Abstract
Objectives:
Skeletal muscle loss or sarcopenia is a frequent complication in heart failure (HF) and contributes to adverse clinical outcomes. We evaluated if age (primary) and chronic disease (secondary) related sarcopenia, that we refer to as compound sarcopenia, impacts clinical outcomes in hospitalized patients with HF.
Design:
Cross sectional study using hospitalized patient data.
Setting:
Data from the Agency for Healthcare Research and Quality through the Healthcare Cost and Utilization Project (HCUP).
Participants.
Hospitalized adult patients with a primary or secondary diagnosis of HF (n=64,476) and a concurrent random 2% sample of general medical population (GMP; n=322,217) stratified by age (<50y, 51–65y, >65y) from the Nationwide Inpatient Sample (NIS) database (years 2010–2014).
Measurements.
In-hospital mortality, length of stay (LoS), cost of hospitalization per admission (CoH), comorbidities and discharge disposition, with and without muscle loss phenotype, were analyzed. Muscle loss phenotype was defined using a comprehensive code set from international classification of diseases-9 (ICD-9).
Results:
Muscle loss phenotype was observed in 8,673(13.5%) patients with HF compared to 5,213(1.6%) GMP across all age strata. In patients with HF, muscle loss phenotype was associated with higher mortality, LoS, and CoH. Patients with HF (>65y) and muscle loss phenotype had higher mortality (adjusted OR: 1.81; 95% CI 1.56–2.10), CoH (adjusted OR 1.48; 95% CI 1.44–1.1.52) and LoS (adjusted OR 1.40; 95% CI 1.37–1.43) compared to >65y GMP with muscle loss phenotype.
Conclusion:
Muscle loss phenotype is more commonly associated with increasing age in hospitalized patients with HF. Clinical outcomes were significantly worse in patients with HF age >65y compared to younger patients with HF and all age strata in GMP with and without a muscle loss phenotype.
Keywords: sarcopenia, heart failure, aging, clinical outcomes, cost of stay, inpatient mortality
Introduction.
With increasing population longevity due to enhanced healthcare1 and improved screening and treatment for common chronic diseases, the prevalence of heart failure(HF) continues to increase2 primarily in older adults and in those with comorbidities3. Hospitalization-related healthcare resource utilization is higher in older patients compared to younger patients4–6 and overall, HF represents the most frequent cause of hospitalization in persons >65 years of age(y)7, 8. The higher frequency of comorbidities with aging further worsens clinical outcomes in patients with HF9, with older patients with HF having increased comorbidities compared to younger patients with HF10, 11. Loss of skeletal muscle mass with aging or “primary sarcopenia” is well recognized to adversely affect healthspan12. Skeletal muscle loss that is frequent in patients with chronic diseases including those with HF is termed “secondary sarcopenia” and contributes to increased mortality, morbidity, decreased physical activity and exercise capacity13–15. Thus, in older patients with chronic diseases, primary and secondary sarcopenia are likely to be present together and may be additive, a condition that we have termed “compound sarcopenia”. The prevalence and clinical course of older patients with compound sarcopenia in HF has not been described.
Sarcopenia in HF adversely affects outcome measures including in-hospital mortality, length of stay(LoS), and cost of hospitalization(CoH)14, 16–19. With aging, hospitalization results in functional decline and difficulty in returning to pre-hospitalization activity levels5, 20. Patients with muscle loss phenotype are also less likely to be discharged home and are more likely to require longer stays in skilled nursing facilities (SNF) or inpatient rehabilitation following hospital discharge21. Adverse outcomes in inpatients are more frequent and severe with increasing age22, 23, but whether differences in clinical outcomes are due to greater frequency of muscle loss phenotype in patients with HF compared to the general medical population(GMP), and whether outcomes are different based on the age of patients with HF, with and without muscle loss are not known8. To be inclusive of all patients with sarcopenia or muscle loss phenotype, we used the International Classification of Diseases, Ninth revision(ICD-9) codes to compile various diagnoses for muscle loss21, 24. Age-stratified outcomes of inpatients with a primary or secondary diagnosis of HF with or without muscle loss phenotype were compared to those in a concurrent random sample of GMP to identify differences associated with secondary or compound sarcopenia. No prior studies have examined the effects of compound sarcopenia on outcomes of hospitalization in older patients with HF. We, therefore, evaluated if a muscle loss phenotype is more frequent and associated with worse clinical outcomes in inpatients with HF of different age strata in a large hospital database in the US.
Methods.
The Nationwide Inpatient Sample(NIS) contains deidentified data from nearly 8 million annual hospitalizations from approximately 1,000 US hospitals and is maintained by the Agency for Healthcare Research and Quality (AHRQ). The participating organizations are listed at https://www.hcup-us.ahrq.gov/db/hcupdatapartners.jsp. The available NIS data from January 1, 2010 to December 31, 2014, including primary and secondary diagnoses, patient demographics, in-hospital mortality, LoS, discharge status, CoH, and severity/comorbidity measures, were analyzed. Readmissions and protected health information including specific laboratory values that are not available within the database were not analyzed. All hospital discharges with a primary or secondary ICD-9 code for HF (398.91, 402.11, 402.91, 404.11, 404.13, 404.91, 404.93, 428.xx) with or without a secondary diagnosis of muscle loss phenotype (n=64,476) as previously described16 were included. To be inclusive of all patients with HF, those with a possible diagnosis of secondary HF due to kidney disease were included in the analyses. However, these patients represented less than 5% of the total sample and our interpretation of the data in HF will not be significantly impacted even if we exclude these patients. A concurrent 2% sample population of hospitalized GMP excluding those with HF (n=322,217) with an approximately 5:1 ratio of GMP to patients with HF served as the control population. In a previous study, a similar analysis was conducted21 in which we excluded patients with cirrhosis from the GMP group. The current analysis utilizes a significantly different GMP group which includes patients with cirrhosis and removes those with congestive heart failure. The Elixhauser comorbidity index, a predictor of in-hospital mortality and discharge disposition, was calculated using ICD-9 codes as previously described16, 25. There is no single ICD code that includes all clinical diagnoses for a muscle loss phenotype and a restrictive use of ICD-9 codes does not capture a large number of patients with skeletal muscle loss, the major constituent of malnutrition and physical frailty26. Therefore, a comprehensive list of diagnoses was used to include patients with a muscle loss phenotype as previously reported21, 24. This approach accounts for differing terminologies when evaluating patients with clinically diagnosed muscle loss. “Sarcopenia” was added to the ICD-10 codes (initiated October 2015), but it refers to aging-related muscle loss or myopathies, but not secondary sarcopenia in chronic disease27. All adult (≥18y) hospitalizations were included and were stratified into three categories based on age at admission: <50y, 51–65y and >65y. The cutoff of 65y was chosen to define the oldest stratum as this is generally the age for retirement in the US and is used by the Center for Medicare and Medicaid Services to define the transition to guaranteed healthcare. To specifically determine if within the older population (age 65y), there were differences based on increasing age, we analyzed critical outcomes in patients 66–75y and >75y. We also compared outcomes in systolic (428.21, 428.23) and diastolic HF (428.31, 428.33)28.
For the outcome of discharge disposition, we defined “routine discharge” (patients sent home from the hospital without additional assistance) and “non-routine discharge” (home with home health aide/support, nursing home, rehabilitation or against medical advice)29. Non-routine discharge is primarily recommended for patients with impaired functional status and was considered a suboptimal outcome, as previously reported16, 24. Hospital location and insurance data were collected to evaluate geographic and socioeconomic differences in outcomes and clinical characteristics.
Statistical analysis.
The primary outcome of the current investigation was in-hospital mortality in patients with HF with or without a muscle loss phenotype across the age-defined strata. Other study outcomes included LoS, CoH and discharge disposition for each admission. Variables that were not normally distributed are presented as median values and interquartile range. Qualitative variables were compared using the chi square test. Continuous variables that were normally distributed were compared using the Student t-test. Data that were not normally distributed, Wilcoxon and box-cox analyses were performed. Associations with outcomes were identified using multivariate logistic and linear regression analyses. Model fit was assessed using R2 and C-Index. Two-tailed analyses were performed with an alpha set at 5% and 95% confidence intervals. Covariates known to affect risk for muscle loss phenotype (including sex, race, chronic lung disease, metastatic cancer, acute kidney injury (AKI), coagulopathy, anemia, alcohol abuse, and solid tumors) were chosen a priori based on a review of the literature24, 30, 31 and adjusted for in our multivariate regression model. A linear regression analysis of the Elixhauser comorbidity score as the dependent variable was performed. In the first analysis, age categories and muscle loss phenotype as independent variables for HF and GMP was performed. We then performed a linear regression analysis where HF/GMP were treated as categorical variables and age as a continuous independent variable with Elixhauser as the dependent variable. All analyses were performed using R version 4.0.0 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS 9.4 software (SAS Institute, Cary, NC).
Our study and methods used are in accordance to the Strengthening the Reporting of Observational Studies in Epidemiology(STROBE) guidelines32. The participant flow chart conforms to the CONSORT statement and is shown in Supplementary Figure 1.
Results.
The clinical, demographic characteristics and outcomes for all inpatients (regardless of muscle loss status) stratified by age are shown in Supplementary Table 1. The prevalence of Black race and Hispanic ethnicity was lower in older GMP and patients with HF (Figure 1, Supplementary Figure 2) and there were more female than male inpatients with HF in each age category. The proportion of inpatients with HF 51–65y with a muscle loss phenotype increased from 2010–2014, unlike in the GMP (Supplementary Figure 3,A,B). The mean age of GMP <50y was significantly lower than in inpatients with HF in the same age group. For the older age groups within the GMP, in-hospital mortality (3.3% in GMP >65y vs 8.5% in HF <50y) and muscle loss phenotype (0.5% in GMP >65y vs 12.3% in HF <50y) was lower compared to the <50y group with HF. In-hospital mortality, LoS, CoH, muscle loss phenotype, number of diagnoses on discharge, and Elixhauser scores were significantly higher (p<0.001) in younger patients (both <50y and 51–65y) with HF when compared to older GMP (>65y). Patients with HF compared to GMP for each age stratum and older GMP compared to younger GMP were less likely to undergo routine discharge and were more likely to transfer to a SNF or require home health care. Comorbidity prevalence including alcohol abuse, AKI and diabetes was significantly higher in patients with HF compared to the GMP for each age stratum. Hypertension was significantly higher (p<0.001) in patients with HF <50y and 51–65y compared to similar aged GMP; however, in those >65y, hypertension was less frequent in patients with HF compared to the GMP (Supplementary Table 1). Detailed co-morbidities, insurance type and hospitalization location for patients with HF and GMP regardless of muscle loss phenotype are shown in Supplementary Table 2.
Figure 1.
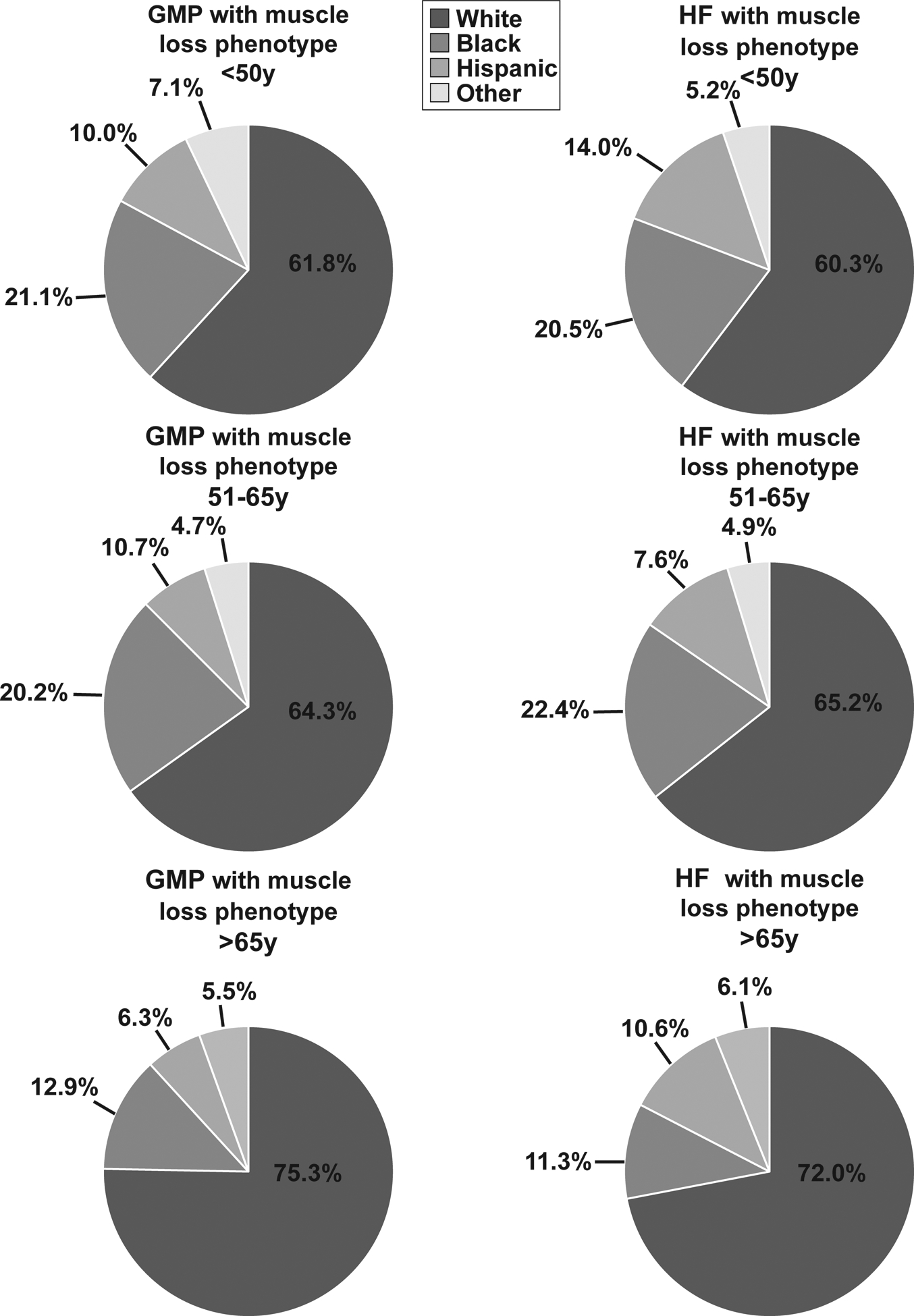
Race and ethnicity proportions among a random 2% inpatient sample of the general medicine population (GMP) and inpatient heart failure patients with muscle loss phenotype from the National Inpatient Sample database stratified by age group.
The clinical and demographic characteristics of GMP and inpatients with HF who had a muscle loss phenotype are shown in Table 1 and Supplementary Table 3. The clinical and demographic information for GMP with and without muscle loss are shown in Supplementary Table 4. Patients with HF with muscle loss phenotype had significantly higher (p<0.01) in-hospital mortality when compared to GMP with muscle loss phenotype in the same age strata. Importantly, in-hospital mortality was significantly higher (p<0.001) in younger patients with HF with muscle loss phenotype compared to all GMP regardless of age and muscle loss status. Patients with HF in the higher age strata had a lower likelihood of routine discharge compared to the younger patients with HF (Table 1). Alcohol use disorder and AKI were significantly more prevalent (p<0.001) in patients with HF with muscle loss than GMP with muscle loss for each comparable age group. Diabetes mellitus (uncomplicated and complicated) was significantly more prevalent in older (>65y) patients with HF with muscle loss compared to similar aged GMP with muscle loss. The prevalence of comorbidities and the composite Elixhauser score were significantly higher (p<0.001) in people with HF with muscle loss than in GMP with muscle loss phenotype in each of the age strata even after adjusting for the presence of HF (Table 1). Additional characteristics including insurance type and geographic distribution of inpatients with muscle loss phenotype are shown in Supplementary Tables 1–6.
Table 1.
Demographic characteristics of hospitalized general medicine population and patients with heart failure with muscle loss phenotype, grouped by age.
| GMP with muscle loss phenotype | HF with muscle loss phenotype | |||||
|---|---|---|---|---|---|---|
| Age categories in years | ≤50 n= 659 |
51–65 n= 1336 |
>65 n= 3218 |
≤50 n= 730 |
51–65 n= 3248 |
>65 n= 4695 |
| Number of Patients | 659 | 1336 | 3,218 | 730 | 3248 | 4,695 |
| Female (%) | 335 (50.8) | 721 (54.0) | 1,500 (46.6) | 418 (57.3) *** | 2,028 (62.4) *** | 2,491 (53.1) *** |
| Age (mean (SD)) | 39.86 (8.50) | 58.60 (4.21) | 78.81 (7.77) | 43.96 (6.34) *** | 58.42 (4.18) | 76.21 (7.04) *** |
| Race (%) | ||||||
| White | 407 (61.8) | 871 (65.2) | 2,423 (75.3) | 440 (60.3) | 2,088 (64.3) | 3,379 (72.0) ** |
| Black | 139 (21.1) | 299 (22.4) | 414 (12.9) | 150 (20.5) | 657 (20.2) | 499 (10.6) ** |
| Hispanic | 66 (10.0) | 101 (7.6) | 203 (6.3) | 102 (14.0) * | 349 (10.7) ** | 529 (11.3) *** |
| Other | 47 (7.1) | 65 (4.9) | 178 (5.5) | 38 (5.2) | 154 (4.7) | 288 (6.1) |
| Length of stay in days (mean (SD)) | 18.09 (19.84)cf | 14.31 (16.70)ci | 10.81 (10.19)fi | 14.54 (19.22) ***bf | 12.83 (13.53) **bi | 11.16 (12.45)fi |
| In-hospital mortality (%) | 40 (6.1)cf | 157 (11.8)c | 372 (11.6)f | 102 (14.0) *** | 484 (14.9) **g | 811 (17.3) ***g |
| CoH in USD (median (IQR)) | 25,329.00 [11,846.00, 56,083.00]cf | 18,619.50 [9,905.25, 40,824.00]ci | 14,088.00 [7,925.25, 26,901.25]fi | 22,278.18 [10,322.53, 45,791.49]cf | 18,245.76 [9,238.09, 38,141.44]ci | 15,425.32 [8,683.92, 29,390.81]fi |
| Number of diagnoses on discharge (mean (SD)) | 21.15 (3.77) | 21.28 (3.73) | 21.07 (3.46) | 21.18 (5.24) | 20.99 (5.36) | 21.02 (5.12) *** |
| Elixhauser score (mean (SD)) | 18.72 (10.63)d | 19.86 (10.89) | 19.82 (10.25)d | 32.05(10.42) *** | 31.96 (10.68) ***g | 32.65 (10.68) ***g |
| Comorbidities (%) | ||||||
| Acute kidney injury | 124 (18.8) | 286 (21.4) | 966 (30.0) | 232 (31.8) *** | 1,159 (35.7) *** | 1,972 (42.0) *** |
| Alcohol abuse | 91 (13.8)f | 204 (15.3)i | 134 (4.2)fi | 297 (40.7) ***cf | 1,121 (34.5) ***ci | 738 (15.7) ***fi |
| Diabetes (uncomplicated) | 101 (15.3)af | 281 (21.0)ag | 791 (24.6)fg | 117 (16.0)cf | 788 (24.3) *ci | 1,380 (29.4) ***fi |
| Diabetes (complicated) | 59 (9.0) | 143 (10.7)i | 213 (6.6)i | 50 (6.8)cd | 398 (12.3)ch | 468 (10.0) ***dh |
| Hypertension | 242 (36.7)cf | 738 (55.2)ci | 2,185 (67.9)fi | 333 (45.6) ***cf | 1,760 (54.2) ***ci | 2,776 (59.1) ***fi |
| Home Discharge | 245 (39.6)cf | 361 (30.7)ci | 431 (15.2)fi | 274 (43.6)cf | 934 (33.9)ci* | 692 (17.9)fi** |
Abbreviations: CoH: Cost of hospitalization per admission, GMP: General medical population, HF: heart failure, LoS: Length of stay, SD: Standard deviation, USD: US dollars.
GMP with muscle loss vs HF with muscle loss, between each age group:
p <0.05,
p <0.01,
p <0.001.
Within disease group, ≤50 vs. 51–65:
p<0.05;
p<0.01;
p<0.001.
Within disease group, ≤50 vs. >65:
p<0.05;
p<0.01;
p<0.001.
Within disease group, 51–65 vs. >65:
p<0.05;
p<0.01;
p<0.001.
A regression analysis of GMP and patients with HF with muscle loss phenotype showed that in-hospital mortality was higher in the patient population with HF with muscle loss in all age groups, but particularly in the <50y group, compared to GMP with muscle loss. LoS was also significantly greater in all age groups (<50y adj OR 1.61, CI: 1.51–1.71; 51–65y adj OR 1.54, CI: 1.50–1.58; >65y adj OR 1.40, CI: 1.37–1.43) (Table 2). Consistent with longer LoS, CoH was also higher for patients with HF with muscle loss.
Table 2.
Regression analysis for hospitalized general medicine and heart failure patients with muscle loss phenotype.
| CHF vs GMP age ≤50 | CHF vs GMP age 51–65 | CHF vs GMP age >65 | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | |
| CoH | 0.86(0.77–0.96) | 1.83 (1.70–1.98) | 0.96(0.90–1.02) | 1.65 (1.59–1.70) | 1.06(1.02–1.11) | 1.48(1.44–1.52) |
| In-hospital mortality | 2.51 (1.73–3.72) | 2.11 (1.35–3.35) | 1.31(1.08–1.60) | 1.48 (1.19–1.84) | 1.60 (1.40–1.82) | 1.81 (1.56–2.10) |
| LoS | 0.81(0.74–0.88) | 1.61 (1.51–1.71) | 0.94(0.89–0.99) | 1.54 (1.50–1.58) | 1.01(0.98–1.04) | 1.40 (1.37–1.43) |
Bold text indicates significance. Abbreviations: CI: Confidence interval; CoH: Cost of hospitalization per admission; GMP: General medical population; LoS: Length of stay; OR: Odds ratio.
Adjusted for sex, race, and comorbidities (acute kidney injury, congestive heart failure, anemia, chronic lung disease, alcohol abuse, coagulopathy, metastatic cancer, cancer with solid tumors, diabetes [uncomplicated], diabetes [complicated]
The clinical and demographic characteristics of inpatients with HF with or without muscle loss phenotype stratified by age are shown in Table 3. There was no difference in the gender distribution of patients with HF based on the presence or absence of muscle loss phenotype in any of the three different age groups. Muscle loss phenotype in patients with HF was associated with significantly higher (p<0.001) in-hospital mortality, LoS, CoH, and number of diagnoses on discharge, and a significantly lower (p<0.001) likelihood of routine discharge (p<0.001) for each age stratum. In-hospital mortality was higher in younger (≤50y) patients with HF with a muscle loss phenotype than in older (>65y) patients with HF without a diagnosis of muscle loss.
Table 3.
Demographic characteristics of hospitalized patients with heart failure with/without muscle loss phenotype, grouped by age.
| HF without muscle loss phenotype | HF with muscle loss phenotype | |||||
|---|---|---|---|---|---|---|
| Age categories in years | ≤50 | 51–65 | >65 | ≤50 | 51–65 | >65 |
| Number of Patients | 5,205 | 22,576 | 28,022 | 730 | 3,248 | 4,695 |
| Female (%) | 3,154 (60.6) | 14,109 (62.5) | 14,519 (51.8) | 418 (57.3) | 2,028 (62.4) | 2,491 (53.1) |
| Age (mean (SD)) | 44.07 (6.19) | 58.39 (4.14) | 75.66 (6.90) | 43.96 (6.34) | 58.42 (4.18) | 76.21 (7.04) *** |
| Race (%) | ||||||
| White | 2,830 (54.4) | 13,944 (61.8) | 20,702 (73.9) | 440 (60.3) ** | 2,088 (64.3) ** | 3,379 (72.0) ** |
| Black | 1,252 (24.1) | 4,642 (20.6) | 2,594 (9.3) | 150 (20.5) * | 657 (20.2) | 499 (10.6) ** |
| Hispanic | 815 (15.7) | 2,926 (13.0) | 3,324 (11.9) | 102 (14.0) | 349 (10.7) *** | 529 (11.3) |
| Other | 308 (5.9) | 1,064 (4.7) | 1,402 (5.0) | 38 (5.2) | 154 (4.7) | 288 (6.1) ** |
| LoS (mean (SD)) | 8.75 (12.32)cf | 8.13 (9.53)ci | 7.69 (8.14)fi | 14.54 (19.22) ***bf | 12.83 (13.53) ***bi | 11.16 (12.45) ***fi |
| In-hospital mortality (%) | 405 (7.8)f | 1805 (8.0)i | 2745 (9.8)fi | 102 (14.0) *** | 484 (14.9) ***g | 811 (17.3) ***g |
| Cost of hospitalization (median (IQR)) | 10,501.11 [5,867.47, 21,261.28]cf | 10,175.32 [5,814.17, 19,472.30]ci | 10,187.96 [5,967.70, 18,184.90]fi | 22,278.18 [10,322.53, 45,791.49] ***cf | 18,245.76 [9,238.09, 38,141.44] ***ci | 15,425.32 [8,683.92, 29,390.81] ***fi |
| Number of diagnoses on discharge (mean (SD)) | 17.60 (5.60)cf | 18.02 (5.46)ci | 18.41 (5.42)fi | 21.18 (5.24) *** | 20.99 (5.36) *** | 21.02 (5.12) *** |
| Elixhauser score (mean (SD)) | 20.33 (10.39)cf | 20.97 (10.24)ci | 22.86 (9.91)fi | 32.05 (10.42) ***cf | 31.96 (10.68) ***ci | 32.65 (10.68) ***fi |
| Comorbidities (%) | ||||||
| Acute kidney injury | 1,634 (31.4) | 7,751 (34.3) | 11,551 (41.2) | 232 (31.8) | 1,159 (35.7) | 1,972 (42.0) |
| Alcohol abuse | 1,746 (33.5)cf | 6,970 (30.9)ci | 4,129 (14.7)fi | 297 (40.7) ***cf | 1121 (34.5) ***ci | 738 (15.7)fi |
| Diabetes (uncomplicated) | 1,301 (25.0)cf | 7852 (34.8)ci | 11,054 (39.4)fi | 117 (16.0) ***cf | 788 (24.3) ***ci | 1,380 (29.4) ***fi |
| Diabetes (complicated) | 498 (9.6)cf | 2,971 (13.2)ci | 3,133 (11.2)fi | 50 (6.8) *cd | 398 (12.3)ch | 468 (10.0) *dh |
| Hypertension | 2,819 (54.2)Cf | 14,256 (63.1)Ci | 18,412 (65.7)fi | 333 (45.6) ***cf | 1,760 (54.2) ***ci | 2,776 (59.1) ***cf |
| Home Discharge | 3,126 (65.1)cf | 11,067 (53.3)ci | 9,019 (35.7)fi | 274 (43.6)cf*** | 934 (33.9)ci*** | 692 (17.9)fi*** |
Abbreviations: CoH: Cost of hospitalization per admission, HF: Heart failure, LoS: Length of stay, USD: US dollars, SD: Standard deviation.
CHF without muscle loss vs CHF with muscle loss, between each age group:
p <0.05,
p <0.01,
p <0.001.
Within phenotype group, ≤50 vs. 51–65:
p<0.05;
p<0.01;
p<0.001.
Within phenotype group, ≤50 vs. >65:
p<0.05;
p<0.01;
p<0.001.
Within phenotype group, 51–65 vs. >65:
p<0.05;
p<0.01;
p<0.001.
A multivariate linear regression model of the Elixhauser comorbidity score comparing age categories and muscle loss phenotype in inpatients with HF (Supplementary Table 7) demonstrated that patients with HF have a significantly higher Elixhauser score in older age categories. Among patients with HF, the adjusted comorbidity score was 0.32 points higher (95% CI 0.08–0.55, p<0.001) for those 51–65y and 1.89 points higher (95% CI 1.66–2.12, p<0.001) for those >65y as compared to the <50y group. In patients with HF and muscle loss phenotype, the Elixhauser score was 9.24 points higher (95% CI 9.07–9.41, p<0.001) than in those without muscle phenotype, even after adjustment for age category. Muscle loss phenotype was similarly associated with a significantly higher Elixhauser comorbidity score in the GMP after adjustment for age category (11.47 points, CI: 11.31–11.62, p<0.001). A multivariate linear regression model comparing the Elixhauser comorbidity scores in those with HF versus GMP in the same age strata showed that patients with HF consistently had higher comorbidity scores. In patients with HF <50y, there was an increase in Elixhauser score by 3.64 points (CI: 3.61–3.67, p<0.001) compared to GMP <50y. In patients with HF in the 51–65y group, there was an increase of 3.65 points (CI: 3.64–3.67, p<0.001) compared to GMP 51–65y, and in patients with HF >65y, there was a 3.67 point increased score compared to GMP >65y (CI: 3.66–3.69, p<0.001) (Supplementary Table 7).
We then compared critical outcomes between patients 66–75y and >75y (Supplementary Table 8). There was increased risk for inpatient mortality, greater LoS and CoH, and a higher Elixhauser co-morbidity index in patients with HF with muscle loss phenotype in both age groups; however, the highest risk for these adverse events was identified in patients with HF and muscle loss >75y. We also observed that there was no significant difference in inpatient mortality, LoS, CoH and Elixhauser co-morbidity index whether patients had systolic or diastolic HF. However, patients >65y with systolic HF were at higher risk for mortality compared to those with diastolic HF (Supplementary Table 9–10).
Discussion.
In this large, national cross-sectional analysis, the prevalence of muscle loss phenotype was higher with increasing age strata among hospitalized patients and was more frequent in patients with HF compared to GMP. Our analyses show an increase over time in hospitalization of patients with HF >50y and that these patients have more frequent muscle loss phenotype than those with HF or GMP <50y. Even though muscle loss phenotype was associated with greater inpatient mortality, LoS, and CoH in both GMP and patients with HF, each outcome was worse in the comparable group in the population with HF than in the GMP.
Sarcopenia in HF is a consequence of complex multifactorial pathophysiologic perturbations. Local and systemic factors contribute to progressive muscle loss in these patients that include decreased oral intake, malabsorption, decreased physical activity, systemic inflammation, oxidative stress, apoptosis, and dysregulated protein homeostasis33. Even though it is challenging to dissect the systemic and local factors that contribute to the molecular and metabolic perturbations in the muscle, identifying shared and unique perturbations for primary age-related and secondary disease-related sarcopenia are important areas of future research.
The increased proportion of older patients with HF was expected because HF is a leading cause of hospitalization in individuals >65y7, 8. However, to date there are no published data examining age-stratified compound sarcopenia or muscle loss phenotype in patients with HF compared to GMP. Primary sarcopenia occurs with aging and our data showed an expected increase in muscle loss phenotype prevalence in older age strata, demonstrating the validity of these analyses. The current data are also consistent with previous analyses showing that sarcopenia was more prevalent in patients with HF compared to GMP13–15, and demonstrates the contributions of secondary sarcopenia in these patients. Overall, secondary sarcopenia contributes to and worsens the clinical consequences of primary, age-associated sarcopenia. The synergistic nature of these muscle loss mechanisms results in a higher proportion of and worse clinical outcomes due to compound sarcopenia in HF compared to other inpatients.
In the present study, patients with HF >65y had higher inpatient mortality, LoS, CoH and comorbidities than patients with HF who were younger (<51y) and age strata matched GMP. Potential reasons for worse outcomes in older patients with HF include greater noncardiac comorbidities like renal failure, diabetes, chronic obstructive pulmonary disease (COPD), and other lower respiratory diseases34. Consistently, older patients with HF had more comorbidities than younger patients with HF. Interestingly, both GMP and patients with HF with a muscle loss phenotype had more comorbidities than those without muscle loss. These data suggest that age, muscle loss, and in HF patients, compound sarcopenia, adversely affect clinical outcomes.
In addition to the combined effects of aging and disease, our observations also show that hospitalized younger patients with HF had clinical outcomes similar to those of older GMP. Younger patients with HF had clinical outcomes similar to those in older GMP, an observation that suggest mechanisms mirroring a form of early aging35, 36. This is supported by our observations that patients with HF in each age stratum had a higher proportion of muscle loss phenotype compared to similar age strata among GMP, reiterating the importance of identifying the mechanisms of secondary sarcopenia in HF. However, within each age stratum, GMP with muscle loss tended to be younger compared to patients with HF and the number of comorbidities was also lower in GMP. Alcohol abuse was higher in patients with HF, which may represent a potential confounding factor for outcome measures, although our regression analyses adjusted for a history of alcohol abuse. Others have reported that patients with HF not only have less muscle mass but also that the muscle is dysfunctional37, 38, which results in physical frailty and places them at higher risk for poor clinical outcomes37, 39.
Our observations that comorbidities are more frequent in GMP and patients with HF with a muscle loss phenotype are consistent with and extend the findings of previous reports that sarcopenic patients with HF commonly exhibit other complications of their disease40. The present association studies do not identify whether the greater prevalence of comorbidities contributes to the muscle loss phenotype or if muscle loss phenotype leads to more comorbidities in patients, highlighting the need for large, multicenter, prospective studies (Figure 2). Recent studies have also shown that older inpatients with decompensated HF have severe limitations in physical function that are likely due to sarcopenia41. Further delineation of the disease-related muscle loss phenotype in older patients (compound sarcopenia) is likely to help improve the clinical management of HF. This understanding could be critical for optimizing patient treatment and rehabilitative strategies in this population. Currently, there are multiple ongoing intervention studies including pharmacotherapy to treat sarcopenia42, 43 and innovative rehabilitation strategies44. However, these interventions are intended to treat primary sarcopenia and their efficacy in secondary/compound sarcopenia is yet to be established. To date, the major issues that interfere with our ability to target primary, secondary, and compound sarcopenia include difficulties in defining the broad effects of muscle loss and thus identifying it reliably in patients14, 45, 46. Due to the continued increase in the population of older patients with HF, the impact of a muscle loss phenotype is likely to have a greater effect on long-term clinical outcomes because sarcopenia impacts the ability to exercise and perform physical activity13, 14. Cardiac rehabilitation programs aim to limit the functional consequences of HF, including muscle loss phenotype, and to reduce the risk for future hospitalizations47. Importantly, compound sarcopenia can limit benefits of cardiac rehabilitation14. Furthermore, our estimate of patients with sarcopenia, which uses ICD-9 codes and administrative data, likely significantly undercounts the true prevalence of secondary sarcopenia in HF, emphasizing the clinical significance of compound sarcopenia in these patients. Even though anorexia, a common symptom in patients with HF48, also contributes to muscle loss, ICD9 codes for anorexia are not a direct measure of muscle mass or function. We therefore, did not use these codes for defining a muscle loss phenotype. Another limitation is that muscle mass or body composition were not directly quantified. Thus, our data lay the foundation for future, prospective studies to directly measure muscle mass with imaging measures and contractile function in older patients hospitalized with HF and develop therapeutic approaches to improve clinical outcomes and increase the efficiency of health care resource utilization.
Figure 2.
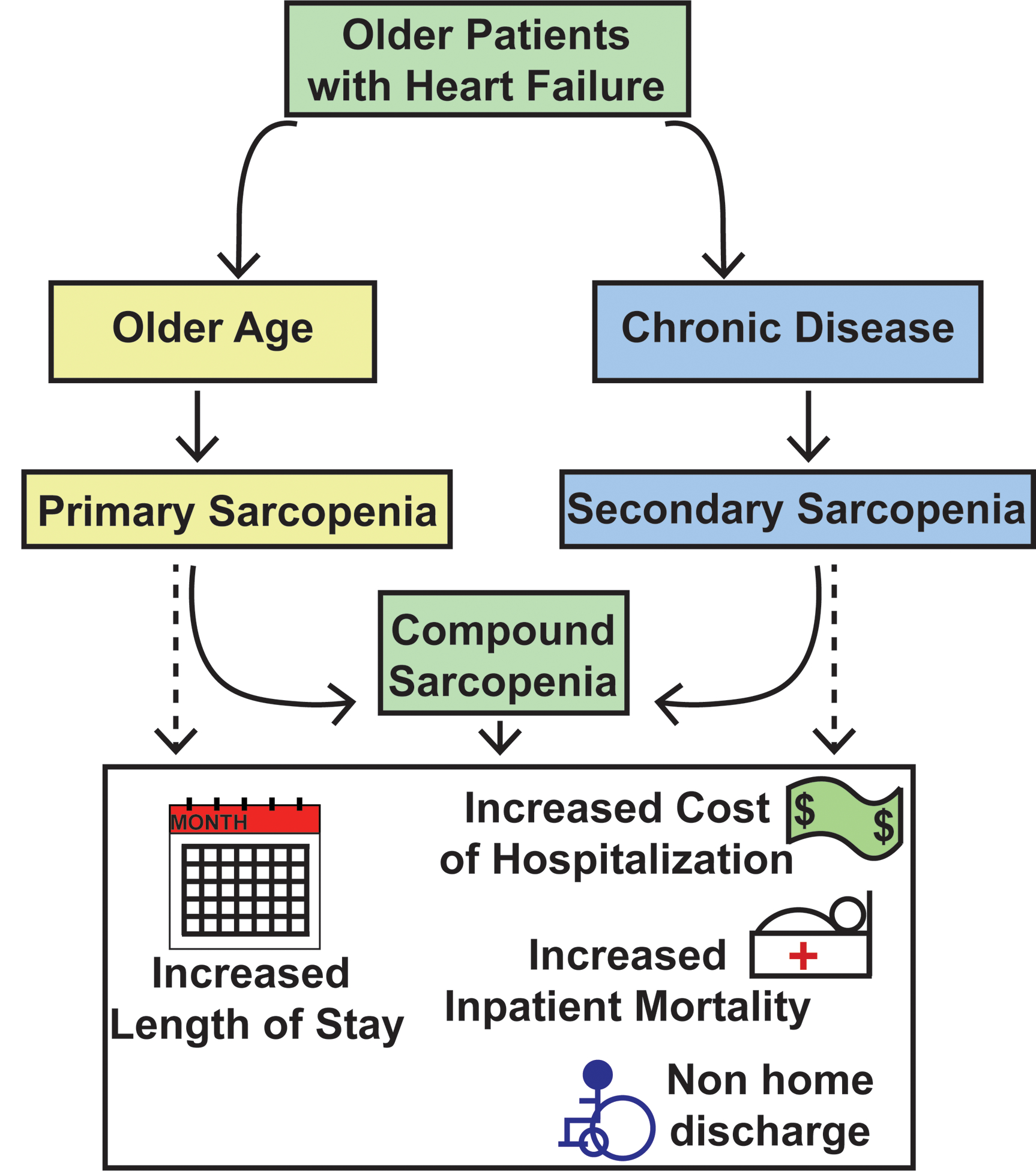
Graphical abstract of the risk factors for sarcopenia and associated outcomes in older patients with heart failure
In conclusion, the presence of a muscle loss phenotype is more frequent with increasing age and is associated with worse clinical outcomes in hospitalized patients but to a greater extent in those with HF. Higher rates of muscle loss are present even in young patients with HF compared to older GMP. Our observations demonstrate the high clinical significance of disease-initiated (i.e. secondary) sarcopenia in addition to primary (i.e. age-related) sarcopenia in hospitalized patients. A better understanding of this combination of primary and secondary, or, compound sarcopenia, will have a direct impact on clinical management as well as resource allocation to identify the mechanisms of compound sarcopenia and serve to inform future exercise and nutrition interventions in these patients.
Supplementary Material
Supplementary Figure 1. CONSORT diagram patient flow chart
Supplementary Figure 2. Race and ethnicity proportions among a random 2% inpatient sample of the general medicine population (GMP) and inpatient heart failure patients from the National Inpatient Sample database stratified by age group.
Supplementary Fig 3A and B. Proportion of GMP and HF population with and without muscle loss phenotype stratified by age group.
Supplementary Table 1. Demographic characteristics for all hospitalized general medicine population and patients with heart failure, grouped by age regardless of muscle loss status.
Supplementary Table 2. Comorbidities, insurance type and geographic distribution of hospitalized general medical and heart failure patients stratified by age.
Supplementary Table 3. Comorbidities, insurance type and geographic distribution of hospitalized general medical and heart failure patients with muscle loss stratified by age.
Supplementary Table 4. Demographic characteristics of hospitalized general medicine patients with and without muscle loss phenotype grouped by age.
Supplementary Table 5. Comorbidities, insurance type and geographic distribution of general medical patients with and without muscle loss phenotype stratified by age.
Supplementary Table 6. Comorbidities, insurance type and geographic distribution of hospitalized heart failure patients with and without muscle loss phenotype stratified by age.
Supplementary Table 7. Linear regression analysis for hospitalized general medicine and HF patients with Elixhauser comorbidity score as the dependent variable. Linear regression analysis comparing hospitalized general medicine and HF patients across age strata with Elixhauser comorbidity score as the dependent variable.
Supplementary Table 8. Regression analysis for hospitalized general medicine and heart failure patients with muscle loss phenotype.
Supplementary Table 9. Sub analysis of patients with Systolic Heart Failure versus Diastolic Heart Failure analyzing comorbidities, insurance type and geographic distribution of hospitalized heart failure patients with and without muscle loss phenotype stratified by age.
Supplementary Table 10. Regression analysis for hospitalized general medicine and heart failure patients with muscle loss phenotype comparing systolic versus diastolic heart failure.
Key points.
Our study is important because heart failure is a frequent cause of hospitalization in older patients.
Primary sarcopenia (muscle loss) with aging compounds secondary sarcopenia of heart failure in older patients.
Compound sarcopenia in older hospitalized heart failure patients worsens clinical outcomes.
Why does this paper matter:
These data show the increase in hospitalized older patients with heart failure with increased health care and post-acute care resource utilization. These data that will lay the foundation for developing public health policies to improve clinical outcomes in older patients with heart failure.
Acknowledgements
Financial -
R21 AR 071046; RO1 GM119174; RO1 DK113196; P50 AA024333; RO1 AA021890; 3U01AA026976 - 03S1; UO1 AA 026976; R56HL141744;UO1 DK061732; 5U01DK062470-17S2 (SD); K12 HL141952 (NW, AA) and the American College of Gastroenterology Clinical Research Award (NW).
Sponsor’s Role-
the sponsor played no role in the design, methods, subject recruitment, data collections, analysis or preparation of this paper.
Footnotes
Conflicts of Interest –
The other authors have no financial conflicts of interest. Personal – The authors have no personal conflicts of interest. Potential Conflicts- the authors have no other potential conflicts of interest.
References
- [1].Ortman JM VV, Hogan H,. An Aging Nation: The Older Population in the United States, Current Population Reports. US Census Bureau. 2014. [Google Scholar]
- [2].Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bosch L, Assmann P, de Grauw WJC, Schalk BWM, Biermans MCJ. Heart failure in primary care: prevalence related to age and comorbidity. Prim Health Care Res Dev. 2019;20: e79–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Antunes AC, Araujo DA, Verissimo MT, Amaral TF. Sarcopenia and hospitalisation costs in older adults: a cross-sectional study. Nutr Diet. 2017;74: 46–50. [DOI] [PubMed] [Google Scholar]
- [5].Hazra NC, Rudisill C, Gulliford MC. Determinants of health care costs in the senior elderly: age, comorbidity, impairment, or proximity to death? Eur J Health Econ. 2018;19: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alemayehu B, Warner KE. The lifetime distribution of health care costs. Health Serv Res. 2004;39: 627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schmidt M, Ulrichsen SP, Pedersen L, Botker HE, Sorensen HT. Thirty-year trends in heart failure hospitalization and mortality rates and the prognostic impact of co-morbidity: a Danish nationwide cohort study. Eur J Heart Fail. 2016;18: 490–499. [DOI] [PubMed] [Google Scholar]
- [8].Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saczynski JS, Go AS, Magid DJ, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013;61: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mohammed SF, Borlaug BA, Roger VL, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5: 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Adams V, Doring C, Schuler G. Impact of physical exercise on alterations in the skeletal muscle in patients with chronic heart failure. Front Biosci. 2008;13: 302–311. [DOI] [PubMed] [Google Scholar]
- [14].Springer J, Springer JI, Anker SD. Muscle wasting and sarcopenia in heart failure and beyond: update 2017. ESC Heart Fail. 2017;4: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fulster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34: 512–519. [DOI] [PubMed] [Google Scholar]
- [16].Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- [17].Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10: 374–379. [DOI] [PubMed] [Google Scholar]
- [18].Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wright SP, Verouhis D, Gamble G, Swedberg K, Sharpe N, Doughty RN. Factors influencing the length of hospital stay of patients with heart failure. Eur J Heart Fail. 2003;5: 201–209. [DOI] [PubMed] [Google Scholar]
- [20].Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51: 451–458. [DOI] [PubMed] [Google Scholar]
- [21].Vural A, Attaway A, Welch N, Zein J, Dasarathy S. Skeletal muscle loss phenotype in cirrhosis: A nationwide analysis of hospitalized patients. Clin Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mudge AM, McRae P, Hubbard RE, et al. Hospital-Associated Complications of Older People: A Proposed Multicomponent Outcome for Acute Care. J Am Geriatr Soc. 2019;67: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Szlejf C, Farfel JM, Curiati JA, Couto Ede B Jr., Jacob-Filho W, Azevedo RS. Medical adverse events in elderly hospitalized patients: a prospective study. Clinics (Sao Paulo). 2012;67: 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Attaway AH, Welch N, Hatipoglu U, Zein JG, Dasarathy S. Muscle loss contributes to higher morbidity and mortality in COPD: An analysis of national trends. Respirology. 2020. [DOI] [PubMed] [Google Scholar]
- [25].Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472: 2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of Cachexia among Cancer Patients Based on Four Definitions. J Oncol. 2009;2009: 693458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7: 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goyal P, Almarzooq ZI, Horn EM, et al. Characteristics of Hospitalizations for Heart Failure with Preserved Ejection Fraction. Am J Med. 2016;129: 635.e615–626. [DOI] [PubMed] [Google Scholar]
- [29].Project HCaU. NIS Description of Data Elements. 2020.
- [30].von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12: 1500–1524. [DOI] [PubMed] [Google Scholar]
- [33].Lena A, Anker MS, Springer J. Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science. Int J Mol Sci. 2020;21: 6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42: 1226–1233. [DOI] [PubMed] [Google Scholar]
- [35].Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol. 2019;74: 313–319. [DOI] [PubMed] [Google Scholar]
- [36].Shinmura K Cardiac Senescence, Heart Failure, and Frailty: A Triangle in Elderly People. Keio J Med. 2016;65: 25–32. [DOI] [PubMed] [Google Scholar]
- [37].Kitzman DW, Haykowsky MJ, Tomczak CR. Making the Case for Skeletal Muscle Myopathy and Its Contribution to Exercise Intolerance in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc. 2017;65: 2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lena A, Anker MS, Springer J. Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Reeves GR, Whellan DJ, Patel MJ, et al. Comparison of Frequency of Frailty and Severely Impaired Physical Function in Patients ≥60 Years Hospitalized With Acute Decompensated Heart Failure Versus Chronic Stable Heart Failure With Reduced and Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2016;117: 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kwak JY, Kwon KS. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann Geriatr Med Res. 2019;23: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morley JE. Pharmacologic Options for the Treatment of Sarcopenia. Calcif Tissue Int. 2016;98: 319–333. [DOI] [PubMed] [Google Scholar]
- [44].Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation Therapy in Older Acute Heart Failure Patients (REHAB-HF) trial: Design and rationale. Am Heart J. 2017;185: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Scherbakov N, Doehner W. Searching for a relevant definition of sarcopenia: results from the cross-sectional EPIDOS study. J Cachexia Sarcopenia Muscle. 2016;7: 100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Drescher C, Konishi M, Ebner N, Springer J. Loss of muscle mass: current developments in cachexia and sarcopenia focused on biomarkers and treatment. J Cachexia Sarcopenia Muscle. 2015;6: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Volterrani M, Iellamo F. Cardiac Rehabilitation in Patients With Heart Failure: New Perspectives in Exercise Training. Card Fail Rev. 2016;2: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Saitoh M, Dos Santos MR, Emami A, et al. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). ESC Heart Fail. 2017;4: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. CONSORT diagram patient flow chart
Supplementary Figure 2. Race and ethnicity proportions among a random 2% inpatient sample of the general medicine population (GMP) and inpatient heart failure patients from the National Inpatient Sample database stratified by age group.
Supplementary Fig 3A and B. Proportion of GMP and HF population with and without muscle loss phenotype stratified by age group.
Supplementary Table 1. Demographic characteristics for all hospitalized general medicine population and patients with heart failure, grouped by age regardless of muscle loss status.
Supplementary Table 2. Comorbidities, insurance type and geographic distribution of hospitalized general medical and heart failure patients stratified by age.
Supplementary Table 3. Comorbidities, insurance type and geographic distribution of hospitalized general medical and heart failure patients with muscle loss stratified by age.
Supplementary Table 4. Demographic characteristics of hospitalized general medicine patients with and without muscle loss phenotype grouped by age.
Supplementary Table 5. Comorbidities, insurance type and geographic distribution of general medical patients with and without muscle loss phenotype stratified by age.
Supplementary Table 6. Comorbidities, insurance type and geographic distribution of hospitalized heart failure patients with and without muscle loss phenotype stratified by age.
Supplementary Table 7. Linear regression analysis for hospitalized general medicine and HF patients with Elixhauser comorbidity score as the dependent variable. Linear regression analysis comparing hospitalized general medicine and HF patients across age strata with Elixhauser comorbidity score as the dependent variable.
Supplementary Table 8. Regression analysis for hospitalized general medicine and heart failure patients with muscle loss phenotype.
Supplementary Table 9. Sub analysis of patients with Systolic Heart Failure versus Diastolic Heart Failure analyzing comorbidities, insurance type and geographic distribution of hospitalized heart failure patients with and without muscle loss phenotype stratified by age.
Supplementary Table 10. Regression analysis for hospitalized general medicine and heart failure patients with muscle loss phenotype comparing systolic versus diastolic heart failure.


