012). For example, prefrontal white-matter connections are consistently much denser in humans relative to other primates (Schoenemann et al., 2005). Grey- and white-matter increases of the prefrontal cortex may have played decisive roles in human cognitive evolution, which led up to the planning, language, attention and social information processing capacities that we have today (Hofman, 2014; Dohmatob et al., 2020).
In light of this evidence at the functional and structural level, the evolutionary trajectory of the human species has probably culminated in the most prominent hemispheric specialization in the entire animal kingdom (Gazzaniga, 2000; Vallortigara and Rogers, 2005). Similarly, the advancement of abilities for complex reasoning, such as required in recursive mental perspective-taking, are distinctly enabled by the way the human brain is organized for information processing. We speculate that a potential fitness gain obtained from stronger lateralization is asymmetrical specialization of neural circuits for enhanced dual processing. Extended functional specialization of one hemisphere to preferentially support a particular class of cognitive functions may maximize efficient, simultaneous information processing. Importantly, this computational design principle can also alleviate redundant computational efforts. More nuanced hemispheric specialization may also enhance advanced neurocognitive operations that depend on simultaneous processing of complementary information flows across both sides of the brain.
As an intermediate standpoint, we are the only species that has given rise to civilization and culture fueled by communication via language (Tomasello, 1999). This human-defining faculty shapes our complex everyday interactions with others and our society at large (Gelman and Roberts, 2017). For example, the ability to read and write books to pass on externalized knowledge to the next generation is a distinctively developed ability in humans, which sets us apart from the behavioral repertoire of other animals. As another example of this uniquely human faculty, the language-dominant left hemisphere has been attributed with integration of the distinct brain modules to form our unified ‘conscious awareness’, including the sense of being the responsible agent of our own actions (Gazzaniga, 2000). The way humans have evolved to solve problems about the world by deliberately hypothesizing, elaborating, and interpreting causal explanations of events may have unique roots in the left hemisphere (Gazzaniga, 2000; Dohmatob et al., 2020).
In the following sections, we discuss key computational strategies of the left and right hemisphere, with their relationships to domain-specific human-defining and domain-general processing in biological and artificial neural networks. We will outline that functionally specialized modules tend to be lateralized for increased overall processing efficiency, and human brains have a lot of such modules.
Asymmetries in biological neural networks
Some aspects of brain asymmetry, including anatomical features of language-related regions in the perisylvian cortex, become apparent already in the fetus in utero (Chi et al., 1977; Hering-Hanit et al., 2001). These observations speak in favor of genetic-developmental programs that are inherently lateralized, and again highlights asymmetry as a fundamental design principle of human brain organization (Kong et al., 2018). Aside from genetic influences, hemispheric asymmetries are generally thought to be shaped by evolutionary, developmental, environmental and in some instances also pathological factors (Toga and Thompson, 2003).
Structural and functional changes in brain organization during childhood provide examples of how developmental factors tie into the gradual maturation of hemispheric asymmetries. During cognitive development in childhood, structural changes of callosal connections, which reflect increased myelination and increasing overall size, allow for optimized neuronal communication between the left and right hemisphere (Danielsen et al., 2020). Such infrastructural refinements are likely related to development of lateralized language function. Structural brain changes may also support the development of related neural modules, which contribute to advanced cognitive capacities that draw on semantic world knowledge (Putnam et al., 2008; Thiel et al., 2006; Westerhausen et al., 2011). With respect to functional changes in hemispheric asymmetries, neuroimaging studies demonstrated that language is symmetrically organized in very young children (ages 4–6). However, the contribution of the right hemisphere was reported to decrease through childhood (Olulade et al., 2020; see also Basser, 1962; Lenneberg, 1967). Early hemispheric symmetry for realizing language-related capacities may reflect the plastic potential for reorganization in case of brain damage (Newport et al., 2017). Indeed, language may be equally affected by left or right perinatal stroke (Lansing et al., 2004). The same argument may explain gradual rather than absolute lateralization for most functions in the adult brain. This notion is also plausible as complete lateralization would increase the vulnerability to lesion-induced deficits and preclude reorganization by plastic recruitment of the non-dominant hemisphere (Thulborn et al., 1999; see Esteves et al., 2021 for a discussion). With respect to the role of environmental factors, active exposure to auditory stimuli facilitates the differentiation of the sounds in the environment during language learning (Benasich et al., 2014; Kuhl, 2004; Werker and Hensch, 2015).
Asymmetries in functional specialization
In general, prototypical kinds of cognitive operations feature hemispheric differentiation in brain organization, although this view is still controversial for some cognitive domains (Turner et al., 2015). The left hemisphere serves a crucial role in communicative speech and language capacities (Friederici, 2011, 2017; Hagoort and Indefrey, 2014), as well as mathematical and logical reasoning (Ashcraft et al., 1992; Goel, 2019). Instead, the right hemisphere is more functionally tuned to attentional reorienting operations (Corbetta and Shulman, 2002), such as in emotional processing (Demaree et al., 2005) as well as affective prosody (Belyk and Brown, 2014) and face processing (Prete et al., 2015; Vakil and Liberman, 2016). Episodic memory processing also features differences in hemispheric specialization, with the left hemisphere being dominant for encoding and the right hemisphere for retrieval (Habib et al., 2003; Johansson et al., 2020; Tulving et al., 1994).
Despite such functional asymmetries, both hemispheres closely interact during many cognitive operations. For example, sophisticated cognitive processes such as language comprehension and logic-based reflection that rely on the contribution of specialized circuits in the left hemisphere (Friederici, 2017; Goel, 2019) typically depend on simultaneous engagement of domain-general cognitive support functions (e.g., Blank and Fedorenko, 2017; Fedorenko et al., 2013). Domain-general support functions such as attention, cognitive control or working memory engage distributed neural networks in both hemispheres (see Asymmetries in human cognition for details).
Reading a book or newspaper requires left-dominant language competence, as well as simultaneous attentional routing that is preferentially supported by the right hemisphere. Different reading strategies require different interactions between these modularized neural systems: when we search for a specific keyword, we may need to ignore and suppress detailed sentence information, requiring inhibition of specific context. In contrast, when we are contemplating the logic of a particular paragraph, we may need to read every single sentence consecutively.
Notably, lateralization of a specific function does not imply that the respective other hemisphere is completely lacking specific processing abilities for that function. Indeed, asymmetries are normally a question of degree rather than strictly binary (Badzakova-Trajkov et al., 2016; Bartolomeo and Thiebaut de Schotten, 2016). As a prominent example, attention is commonly believed to be distributed across both hemifields in the intact human brain. However, the right inferior parietal lobule typically dominates over its left counterpart during bilateral attentional processes (Cicek et al., 2007). Accordingly, healthy participants usually show an attentional bias towards the left hemifield, a phenomenon called pseudoneglect (Bowers and Heilman, 1980; Zago et al., 2017). In neurological patients, this observation is consistent with a greater severity of attentional impairments after right relative to left parietal damage (Weintraub and Mesulam, 1987; see next section for details).
As such, not all cognitive functions are necessarily lateralized. For example, decision making tends to engage the prefrontal cortex in both hemispheres (Domenech and Koechlin, 2015). As discussed below, more domain-general functions appear to feature more bilateral recruitment. Indeed, neuroimaging studies provide evidence for distinct sub-specialization within the larger prefrontal cortex during decision making. The posterior medial part was associated with domain-general aspects of decision making and cognitive control, including conflict monitoring, error detection and confidence representation across a variety of tasks (Botvinick et al., 2004; Gehring et al., 1993; Ridderinkhof et al., 2004). In contrast, the anterior prefrontal cortex was related to tracking the reliability of specific alternative strategies during decision making (e.g., Donoso et al., 2014; Dohmatob et al., 2020). Recent work points to the coexistence of domain-agnostic and domain-specific representations of confidence in neighboring prefrontal subregions (Morales et al., 2018).
Asymmetries in structural organization
Primacy of functional dedication in both hemispheres in the human cerebral cortex is probably grounded in characteristic divergence of structural properties. Although both hemispheres are similar in total weight and volume, the spatial distribution of tissue differs markedly in the left and right hemisphere (Toga and Thompson, 2003). Considering structural differentiation in the entire brain, perisylvian regions, extending into the inferior parietal lobule, have shown the most prominent asymmetries, with larger volumes in the left hemisphere (Amunts et al., 1999; Falzi et al., 1982; Steinmetz, 1996). This feature of the human brain is likely related to the left-hemispheric dominance for language. Likewise, left-biased asymmetry in fiber density has been demonstrated for the arcuate fasciculus. This major white-matter pathway is known to connect language-related regions in the human brain (Buchel et al., 2004; Catani et al., 2007; Lebel and Beaulieu, 2009; Takao et al., 2011).
Functional asymmetries depend on specific contextual demands
It is one of the most established insights from neuroscience research that the left hemisphere harbors the superior language processor (Broca, 1865; Wernicke, 1874). Yet, this notion does not imply that the right hemisphere is invariably word-blind (Lindell, 2006). Indeed, meta-linguistic processing aspects such as metaphor processing or the processing of subordinate meanings of ambiguous words rely on a stronger contribution of right-hemispheric regions (see Hartwigsen and Siebner, 2012). In some cases, lateralization depends on the specific function of a given linguistic process.
This insight is epitomized by prosody, the change in rhythm and melody of speech (Cutler et al., 1997). Emotional prosody usually shows strong lateralization to the right hemisphere (e.g., Heilman et al., 1984; Hoekert et al., 2010; van Rijn et al., 2005). Likewise, the right hemisphere has been found to be involved in speech processing depending on the presence of pitch information (Meyer et al., 2002; Meyer et al., 2004). In contrast, linguistic prosody tends to be lateralized to the left hemisphere (Friederici and Alter, 2004; van der Burght et al., 2019; van Lancker, 1980; Belyk and Brown, 2014 for a meta-analysis). For example, functional neuroimaging work has demonstrated increased activity in the left prefrontal cortex when prosodic cues guide sentence comprehension (van der Burght et al., 2019). In contrast, when prosodic cues are superfluous for establishing the sentence structure, neural activity responses were lateralized to the right prefrontal cortex. Moreover, comprehension of meaningful words strongly engages the left hemisphere, while non-verbal semantic processing of meaningful environmental sounds depends especially on the right hemisphere (e.g., Butler et al., 2009; Thierry et al., 2003). Collectively, these results show that hemispheric asymmetries in the processing of meaningful stimuli strongly depend on the amount of linguistic information and its relevance for guiding language comprehension. This is an example of how flexible recruitment of specialized modules in our brain helps to master complex cognitive operations which depend on both, hemispheric specialization, and interhemispheric interactions. Indeed, some authors have argued that rapid excitatory and inhibitory interactions between neural circuits in both hemispheres are necessary for realizing particularly complex neural operations (Carson, 2020; Gunturkun and Ocklenburg, 2017; O’Donnell et al., 2017).
Probing the functional relevance of hemispheric specialization
How can we get a handle on the functional relevance of hemispheric specialization in humans? One opportunity is to study patients with circumscribed brain lesions in cognitive key regions. The anterior inferior parietal lobule is particularly pregnant to reveal new insights in brain lateralization. This is because this region is a hotspot of asymmetry in the human cortical mantle (Toga and Thompson, 2003).
Stroke-induced brain lesions in the left temporo-parietal cortex typically lead to impairments in language comprehension, semantic reflection and communicative abilities – known as Wernicke’s aphasia (Geschwind, 1970; Lichtheim, 1885; Wernicke, 1874; see Fridriksson et al., 2018). These neurological patients are usually able to speak fluently. However, circumscribed brain lesions around the temporo-parietal Sylvian fissure typically cause difficulties in the comprehension of spoken or written language. Patients may be unable to verbally describe pictures which require complex scene understanding. Depending on the severity of the neurological disorder, some patients may even be unable to articulate semantic concepts that occur in simple pictures. The semantic impairments substantially affect the abilities for interpersonal communication and social interactions in patients with Wernicke’s aphasia.
In contrast to impairments of human language and reasoning after brain lesions in the left hemisphere, selective tissue damage to the right anterior inferior parietal lobule is typically associated with specific impairments in visuospatial awareness and attentional routing processes – called hemispatial neglect (Corbetta and Shulman, 2011; Vallar and Calzolari, 2018). This neuronal damage causes the inability to process environmental information in the left visual field. Neurological patients with neglect typically behave as if the left side of their visual space did not exist. Hence, such individuals may ignore the left side of their body when taking a shower, or may shave only the right side of their face. While walking, neglect patients often collide with objects on their left side. Further, these patients may ignore and leave untouched food on the left side of the plate, as another example of the affected visuo-spatial capacities that are a consequence of damage to the right hemisphere. The proposed double dissociation in the functional relevance of the left hemisphere for language and the right hemisphere for visuospatial awareness and attentional reorientation receives support from a recent multi-site cohort study in >1,000 patients the early phase after stroke (Bonkoff et al., 2021; Figure 1).
Figure 1. Brain damage has hemisphere-specific consequences on specific cognitive capacities in 1080 stroke patients.

Causal evidence that circumscribed tissue damage leads to selective cognitive impairments in visuospatial function (RC, Rey Complex Figure Test), global cognition including space and time (MMSE, Korean Mini-Mental State Examination), language performance (BN, Boston Naming Test), and memory function (SVL, Seoul Verbal Learning Test). To achieve these single-patient predictions of clinical outcomes, the Bayesian hierarchical model relied on information of brain segmentation of lost grey-matter as input features. A) Heatmaps indicate the associations of each anatomical region (Harvard-Oxford atlas) with lost (blue color) or preserved (red color) cognitive performance in patients for different clinical assessments. B) Predictive accuracy (coefficient of determination R2 estimated via posterior predictive checks) for the four cognitive scores along with scatterplots of actual (x axis) and predicted (y axis) cognitive performance. C) Brain renderings of the brain region-wise predictive relevance. Coordinates of axial brain slices in Montreal Neurological Institute (MNI) reference space. The population-level evidence shows that language-related and attentional-routing-related cognitive capacities are biased to the left versus right brain side. Reprinted with permission from Bonkhoff et al., Brain Comms, in press.
A central clinical question with respect to functional specialization is how the brain circuitry gradually adapts to recover from stroke and other tissue lesions in either hemisphere. We propose that functional specialization within each hemisphere and functional competition between both hemispheres shapes compensation when recovering from brain damage. Following tissue damage to the language network in the left hemisphere, recruitment of ipsilesional regions is usually associated with better recovery of function than recruitment of homologous regions in the right hemisphere (Winhuisen et al., 2005, see Hartwigsen and Saur, 2019; Saur and Hartwigsen, 2012 for review). The role of the non-dominant hemisphere in stroke recovery remains debated (e.g., Hartwigsen and Volz, 2021; Turkeltaub et al., 2011; Volz and Grefkes, 2016). Some studies in the language domain argue that right-hemispheric regions may be integrated, especially in case of large-scale damage to the language-dominant left hemisphere (Crosson et al., 2007; Heiss and Thiel, 2006). Indeed, longitudinal neuroimaging work shows that stronger recruitment of right prefrontal regions is associated with better language recovery (Saur et al., 2006; Stockert et al., 2020), at least in the so-called critical period (Krakauer, 2015) early after stroke.
How does the non-dominant hemisphere contribute to language recovery after large brain lesions? Recent work suggests that increased activity in right prefrontal regions during stroke recovery likely reflects domain-general cognitive control processes rather than language specific operations (Brownsett et al., 2014). There is increasing evidence that stronger recruitment of domain-general networks is associated with better language recovery after stroke (Geranmayeh et al., 2014; Geranmayeh et al., 2017). Many complex cognitive processes such as semantic reasoning, problem solving and planning require recruitment of diverse domain-general processes to realize their operation (Blank and Fedorenko, 2017).
At the neural level, this is reflected in the recruitment of multiple distinct, interacting brain networks (Petersen and Sporns, 2015). The more complex or demanding a cognitive process, the more disparate the set of cognitive processes that are typically co-recruited by default (see Diachek et al., 2020; Fedorenko and Blank, 2020; Fedorenko et al., 2013). Yet, engagement of such domain-general processes is unlikely to fully compensate for loss of elaborate mental performances that hinge on hemispherically specialized brain circuits (Hartwigsen, 2018; Heiss and Thiel, 2006). Compensatory mechanisms between left-hemispheric and right-hemispheric functional networks likely represent an overarching principle of neural organization that may be observed across diverse cognitive domains, such as language, abstract problem solving, imagination and planning (cf. Dohmatob et al., 2020).
Aside from the study of patients with brain lesions, functional neuroimaging in healthy volunteers is one of the key techniques to investigate hemispheric asymmetries in cognition (Ocklenburg et al., 2020). For example, a recent functional neuroimaging study demonstrated task-specific hemispheric specialization within the human inferior parietal lobule (IPL) (Numssen et al., 2021). The anterior part of the left IPL was strongest associated with a semantics/language task, while the anterior part of the right IPL showed the strongest predictive relevance for attentional reorienting. In contrast, both posterior IPL subregions showed the strongest association with social cognitive processing in the same set of healthy volunteers (Figure 2). Distinct domain-specific neural response patterns were reflected in distinct coupling patterns of the respective subregions. This observation pointed to stronger unilateral coupling for the anterior language and attention related regions and more complex bilateral coupling patterns for the posterior regions.
Figure 2. Hemispheric asymmetries in the inferior parietal lobule (IPL) characterize the neural substrates of human-defining cognitive domains.
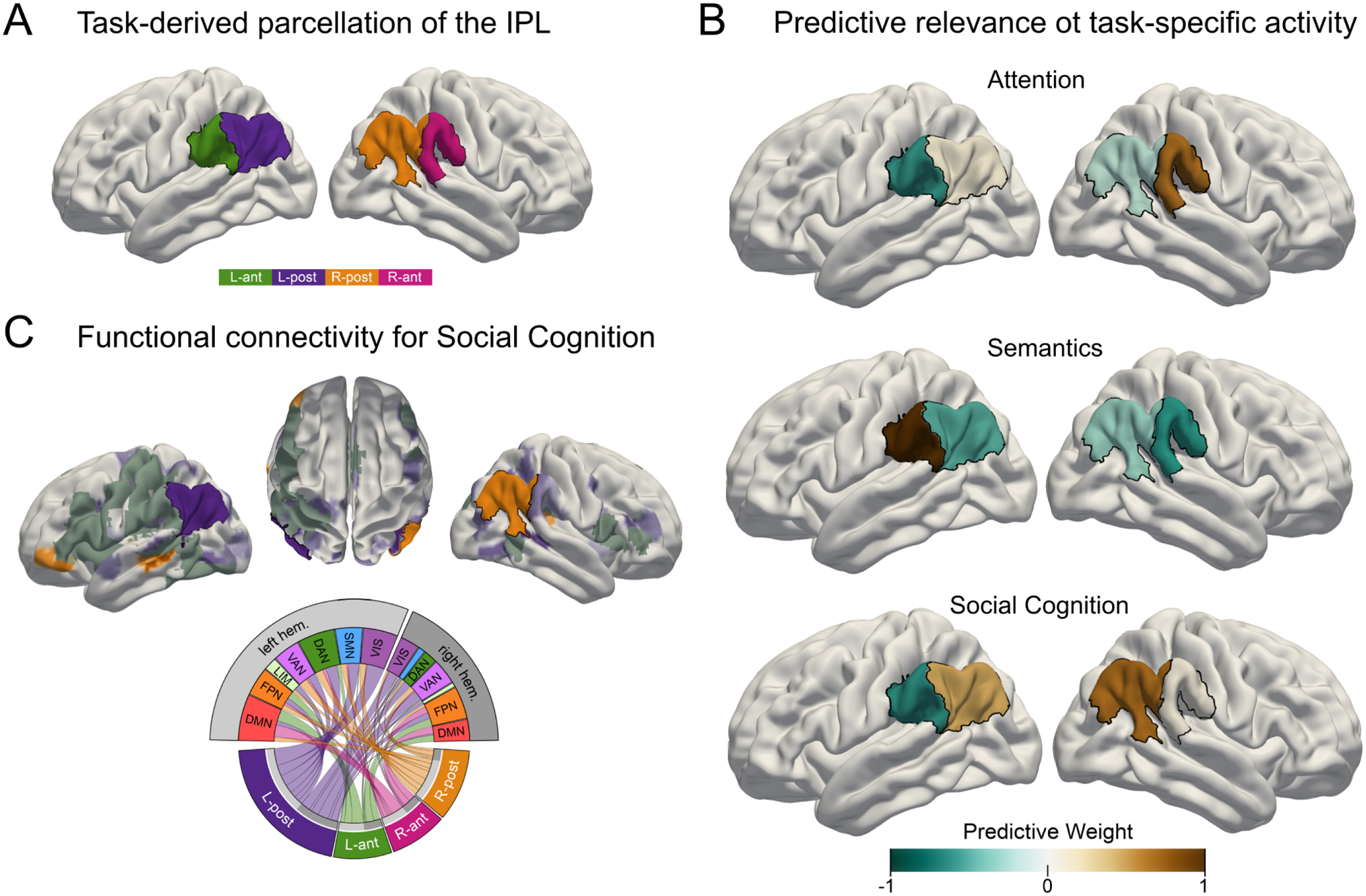
A) IPL parcellation extracted from neural activity responses across attention, semantics and social cognition tasks in the same sample of healthy volunteers during functional brain scanning. Both IPL regions were divided into an anterior and a posterior subregion. B) In these task experiments, the IPL region showed a functional triple dissociation: the right anterior subregion had the strongest predictive relevance for attentional reorienting. The left anterior subregion had the strongest predictive relevance for lexical decisions. In contrast, both posterior subregions showed the strongest relevance with a social cognitive task that requires contemplating other individuals’ mental states. C) Task-evoked functional connectivity profile for the social cognition task. Both posterior subregions showed spatially distributed connectivity patterns with brain-wide cortical networks. Left anterior IPL: L-ant, right anterior IPL: R-ant, left posterior IPL: L-post, right posterior IPL: R-post. Networks: DAN: dorsal attention network, DMN: default mode network, FPN: fronto-parietal network, LIM: limbic network, VAN: ventral attention network, VIS: visual network, SMN: somatomotor network. Adapted and reprinted with permission from Numssen et al., eLife 2021.
Collectively, these results support the idea of hemispheric asymmetries for language and attentional allocation. The bilateral recruitment of the IPL for social cognition converges with previous meta-analyses demonstrating that advanced social cognitive functions, such as the capacity to infer others’ thoughts, beliefs, and behavioral dispositions, engage the IPL in both hemispheres (Bzdok et al., 2016; Bzdok et al., 2013; Schurz et al., 2020). The contribution of both hemispheres to social cognition may reflect the increased complexity of social cognitive operations, engaging both semantically tuned processes in the left hemisphere (Bzdok et al., 2016) as well as affectively tuned processes in the right hemisphere (Bzdok et al., 2013).
Asymmetries in human cognition
We speculate that there may be a connection between aspects of hemispheric specialization for different cognitive functions (cf. above) and Kahneman’s System 1 and System 2 (Kahneman, 2011). System 1 describes fast modes of cognition such as automatic gut responses to seeing another person’s angry facial expression or being surprised by a sudden harsh noise, without much conscious access or the results of deeper reflection. According to Kahneman, such impressions and feelings drive much routinized behavior in everyday life. For example, we often trust our gut feeling when judging other people or deciding if we want to either further engage in a cooperation or distance ourselves. Kahneman argues that the majority of action-perception cycles throughout our days are mainly paced by System 1 cognition, maybe in a kind of autopilot. Important to our present considerations, sensory perception, reactive processing and mounting stereotypical motor responses are behaviors that humans share with many other animals (see Gunturkun and Ocklenburg, 2017).
It is the System 2 mode of thinking that drives the types of cognition that are unique to the human species: deliberate reasoning and detailed reconsideration to override prepotent emotional responses and actions, effortful rationalization, and manipulating representations of people’s world view (Hofman, 2015). Such cognitive operations may prevail when solving a creative planning problem such as “Which alternative street paths could I drive back home, given unexpected construction on the road?”. Contemplating candidate solutions to such problems usually takes more time than problems that can be solved exclusively with cognitive processes that belong to System 1. When concentrating on figuring out a challenging planning and navigation task, humans are less likely to be triggered or distracted by visual and auditory cues or sources of affective arousal from the environment (e.g., ‘Invisible Gorilla’ Test; Chabris and Simons, 2010).
System 2 cognition is thought to reject, alter, or overcome impressions, intuitions, feelings, and reactive tendencies that are issued by System 1 (Kahneman, 2011). System 2 can also fully endorse and select from the bottom-up inputs provided by System 1. The division of labor between the two cognitive modes likely optimizes everyday life in terms of allocated resources and spent cognitive effort. Both System 1 and 2 probably work hand-in-hand and are permanently active in degrees. System 1 may be sufficient for handling most routine decisions. System 2 may come into play as we encounter unusual objects, new situations and other cognitively challenging tasks.
To efficiently execute advanced cognitive operations, System 2 routinely exerts tight governance over various System 1 processes. Instead, System 1 processes do not typically depend on System 2 operations. One example for the strong interaction between both systems would be mentally browsing candidate driving routes (outside of one’s visual environment). This operation requires recruitment of System 2 for coordination between System 1 modules, involving retrieval of structured world knowledge, visual imagination, planning, as well as attentional and working-memory processes. In contrast, executing familiar everyday actions, such as driving straight on an already chosen and known driving path do not require effortful reasoning or sophisticated semantic representations.
Notably, to the best of our knowledge, System 1 and System 2 have not been explicitly mapped onto specific brain regions (Kahneman, 2011; Evans and Stanovich, 2013; Kannengiesser and Gero, 2019). The exact mapping of particular cognitive processes to System 1 and System 2 cognition can also be challenging. For example, there are different facets of attentional routing mechanisms, including conscious and automatic forms of attention (Turatto et al., 2000). According to Kahneman, System 2 typically depends on conscious attention. Instead, System 1 (e.g., in visual object detection) is believed to operate with automatic or subliminal attention, without the need to deliberately focus on a specific aspect of one’s internal or external landscape (e.g., processing objects that occur outside of the currently focused field of view).
Can we link System 1 and System 2 to different specialized circuits in the human brain? We are tempted to draw some tentative parallels to hemispheric specialization. We speculate that System 1 may be reflected in localized processing of distinct regions or networks. Depending on the specific subserved process, these local neural systems may engage circuits in the left hemisphere, right hemisphere, or both conjointly. As cognitive operations grow more complex, they probably prompt recruitment of more effortful System 2 cognition. System 2 engagement, in turn, may involve recruitment of both specialized networks and domain-general support functions.
For example, language comprehension is known to invoke a collection of functionally specialized circuits that are biased to the left hemisphere (e.g., Binder et al., 2009; Binder et al., 1997; Fedorenko et al., 2012; see also discussion above). With rising task demands, language comprehension is increasingly supported by domain-general cognitive and executive control functions to enable efficient processing (Fedorenko et al., 2013; Duncan and Owen 2000). At the neural level, domain-general process engagement is reflected by increased recruitment of bilateral domain-general networks, especially the ‘multiple-demand network’ for cognitive control (Duncan, 2010). The multiple-demand network includes brain regions that respond to increasing task demands across many cognitive domains and is involved in the planning, top-down control, and regulation of cognition and goal-directed behavior (Dosenbach et al., 2006; Dosenbach et al., 2007). This network engagement was reported to scale as a function of task difficulty across a variety of cognitive operations (Duncan and Owen, 2000; Fedorenko et al., 2013). Previous work demonstrated that simple story listening, which closely matches natural language processing, mainly recruits the specialized left-lateralized language network (Blank and Fedorenko, 2017; Diachek et al., 2020). In contrast, more complex listening scenarios, such as listening to speech in noisy environments, strongly engage additional multiple-demand systems (e.g., Peelle, 2018; Vaden et al., 2013; Wild et al., 2012).
By analogy to Kahneman’s cognitive systems, listening to a story is a relatively automatic and routinized process which is probably mainly supported by System 1. In contrast, increased task demands under challenging listening conditions lead to higher cognitive load. We reason that such effortful, System 2-like task processing requires interaction of the specialized language network with the bilateral multiple-demand network in the example above. To be clear, we do not mean to argue that language equals System 1 processing, nor that increased cognitive control equals System 2 cognition. We rather suggest that System 1 cognition translates to all automatic, overlearned processes which may engage relatively local specialized networks. In contrast, System 2 cognition would engage effortful cognitive processes that rely on more distributed processing of collective networks and flexible interactions between these specialized neural systems (Figure 3).
Figure 3. Two examples of cognitive problem solving that engage preferentially System 1 (left) or also System 2 (right).

A) Finding the solution to this problem is easy and quick. System 1 answers this question without prompting in one second at most. Human observers do not necessarily know how to describe the average length in centimeters. However, we are accurate in adjusting the length of another line to match the average. Involvement of System 2 is not needed to form an impression of the norm of length for an array. System 1 achieves this task automatically and effortlessly, just as it recognizes the color of the lines and the fact that the lines are not parallel to each other. B) A glance provides an immediate impression of many features of the figure. We realize that the two towers are equally tall and that they are more similar to each other than the tower on the left is to the array of blocks in the middle. However, the observer does not immediately know that the number of blocks in the left-hand tower is the same as the number of blocks arrayed on the floor. To confirm that the numbers are the same, we would need to deliberately count the two sets of blocks and compare the results, an activity that requires recruitment of System 2. Adapted and reprinted from Kahneman, 2011.
However, it remains incompletely understood how information is efficiently relayed between System 1 and System 2 (Shleifer, 2012). Some authors proposed such information transfer between neural modules to be enabled by the global workspace (Dehaene et al., 1998; Mashour et al., 2020). In short, the global workspace theory was introduced to make steps towards explaining conscious processing (Baars, 1988). This conceptual framework was later extended to incorporate neural processes underlying effortful cognitive tasks (Dehaene et al., 1998). These authors distinguish two computational spaces: one global workspace composed of distributed and heavily interconnected neural circuits with long-range connections, and a set of specialized modules committed to perceptual, motor and cognitive processing (Dehaene et al., 1998; Mashour et al., 2020). Workspace neurons are mobilized in effortful tasks for which the specialized processors dissatisfy the needs to reach the processing goal. Thereby, they mobilize or suppress the contribution of specific processor neurons and enable conscious processing (see also Baars, 1988).
Supported by evidence from computational modeling, the global workspace appears to be central during the acquisition of novel tasks and effortful execution. The governance of the global workspace fades during highly overlearned tasks, but resumes sharply after erroneous task responses (Dehaene et al., 1998). As such, the global workspace satisfies key requirements for coordinating between System 1 and System 2: It is absent during routine tasks (requiring System 1 only) and takes importance when a novel non-routine task is introduced (requiring System 2). As an important prediction from this framework, the global workspace is recruiting distributed brain regions that have ample long-distance connections (Dehaene et al., 1998). The global workspace is thought to integrate multiple specialized brain regions in a coordinated and flexible manner during conscious processing. Thereby, the global workspace enables information transfer between specialized modules in both hemispheres, by integrating information across distributed neural processors (Mashour et al., 2020). Since distributed workspace neurons are mobilized in effortful tasks for which specialized processors do not suffice, we reason that workspace regions may overlap to some degree with domain-general regions of the multiple-demand network. Indeed, it was suggested that, among other regions, the dorsolateral prefrontal and anterior cingulate cortex contribute to the workspace (Dehaene et al., 1998). These regions have been associated with effortful task processing (Cohen et al., 1997; Pardo et al., 1990).
In short, the global workspace is thought to act as a central router that mediates intermediate processing outcomes to be amplified, sustained, and broadcasted to specialized neural modules that act as processing partners (Mashou et al., 2020).
Asymmetries in artificial neural networks
Various types of pattern-recognition tasks are solved by the human brain in less than one second – without effort, deliberate concentration or conceptual elaboration. We quickly detect other people’s emotional experiences and identity from parsing cues in their faces, mimics and body gestures (cf. Bzdok et al., 2012). We easily recognize a friend and her current affective state from the sound of her voice on the phone; without visual input from her facial or body expressions (cf. Hensel et al., 2015). We rely on automatic and fast memory retrieval to know whether we have heard a particular music melody before. This comes easy although we have been exposed to many thousands of different music pieces over our lifetime.
All these pattern-recognition computations can be carried out by the human brain in dozens or few hundreds of milliseconds (Dehaene et al., 2017). Notably, it is also these kinds of visual and auditory signal detection problems that have been revolutionized by deep learning algorithms over the last 10–15 years (Goodfellow et al., 2016). In some tasks, new deep learning architectures have even exceeded human-level performance (Ghahramani, 2015; Jordan and Mitchell, 2015; LeCun et al., 2015). In the terminology developed above, the artificial neural networks invented so far have proven effective in solving problems that are largely reminiscent of Kahneman’s System 1 cognition. Designing artificial neural network algorithms to take on tasks that involve Kahneman’s System 2 cognition remains more daunting. To tackle high-level problems, humans often require more than 1 second (Lake et al., 2017). For example, today’s deep learning architectures still struggle to understand the intentions of agents or causal implications in complex visual scenes based on images or photos, partly because of lacking modules that are specialized in representing explicit world knowledge (cf. Bengio, 2017; Marcus and Davis, 2019).
Existing deep learning systems excel at narrow tasks for which we have a sufficiently massive dataset of representative and correctly labeled instances or data points. However, if these artificial neural networks are trained to solve a task at hand using for example medical images from one hospital, their performance on the same kind of medical images from another hospital may be much worse (sometimes called ‘distribution drift’) (Tsymbal, 2004). This is due to changes in why, how, and when these medical images of human individuals are acquired. Such changing data distributions may be due to the practices or equipment that diverge between the two hospitals. The ability to generalize well or quickly out-of-distribution, from example observations freshly acquired from alternative sources, is much stronger in humans than in current artificial intelligence systems (Goyal and Bengio, 2020). This realization begs the question of how humans deal with newly encountered settings. Humans appear to resort to System 2 abilities (such as required for deliberate planning) to reason about such changes in the distribution in their environment and adjust their predictions accordingly. This partial robustness of human judgment to volatility in the world is stimulating deep learning research by inspiration from the cognitive neuroscience of System 2 abilities (Bengio, 2017; Goyal and Bengio, 2020).
Importantly, we do not argue that current artificial intelligence systems lack all aspects of what constitutes System 2 abilities. For example, the similarity question in Figure 3B represents a counting example in which today’s artificial intelligence systems have been reported to outperform humans. Rather, today’s artificial neural networks struggle in many more elaborate tasks, which require common-sense abstraction or generalization to new categories of encountered problems. For example, the task “play a song in C minor” necessitates System 2 thinking but would not be unfeasible for humans with musical training. In contrast, current artificial intelligence systems struggle with many such creative tasks.
For several decades, there has been vibrant crosstalk between the design of artificial intelligence systems and neuroscientific ideas about how the brain and its functionally dedicated modules work. Many such fruitful analogies were largely inspired by hierarchical processing cascades in the early sensory cortices of the primate brain. For example, principal component analysis and convolutional neural network algorithms applied to images of natural scenes do recover atomic features, such as edges and shapes. These image-derived features are indeed similar to current knowledge of the consecutive processing stages of environmental stimuli in the visual cortex (Hubel and Wiesel, 1962; Olshausen and Field, 1996; Yamins et al., 2014).
We emphasize that, up to now, the design of artificial neural network architectures has successfully mimicked several computational design principles that are not specific to human cognition, such as visuo-spatial object recognition, attentional selection, and raw memory retrieval. These influences from neuroscience on developments in the deep learning community are more naturally placed in Kahneman’s System 1 mode of cognition. As a logical next step, deep learning will probably thrive on new algorithms that capitalize on incorporating more advanced forms of human neurocognition and their functional interaction with brain-wide neural systems. New impulses may allow for a more complete understanding of how human-defining cognition is instantiated by brain circuits. Critical insights may be derived from the distributed representation across and functional asymmetry between two architecturally almost identical subsystems – the modular circuits from biological neural networks that together form the left and right brain hemisphere.
Stronger functional compartmentalization of artificial neural networks can yield many benefits, which are exemplified in the earlier idea of ‘capsules’ (Sabour et al., 2017). More generally, modular neural networks (Andreas et al., 2016; Goyal et al., 2019; Jacobs et al., 1991) are increasingly proposed that have modules that communicate not scalars but bundles of network activations. Building more functionally modularized infrastructure into the modeling architecture allowed learning more robust internal representations or concepts, with the ability to tackle novel settings by dynamically combining the pieces of knowledge contributed by different modules.
Decomposition of knowledge into independent mechanisms has been argued to be a key checkpoint on our path towards capturing causal structure with learning algorithms (Goyal and Bengio, 2020; Goyal et al., 2019; Peters et al., 2017). Distinct modules could implement such a specialization. The global workspace theory (Baars, 1988; Dehaene et al., 2017; see above) proposes that conscious processing involves the selective participation of only a few of the available expert modules at a time, then broadcasting information products to the rest of the brain through a communication bottleneck. Conscious attention selects which module and which conscious content is gated through this bottleneck and remains available shortly in working memory. In this way, with certain parallels to attention processes in brain and cognition (cf. above), computational attention mechanisms offer an algorithmic opportunity to negotiate between incoming bottom-up information and governing top-down modulation from deeper processing layers (Mittal et al., 2020). As argued recently (Bengio, 2017; Goyal and Bengio, 2020; Goyal et al., 2019), this dynamic selection of computational modules may bolster the ability to generalize in systematic and robust ways which the homogeneous and static architecture of more traditional deep learning systems does not enjoy (Mittal et al., 2020). In addition, this localization and factorization of knowledge into the appropriate modules can confer an advantage in terms of effective adaptation to causal understanding of and contingent action on the environment (Bengio et al., 2020).
The exchange of intermediate computing products between dedicated information processing modules can be controlled through an attention mechanism that “spotlights” pieces of information for active read-out. Coherent interpretation of input information can be achieved by algorithmic focus on a single or a small number of elements at once – a key ingredient that can be instrumental for realizing human-level artificial intelligence. Global-workspace components integrated into emerging deep neural network architectures get to decide where a given module reads information outputs from (Goyal et al., 2019). At a given time, only a subset of the functionally specialized modules, which compose the artificial neural network, are in sync with other parts of the modeling architecture (Figure 4). This mimics the realization from neuroscience research that performance gains in neural processing are obtained from imposing a bottleneck: any module cannot communicate with any other module all the time. Instead, purposeful scarcity of information flows is imposed by competition between expert modules to solve the down-stream learning goal. Indeed, human minds typically can hold only 7 +/−2 elements in working memory (Baars, 1988; Miller, 1956).
Figure 4. Flexible information processing in artificial neural networks through a global workspace.

Unconstrained pairwise communication between modules can be replaced by deliberately imposing a bottleneck on information communication via a shared workspace. Only a constrained subset of expert modules can “talk to” other expert modules at any given time (Mittal et al., 2020). Thus, only a few pieces of information are considered by the learning architecture in any given moment – a rough analogue to what is “consciously perceived”. Actively communicating expert modules are shown in blue. In a two-step process, expert modules first compete against each other for write permission to the shared workspace. Then, the ensuing processing outputs from a (purposefully restricted) module collaboration is made available to all modules of the artificial neural network architecture. The Figure shows example extensions of backbone architectures (top row) by equipping them with a global processing workspace (bottom row). RIM: Recurrent independent mechanisms, TIM: transformers with independent mechanisms, SW: shared workspace. Reproduced with permission from Goyal et al., 2021.
The collaboration of engaged functionally specialized modules is permitted to write into the shared workspace. The information, issued by the channeled module interaction, is then broadcasted for reuse by the entire modeling architecture. Every other module can collect any piece of information from that common buffer of intermediate processing outputs. The more parsimonious the set of dynamically interacting recurrent mechanisms, the bigger the advantage from compartmentalized information processing (Bengio, 2017). In this way, attention mechanisms can also assist in consulting and integrating declarative world knowledge from semantic expert modules. Their implication in processing cascades in artificial neural networks in turn may be a necessary step for realizing ‘elements of a conscious thought’ or for solving problems that ‘require a sequence of conscious steps’ (Bengio, 2017). Indeed, the election of pieces of information to be picked for global dissemination has been proposed to be a crucial aspect of how consciousness is instantiated in the human brain, and may be in machines (Dehaene et al., 2017). These advances in architecture design have led to more robust hidden representations and promising performance gains in a variety of challenging prediction tasks, especially those involving out-of-distribution generalization (Lake et al., 2017). Such brain-inspired enhancements of deep neural network computation can make strides towards human-defining reasoning, planning, and decision-making by algorithmic inference.
The negotiation between bottom-up processing of sensory inputs and top-down modulation calibrated by semantic world knowledge could make progress towards learning agents with improved performance at a variety of advanced tasks. Decomposable pieces of high-level semantic world knowledge can be flexibly induced, adjusted and recombined for binding with information products from other neural network modules – including not only visual object recognition but also attention mechanisms. Importantly, attending and composing the appropriate modules, and abstract entities they represent, has the potential for systematic generalization in non-stationarity environmental dynamics, that is, solidified out-of-distribution extrapolation (Goyal & Bengio, 2020). Such intersections between designated processing modules may make steps towards realizing aspects of causal reasoning, and more System 2-like deep learning systems that robustify generalization from one context to future contexts.
It has been argued that even the most recent deep learning systems remain limited to what corresponds to nonconscious processing in the biological neural networks of the human brain (Dehaene et al., 2017). Progress has recently been made in extremely large artificial neural networks for processing natural human language. These architectures were composed of ~175 billion model parameters, making them several orders of magnitude bigger than its predecessors (Mindt and Montemayor, 2020; Radford et al., 2018). Generative Pre-trained Transformer 3 (GPT-3) was released in mid-2020, after ingesting a diverse series of massive text corpora (as much as >40 TB). The trained learning system can produce human-like text in a variety of contexts, including ad-hoc conversation and completing the next sentence. In many cases, text created by GPT-3 is hard to tell apart from text that was written by humans. However, critics have flagged inaccurate world representation as a critical flaw in the GPT-3 architecture. The generated text passages were typically perfect in appearance. Yet, discrepancies repeatedly loomed in world understanding and common-sense logic, such as reflected in poor social, physical, biological, or causal reasoning (Marcus and Davis, 2020). Moreover, such state-of-the-art learning systems tend to have difficulty in connecting semantic information with long-term dependencies, such as following the narrative in longer stories (cf. Pereira et al., 2018). Hence, today’s language models mostly adhere to the brute-force agenda of interpolating between swaths of encountered language production patterns, rather than extrapolating principled rules from them (cf. Dreyfus, 1972).
Many authors have advocated that we need to go beyond learning superficial internal representations of happenstance word correlations. To this end, next-generation artificial intelligence systems may have to incorporate a special semantic or symbolic module that can dynamically liaise with other expert modules (cf. Chomsky, 1965; Fodor and Pylyshyn, 1988; Marcus and Davis, 2019; Pinker, 1994). A form of a-priori knowledge or set of priors can prove pivotal to go beyond narrow-sense artificial intelligence by discovering and manipulating strong candidate representations as a cornerstone to understand meaning in the external world (Chollet, 2019; Marcus, 2020; Marcus and Davis, 2019). In short, even today’s best-performing artificial intelligence systems have little grasp of and are untethered from reality; at least they are not cognizant of world structure in the way humans understand it. Hence, it may be no accident that it is precisely language understanding which is the human-defining capacity that displays such a strong hemispheric asymmetry between the left and right brain hemisphere.
Conclusions and Outlook
The human central nervous system may be the best existence proof for the feasibility of artificial general intelligence. This begs the question of the computational design principles of how processing modules of the human mind and brain are anchored in hemispheric asymmetry. After all, in nature and technology, intelligent systems often arise from flexible interaction between a small set of simple system components (Braitenberg, 1986; Fodor, 1983; Minsky, 1988). Such information processing systems may better capture pertinent structure in the world and provide a blueprint for more robust generalization to new environmental changes and challenges.
In particular, evolutionarily younger parts in the human inferior parietal lobule display some of the widest structural and functional divergences between the left and right hemisphere in the human brain. The functional role of the right IPL is tuned to serve attentional routing for information relay between specialized modules in our biological neural networks: this finds an algorithmic counterpart in attention-directing mechanisms that are increasingly boosting the capabilities of modern artificial neural network systems. In contrast, the functional role of the left IPL in language understanding, semantics-related reasoning and binding knowledge of world structure, which reflects System 2 thinking, is still awaiting an algorithmic counterpart in future developments that can propel deep learning.
Hence, thrusts forward in understanding the division of labor between the left and right brain hemisphere bear the potential to stimulate new innovations in deep learning. Emerging artificial intelligence tools may in turn unlock new neuroscience insights from big data, percolating into novel treatment interventions in various brain disorders with a disbalance in hemispheric asymmetry.
Acknowledgments
We are indebted to Ralph Adolphs, Lynn Paul, Valentina Borghesani and Karin Saltoun for helpful comments on a previous version of this work. GH was funded by the Max Planck Society (MPG) and the German Research Foundation (DFG). DB was funded by the Canada CIFAR Artificial Intelligence Chairs program, the Healthy Brains, Healthy Lives initiative (CFREF), the Canadian Institute of Health Research (CIHR), the National Institutes of Mental Health of the USA (NIH), and Google.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Gesa Hartwigsen, Max Planck Institute for Human Cognitive and Brain Sciences, Lise Meitner Research Group Cognition and Plasticity, Leipzig, Germany.
Yoshua Bengio, Mila and University of Montreal, Montreal, Canada.
Danilo Bzdok, Montreal Neurological Institute, McConnell Brain Imaging Centre, Faculty of Medicine, McGill University, Montreal, Canada; Department of Biomedical Engineering, Faculty of Medicine, McGill University, Montreal, Canada; School of Computer Science, McGill University, Montreal, Canada; Mila, Montreal, Canada.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, and Zilles K (1999). Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412, 319–341. [DOI] [PubMed] [Google Scholar]
- Andreas J, Rohrbach M, Darrell T, and Klein D (2016). Neural module networks. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, 39–48. [Google Scholar]
- Ashcraft MH, Yamashita TS, and Aram DM (1992). Mathematics performance in left and right brain-lesioned children and adolescents. Brain Cogn 19, 208–252. [DOI] [PubMed] [Google Scholar]
- Baars B (1988). A cognitive theory of consciousness. New York: Cambridge University Press. 2nd Edition. [Google Scholar]
- Badzakova-Trajkov G, Corballis MC, and Haberling IS (2016). Complementarity or independence of hemispheric specializations? A brief review. Neuropsychologia 93, 386–393. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, and Thiebaut de Schotten M (2016). Let thy left brain know what thy right brain doeth: Inter-hemispheric compensation of functional deficits after brain damage. Neuropsychologia 93, 407–412. [DOI] [PubMed] [Google Scholar]
- Basser LS (1962). Hemiplegia of early onset and the faculty of speech with special reference to the effects of hemispherectomy. Brain 85, 427–460. [DOI] [PubMed] [Google Scholar]
- Belyk M, and Brown S (2014). Perception of affective and linguistic prosody: an ALE meta-analysis of neuroimaging studies. Soc Cogn Affect Neurosci 9, 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Choudhury NA, Realpe-Bonilla T, and Roesler CP (2014). Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping. J Neurosci 34, 13349–13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengio Y (2017). The Consciousness Prior. ArXiv: 1709.08568.
- Bengio Y, Deleu T, Rahaman N, Ke R, Lachapelle S, Bilaniuk O, Goyal A, and Pal C (2020). A meta-transfer objective for learning to disentangle causal mechanisms. In ICML. [Google Scholar]
- Binder JR, Desai RH, Graves WW, and Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19, 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, and Prieto T (1997). Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisazza A, Rogers LJ, and Vallortigara G (1998). The origins of cerebral asymmetry: a review of evidence of behavioural and brain lateralization in fishes, reptiles and amphibians. Neurosci Biobehav Rev 22, 411–426. [DOI] [PubMed] [Google Scholar]
- Blank IA, and Fedorenko E (2017). Domain-General Brain Regions Do Not Track Linguistic Input as Closely as Language-Selective Regions. J Neurosci 37, 9999–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff AK, Lim J-S, Bae H-J, Weaver NA, Kuijf HJ, Biesbroek JM, Rost NS, and Bzdok D, (in press). Generative lesion pattern decomposition of cognitive impairment after stroke. Brain Comms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, and Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8, 539–546. [DOI] [PubMed] [Google Scholar]
- Bowers D, and Heilman KM (1980). Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia 18, 491–498. [DOI] [PubMed] [Google Scholar]
- Braitenberg V (1986). Vehicles: Experiments in synthetic psychology. MIT press. [Google Scholar]
- Broca P (1865). Sur la faculte du langage articule. Bulletin de la Societe d’Anthropologie de Paris 6, 377–393. [Google Scholar]
- Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, and Wise RJ (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain 137, 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, and Koch MA (2004). White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex 14, 945–951. [DOI] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, and Gorno-Tempini ML (2009). The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol 22, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Hartwigsen G, Reid A, Laird AR, Fox PT, and Eickhoff SB (2016). Left inferior parietal lobe engagement in social cognition and language. Neurosci Biobehav Rev 68, 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Hoffstaedter F, Turetsky BI, Zilles K, and Eickhoff SB (2012). The modular neuroarchitecture of social judgments on faces. Cereb Cortex 22, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Schilbach L, Jakobs O, Roski C, Caspers S, Laird AR, Fox PT, Zilles K, and Eickhoff SB (2013). Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage 81, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Bisazza A, and Vallortigara G (1995). Lateralization of predator-evasion response in a teleost fish (Girardinus falcatus). Neuropsychologia 33, 1637–1646. [DOI] [PubMed] [Google Scholar]
- Carson RG (2020). Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J Physiol 598, 4781–4802. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, and Jones DK (2007). Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104, 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris C, and Simons D (2010). The invisible gorilla: And other ways our intuitions deceive us. Crown Publishers/Random House. [Google Scholar]
- Chi JG, Dooling EC, and Gilles FH (1977). Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol 34, 346–348. [DOI] [PubMed] [Google Scholar]
- Chollet F (2019). On the Measure of Intelligence. arXiv: 1911.01547.
- Chomsky N (1965). Aspects of the Theory of Syntax. New York: MIT Press. [Google Scholar]
- Cicek M, Gitelman D, Hurley RS, Nobre A, and Mesulam M (2007). Anatomical physiology of spatial extinction. Cereb Cortex 17, 2892–2898. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, and Smith EE (1997). Temporal dynamics of brain activation during a working memory task. Nature 386, 604–608. [DOI] [PubMed] [Google Scholar]
- Corballis MC (2009). The evolution and genetics of cerebral asymmetry. Philos Trans R Soc Lond B Biol Sci 364, 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC (2019). Evolution of cerebral asymmetry. Prog Brain Res 250, 153–178. [DOI] [PubMed] [Google Scholar]
- Corbetta M, and Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, and Shulman GL (2011). Spatial neglect and attention networks. Annu Rev Neurosci 34, 569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang YL, Moore AB, Raymer AM, Briggs RW, Sherod MG, et al. (2007). Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev 17, 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A, Dahan D, and van Donselaar W (1997). Prosody in the comprehension of spoken language: a literature review. Lang Speech 40 (Pt 2), 141–201. [DOI] [PubMed] [Google Scholar]
- Danielsen VM, Vidal-Pineiro D, Mowinckel AM, Sederevicius D, Fjell AM, Walhovd KB, and Westerhausen R (2020). Lifespan trajectories of relative corpus callosum thickness: Regional differences and cognitive relevance. Cortex 130, 127–141. [DOI] [PubMed] [Google Scholar]
- Davis SW, and Cabeza R (2015). Cross-hemispheric collaboration and segregation associated with task difficulty as revealed by structural and functional connectivity. J Neurosci 35, 8191–8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Kerszberg M, and Changeux JP (1998). A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci U S A 95, 14529–14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Lau H, and Kouider S (2017). What is consciousness, and could machines have it? Science 358, 486–492. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, and Harrison DW (2005). Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev 4, 3–20. [DOI] [PubMed] [Google Scholar]
- Diachek E, Blank I, Siegelman M, Affourtit J, and Fedorenko E (2020). The Domain-General Multiple Demand (MD) Network Does Not Support Core Aspects of Language Comprehension: A Large-Scale fMRI Investigation. J Neurosci 40, 4536–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekamp B, Regolin L, Gunturkun O, and Vallortigara G (2005). A left-sided visuospatial bias in birds. Curr Biol 15, R372–373. [DOI] [PubMed] [Google Scholar]
- Dohmatob E, Dumas G, Bzdok D, 2020. Dark Control: The Default Mode Network as a Reinforcement Learning Agent. Hum Brain Mapp 41, 3318–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech P, and Koechlin E (2015). Executive control and decision-making in the prefrontal cortex. Current Opinion in Behavioral Sciences 1, 101–106. [Google Scholar]
- Donoso M, Collins AG, and Koechlin E (2014). Human cognition. Foundations of human reasoning in the prefrontal cortex. Science 344, 1481–1486. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, and Petersen SE (2006). A core system for the implementation of task sets. Neuron 50, 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A 104, 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus H (1972). What computers can’t do: The limits of artificial intelligence. New York: Harper & Row. [Google Scholar]
- Dunbar RI, and Shultz S (2007). Evolution in the social brain. Science 317, 1344–1347. [DOI] [PubMed] [Google Scholar]
- Duncan J (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14, 172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J, and Owen AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23, 475–483. [DOI] [PubMed] [Google Scholar]
- Esteves M, Ganz E, Sousa N, and Leite-Almeida H (2021). Asymmetrical Brain Plasticity: Physiology and Pathology. Neuroscience 454, 3–14. [DOI] [PubMed] [Google Scholar]
- Esteves M, Lopes SS, Almeida A, Sousa N, and Leite-Almeida H (2020). Unmasking the relevance of hemispheric asymmetries-Break on through (to the other side). Progress in Neurobiology 192, 101823. [DOI] [PubMed] [Google Scholar]
- Evans JS, and Stanovich KE (2013). Dual-Process Theories of Higher Cognition: Advancing the Debate. Perspect Psychol Sci 8, 223–241. [DOI] [PubMed] [Google Scholar]
- Falzi G, Perrone P, and Vignolo LA (1982). Right-left asymmetry in anterior speech region. Arch Neurol 39, 239–240. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, and Blank IA (2020). Broca’s Area Is Not a Natural Kind. Trends Cogn Sci 24, 270–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, and Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A 110, 16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Nieto-Castanon A, and Kanwisher N (2012). Lexical and syntactic representations in the brain: an fMRI investigation with multi-voxel pattern analyses. Neuropsychologia 50, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor JA, and Pylyshyn ZW (1988). Connectionism and cognitive architecture: a critical analysis. Cognition 28, 3–71. [DOI] [PubMed] [Google Scholar]
- Fodor JAT (1983). The modularity of mind. MIT press. [Google Scholar]
- Fridriksson J, den Ouden DB, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, and Bonilha L (2018). Anatomy of aphasia revisited. Brain 141, 848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: from structure to function. Physiol Rev 91, 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2017). Evolution of the neural language network. Psychon Bull Rev 24, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, and Alter K (2004). Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang 89, 267–276. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, and Braun AR (1998). Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke’s brain language area homolog. Science 279, 220–222. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS (1995). Principles of human brain organization derived from split-brain studies. Neuron 14, 217–228. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS (2000). Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123 (Pt 7), 1293–1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, and Donchin E (1993). A neural system for error detection and compensation. Psychol Sci 4, 385–390. [Google Scholar]
- Gelman SA, and Roberts SO (2017). How language shapes the cultural inheritance of categories. Proc Natl Acad Sci U S A 114, 7900–7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc E, Bergmann J, Singer W, and Kohler A (2011). Interhemispheric connections shape subjective experience of bistable motion. Curr Biol 21, 1494–1499. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SL, and Wise RJ (2014). Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain 137, 2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Chau TW, Wise RJS, Leech R, and Hampshire A (2017). Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain 140, 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N (1970). The organization of language and the brain. Science 170, 940–944. [DOI] [PubMed] [Google Scholar]
- Geschwind N, and Galaburda AM (1985). Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol 42, 428–459. [DOI] [PubMed] [Google Scholar]
- Ghahramani Z (2015). Probabilistic machine learning and artificial intelligence. Nature 521, 452–459. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Goyal MS, Preuss TM, Raichle ME, and Van Essen DC (2014). Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage 93 Pt 2, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V (2019). Hemispheric asymmetry in the prefrontal cortex for complex cognition. In Handbook of clinical neurology, D’Esposito M, and Grafman JH, eds. (Elsevier; ), pp. 179–196. [DOI] [PubMed] [Google Scholar]
- Goodfellow IJ, Bengio Y, and Courville A (2016). Deep Learning (USA: MIT Press; ). [Google Scholar]
- Goyal A, and Bengio Y (2020). Inductive Biases for Deep Learning of Higher-Level Cognition. ArXiv, 2011.15091.
- Goyal A, Didolkar A, Lamb A, Badola K, Ke NR, Rahaman N, Binas J, Blundell C, Mozer M, and Bengio Y (2021). Coordination Among Neural Modules Through a Shared Global Workspace. arXiv 2103.01197
- Goyal A, Lamb A, Hoffmann J, Sodhani S, Levine S, Bengio Y, and Scholkopf B (2019). Recurrent Independent Mechanisms. ArXiv: 1909.10893.
- Gunturkun O, and Ocklenburg S (2017). Ontogenesis of Lateralization. Neuron 94, 249–263. [DOI] [PubMed] [Google Scholar]
- Gunturkun O, Strockens F, and Ocklenburg S (2020). Brain Lateralization: A Comparative Perspective. Physiol Rev 100, 1019–1063. [DOI] [PubMed] [Google Scholar]
- Habib R, Nyberg L, and Tulving E (2003). Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn Sci 7, 241–245. [DOI] [PubMed] [Google Scholar]
- Hagoort P, and Indefrey P (2014). The neurobiology of language beyond single words. Annu Rev Neurosci 37, 347–362. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G (2018). Flexible Redistribution in Cognitive Networks. Trends Cogn Sci 22, 687–698. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, and Saur D (2019). Neuroimaging of stroke recovery from aphasia - Insights into plasticity of the human language network. Neuroimage 190, 14–31. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G, and Siebner HR (2012). Probing the involvement of the right hemisphere in language processing with online transcranial magnetic stimulation in healthy volunteers. Aphasiology 26, 1131–1152. [Google Scholar]
- Hartwigsen G, and Volz LJ (2021). Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. Neuroimage 224, 117449. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Speedie L, and Coslett HB (1984). Comprehension of affective and nonaffective prosody. Neurology 34, 917–921. [DOI] [PubMed] [Google Scholar]
- Heiss WD, and Thiel A (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang 98, 118–123. [DOI] [PubMed] [Google Scholar]
- Hensel L, Bzdok D, Muller VI, Zilles K, and Eickhoff SB (2015). Neural correlates of explicit social judgments on vocal stimuli. Cereb Cortex 25, 1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering-Hanit R, Achiron R, Lipitz S, and Achiron A (2001). Asymmetry of fetal cerebral hemispheres: in utero ultrasound study. Arch Dis Child Fetal Neonatal Ed 85, F194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, and Van Essen D (2010). A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci 30, 2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley LB, Marco EJ, Brown EG, Bukshpun P, Gold J, Hill S, Findlay AM, Jeremy RJ, Wakahiro ML, Barkovich AJ, et al. (2016). The Contribution of the Corpus Callosum to Language Lateralization. J Neurosci 36, 4522–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekert M, Vingerhoets G, and Aleman A (2010). Results of a pilot study on the involvement of bilateral inferior frontal gyri in emotional prosody perception: an rTMS study. BMC Neurosci 11, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA (2014). Evolution of the human brain: when bigger is better. Front Neuroanat 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA (2015). Evolution of the human brain: from matter to mind. In Handbook of Intelligence: Evolutionary Theory, Historical Perspective, and Current Concepts Goldstein S, Princiotta D, and Naglieri JA, eds.. New York: Springer Science+Business Media. [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, and MacGregor LA (1998). Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI). Neuroreport 9, 2913–2918. [DOI] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Jordan MI, Nowlan SJ, and Hinton GE (1991). Adaptive Mixtures of Local Experts. Neural Comput 3, 79–87. [DOI] [PubMed] [Google Scholar]
- Johansson J, Salami A, Lundquist A, Wahlin A, Andersson M, and Nyberg L (2020). Longitudinal evidence that reduced hemispheric encoding/retrieval asymmetry predicts episodic-memory impairment in aging. Neuropsychologia 137, 107329. [DOI] [PubMed] [Google Scholar]
- Jordan MI, and Mitchell TM (2015). Machine learning: Trends, perspectives, and prospects. Science 349, 255–260. [DOI] [PubMed] [Google Scholar]
- Kaas JH (2000). Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain and Mind 1, 7–23. [Google Scholar]
- Kaas JH, and Herculano-Houzel S (2017). What Makes the Human Brain Special: Key Features of Brain and Neocortex. In The Physics of the Mind and Brain Disorders, Opris I, and Casanova MF, eds. Cham, Switzerland: Springer. [Google Scholar]
- Kahneman D (2011). Thinking, fast and slow. MacMillan. [Google Scholar]
- Kannengiesser U, and Gero JS (2019). Empirical evidence for Kahneman’s system 1 and system 2 thinking in design. In: Human Behaviour in Design. [Google Scholar]
- Karolis VR, Corbetta M, and Thiebaut de Schotten M (2019). The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat Commun 10, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XZ, Mathias SR, Guadalupe T, Group ELW, Glahn DC, Franke B, Crivello F, Tzourio-Mazoyer N, Fisher SE, Thompson PM, and Francks C (2018). Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc Natl Acad Sci U S A 115, E5154–E5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW (2015). The applicability of motor learning to neurorehabilitation. In Oxford Textbook of Neurorehabilitation Dietz V, and Ward NS, eds. Oxford University Press, pp. 55–63. [Google Scholar]
- Kuhl PK (2004). Early language acquisition: cracking the speech code. Nat Rev Neurosci 5, 831–843. [DOI] [PubMed] [Google Scholar]
- Lake BM, Ullman TD, Tenenbaum JB, and Gershman SJ (2017). Building machines that learn and think like people. Behav Brain Sci 40, e253. [DOI] [PubMed] [Google Scholar]
- Lansing AE, Max JE, Delis DC, Fox PT, Lancaster J, Manes FF, and Schatz A (2004). Verbal learning and memory after childhood stroke. J Int Neuropsychol Soc 10, 742–752. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, and Sejnowski TJ (2003). Communication in neuronal networks. Science 301, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, and Beaulieu C (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30, 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCun Y, Bengio Y, and Hinton G (2015). Deep learning. Nature 521, 436–444. [DOI] [PubMed] [Google Scholar]
- Lenneberg EB (1967). Biological Foundations of Language. New York: Wiley. [Google Scholar]
- Lennie P (2003). The cost of cortical computation. Curr Biol 13, 493–497. [DOI] [PubMed] [Google Scholar]
- Levy WB, and Baxter RA (1996). Energy efficient neural codes. Neural Comput 8, 531–543. [DOI] [PubMed] [Google Scholar]
- Lichtheim L (1885). On aphasia. Brain 7, 433–484. [Google Scholar]
- Lindell AK (2006). In your right mind: right hemisphere contributions to language processing and production. Neuropsychol Rev 16, 131–148. [DOI] [PubMed] [Google Scholar]
- Marcus G (2020). The next decade in AI: four steps towards robust artificial intelligence. arXiv: 2002.06177. [Google Scholar]
- Marcus G, and Davis E (2019). Rebooting AI: Building artificial intelligence we can trust. Vintage. [Google Scholar]
- Marcus G, and Davis E (2020). GPT-3, Bloviator: OpenAI’s language generator has no idea what it’s talking about. MIT Technology Review. [Google Scholar]
- Mashour GA, Roelfsema P, Changeux JP, and Dehaene S (2020). Conscious Processing and the Global Neuronal Workspace Hypothesis. Neuron 105, 776–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, and von Cramon DY (2002). FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp 17, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Steinhauer K, Alter K, Friederici AD, and von Cramon DY (2004). Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang 89, 277–289. [DOI] [PubMed] [Google Scholar]
- Miller GA (1956). The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev 63, 81–97. [PubMed] [Google Scholar]
- Mindt G, and Montemayor C (2020). A Roadmap for Artificial General Intelligence: Intelligence, Knowledge, and Consciousness. Mind and Matter 18, 9–37. [Google Scholar]
- Minsky M (1988). Society of mind. Simon and Schuster. [Google Scholar]
- Mittal S, Lamb A, Goyal A, Voleti V, Shanahan M, Lajoie G, Mozer MU, and Bengio Y (2020). Learning to combine top-down and bottom-up signals in recurrent neural networks with attention over modules. In: Proceedings of the 37th International Conference on Machine Learning (PMLR). [Google Scholar]
- Morales J, Lau H, and Fleming SM (2018). Domain-General and Domain-Specific Patterns of Activity Supporting Metacognition in Human Prefrontal Cortex. J Neurosci 38, 3534–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport EL, Landau B, Seydell-Greenwald A, Turkeltaub PE, Chambers CE, Dromerick AW, Carpenter J, Berl MM, and Gaillard WD (2017). Revisiting Lenneberg’s Hypotheses About Early Developmental Plasticity: Language Organization After Left-Hemisphere Perinatal Stroke. Biolinguistics (Nicos) 11, 407–422. [PMC free article] [PubMed] [Google Scholar]
- Numssen O, Bzdok D, and Hartwigsen G (2021). Functional specialization within the inferior parietal lobes across cognitive domains. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell C, Goncalves JT, Portera-Cailliau C, and Sejnowski TJ (2017). Beyond excitation/inhibition imbalance in multidimensional models of neural circuit changes in brain disorders. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S, Berretz G, Packheiser J, and Friedrich P (2020). Laterality 2020: entering the next decade. Laterality, 1–33. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, and Field DJ (1996). Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Seydell-Greenwald A, Chambers CE, Turkeltaub PE, Dromerick AW, Berl MM, Gaillard WD, and Newport EL (2020). The neural basis of language development: Changes in lateralization over age. Proc Natl Acad Sci U S A 117, 23477–23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA (2016). Functional definitions of parietal areas in human and non-human primates. Proc Biol Sci 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, and Raichle ME (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A 87, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, and Sherr EH (2007). Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 8, 287–299. [DOI] [PubMed] [Google Scholar]
- Peelle JE (2018). Listening Effort: How the Cognitive Consequences of Acoustic Challenge Are Reflected in Brain and Behavior. Ear Hear 39, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F, Lou B, Pritchett B, Ritter S, Gershman SJ, Kanwisher N, Botvinick M, and Fedorenko E (2018). Toward a universal decoder of linguistic meaning from brain activation. Nat Commun 9, 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Janzing D, and B. S (2017). Elements of causal inference: foundations and learning algorithms. MIT Press. [Google Scholar]
- Petersen SE, and Sporns O (2015). Brain Networks and Cognitive Architectures. Neuron 88, 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinker S (1994). The Language Instinct: How the Mind Creates Language (New York: William Morrow and Company; ). [Google Scholar]
- Prete G, Marzoli D, and Tommasi L (2015). Upright or inverted, entire or exploded: right-hemispheric superiority in face recognition withstands multiple spatial manipulations. PeerJ 3, e1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam MC, Wig GS, Grafton ST, Kelley WM, and Gazzaniga MS (2008). Structural organization of the corpus callosum predicts the extent and impact of cortical activity in the nondominant hemisphere. J Neurosci 28, 2912–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A, Narasimhan K, Salimans T, and Sutskever I (2018). Improving Language Understanding by Generative Pre-Training. openai-assets preprint.
- Ridderinkhof KR, Ullsperger M, Crone EA, and Nieuwenhuis S (2004). The role of the medial frontal cortex in cognitive control. Science 306, 443–447. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, and Simard PY (1994). Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex 4, 331–343. [DOI] [PubMed] [Google Scholar]
- Rogers LJ (2014). Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 52, 555–571. [DOI] [PubMed] [Google Scholar]
- Sabour S, Frosst N, and Hinton GE (2017). Dynamic routing between capsules. Adv Neural Inf Process Sys 30, 3856–3866. [Google Scholar]
- Saur D, and Hartwigsen G (2012). Neurobiology of language recovery after stroke: lessons from neuroimaging studies. Arch Phys Med Rehabil 93, S15–25. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, and Weiller C (2006). Dynamics of language reorganization after stroke. Brain 129, 1371–1384. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Morosan P, and Zilles K (1999). Observer-independent method for microstructural parcellation of cerebral cortex: A quantitative approach to cytoarchitectonics. Neuroimage 9, 165–177. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, and Pandya DN (2006). Fiber Pathways of the Brain. Oxford University Press. [Google Scholar]
- Schoenemann PT, Sheehan MJ, and Glotzer LD (2005). Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci 8, 242–252. [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, Sallet J, and Kanske P (2020). Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol Bull. [DOI] [PubMed] [Google Scholar]
- Seghier ML (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Teffer K, Buxhoeveden DP, Park MS, Bludau S, Amunts K, Travis K, and Buckwalter J (2011). Spatial organization of neurons in the frontal pole sets humans apart from great apes. Cereb Cortex 21, 1485–1497. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Hof PR, and L. HR (2003). Variability in Broca’s area homologue in great apes: Implications for language evolution. Anatomical Record 271 276–285. [DOI] [PubMed] [Google Scholar]
- Shleifer A (2012). Psychologists at the Gate: A Review of Daniel Kahneman’s Thinking, Fast and Slow. Journal of Economic Literature 50, 1–12. [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, and Hilgetag CC (2004). Organization, development and function of complex brain networks. Trends Cogn Sci 8, 418–425. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ, and Kotter R (2007). Identification and classification of hubs in brain networks. PLoS One 2, e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz H (1996). Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci Biobehav Rev 20, 587–591. [DOI] [PubMed] [Google Scholar]
- Stockert A, Wawrzyniak M, Klingbeil J, Wrede K, Kummerer D, Hartwigsen G, Kaller CP, Weiller C, and Saur D (2020). Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain 143, 844–861. [DOI] [PubMed] [Google Scholar]
- Sun T, and Walsh CA (2006). Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci 7, 655–662. [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, Yoshioka N, and Ohtomo K (2011). Gray and white matter asymmetries in healthy individuals aged 21–29 years: a voxel-based morphometry and diffusion tensor imaging study. Hum Brain Mapp 32, 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teffer K, and Semendeferi K (2012). Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res 195, 191–218. [DOI] [PubMed] [Google Scholar]
- Thiel A, Schumacher B, Wienhard K, Gairing S, Kracht LW, Wagner R, Haupt WF, and Heiss WD (2006). Direct demonstration of transcallosal disinhibition in language networks. J Cereb Blood Flow Metab 26, 1122–1127. [DOI] [PubMed] [Google Scholar]
- Thierry G, Giraud AL, and Price C (2003). Hemispheric dissociation in access to the human semantic system. Neuron 38, 499–506. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, and Just MA (1999). Plasticity of language-related brain function during recovery from stroke. Stroke 30, 749–754. [DOI] [PubMed] [Google Scholar]
- Toga AW, and Thompson PM (2003). Mapping brain asymmetry. Nat Rev Neurosci 4, 37–48. [DOI] [PubMed] [Google Scholar]
- Tomasello M (1999). The cultural origins of cognition. Cambridge, MA: Harvard University Press. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, and Moll H (2005). Understanding and sharing intentions: the origins of cultural cognition. Behav Brain Sci 28, 675–691; discussion 691–735. [DOI] [PubMed] [Google Scholar]
- Tsymbal A, The problem of concept drift: Definitions and related work. Technical Report. 2004, Department of Computer Science, Trinity College: Dublin, Ireland. [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, and Houle S (1994). Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A 91, 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turatto M, Benso F, Facoetti A, Galfano G, Mascetti GG, and Umilta C (2000). Automatic and voluntary focusing of attention. Percept Psychophys 62, 935–952. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, and Hamilton RH (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology 76, 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BO, Marinsek N, Ryhal E, and Miller MB (2015). Hemispheric lateralization in reasoning. Ann N Y Acad Sci 1359, 47–64. [DOI] [PubMed] [Google Scholar]
- Vaden KI Jr., Kuchinsky SE, Cute SL, Ahlstrom JB, Dubno JR, and Eckert MA (2013). The cingulo-opercular network provides word-recognition benefit. J Neurosci 33, 18979–18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E, and Liberman H (2016). Perceptual asymmetry during free viewing of words and faces: The effect of context on recognition. Brain Cogn 109, 43–49. [DOI] [PubMed] [Google Scholar]
- Valenti A, Sovrano VA, Zucca P, and Vallortigara G (2003). Visual lateralisation in quails (Coturnix coturnix). Laterality 8, 67–78. [DOI] [PubMed] [Google Scholar]
- Vallar G, and Calzolari E (2018). Unilateral spatial neglect after posterior parietal damage. In Handbook of clinical neurology, Vallar G, and Coslett HB, eds. Elsevier, pp. 287–312. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, and Andrew RJ (1994). Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav Processes 33, 41–57. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Chiandetti C, and Sovrano VA (2011). Brain asymmetry (animal). Wiley Interdiscip Rev Cogn Sci 2, 146–157. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Pagni P, and Sovrano VA (2004). Separate geometric and non-geometric modules for spatial reorientation: evidence from a lopsided animal brain. J Cogn Neurosci 16, 390–400. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, and Rogers LJ (2005). Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28, 575–589; discussion 589–633. [DOI] [PubMed] [Google Scholar]
- van der Burght CL, Goucha T, Friederici AD, Kreitewolf J, and Hartwigsen G (2019). Intonation guides sentence processing in the left inferior frontal gyrus. Cortex 117, 122–134. [DOI] [PubMed] [Google Scholar]
- Van Essen DC (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, and Dierker DL (2007). Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 56, 209–225. [DOI] [PubMed] [Google Scholar]
- van Lancker D (1980). Cerebral lateralization of pitch cues in the linguistic signal. Paper in Linguistics 13, 201e277. [Google Scholar]
- van Rijn S, Aleman A, van Diessen E, Berckmoes C, Vingerhoets G, and Kahn RS (2005). What is said or how it is said makes a difference: role of the right fronto-parietal operculum in emotional prosody as revealed by repetitive TMS. Eur J Neurosci 21, 3195–3200. [DOI] [PubMed] [Google Scholar]
- Ventolini N, Ferrero EA, Sponza S, Della Chiesa A, Zucca P, and Vallortigara G (2005). Laterality in the wild: preferential hemifield use during predatory and sexual behaviour in the black-winged stilt Anim Behav 69, 1077–1084. [Google Scholar]
- Volz LJ, and Grefkes C (2016). Basic Principles of rTMS in Motor Recovery After Stroke. In Therapeutic rTMS in Neurology, Platz T, ed. Cham: Springer, pp. 23–37. [Google Scholar]
- Weintraub S, and Mesulam MM (1987). Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Arch Neurol 44, 621–625. [DOI] [PubMed] [Google Scholar]
- Werker JF, and Hensch TK (2015). Critical periods in speech perception: new directions. Annu Rev Psychol 66, 173–196. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1874). Der aphasische Symptomencomplex. Breslau: Cohn and Weigert. [Google Scholar]
- Westerhausen R (2019). A primer on dichotic listening as a paradigm for the assessment of hemispheric asymmetry. Laterality 24, 740–771. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, and Wittling W (2006). The association of macro- and microstructure of the corpus callosum and language lateralisation. Brain Lang 97, 80–90. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Luders E, Specht K, Ofte SH, Toga AW, Thompson PM, Helland T, and Hugdahl K (2011). Structural and functional reorganization of the corpus callosum between the age of 6 and 8 years. Cereb Cortex 21, 1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CJ, Yusuf A, Wilson DE, Peelle JE, Davis MH, and Johnsrude IS (2012). Effortful listening: the processing of degraded speech depends critically on attention. J Neurosci 32, 14010–14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, and Heiss WD (2005). Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 36, 1759–1763. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Aust U, Huber L, Hausmann M, and Gunturkun O (2007). Lateralized cognition: asymmetrical and complementary strategies of pigeons during discrimination of the “human concept”. Cognition 104, 315–344. [DOI] [PubMed] [Google Scholar]
- Yamins DL, Hong H, Cadieu CF, Solomon EA, Seibert D, and DiCarlo JJ (2014). Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc Natl Acad Sci U S A 111, 8619–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago L, Petit L, Jobard G, Hay J, Mazoyer B, Tzourio-Mazoyer N, Karnath HO, and Mellet E (2017). Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychologia 94, 75–83. [DOI] [PubMed] [Google Scholar]


