Abstract
Rationale:
Renin-expressing cells are myoendocrine cells crucial for survival. They have been postulated to possess a pressure-sensing mechanism, a baroreceptor, that can detect slight changes in blood pressure and respond with precise and synchronized amounts of renin synthesized and released to the circulation to maintain blood pressure and fluid-electrolyte balance. The location and nature of this puzzling pressure-sensing structure have remained unknown since it was originally suggested over sixty years ago.
Objective:
To elucidate the location and structure of the renin cell baroreceptor.
Methods and Results:
We used a variety of genetically modified mice whereby renin cells were exposed in vivo to either low or high arterial pressure. In addition, we applied direct mechanical stimuli, i.e., pneumatic pressure or stretch, directly to renin cells cultured under different conditions and substrata. Changes in perfusion pressure and/or direct mechanical stimuli induced significant changes in renin gene expression and the phenotype of renin cells. Importantly, the experiments show that the pressure-sensing mechanism (the baroreceptor) resides in the renin cells; it requires initial extracellular sensing by integrin β1 at the renin cell membrane and is transduced to the nuclear membrane and chromatin by lamin A/C.
Conclusions:
These studies show that the enigmatic baroreceptor is a nuclear mechanotransducer that resides in the renin cells per se and is responsible for the sensing and transmission of extracellular physical forces directly to the chromatin of renin cells via lamin A/C to regulate renin gene expression, renin bioavailability, and homeostasis.
Keywords: Renin, blood pressure, perfusion, gene expression, mechanotransduction
Subject Terms: ACE/Angiotensin Receptors/Renin Angiotensin System, Nephrology and Kidney
INTRODUCTION
Renin-expressing cells are essential for survival, perfected throughout evolution to maintain blood pressure (BP) and fluid-electrolyte homeostasis1. In adult mammals, renin cells are strategically located at the juxtaglomerular (JG) tip of the afferent arterioles as they enter the glomeruli2. Renin cells are sensors endowed with the ability to detect and respond to minute changes in BP and the composition and volume of the extracellular fluid. In response to a decrease in perfusion pressure, renin cells synthesize and release renin to the circulation resulting in the restoration of BP1. An increase in pressure, on the other hand, results in decreased renin release, thus preventing the development of hypertension1. How renin cells sense and respond to changes in pressure is unknown. In 1957 Tobian and coworkers3 postulated the existence of a pressure sensing-mechanism (denominated the baroreceptor) in the control of renin release. However, the nature and location of this baroreceptor and how it controls renin expression are unclear.
Using a variety of novel in vivo and in vitro approaches, we tested the hypothesis that this pressure-sensing mechanism, the enigmatic baroreceptor, is a nuclear mechanotransducer that resides in the renin cells and is responsible for the sensing and transmission of extracellular physical forces directly to the chromatin of renin cells to regulate renin gene expression, renin bioavailability, and renin cell phenotype.
METHODS
Detailed methods are available in the Online Supplement. Please, see also the Major Resources Table in the Supplemental Materials.
Data Availability.
All supporting data are available within the article and its supplementary files. The RNA-seq and ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing) data sets generated in this study can be accessed at the GEO public repository using the accession number GSE157699 and GSE167522, respectively. R codes are available on Zenodo at https://doi.org/10.5281/zenodo.4672146. Additional information will be available upon request to the corresponding authors.
RESULTS
Perfusion pressure controls the expression of the renin gene.
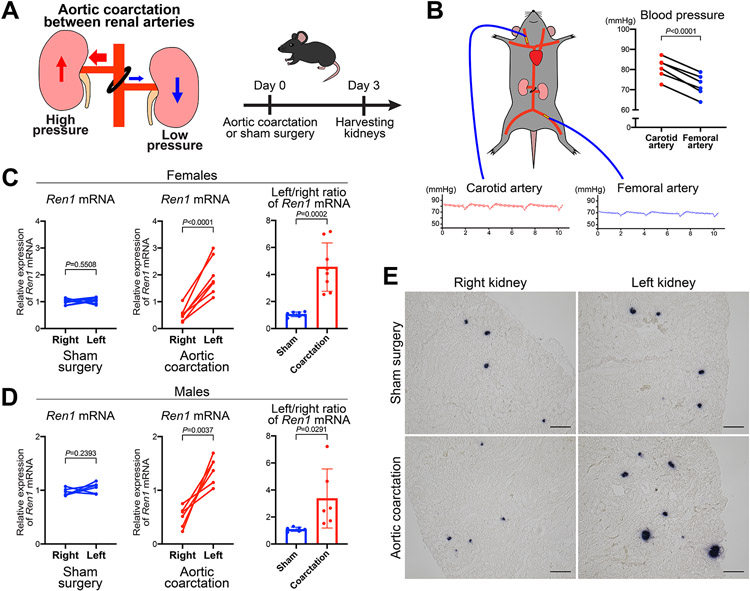
To investigate whether changes in perfusion pressure affect the expression of the renin gene, we implemented a model of aortic coarctation (AoCo) between the roots of the right and left renal arteries (Figure 1A). In this model, the right and left kidneys are subjected to high and low perfusion pressure, respectively, allowing the study of the kidneys from the same mouse simultaneously, eliminating effects due to genetic background and individual differences. We confirmed this surgical model’s validity by simultaneously measuring the intraarterial BP above (carotid artery) and below (femoral artery) the constriction three days after the AoCo. BP in the carotid arteries was significantly higher than in the femoral arteries in mice subjected to the AoCo, (Figure 1B). Three days after the AoCo, we extracted RNA from the renal cortices. Using quantitative reverse transcription PCR (qRT-PCR) for Ren1, there was no difference between the right and left renal cortices of mice subjected to sham surgeries. However, the renal cortices from mice subjected to the AoCo showed significant differences in Ren1 mRNA in both female and male mice: lower expression in the right kidneys (exposed to higher perfusion pressure) and higher expression in the left kidneys (exposed to lower perfusion pressure) (Figure 1C, D). Ren1 expression in the right kidneys and left kidneys with AoCo was significantly lower in the right and higher in the left than in mice subjected to sham surgeries (Online Figure I). By in situ hybridization (ISH), the intensity and extension of the Ren1 mRNA signals at the JG area were lower in the right and higher in the left kidneys of the mice subjected to AoCo, compared to the kidneys from mice with sham surgeries (Figure 1E). Overall, our in vivo model to study the effects of changes on renal perfusion pressure showed that the Ren1 expression in renin cells was decreased by high perfusion pressure and increased by low perfusion pressure, respectively.
Figure 1. Changes in perfusion pressure induce modifications in Ren1 expression.
A, Schematic of the aortic coarctation (AoCo) surgical model. The ligation between the roots of the right and left renal arteries results in an increase in perfusion pressure in the right kidney and a decrease in the left kidney. Kidneys were harvested 72 hours after the AoCo or sham surgeries. B, Simultaneous blood pressure (BP) measurement under anesthesia from the carotid and femoral arteries of mice with AoCo. The BP was significantly different between upper arteries (carotid arteries) and lower arteries (femoral arteries) of AoCo (n=6, paired t-test). C, Changes in Ren1 expression in kidneys subjected to AoCo in female mice by quantitative reverse transcription PCR (qRT-PCR) showed a significant difference in Ren1 mRNA between the right and left renal cortices from mice with AoCo (n=8, paired t-test), whereas Ren1 mRNA was not different between right and left renal cortices from mice with sham surgeries (n=7, paired t-test). With AoCo, the left/right ratio of Ren1 mRNA was significantly higher than the one of sham surgeries (Student’s t-test). D, Changes in Ren1 expression in kidneys subjected to AoCo in male mice by qRT-PCR showed a significant difference in Ren1 mRNA between the right and left renal cortices from mice with AoCo (n=6, paired t-test), whereas Ren1 mRNA was not different between right and left renal cortices from mice with sham surgeries (n=6, paired t-test). With AoCo, the left/right ratio of Ren1 mRNA was significantly higher than the one of sham surgeries (Student’s t-test). E, In situ hybridization for Ren1 mRNA in the kidneys. The intensity and extension of the Ren1 mRNA signals at the juxtaglomerular areas were not different between the right and left kidneys from mice with sham surgery. With AoCo, signals in right kidneys were decreased, and signals in left kidneys were significantly increased, compared to sham surgeries. Scale bar, 100 μm. All data are reported as means ± standard deviation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Renin cells switch phenotype in response to changes in perfusion pressure.
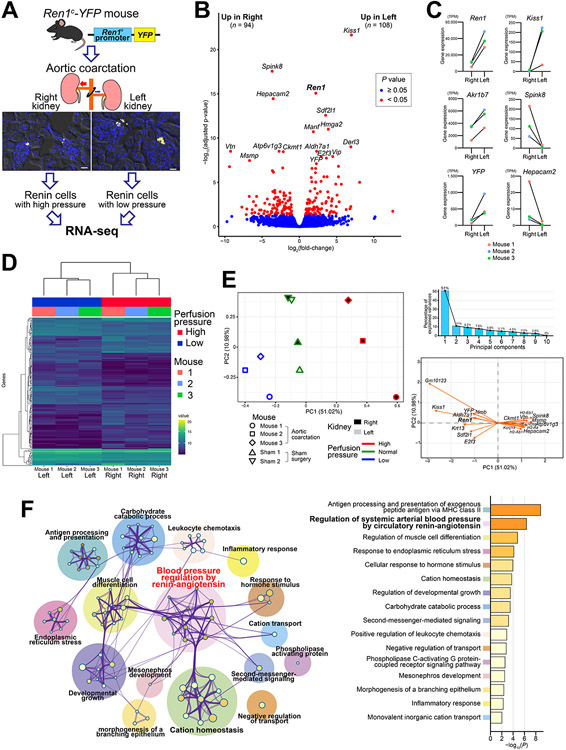
To gain insight into the mechanisms involved in the transformation of renin cells in response to changes in perfusion pressure, we performed a transcriptome analysis of renin cells in the kidneys with AoCo (Figure 2A). We used the Ren1c-YFP mice that express a yellow fluorescent protein (YFP) under the control of 4.9 kb of the 5’ regulatory region of the renin gene4 and isolated YFP-positive cells using fluorescence-activated cell sorting5. With AoCo, the percentage of YFP-positive cells captured from the left renal cortices was significantly higher than from the right renal cortices (Online Figure II). In kidney sections from these mice, the intensity and extension of YFP signals at the JG area were lower in the right and higher in the left kidneys (Figure 2A). These results suggest that with high perfusion pressure, expression of the YFP gene decreased to undetectable levels in some renin cells, whereas with low perfusion pressure, new cells turned on the expression of YFP and renin. Furthermore, the data indicate the 4.9 kb of DNA sequence at the 5’ Ren1 regulatory region is sufficient to confer responses to changes in perfusion pressure.
Figure 2. Renin cells change their transcription programs in response to changes in perfusion pressure.
A, RNA-seq of renin cells from the kidneys subjected to aortic coarctation (AoCo) surgery. YFP-positive cells from Ren1c-YFP mice were isolated using fluorescence-activated cell sorting. Each kidney cortex was processed separately. Scale bar, 20 μm. B, Volcano plot of RNA-seq analysis. The 202 differentially expressed genes (DEGs) between renin cells in the right kidneys and left kidneys were shown in red. C, Representative genes that showed differential expression between renin cells in the right and left kidneys. Data are shown in transcripts per million (TPM). D, Heatmap using the expression levels of DEGs showed clear segregation of renin cells in the right and left kidneys, indicating marked differences in gene expression in response to changes in perfusion pressure. E, Principal component analysis (PCA) by the DEGs of renin cells from kidneys subjected to AoCo and sham surgeries. PC1 clearly segregated renin cells with low, normal, and high perfusion pressure. PC1 represented 51.02 % of the variances. The top 20 genes that contributed to PC1 were shown in the variable correlation plot. F, Enrichment analysis using Gene Ontology with the DEGs between renin cells in the right and left kidneys. The enriched categories were shown with the network analysis.
We performed RNA-seq using YFP-positive renin cells from kidneys of 3 mice with AoCo and 2 mice with sham surgeries. Differential gene expression profiling using the paired analysis to compare gene expression between the right and left kidneys from the AoCo group revealed 202 differentially expressed genes (DEGs) (Figure 2B, Online Figure III-A, Online Table I, II). We confirmed the difference in expression of those newly-detected genes in renin cells subjected to AoCo using ISH (Online Figure IV). Of those DEGs, 94 were expressed higher in the right, and 108 were higher in the left kidneys (Figure 2B). In agreement with the above qRT-PCR and ISH data, Ren1 expression in renin cells was higher in the left than in the right kidney. This shows that in addition to an increase in the number of renin-expressing cells (more YFP-positive cells), there was also an increase in Ren1 expression by these cells in response to low perfusion pressure. Because YFP-positive cells are all renin-expressing cells, as expected, Ren1 was the most highly expressed in both high and low perfusion pressure conditions (Online Figure III-A). Kiss1, which is located close to Ren1 on chromosome 1, showed higher expression in renin cells from kidneys with low perfusion pressure and had the smallest P-value among DEGs (Figure 2B, Online Figure IV-C), although the absolute value of the expression was much lower than Ren1 (Figure 2C). This is consistent with previous reports that KISS1 and REN in humans exhibit similar expression patterns6. Akr1b7, a previously established renin cell marker gene7, was also highly expressed in renin cells, and its expression levels were significantly different between cells from the right and left kidneys (Figure 2C), further confirmed by ISH (Online Figure V). We also detected 87 and 122 DEGs from high vs. normal perfusion pressure and normal vs. low perfusion pressure, respectively (Online Figure VI).
The identified DEGs by paired analysis from the AoCo group clearly separated the samples from the right and left kidneys (Figure 2D). Furthermore, when we included the renin cells from mice with sham surgeries, the DEGs also showed a clear separation of the samples according to the perfusion pressure with the sham group placed in between the ones from the right and left kidneys from the AoCo group (Online Figure III-B). Principal component analysis (PCA) also clearly segregated the samples according to changes in perfusion pressure (Figure 2E). The top principal component (PC1) represented more than half of the variances. PC1 was strongly associated with the perfusion pressure, whereas PC2 showed the differences between each mouse. We identified genes contributing to PC1 that are particularly important for the regulation of renin cells in response to changes in perfusion pressure, including Ren1 (Figure 2E). The gene with the smallest P-value with high perfusion pressure, serine protease inhibitor Kazal-type 8 (Spink8), may inhibit yet undefined proteases that convert inactive prorenin to active renin8. Genes of vacuolar ATPase were significantly higher with high perfusion pressure (Online Figure IV-A, VII). The vacuolar ATPase is associated with (pro)renin receptor9 and related to autophagy10 that may play a role in removing excessive renin in renin cells exposed to high perfusion pressure. Of genes upregulated with low perfusion pressure, stromal cell-derived factor 2-like 1 (Sdf2l1)11 (Online Figure IV-E) and mesencephalic astrocyte-derived neurotrophic factor (Manf)12 are related to endoplasmic reticulum stress, and aldehyde dehydrogenase family 7, member A1 (Aldh7a1) (Online Figure IV-B) is related to osmotic stress13. These changes likely reflect the transformation of renin cells to an endocrine phenotype in response to intracellular stress14. E2F transcription factor 3 (E2f3) (Online Figure IV-F), a member of the E2F family which was increased with low perfusion pressure, may have direct and/or indirect effects on renin expression15.
Gene Ontology (GO) enrichment analysis of biological processes identified several biological pathways related to changes in perfusion pressure (Figure 2F). As expected, pathways associated with BP regulation by the renin-angiotensin system were among the top biological pathways encountered. In addition, pathways for the development and differentiation of muscle cells were detected, which might reflect the phenotypical switching between renin cells and smooth muscle cells of the renin lineage. Consistent with this, smooth muscle cell-related genes, such as vitronectin (Vtn)16 (Online Figure IV-D) and creatine kinase, mitochondrial 1 (Ckmt1)17 were higher in renin cells with high perfusion pressure. Vitronectin is an adhesive glycoprotein of the extracellular matrix (ECM) that promotes cell adhesion, migration, and proliferation through its binding to integrins16. Similarly, talin2 (Tln2), which activates integrins18, and HEPACAM family member 2 (Hepacam2), which is involved in cell-ECM interactions with F-actin19, were increased with high perfusion pressure. Further, the expression of cytoskeletal components was increased in renin cells under high perfusion pressure, raising the possibility that cytoskeletal remodeling20 is an adaptation to changes in perfusion pressure.
The cAMP pathway is crucial for the control and maintenance of renin-expressing cells4. Neuromedin B (Nmb)21 and Vip (vasoactive intestinal polypeptide)22, which increase intracellular cAMP, were upregulated with low perfusion pressure. A gap junction protein, Gja5 encoding connexin 40, which is associated with Ren1 expression23, was significantly higher with low perfusion pressure (Online Table II). These changes support a phenotype switch from contractile to endocrine in response to decreased perfusion pressure.
Overall, we identified novel genes in renin cells that are regulated by changes in perfusion pressure. Renin cells are able to sense mechanical signals and respond transcriptionally by inhibiting or promoting the endocrine phenotype. It would be interesting to examine whether these phenotypic changes are manifested earlier in response to either activation or suppression of the baroreceptor mechanism. It is likely that chromatin events occur rapidly after changes in perfusion pressure and get firmly established with time.
Mechanical stimuli affect Ren1 expression in vitro.
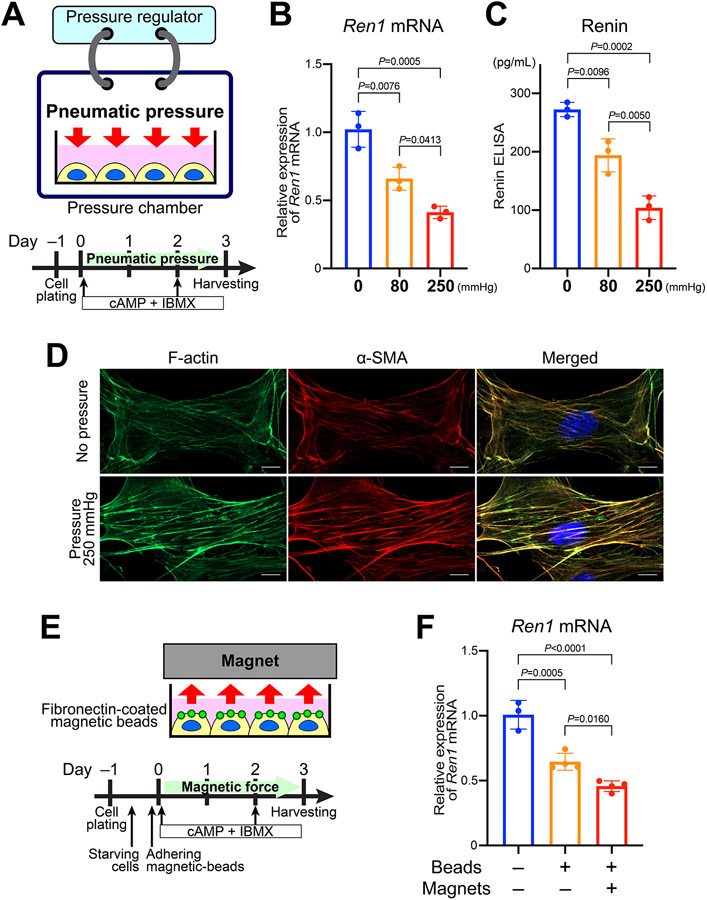
To test the direct effects of changes in pressure or mechanical stimulation on renin cells, we performed novel in vitro experiments using pneumatic chambers that can change the pressure applied to renin cells in culture (Figure 3A). We used our renal afferent arteriolar smooth muscle cell line (CFP/YFP cells) derived from mice where cyan fluorescent protein (CFP) is a renin lineage reporter4 and YFP marks cells that actively express renin24. These cells express Ren1 mRNA when stimulated by cAMP + IBMX, although its expression without stimulation is very low. We continuously applied pneumatic pressure of 80 mmHg and 250 mmHg to the cells and harvested the cells 72 hours after the treatment. By applying the pneumatic pressure to the renin cells, the expression of Ren1 induced by cAMP + IBMX was significantly inhibited in a pressure-dependent manner (Figure 3B). Renin protein secretion also decreased by the application of pressure (Figure 3C). Staining for F-actin and α-smooth muscle actin confirmed a significant increase in the intensity of cytoskeletal actin filament in the cells exposed to pneumatic pressure (Figure 3D). This result suggests that as the cells decrease renin expression, they become more contractile and shift towards a myocrine phenotype in response to mechanical stimuli.
Figure 3. Mechanical stimuli to renin cells in vitro inhibit Ren1 expression.
A, Schematic of the pneumatic pressure model. We used renin cells stimulated by cAMP + IBMX. Cells were harvested after 3 days of stimulation and pressure application. B, Quantitative reverse transcription PCR (qRT-PCR) showed a decrease in Ren1 mRNA expression in a pressure-dependent manner (n=3, one-way ANOVA followed by Tukey’s multiple comparison test). The data are representative of three independent experiments. C, ELISA for renin in the culture medium showed that secretion of renin protein from renin cells also decreased in response to the pneumatic pressure (n=3, one-way ANOVA followed by Tukey’s multiple comparison test). D, F-actin staining by phalloidin and immunocytochemistry for α-smooth muscle actin (α-SMA) showed the increased intensity of actin filaments in cultured renin cells subjected to pneumatic pressure. Scale bar, 10 μm. E, Schematic of the magnetic force model. Fibronectin-coated magnetic beads adhered to the cells of the renin lineage. The renin cell phenotype was induced by cAMP + IBMX, and tensile force was applied by placing permanent magnets above the cells. F, qRT-PCR showed a decrease in Ren1 mRNA expression by applying fibronectin-coated magnetic beads. The Ren1 mRNA expression decreased further when magnets were placed above the cells to apply the force (n≥3, one-way ANOVA followed by Tukey’s multiple comparison test). The data are representative of three independent experiments. All data are reported as means ± standard deviation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
We also tested the influence of a different type of mechanical stimulus on renin cells using a magnetic force (Figure 3E). Fibronectin is the major ligand for integrin β1 which is highly expressed in renin cells25. Taking advantage of this ability of fibronectin to bind integrins, we applied fibronectin-coated magnetic beads to the CFP/YFP cells, which resulted in decreased Ren1 mRNA expression. The expression of Ren1 was further inhibited when we applied tensional force to the cells by placing a permanent magnet above the cells (Figure 3F). Overall, these results indicate that renin cells respond directly to mechanical stimuli with changes in the expression of the renin gene and concomitant changes in renin release.
Operation of the renal baroreceptor is mediated by integrin β1 transduction in response to changes in perfusion pressure into renin cells.
We then investigated the core components of the mechanotransduction mechanism in renin cells which regulate the changes in gene expression by perfusion pressure. The results of RNA-seq and in vitro magnetic force experiments suggested the involvement of integrins. Integrins are a large family of heterodimeric transmembrane glycoproteins that function in both cell-ECM and cell-cell adhesion and are involved in the bi-directional flow of signals from the extracellular environment to the cell interior26. Integrin dimers are composed of distinct α and β subunits. Our RNA-seq data showed that the integrin β1 gene (Itgb1) was the most highly expressed β integrin in renin cells during high, normal, and low perfusion pressure conditions (Online Figure VIII-A). And the integrin αV gene was the highest in the α subfamily (Online Figure VIII-B). By immunoprecipitation with an integrin β1 antibody following Western blot, we found integrin αV and α5 were present in the renin cells, forming heterodimers with integrin β1 (Online Figure VIII-C).
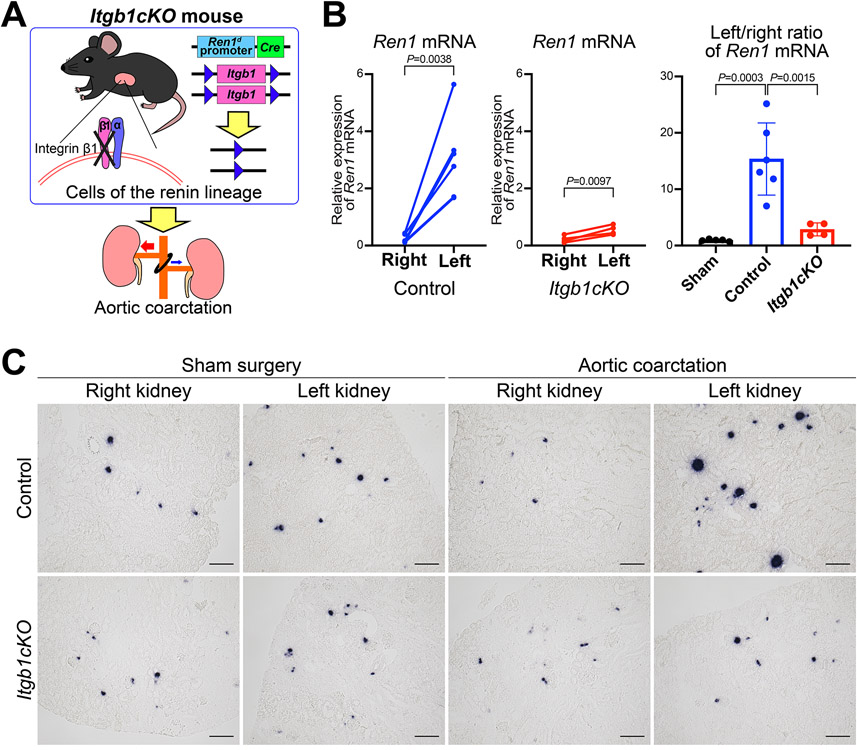
To test whether integrin β1 plays a role in renin cells in response to changes in perfusion pressure, we used a conditional knockout mouse model of integrin β1 in cells of the renin lineage (Itgb1cKO), which we recently generated25 (Figure 4A). We performed AoCo surgeries on Itgb1cKO mice at 4 weeks of age. BP after three days of AoCo in Itgb1cKO mice was significantly lower than in control mice (Online Figure IX). When subjected to AoCo, the decrease in Ren1 mRNA expression (by qRT-PCR) in the right kidneys and the increase in Ren1 mRNA in the left kidneys were significantly blunted in Itgb1cKO mice when compared to controls (Figure 4B). ISH for Ren1 mRNA showed that Ren1 expression did not change in response to the AoCo in the Itgb1cKO mice when compared to control or sham groups (Figure 4C). These data suggest that integrin β1 in renin cells may represent one of the first steps in a mechanotransduction mechanism in response to changes in perfusion pressure, and without β1 integrin, the renin cells cannot respond appropriately to changes in perfusion pressure.
Figure 4. Itgb1 gene knockout in cells of the renin lineage inhibited the response to changes in perfusion pressure.
A, Mice with conditional deletion of the Itgb1 gene in cells of the renin lineage (Itgb1cKO) and control mice were subjected to aortic coarctation (AoCo). B, Itgb1cKO mice showed impaired responses in Ren1 expression to changes in perfusion pressure. Quantitative reverse transcription PCR showed significant differences in Ren1 mRNA between the right and left renal cortices from the control mice (n=6, paired t-test) and from the Itgb1cKO mice (n=4, paired t-test), respectively. The left/right ratio of Ren1 mRNA of Itgb1cKO mice was significantly lower than the one of control animals (one-way ANOVA followed by Tukey’s multiple comparison test). C, in situ hybridization for Ren1 mRNA in the kidneys from Itgb1cKO mice subjected to the AoCo. For Itgb1cKO mice, the decrease in Ren1 mRNA in the right kidneys and the increase in that in the left kidneys were milder than those in control mice, respectively. Scale bar, 100 μm. All data are reported as means ± standard deviation. **P<0.01, ***P<0.001.
Lamin A/C conveys mechanical stimuli to chromatin via a nuclear mechanotransduction mechanism.
The nuclear lamina mediates the transmission of mechanical forces from the cytoskeleton to the nucleus27. Lamins are the major architectural proteins of the nucleus and compose a filamentous protein network underneath the inner membrane that connects to the chromatin28,29. Nuclear lamins are divided into A and B types on the basis of structural and protein features and expression patterns30. The Lmna gene, which encodes lamin A and its splice variant lamin C, is expressed at high levels in renin cells regardless of changes in perfusion pressure, whereas the expression of Lmnb1 and Lmnb2 that encode lamin B is very low (Online Figure X-A). Analyzing the data of our chromatin immunoprecipitation sequencing24, we found that the Lmna gene locus is associated with one of the 91 super-enhancers specific for renin cells, and it ranked in the fifteenth position for H3K27ac enrichment in recruited native renin cells (Online Figure X-B). With ATAC-seq, we found that the Lmna gene locus is epigenetically active, shown by its open chromatin signals in the several physiological stages of the native renin cells (Online Figure X-C). Hence, lamin A/C is prominent in renin cells and may be one of the crucial components of the nuclear mechanotransducer in controlling the expression of the renin gene and the identity and renin cell fate.
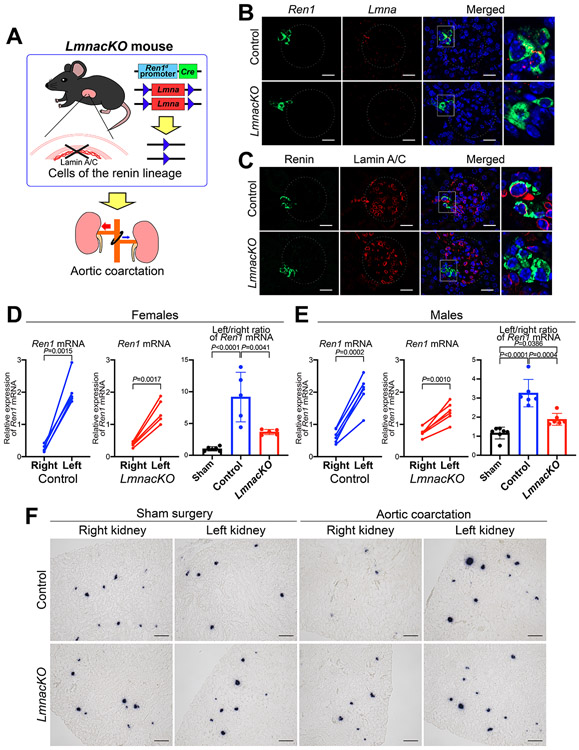
To investigate the role of lamin A/C in renin cells, we generated mice with conditional deletion of the Lmna gene in cells of the renin lineage (LmnacKO) by crossing Ren1dCre mice31 and Lmna floxed mice32 (Figure 5A). We confirmed the deletion of the Lmna gene using fluorescence ISH for Lmna mRNA and Ren1 mRNA (Figure 5B) and immunohistochemistry (Online Figure XI-A) and immunofluorescence staining (Figure 5C) for lamin A/C and renin. Lmna mRNA and lamin A/C were clearly observed in renin expressing cells at JG areas in the control mice, whereas LmnacKO mice did not show signals in renin expressing cells (Figure 5B, C, Online Figure XI-A). LmnacKO mice were born at the expected mendelian ratio, developed normally, and showed no alterations in renal function, renin expression, or kidney morphology under basal conditions (Online Figure XI-B-F). To test how renin cells lacking lamin A/C react to changes in perfusion pressure, we performed AoCo on LmnacKO mice (Figure 5A). AoCo induced significant differences in Ren1 mRNA between the right and left renal cortices in both control and LmnacKO mice. Simultaneous BP measurements from the carotid and femoral arteries in these mice showed significant differences between BPs above and below the coarctation in both the control and LmnacKO mice. However, the pressures were not different between the control and LmnacKO mice (Online Figure XII), suggesting that the kidneys in the LmnacKO mice were exposed to the same BP changes as the control mice. However, the expression changes shown by the left/right ratio of Ren1 mRNA were significantly lower in LmnacKO mice than those of control mice (in both female and male mice) (Figure 5D, E). ISH showed that the decrease in Ren1 mRNA at JG areas in the right kidneys and the increase in the left kidneys were milder in the LmnacKO mice than those of the control mice, respectively (Figure 5F). Taken together, LmnacKO mice showed a diminished ability to respond to changes in perfusion pressure resulting from AoCo, underscoring the role of lamin A/C in transcriptional regulation of the renin gene in response to changes in perfusion pressure.
Figure 5. Lmna gene knockout in cells of the renin lineage inhibited the response to changes in perfusion pressure.
A, Mice with conditional deletion of the Lmna gene in cells of the renin lineage (LmnacKO) and their controls were subjected to aortic coarctation (AoCo). B, Fluorescence in situ hybridization (ISH) for Ren1 mRNA (green), Lmna mRNA (red), and DAPI (blue). Lmna was highly expressed in the renin-expressing cells in the control mice. There was no Lmna expression in renin-expressing cells in LmnacKO mice. Dashed circles indicate glomeruli. Scale bar, 20 μm. C, Immunofluorescence staining for renin (green), lamin A/C (red), and Hoechst (blue). Lamin A/C was detected in the nuclei of renin cells in control mice and absent in the nuclei of renin cells in LmnacKO mice while preserved in non-renin cells. Dashed circles indicate glomeruli. Scale bar, 20 μm. LmnacKO showed impaired responses of Ren1 expression to changes in perfusion pressure in both female (D) and male mice (E). Quantitative reverse transcription PCR showed significant differences in Ren1 mRNA between the right and left renal cortices from control mice (n=5 for females and n=6 for males, paired t-test) and from LmnacKO mice (n=5 for females and n=6 for males, paired t-test). The left/right ratio of Ren1 mRNA from LmnacKO mice was significantly lower than the one from control animals (one-way ANOVA followed by Tukey’s multiple comparison test). F, ISH for Ren1 mRNA in kidneys from LmnacKO mice subjected to the AoCo. In LmnacKO mice, the decrease in Ren1 mRNA in the right kidneys and the increase in the left kidneys were milder than those from the control mice, respectively. Scale bar, 100 μm. All data are reported as means ± standard deviation. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Lamin A/C regulates Ren1 expression in response to mechanical stimuli in vitro.
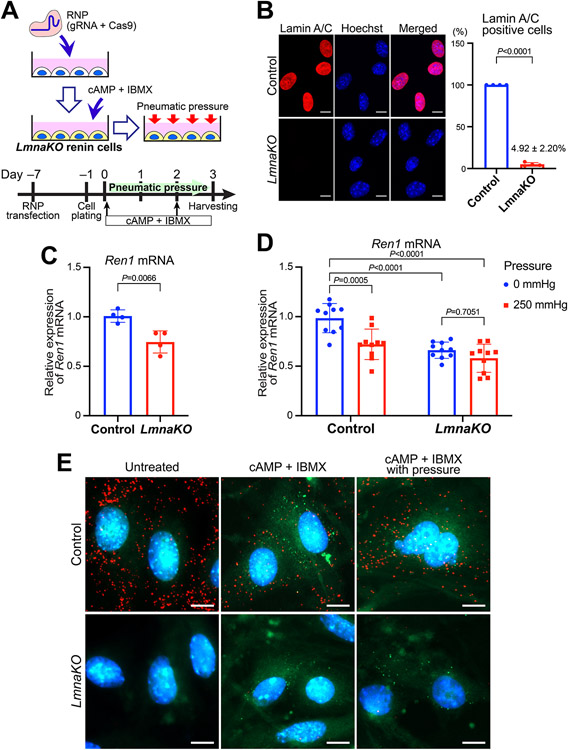
To further investigate the role of lamin A/C in the regulation of Ren1 expression, we knocked out the Lmna gene in the CFP/YFP cells using CRISPR-Cas9 (Figure 6A). We targeted the exon 2 of Lmna to knockout all the transcript variants of the gene (Online Figure XII-A). Following CRISPR-Cas9 ribonucleoproteins transfection, we obtained an efficient knockout of Lmna (LmnaKO), confirmed by PCR and Sanger sequencing of genomic DNA from the cells and qRT-PCR for Lmna mRNA (Online Figure XIII-B-D). By immunocytochemistry, only 4.92 ± 2.20% of the renin cells showed the signals of lamin A/C in the LmnaKO cell pools (Figure 6B), indicating sufficient efficiency of the knockout (~95%) for further analysis.
Figure 6. Lamin A/C regulates the expression of Ren1 in response to mechanical stimuli.
A, Schematic of the knockout of the Lmna gene (LmnaKO) using CRISPR-Cas9. The ribonucleoproteins (RNPs), made with gRNAs for the exon 2 of Lmna and Cas9, were transfected to the renin lineage cells. Seven days after the transfection, cells were stimulated with cAMP + IBMX to regain the renin phenotype. Pneumatic pressure was applied as described above. B, Immunocytochemistry for Lamin A/C to measure the efficiency of the Lmna gene knockout. The percentage of the Lamin A/C positive cells was significantly lower in the LmnaKO renin cells (n=4, Student’s t-test). Scale bar, 10 μm. Averages of three fields per experiment from four independent experiments were used. C, The Ren1 mRNA expression in the LmnaKO renin cells induced by cAMP + IBMX was significantly lower than in control renin cells by quantitative reverse transcription PCR (qRT-PCR) (n=4, Student’s t-test). The data are representative of three independent experiments. D, LmnaKO in cultured renin cells inhibited the decrease in Ren1 expression by the pneumatic pressure. qRT-PCR showed significant reductions of Ren1 mRNA in control renin cells subjected to pneumatic pressure, whereas there was no significant difference in LmnaKO renin cells (n=15, two-way ANOVA followed by Sidak’s multiple comparison test). Data are from three independent experiments with 3–4 replicates per condition. E, Fluorescence in situ hybridization in cultured renin cells for Lmna mRNA (red), Ren1 mRNA (green), and DAPI (blue). The Ren1 expression (shown as green dots) was induced by cAMP + IBMX, and it was lower in LmnaKO renin cells compared to control. Control renin cells showed a decrease in the Ren1 expression by the pneumatic pressure. LmnacKO renin cells did not affect Ren1 mRNA expression in response to pneumatic pressure. Scale bar, 10 μm. All data are reported as means ± standard deviation. **P<0.01, ***P<0.001, ****P<0.0001.
LmnaKO renin cells showed lower expression of Ren1 when compared to the control renin cells upon stimulation with cAMP + IBMX (Figure 6C). The application of pneumatic pressure showed significant reductions of Ren1 mRNA (by qRT-PCR) in control renin cells. In contrast, the LmnaKO renin cells did not show changes in Ren1 expression (Figure 6D). Next, by fluorescence ISH, Ren1 expression was also lower in LmnaKO renin cells compared to controls (Figure 6E). Whereas control renin cells showed a marked decrease in the Ren1 expression in response to pneumatic pressure, in LmnaKO renin cells, the reduction was less pronounced (Figure 6E, Online Figure XIV). These results demonstrate that in vivo and in vitro, lamin A/C plays an essential role in renin cells in the control of Ren1 expression in response to external mechanical stimuli.
Lamin A/C regulates chromatin organization in renin cells.
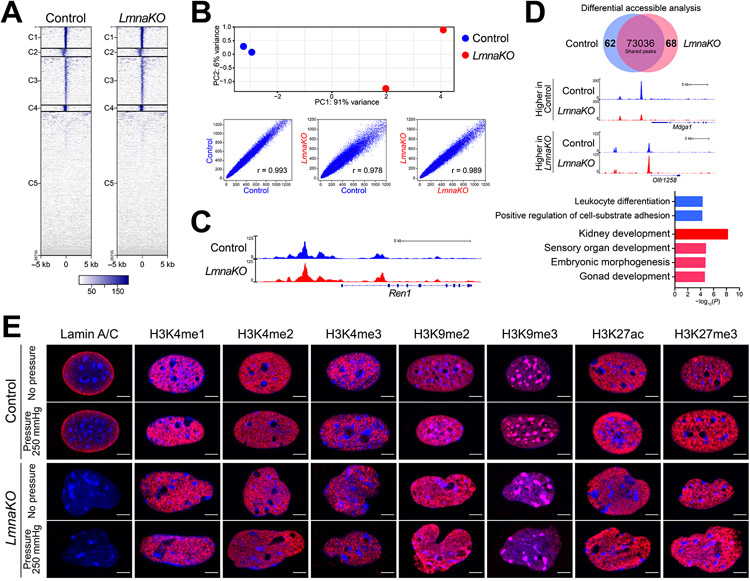
To test the effect of Lmna knockout on chromatin structure, we performed ATAC-seq in the CFP/YFP cells 7 days after the CRISPR-Cas9 reaction. Using 2 replicates of control cells and 2 replicates of LmnaKO cells, we identified 81,394 and 78,833 accessible chromatin regions in control cells and LmnaKO cells, respectively. Control and LmnaKO cells shared a similar overall distribution of accessible chromatin (Figure 7A). However, the PCA of the accessible chromatin loci showed clear separation of control and LmnaKO cells, which was supported by the Pearson correlation coefficient (Figure 7B). Although the cells tested were not stimulated by cAMP +IBMX, the region upstream of the Ren1 gene was open in both cases (Figure 7C). The position of the peaks was the same as previously shown24, and there was no difference between the control and LmnaKO cells at this locus. These results suggest that the Ren1 gene locus in these cells is poised without stimulation and is activated by cAMP with the chromatin open. With differential accessible analysis, we identified 62 and 68 peaks that are significantly different between the control and LmnaKO cells (Online Table V). GO enrichment analysis of biological processes identified that the peaks that were changed by LmnaKO were related to cellular development, including kidney and nephron (Figure 7D). These data indicate that lamin A/C in renin cells interact with chromatin to regulate its organization, and loss of lamin A/C leads to changes in chromatin accessibility with unanticipated changes in developmental fates.
Figure 7. Lamin A/C regulates chromatin organization in renin cells.
A, Heatmaps showing normalized ATAC-seq signal intensity around the transcription start site (TSS) in control cells and cells with deletion of the Lmna gene (LmnaKO). B, LmnaKO cells showed a difference in accessible chromatin compared to controls. Principal component analysis (PCA) with identified ATAC-seq peaks in 2 replicates of each group and the Pearson correlation coefficient clearly separated samples of each group. C, Images of peaks of the accessible chromatin at the Ren1 regulatory locus. D, The differential accessible analysis identified 62 and 68 peaks in the control and LmnaKO cells, respectively. The images show representative loci of the differential accessible regions. Gene Ontology (GO) analysis identified enriched categories on genes associated with peaks significantly higher in control (blue) and higher in LmnaKO (red). E, Immunocytochemistry for lamin A/C, H3K4me1, H3K4me2, H3K4me3, H3K9me2, H3K9me3, H3K27ac, and H3K27me3 shown in red and nuclear staining with Hoechst in blue in renin cells with or without LmnaKO subjected to pneumatic pressure. Scale bar, 5 μm.
The ATAC-seq result suggests that loss of Lmna in renin cells induces partial chromatin structural changes under basal conditions. However, with the current technology, it is not possible to perform ATAC-seq with cells receiving mechanical stimuli, as cells are released from stimulation during the procedure. Therefore, to investigate the role of lamin A/C in chromatin organization in renin cells that are receiving mechanical stimuli, we performed immunocytochemistry for epigenetic marks involved in chromatin configuration on cells immediately fixed after the pneumatic pressure treatment. The LmnaKO renin cells showed dysmorphic nuclei, with unsmooth, irregular nuclear peripheries, compared to the control cells even without pneumatic pressure (Figure 7E). Staining for several histone modification marks showed heterogeneous distributions of the histone marks and heterochromatin. Particularly, histone H3 lysine 9 dimethylated (H3K9me2) chromatin at the nuclear lamina was not distributed equally in the LmnaKO renin cells, consistent with previous reports of human cells with LMNA mutation33. These nuclear abnormalities were enhanced by applying pneumatic pressure (Figure 7E, Online Figure XV). These data indicate that lamina-chromatin interactions are disrupted by LmnaKO, which induces failure to adjust transcription of the genes, including Ren1, appropriately by changes in perfusion pressure.
Taken together, we identified that lamin A/C is essential to maintain the chromatin structure in renin cells, which is essential for nuclear mechanotransduction. Loss of lamin A/C in renin cells prevents their ability to respond appropriately to mechanical stimuli by affecting gene transcription. It is likely that loss of Lmna prevents renin cells from modifying the chromatin in response to stimuli and thus blocks upregulation of renin gene expression.
DISCUSSION
In 1957, Tobian and coworkers postulated the existence of a pressure sensing mechanism that controlled renin release and thus BP homeostasis3. In this tightly regulated mechanism, high perfusion pressure suppresses, whereas low perfusion pressure stimulates renin release from renin cells34-36. The sensing mechanism was denominated the renal “baroreceptor,” and it is believed to regulate both renin secretion and transcription of the renin gene. Both effects, release and transcription are likely to operate in concert, although this needs to be studied in detail. The structure and location of the baroreceptor have remained an enigma for over sixty years37. In this study, we focus our efforts on investigating the effects of mechanical/pressure changes on renin gene expression. We show here that the pressure sensing mechanism resides within the renin cells, and it is a complex mechanotransducer: changes in pressure are sensed by integrin β1, likely dimerizing with αV or α5, and transmitted to the cell’s nucleus via lamin A/C which induces chromatin architectural changes that ultimately control Ren1 expression and the identity of the renin cells (Figure 8).
Figure 8. The nuclear mechanotransduction mechanism in renin cells.
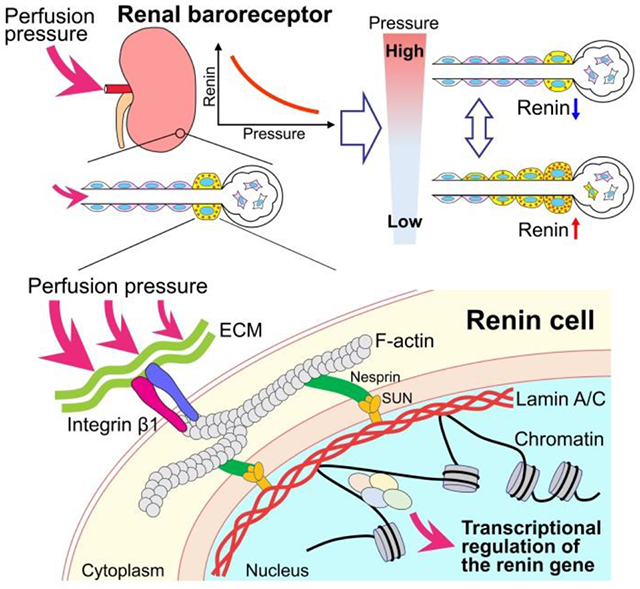
DNA is connected to the extracellular environment through an array of proteins. The perfusion pressure change may be transmitted from the extracellular matrix (ECM) to the cytoskeletal elements by integrin and transduced to the lamin A/C at the inner nuclear membrane in renin cells, likely through F-actin, nesprin, and SUN domains. Lamin A/C may directly interact with chromatin and DNA and induce chromatin architectural changes to regulate the renin gene expression. Thus, changes in perfusion pressure transmit directly to the renin cells and the renin gene via a nuclear mechanotransduction mechanism. Intermediate molecules remain to be identified.
The ability of a cell to respond to mechanical forces is crucial for numerous biological processes. It has become evident in recent years that external mechanical forces induce changes in nuclear structure and composition, chromatin organization, and gene expression28. Although it is difficult to completely recreate the physical conditions of the cells in the afferent arterioles, to study mechanotransduction in renin cells, we used novel in vivo and in vitro approaches. The in vitro experiments we performed have not previously been attempted and represent a first step in understanding how physical stimuli modify the expression of the renin gene and the overall phenotype of the cells. With these in vitro experiments, application of pneumatic pressure and magnetic forces -via fibronectin-coated magnetic beads, we found that expression of the renin gene is controlled by a direct mechanical stimulus. To extend these findings in vivo, we established a surgical model of AoCo between the roots of both right and left renal arteries, which allowed the comparison between cells exposed to high and low perfusion pressure in the same animal, thus eliminating the possibility of spurious changes due to inter-animal variability or genetic background, a clear advantage when compared to traditional rat surgery models38,39.
Several cell surface protein assemblies have been characterized that can sense physical and chemical signals20. In this study, we identified integrin β1 as one of the critical components of sensing and transduction of extracellular mechanical signals into renin cells (Figure 8). Integrins are heterodimeric cell surface receptors ensuring the mechanical connection between cells and the ECM, conveying externally applied forces to the intracellular complex40. It has been shown that forces are transmitted from the cell surface to the nucleus through integrins, the actin cytoskeleton, the LINC complex, and then lamin–chromatin interactions, which in turn regulate chromatin architecture and gene transcription41. Thus, extracellular forces exerted on the nucleus by the cytoskeleton may influence nuclear morphology, chromatin organization, and gene transcription42. Low responsiveness of the renin gene to AoCo in Itgb1cKO mice suggests that an integrin with a β1 subunit is one of the critical sensors in renin cells that perceive changes in perfusion pressure and regulate Ren1 expression.
Mechanotransduction transmits mechanical signals to the nucleus to regulate gene expression43. We found that lamin A/C inside the nuclei is essential to regulate Ren1 expression in renin cells in response to changes in perfusion pressure (Figure 8). Whereas in animals without AoCo, Ren1 expression in LmnacKO mice was not different from controls, the reactivity to AoCo for Ren1 expression was significantly impaired in the LmnacKO mice. Further, in vitro experiments showed a similar effect of lamin A/C on cells exposed to a mechanical stimulus. Together, these results demonstrate the novel finding that lamin A/C in renin cells directly controls Ren1 expression. The nuclear lamina anchors to the inner nuclear membrane, DNA, and chromatin44. Lamins B1 and B2 are usually localized at the peripheral nuclear lamina, where they interact with extended chromatin domains called lamina-associated domains (LADs)20,27. However, LADs are not static, and unlike lamin B which is typically associated with heterochromatin, lamin A/C is associated with gene-rich and transcriptionally active euchromatic regions44,45. Lamin A/C interacts with chromatin through LADs and influences gene expression in a cell type-specific manner46. Hence, it is possible that lamin A/C attaches to specific genomic loci in renin cells and mechanically changes the three-dimensional structure of the chromatin related to Ren1 expression. Our ATAC-seq and immunocytochemistry for several histone marks on LmnaKO cells indicated that the chromatin structure in renin cells is affected by deletion of lamin A/C. Recently, we found that renin cells possess unique chromatin states characterized by open chromatin and the presence of super-enhancers that convey the physiologic status of the organism and are responsible for the transformation of renin cell descendants to the renin phenotype24. These findings suggest that the nuclear mechanotransduction in renin cells induces chromatin architectural changes and formation of the super-enhancers at the renin gene. Further studies will be required to explain how lamin A/C regulates Ren1 expression in renin cells through chromatin modification initiated by changes in perfusion pressure.
In addition to changes in the expression of Ren1, our RNA-seq data from sorted renin cells showed clear differences in the repertoire of genes expressed by renin cells obtained from the right and left kidneys exposed to high or low perfusion pressure, respectively. Interestingly, cells exposed to low perfusion pressure expressed a variety of genes directly related to the endocrine phenotype (Ren1, Akr1b7, Sdf2l1), whereas cells exposed to high perfusion pressure maintained a contractile phenotype (Vtn, Ckmt1) while diminishing their endocrine characteristics (Spink8, Atp6v1g3). We have previously shown that in response to physiological challenges, renin cells possess the innate ability or plasticity to switch phenotypes from a smooth muscle-contractile phenotype to an epitheloid-granular phenotype1. We show here that the memory of the renin phenotype is controlled in vivo and in vitro by a nuclear mechanotransducing mechanism that controls the identity and plasticity of renin cells.
In summary, we elucidated the structure and location of the renal baroreceptor, a pressure sensing and mechanotransducing mechanism in renin cells that conveys mechanical signals directly to the chromatin to control renin expression and renin cell identity to maintain homeostasis.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
The existence of a pressure sensing-mechanism (the baroreceptor) that controls renin release has been postulated for a long time. However, the nature and location of this baroreceptor and how it controls renin expression are unclear.
Renin cells synthesize and release renin to the circulation in response to a decrease in perfusion pressure.
By increased perfusion pressure, renin cells decrease renin release preventing the development of hypertension.
What New Information Does This Article Contribute?
The baroreceptor is located in the renin cell and consists of a nuclear mechanotransducer that controls renin expression.
Changes in perfusion pressure are sensed by integrin β1 and transmitted to the renin cell’s nucleus via lamin A/C, which induces chromatin architectural changes that control the expression of the renin gene.
Renin cells are crucial for survival. They are sensors with the exquisite ability to detect changes in arterial blood pressure and respond to those changes with exact amounts of renin released to the circulation to maintain blood pressure and fluid-electrolyte homeostasis. The location and nature of this unique pressure-sensing structure have remained unknown since it was originally suggested as a “baroreceptor” over sixty years ago. Our studies show that the enigmatic baroreceptor is a novel nuclear mechanotransducer that resides in the renin cells per se and is responsible for the sensing and transmission of extracellular physical forces directly to the chromatin to regulate renin gene expression, circulating renin, and homeostasis.
ACKNOWLEDGMENTS
The authors thank Dr. Yixian Zheng (Carnegie Institution for Science) for kindly providing the Lmna floxed mouse, Alexandre G. Martini for RNA-seq data handling, and Xiuyin Liang, Tiffany J. Southard, Devon L. Farrar, and Fang Xu for excellent technical assistance.
SOURCES OF FUNDING:
This work was supported by National Institutes of Health Grants P50 DK 096373 and R01 DK 116718 (to R. A. G.), and R01 DK 116196, R01 DK 096373, and R01 HL 148044 (to M. L. S. S.-L.), and the Japan Society for the Promotion of Science Overseas Research Fellowships to (H. W.).
Nonstandard Abbreviations and Acronyms:
- AoCo
Aortic coarctation
- ATAC-seq
Assay for Transposase-Accessible Chromatin using sequencing
- BP
Blood pressure
- CFP
Cyan fluorescent protein
- DEGs
Differentially expressed genes
- ECM
Extracellular matrix
- GO
Gene Ontology
- ISH
in situ hybridization
- JG
Juxtaglomerular
- LADs
Lamina-associated domains
- PC
Principal component
- PCA
Principal component analysis (PCA)
- qRT-PCR
Quantitative reverse transcription PCR
- YFP
Yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
DISCLOSURES
None.
REFERENCES
- 1.Gomez RA, Sequeira-Lopez MLS. Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol. 2018;14:231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez RA, Sequeira-Lopez ML. Novel functions of renin precursors in homeostasis and disease. Physiology (Bethesda). 2016;31:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobian L, Tomboulian A, Janecek J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959;38:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pentz ES, Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294:H699–H707. [DOI] [PubMed] [Google Scholar]
- 5.Oka M, Medrano S, Sequeira-López MLS, Gómez RA. Chronic stimulation of renin cells leads to vascular pathology. Hypertension. 2017;70:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vazquez-Alaniz F, Galaviz-Hernandez C, Marchat LA, Salas-Pacheco JM, Chairez-Hernandez I, Guijarro-Bustillos JJ, Mireles-Ordaz A. Comparative expression profiles for KiSS-1 and REN genes in preeclamptic and healthy placental tissues. Eur J Obstet Gynecol Reprod Biol. 2011;159:67–71. [DOI] [PubMed] [Google Scholar]
- 7.Lin EE, Pentz ES, Sequeira-Lopez ML, Gomez RA. Aldo-keto reductase 1b7, a novel marker for renin cells, is regulated by cyclic AMP signaling. Am J Physiol Regul Integr Comp Physiol. 2015;309:R576–R584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. [DOI] [PubMed] [Google Scholar]
- 9.Trepiccione F, Gerber SD, Grahammer F, et al. Renal Atp6ap2/(pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol. 2016;27:3320–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pamarthy S, Kulshrestha A, Katara GK, Beaman KD. The curious case of vacuolar ATPase: regulation of signaling pathways. Mol Cancer. 2018;17:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasako T, Ohsugi M, Kubota N, et al. Hepatic Sdf2l1 controls feeding-induced ER stress and regulates metabolism. Nat Commun. 2019;10:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu S, Di Z, He Y, et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress. J Neuroinflammation. 2019;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JS, Hsu JW, Park SY, et al. ALDH7A1 inhibits the intracellular transport pathways during hypoxia and starvation to promote cellular energy homeostasis. Nat Commun. 2019;10:4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh J, Matkovich SJ, Riek AE, et al. Macrophage secretion of miR-106b-5p causes renin-dependent hypertension. Nat Commun. 2020;11:4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martí-Pàmies I, Cañes L, Alonso J, Rodríguez C, Martínez-González J. The nuclear receptor NOR-1/NR4A3 regulates the multifunctional glycoprotein vitronectin in human vascular smooth muscle cells. FASEB J. 2017;31:4588–4599. [DOI] [PubMed] [Google Scholar]
- 17.Karamat FA, Oudman I, Ris-Stalpers C, Afink GB, Keijser R, Clark JF, van Montfrans GA, Brewster LM. Resistance artery creatine kinase mRNA and blood pressure in humans. Hypertension. 2014;63:68–73. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moh MC, Tian Q, Zhang T, Lee LH, Shen S. The immunoglobulin-like cell adhesion molecule hepaCAM modulates cell adhesion and motility through direct interaction with the actin cytoskeleton. J Cell Physiol. 2009;219:382–391. [DOI] [PubMed] [Google Scholar]
- 20.Uhler C, Shivashankar GV. Regulation of genome organization and gene expression by nuclear mechanotransduction. Nat Rev Mol Cell Biol. 2017;18:717–727. [DOI] [PubMed] [Google Scholar]
- 21.Mo C, Huang L, Cui L, Lv C, Lin D, Song L, Zhu G, Li J, Wang Y. Characterization of NMB, GRP and their receptors (BRS3, NMBR and GRPR) in chickens. J Mol Endocrinol. 2017;59:61–79. [DOI] [PubMed] [Google Scholar]
- 22.An S, Irwin RP, Allen CN, Tsai C, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerl M, Vöckl J, Kurt B, van Veen TA, Kurtz A, Wagner C. Inducible deletion of connexin 40 in adult mice causes hypertension and disrupts pressure control of renin secretion. Kidney Int. 2015;87:557–563. [DOI] [PubMed] [Google Scholar]
- 24.Martinez MF, Medrano S, Brown EA, et al. Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J Clin Invest. 2018;128:4787–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed TH, Watanabe H, Kaur R, Belyea BC, Walker PD, Gomez RA, Sequeira-Lopez MLS. Renin-expressing cells require β1-integrin for survival and for development and maintenance of the renal vasculature. Hypertension. 2020;76:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. [DOI] [PubMed] [Google Scholar]
- 27.Janota CS, Calero-Cuenca FJ, Gomes ER. The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol. 2020;63:204–211. [DOI] [PubMed] [Google Scholar]
- 28.Kirby TJ, Lammerding J. Emerging views of the nucleus as a cellular mechanosensor. Nat Cell Biol. 2018;20:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dittmer TA, Misteli T. The lamin protein family. Genome Biol. 2011;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Zheng Y. Generation and characterization of a conditional deletion allele for Lmna in mice. Biochem Biophys Res Commun. 2013;44:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Termglinchan V, Diecke S, et al. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature. 2019;572:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skinner SL, Mccubbin JW, Page IH. Renal baroreceptor control of acute renin release in normotensive, nephrogenic and neurogenic hypertensive dogs. Circ Res. 1964;15:522–531. [DOI] [PubMed] [Google Scholar]
- 35.Blaine EH, Davis JO, Prewitt RL. Evidence for a renal vascular receptor in control of renin secretion. Am J Physiol. 1971;220:1593–1597. [DOI] [PubMed] [Google Scholar]
- 36.Kirchheim H, Ehmke H, Persson P. Physiology of the renal baroreceptor mechanism of renin release and its role in congestive heart failure. Am J Cardiol. 1988;62:68E–71E. [DOI] [PubMed] [Google Scholar]
- 37.Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor?: the connexin hypothesis. Kidney Int. 2009;75:460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tufro-McReddie A, Chevalier RL, Everett AD, Gomez RA. Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol. 1993;264:R696–R702. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Iturbe B, Quiroz Y, Kim CH, Vaziri ND. Hypertension induced by aortic coarctation above the renal arteries is associated with immune cell infiltration of the kidneys. Am J Hypertens. 2005;18:1449–1456. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann M, Kukkurainen S, Hytönen VP, Wehrle-Haller B. Cell adhesion by integrins. Physiol Rev. 2019;99:1655–1699. [DOI] [PubMed] [Google Scholar]
- 41.Tajik A, Zhang Y, Wei F, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater. 2016;15:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol. 2015;427:695–706. [DOI] [PubMed] [Google Scholar]
- 43.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briand N, Collas P. Laminopathy-causing lamin A mutations reconfigure lamina-associated domains and local spatial chromatin conformation. Nucleus. 2018;9:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gesson K, Rescheneder P, Skoruppa MP, von Haeseler A, Dechat T, Foisner R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 2016;26:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017;169:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are available within the article and its supplementary files. The RNA-seq and ATAC-seq (Assay for Transposase-Accessible Chromatin using sequencing) data sets generated in this study can be accessed at the GEO public repository using the accession number GSE157699 and GSE167522, respectively. R codes are available on Zenodo at https://doi.org/10.5281/zenodo.4672146. Additional information will be available upon request to the corresponding authors.