Abstract
Nanomaterial-based drug delivery systems (DDSs) increase the efficacy of various therapeutics, and shear stress has been shown to be a robust modulator of payload release. In the past few decades, a deeper understanding has been gained on the effects of flow in the body and its alteration in pathological microenvironments. More recently, shear-responsive nanomaterial DDSs have been developed. Here we review studies on this subject mainly from the last decade, focusing on innovations of the material design and mechanisms of the shear response. The two most popular shear-controlled drug carriers distinguished by different release mechanisms, i.e. shear-deformable nanoparticles (NPs) and shear-dissociated nanoparticle aggregates (NPAs) are surveyed. The influence of material structures on their properties such as drug loading, circulation time and shear sensitivity are discussed. We further inspect the drug development stages, therapeutic effects, limitations and potential of these DDSs. The reviewed research emphasizes the advantages and significance of nanomaterial-based shear-sensitive DDSs in the field of targeted drug delivery. We also believe that efforts to rationally design nanomaterial DDSs responsive to shear may prompt a new class of diagnostics and therapeutics for signaling and rectifying pathological flows in the body.
Keywords: drug delivery systems (DDSs), shear-sensitive, advanced nanomaterials, deformable nanoparticles, nanoparticle aggregates
Graphical Abstract
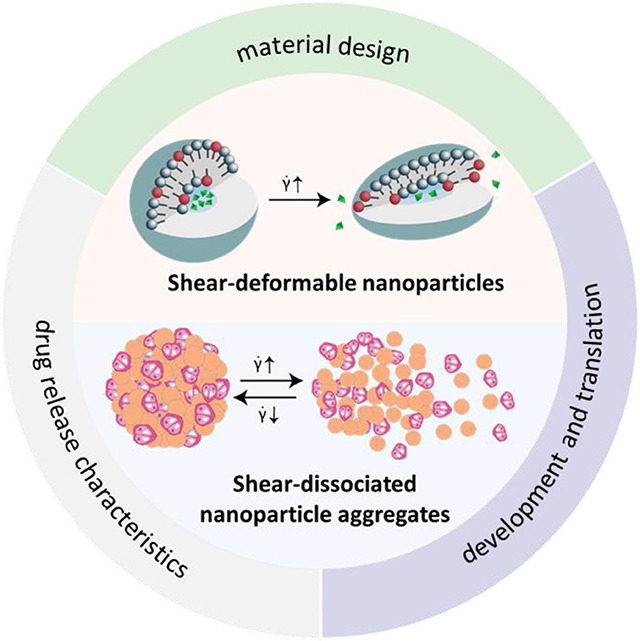
Recent developments of shear-responsive nanomaterial drug delivery systems (DDSs) are reviewed in this paper. We focused on the innovation of the material design and shear-response mechanism, and reviewed drug release characteristics such as loading capacity, circulation time and shear sensitivity. The stages of development, therapeutic effects as well as the limitations and potentials of these DDS are also discussed.
1. Introduction
Nanomaterial-based drug delivery systems (DDSs) have been under rapid development over the past decade and have been shown to greatly improve the targeting precision and efficiency of various therapeutics. These nanomaterials, including liposomes, polymeric nanoparticles (NPs), nanogels and functionalized hydrogels etc., are engineered to exhibit unique structural, physical and chemical properties for desirable biological responses.[1–6] In particular, stimuli-responsive nanomedicines are designed to deliver drugs under environmental triggers. While efforts have been conventionally focused on nanomaterials responsive to chemical and biological cues, increasing interest has been generated in nanomedicines sensitive to mechanical stimulations, owing to recent progress in mechanobiology and better understanding of the mechanical properties of normal versus pathological tissues.[1, 7–9]
One type of mechanical force studied widely in the realm of nanotherapeutics is shear stress. As one of the fundamental forces associated with blood flow, shear stress exists and plays an important role in regulating biomolecular processes such as platelet aggregation and endothelial cell function. Blood flow is often modeled as laminar flow, in which fluid particles follow smooth paths in layers, i.e. laminae, with little or no mixing between adjacent layers. Shear stress is defined as the friction force per unit area between laminae. [10–12] As it pertains to fluid flow, shear stress is intrinsic to the cardiovascular system, and definitive changes in the shear magnitude and pattern are often manifested in bleeding and cardiovascular diseases.[13] These changes have conventionally served as diagnostic markers and have recently been researched as stimuli to trigger drug delivery. Physiological blood flow normally generates a shear stress of 10–70 dyn/cm2 in arteries and 1–6 dyn/cm2 in veins. On the other hand, shear stress could increase up to 1,000 dyn/cm2 as a result of hemorrhages and cardiovascular pathologies and is often accompanied by turbulent flow.[14, 15] These high shear sites have drawn increasing research attention in recent years. For example, studies have shown that at sites of injured blood vessels, high shear activates the blood clotting factor Von Willebrand Factor (VWF) and triggers platelet aggregation, which leads to rapid arterial occlusion.[16,17] It has also been reported that high shear is related to cardiovascular diseases such as stenosis, thrombosis and intraplaque hemorrhage.[18,19] Furthermore, vascular narrowing has been found as a common feature for a number of fatal diseases such as atherosclerosis, stroke, acute coronary syndrome and myocardial infarction.[20–22] Thus, hemodynamic shear stress related to constrained vessels has been identified as an important pathological condition. A number of nanomaterial-based DDSs have therefore been developed to act in altered shear environments and target pathological conditions. Compared to conventional systems, shear controlled nano-DDSs offer the potential of high efficiency, low possible side effects and ease of modeling, etc.[23–25] Their development has been reviewed in previous papers.[23, 26] In addition to blood flow, the application and reversal of high shear during drug injection have been capitalized by shear-thinning gels to deliver scaffolds and pharmaceuticals to specific tissues and organs. These materials have reversible phase transformations in response to shear and have utility in localized drug delivery and tissue engineering.[23,26]
Shear-sensitive DDSs are increasingly researched given their significant potential in treating cardiovascular diseases and hemorrhages. While a few other physical stimuli such as light, heat and ultrasound have been proposed or implemented for treating these conditions, shear stress as a mechanical trigger has the advantage of being endogenous and site-specific to the bleeding or pathological location.[27–29] This review provides a critical literature survey of research from the past decade on shear-triggered nanocarriers for drug delivery. This review will primarily focus on the formulations of the nano systems and their shear response mechanisms, as opposed to previous reviews which focus more on the physiological and translational aspects of shear-sensitive DDSs.[22,23,26,30] While these materials has been researched for a few decades, earlier work mostly focused on characterizing the shear sensitivity of nanomaterial DDSs originally designed for purposes other than the shear response. Two seminal works in 2012, describing shear-deformable NPs based on lenticular liposomes and shear-dissociated NPAs respectively, led to nanomaterials engineered specifically for applications of shear-triggered release.[14,31] Inspired by these two types of nanomaterials, recent progress has been made to tune shear sensitivity to specific applications. Accordingly, the literature in this review is grouped into deformable NP and dissociable NPA systems. We inspect the engineering considerations and performances of materials in each group (Table 1). We further discuss the potential and challenges of shear-activated DDSs as well as provide future perspectives on other suitable material designs.
Table 1.
A summary of major deformable-nanoparticle-based shear-sensitive drug delivery systems.
| Drug carrier | Spherical liposome | Lenticular liposome | Nanogel | Micellar composite hydrogel |
| Payload | Eptifibatide[39] | Carboxyfluorescein for imaging[31] | Plasmalemma vesicle associated protein (PLVAP) antibody[58] | Simvastatin[71] |
| Drug target | Thrombosis | Constricted arteries with atherosclerosis | PLVAP in lung cells | Inflammatory macrophages at the thrombotic site |
| Drug development phase | Prevention of thrombotic occlusion in mice | Delivery of an imaging dye in an in vitro arterial model | Delivery of an antibody in mice | Inhibition of thrombosis in mice and rabbits |
| Carrier materials | Phosphatidylcholine with Brij 76 | 1,3-dipalmitamidopropan-2-yl 2-(trimethylammonio)ethyl phosphate (Pad–PC–Pad) | Lysozyme-dextran | Hyaluronic acid (HA) modified with glycidyl methacrylate |
| Carrier size | 200 nm | 100 nm | 150–300 nm | Micelles: 100 nm |
| Shear range of release | > 1500 s−1 | ~40 Pa (2 Pa represents a healthy artery) | > 15 s−1 | 55% and 75% narrow flow states |
2. Shear-deformable nanoparticles (NPs)
Shear-deformable NPs, one of the major types of shear-triggered drug carriers, rely on physical deformation of NPs and nanovesicles caused by elevated shear stress to release the cargo. Changes in the surface-area-to-volume ratio during deformation, which are often reversible, induce defects on the particle surface and lead to drug release. In this group of DDSs, liposomes and micelles are central materials because of their flexible, self-assembled surface structure, while deformable polymeric nanoparticles have also been explored as drug carriers for crossing biological barriers.
2.1. Spherical liposomes
Liposomes are nano- to micron-sized spherical vesicles consisting of at least one phospholipid bilayer. They have been extensively used as drug carriers for their high biocompatibility, biodegradability, low toxicity and ability to trap both hydrophilic and lipophilic drugs.[28] One of the best examples of a drug-encapsulated liposome is the FDA-approved anthracycline-based anti-cancer chemotherapy drug Doxil.[32] More recently, studies on lipid-based nanoparticles (LNPs) further improved liposomal performance by assembling non-lipid components such as polymer chains and NPs into liposome membranes to stabilize the liposomes from fusion. These LNPs with hybrid core-shell structures have unique properties beyond nanomaterials of single components. Using cellular membrane components to functionalize synthetic NPs has also enabled a new field of biomimetic drug delivery. [33]
While various studies have demonstrated that shear flow could regulate drug delivery of liposomal carriers by affecting their cell uptake or binding, evidence has emerged on shear-controlled drug release of spherical liposomes.[34–37] Early attempts that applied shear to trigger cargo release from liposomes found that transient pore formation dominated the mechano-induced leakage of liposomes. Moreover, these pores were produced more easily in heterogeneous domains on the lipid bilayer.[37] After shearing egg phosphatidylcholine (EPC) vesicles of 100 nm size in the presence of detergent polyethylene glycol octadecyl ether (Brij 76), fusion and leakage were observed at shear rates above 5000 s-1. The detergent molecules bear a large head group and partially segregate within the bilayer (Figure 1A). The detergent rich domain then lowers the local membrane tension and promotes pore formation when shear is applied on the liposome. However, the shear rate threshold to trigger drug release, 5000 s−1, is high compared to that observed at the thrombus site, 1000 s-1. This high threshold makes this liposomal DDS less effective for its target application. In addition, 100 nm vesicles formed in the absence of detergent showed little drug release from shear stimulation. The need of detergent limits the in vivo applications of this type of liposome.[38]
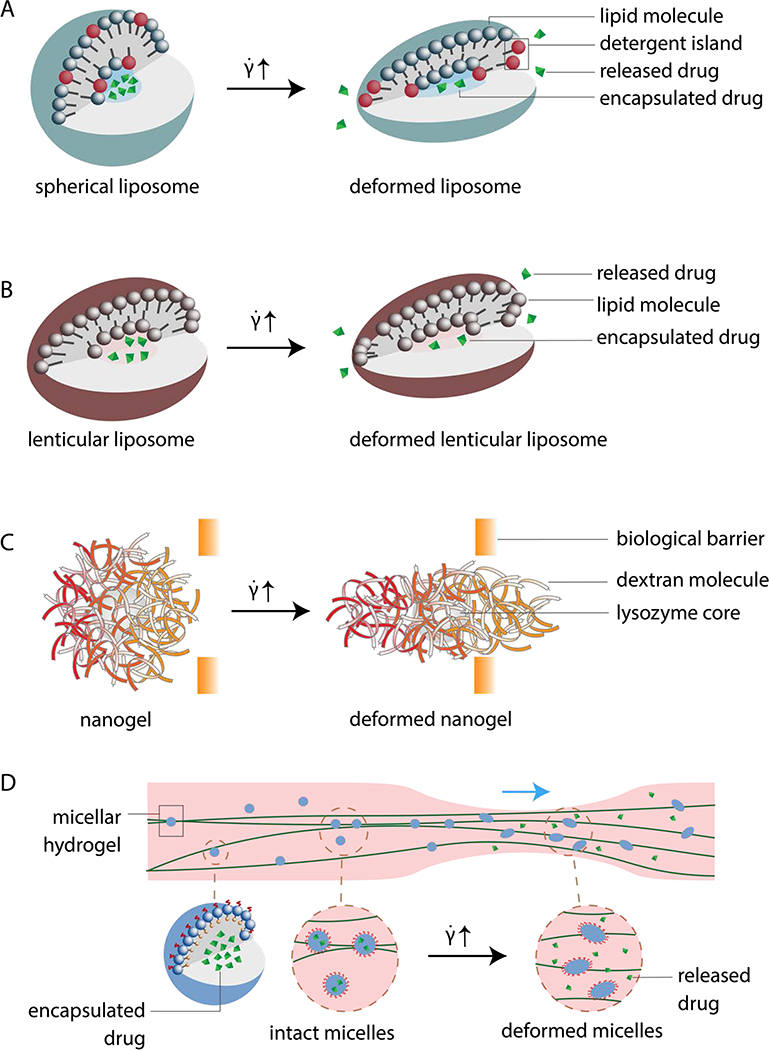
Figure 1.
Schematics of different designs of shear-deformable nanoparticles (NPs). (A) Spherical liposomes remain globular under no or low flow with drugs encapsulated inside the vesicles (left). As shear flow exceeds a threshold, the liposomes are deformed and release drugs from the pores on the lipid membrane (right). Black colored molecules in the liposome bilayer represent detergent molecules, which disperse evenly in the spherical vesicle, but segregate upon shear and create pores on the membrane. Modified from [38]. (B) Lenticular liposomes (left) deform under a lower shear rate threshold than that of spherical liposomes. Drugs encapsulated inside the lenticular liposomes are released through pores formed on the lipid membrane by shear-induced particle deformation (right). Redrawn from [31]. (C) Lysozyme-dextran nanogel with a lysozyme core concealed by a loose dextran shell. The spherical NP (left) is stretched under increased shear flow (right) to pass porous biological barriers for drug delivery purposes. Modified from [58]. (D) Shear-deformable micelles as part of a shear-thinning hydrogel moving through a thrombus site in circulation. Spherical micelles (left) deform under increased shear flow at the constricted blood vessel site and releases drugs. Modified from [71].
Recently, Molloy et al. tested shear-triggered drug release using spherical liposomes. Phosphatidylcholine (PC)-based liposomes were synthesized to encapsulate high dose of eptifibatide for inhibiting platelet aggregation at stenotic sites.[39] The antiplatelet drug was expected to release through shear-induced pore formation of the liposomes,. The authors confirmed this mechanism by adding detergent to partially destabilize the liposome. The detergent Brij 76 increased the permeability of the lipid bilayer PC membrane, thus lowering the shear threshold for drug release, consistent with the mechanism first reported by Bernard et al.[40] In Molloy’s study, increasing the detergent concentration led to the reduction of platelet aggregation under the same shear rate, indicating that the composition of the lipid bilayer determines its shear response. Molloy et al. also found that the shear rate threshold for the nano-capsules without detergent was 3,000 s−1, above which eptifibatide effectively prevented thrombus formation in a narrow microfluidic channel. Further in vitro studies performed by flowing whole blood containing the liposomes over collagen matrix at different shear rates showed the promise of these particles to deliver anti-thrombus drugs under shear activation. Additionally, in vivo mouse studies supported the drug carrier’s ability to target areas of high shear and reduce thrombus formation without prolonging systemic bleeding. Altogether, spherical liposomes show a great potential as a highly efficient, shear-activated DDS to protect blood vessels against occlusion while maintaining a low systemic concentration of drugs. However, modeling flow in microfluidic platforms oversimplify the flow conditions, and the results may not be directly translatable to in vivo outcomes. In vivo evaluations in humans have yet to occur, suggesting the need for clinical research to compare these DDSs to current FDA-approved antithrombotic or vasodilatory therapeutics.
2.2. Lenticular liposomes
The development of lenticular-shaped liposomes was a major milestone in the advancement of shear-triggered DDSs for treating cardiovascular diseases. Traditional liposomes are generally spherical, which is an energy-minimized shape.[40] However, as discussed above, spherical liposomes based on common phospholipids such as PC require a high shear rate to trigger drug release.[38,39] One way to increase the shear sensitivity of liposomes is to change their morphology. Early studies suggested faced liposomes based on the PC chemistry could be formed by packing the lipids hexagonally with cationic and anionic surfactants. However, these liposomes are only stable under certain environments such as those with specific ionic concentrations, which are unsuitable for physiological drug delivery applications. [41,42] Alternatively, polyhedral-shaped vesicles have been formed by introducing artificial amides into natural phospholipids to stabilize the vesicle structure in the gel state, which possesses a large bending modulus. [31] In the polyhedrons, edges are formed by line defects to reduce the curvature energy, as it takes less energy to keep the edges than to constantly bend the vesicles into a spherical shape. Simulations have been used to explain the polyhedral morphology based on the defect types and spontaneous curvature of phospholipid monolayers.[40] Interestingly, amide groups were first introduced into natural phospholipids to improve their resistance to phospholipase cleavage, resulting in better liposome stability. Amide-containing liposomes have been shown to have prolonged circulation time and have been proposed to deliver anti-HIV and anticancer agents.[43, 44] Symmetrical 1,3-diamidophospholipids carrying hydrogenated alkyl chains have been reported to further improve liposomal stability.[45] The finding that amide groups enable the formation of lenticular liposomes indicates another function of this chemistry as a critical element to promote the shear response of liposomes.
Using amide-bearing 1,3-dipalmitamidopropan-2-yl 2-(trimethylammonio)ethyl phospholipid (Pad-PC-Pad), Holmes et al. constructed lenticular, shear-responsive liposomal structures for the first time.[31] These non-spherical liposomes are stable in the static state, while they deform easily under shear, leading to drug release from transiently formed pores (Figure 1B). The Pad-PC-Pad liposomes used in this seminal work were ~100 nm in size and were prepared by an extrusion method, which reduced the tendency of Pad-PC-Pad to form flat membranes. The lenticular morphology is an intermediate state to minimize the energy between a spherical vesicle and a flat surface. The non-spherical morphology leads to instabilities along the equator upon exposure to shear stress, which induces drug leakage from the liposomes. Thus, the unique shape improves the shear sensitivity of the liposomal system compared to spherical liposomes. In the work by Holmes et al., controlled release from the lenticular liposomes was demonstrated in vitro using carboxyfluorescein as the cargo and microfluidic channels to model healthy and 75% constricted blood vessels. The wall shear stress was 2 Pa to represent the healthy, physiological level and 40 Pa to represent that in a pathological condition. The release of carboxyfluorescein was found to be 27% greater in the pathological flow than in the physiological one, showing the promise of shear-triggered, localized drug release from lenticular liposomes. Preclinical studies confirmed the safety of this drug carrier, as immune responses to Pad-PC-Pad liposomes containing nitroglycerin had similar profiles to those produced by FDA-approved liposomal nanocarriers in in vitro human sera immunoassays and in vivo porcine models.[46] These studies demonstrate a great potential of Pad-PC-Pad liposomes for shear-controlled release in vivo.
Fundamental studies of liposomes made of symmetric artificial 1,3-diamidophospholipids identified their structures as monolayer and bilayer vesicles, which have been further related to the stability of faceted structures. These synthetic phospholipids tend to form a flat bilayer sheet below the phase transition temperature (Tm) but favor a spherical shape above Tm. Upon cooling, the phospholipid assembly changes into the subgel phase, a slowly formed highly ordered phase first found in PC bilayers, and packs into a herringbone structure from a spherical geometry. The liposome then relaxes into a cuboid shape that maximizes flat faces and minimizes defect lines.[47] A separate study suggested that the strong hydrogen bonding among the head groups of the synthetic lipids was responsible for the observed faceted shapes.[48] The facet formation was controllable by factors such as cooling rates and liposome preparation methods.[49] These fundamental studies ultimately promote the inclusion of 1,3-diamidophospholipids in the rational design of shear-triggered liposomal DDSs.
Although many challenges exist, non-spherical nano-carriers appear to be one of the most promising types of material for shear-controlled DDSs. Recent efforts have provided a more mechanistic understanding of drug release from artificial 1,3-diamidophospholipid-containing liposomes and have further improved their shear responses. For example, Buscema et al. recently characterized the structural changes of faceted Pad-PC-Pad liposomes under shear through spatially resolved small-angle X-ray scattering (SAXS) in a microfluidic device mimicking a stenotic blood vessel.[50] Their results indicated that the gradient pressure force upstream and downstream of the stenotic sites, rather than the wall shear stress in the blood vessel, provoked the structural changes of the liposomes and subsequent drug release. In an effort to improve their stability in the human body, Pad-PC-Pad liposomes have been further modified. It has been recognized that Pad-PC-Pad has a main phase transition, i.e. the transition between the liquid crystalline phase and the gel phase, around 37 °C, above which encapsulated cargo is fully released because of the membrane leakiness. To increase the transition temperature while maintaining the shear sensitivity, a C17 analogue of Pad-PC-Pad named 1,3-diheptadecanamidopropan-2-yl (2(trimethylammonio)ethyl) phosphate (Rad-PC-Rad) has been synthesized and characterized, showing a main phase transition temperature of 44.7 °C.[51] Minimal immune toxicity of the Rad-PC-Rad liposomes has also been confirmed in vitro.[52]
Besides artificial 1,3-diamidophospholipids, polyoxyethylene (2) cetyl ether (Brij 52), a non-ionic surfactant, has been hypothesized in a recent paper by Arjmand et al. to serve as a shear-activated antithrombotic agent carrier. The proposed work aims to create a DDS that is inspired by platelet activation occurring in response to high shear at sites of thrombosis. Their design includes constructing non-spherical Brij 52 vesicles that break down or at least distort under elevated shear rates and are PEGylated to prolong half-life of the carrier. They also surmised that more specific drug release activation can be achieved at sites of thrombus by vesicle attachment of either the A1 domain of Von Willebrand Factor or modified Arginine-Glycine-Aspartate (RGD) peptides that bind with platelets. This proposed work provided a new design of a niosomal-vesicle-based shear-deformable drug delivery system, which may lead to more advanced drug carriers for antithrombotic pharmacotherapy.[53]
2.3. Nanogels (NGs)
Nanogels (NGs) often refer to highly hydrated, cross-linked polymeric NPs. Their flexibility allows easy deformation in response to a mechanical force compared to conventional polymeric NPs. As materials for drug release applications, NGs have a high drug loading capacity and are easy to synthesize. Compared to liposomes, NGs have prolonged circulation time due to minimal nonspecific interactions with proteins and cells, making them ideal candidates as drug carriers.[54,55] Drug release from NGs involves various mechanisms including passive diffusion, particle degradation, deionization, ion displacement and structural changes of the NG polymer chains induced by external energy. In addition, the volume change of the NG and subsequent release profile can often be controlled through both the NG composition, such as the degree of cross-linking and the introduction of cleavable cross-linkers, and environmental cues, such as solution pH, ionic strength or temperature.[56]
Because NGs behave as soft gels in the swollen state, they can easily deform and navigate the extracellular matrix and crowded cellular environment under forces such as shear stress. This deformation can be regulated by the cross-linker and polymer backbone properties.[57] While the size and geometrical constraints have long been observed to limit the penetration of conventional NPs in vivo, mechanically deformable NGs are appealing materials to overcome these limitations.
Recently, Myerson et al. published the first experimental work where the deformation of NGs induced by shear enabled targeted drug delivery to topologies inaccessible to rigid NPs (Figure 1C).[58] The authors designed lysozyme-dextran NGs as the drug carrier and further coated them with antibodies to target the lung plasmalemma vesicle associated protein (PLVAP), a protein sequestered in small invaginations on lung cells. The material has a low cross-linking density and thus a high mechanical deformability. This DDS is relevant for a niche drug target affecting inflammatory signaling and transcytosis in the pulmonary system. Unlike a rigid NP, the NG could elongate upon high shear to access small concavities, also known as caveolae. It was found that NGs ranging from 150–300 nm deformed and passed through filters with 100nm pores under a range of shear flows: sub-physiological (15 s−1), venous (400 s−1) and arterial shear flows (1000 s−1). Furthermore, in an in vivo mouse model, PLVAP antibody-coated NGs successfully targeted lung cell caveolae at 40% of the binding level observed for free PLVAP antibodies, while rigid polystyrene NPs of similar sizes did not bind to caveolae. The promising results of targeted drug delivery to nanostructures on cells warrant further studies to determine the cargo release efficiency after the engagement of NGs and PLVAPs. While it remains in question whether NG DDSs can target nanostructures other than caveolae, increasing attention has been drawn to deformable NGs to advance the material for shear activation and targeted drug delivery applications.[59]
2.4. Micellar hydrogels
Shear-thinning and self-healing hydrogels have long been recognized as excellent carriers for minimally invasive drug delivery and conformal scaffold applications, owing to their ability to flow on applied stress and rapidly self-heal in the absence of shear. They have been extensively studied and well-reviewed in recent literature.[60–63] Recent efforts to incorporate nanomaterials such as micelles have further advanced shear-thinning gels for drug release applications.[64] As the name suggests, micellar hydrogels refer to a class of materials where micelles are embedded into hydrogels. Compared to conventional shear-thinning hydrogels, some unique properties of micellar hydrogels include: 1) improved strength, 2) dual drug release modes, for example, independent release of two drugs encapsulated in the micelles and dispersed in the hydrogel, 3) sustained responses to multiple stimuli and 4) broader spectrum of embedded drugs.[65–69]
In micellar hydrogels, micelles serve as self-assembled cross-linkers that improve the strength and toughness of hydrogels, while their cross-linking is often reversible.[70] In addition, the micelles can sequester hydrophobic drug molecules in their core. Among the published works on micellar hydrogel DDSs, one study demonstrated drug release from micellar hydrogels activated by shear stress.[71] In this work, micelles from an amphiphilic block copolymer (CBC) were cross-linked by hyaluronic acid macromers (HAGMAs) to form a micelle hydrogel composed of the hyaluronic acid amphiphilic block copolymer (HACBC) (Figure 1D). When injected into a stenotic vessel, the micelle components in HACBC were deformed by mechanical stress to effectively release Simvastatin, which could inhibit inflammatory macrophages at thrombotic sites. Tests of drug release were first carried out in simulated artificial blood vessels mimicking 4 states: static, normal flow, 55 % narrow flow and 75 % narrow flow. Under these conditions, the release rate of Simvastatin increased as the vessel narrowed. After 48 hours, the percentage of released drug was found to be around 20% in the static state, 26.6% under the normal flow condition, 58.2% and 73.6% under the 55% and 75% narrow flow states respectively. Drug release from the micellar hydrogel, therefore, significantly increased with the degree of vascular occlusion, which has elevated shear stress in the vessel. In vivo tests in a rabbit model reported no obvious toxicity of the micellar hydrogel in internal organs. Similar to NG-based DDSs, micellar hydrogel DDSs responsive to shear are still in a very early stage of development. Better understanding and control of the viscoelasticity properties as well as shear responses are still needed before advancing the materials to clinical evaluations.
3. Shear-sensitive nanoparticle aggregates (NPAs)
In the same year that Holme’s seminal work on shear-deformable lenticular liposomes was published, another critical shear-sensitive DDS, known as nanoparticle aggregates (NPAs), was reported for the treatment of cardiovascular diseases. These aggregates are similar to lenticular liposomes in that their drug release is triggered by high shear stress. However, instead of delivering therapeutics by deformation, the NPAs dissociate into individual NPs to deliver antithrombotic drugs.[14] This construction has since remained popular in the field of shear-sensitive DDSs. NPAs and related carriers have been designed to deliver drugs in occlusive vessels by autonomous dispersion and in damaged tissue sites by disaggregating during injection and re-aggregating afterwards.[14,72] The broader topic of NP aggregation has also been studied extensively, as the phenomenon and its prevention are of common interest among many researchers. This has led to findings that aggregation is dependent on the NP mass, fractal dimension, coating and cross-linker properties.[73–76]
When individual NPs decrease in size, the total surface energy of the system increases, causing particles to form aggregates in order to minimize the system’s free energy. These aggregates form from particle collision in a liquid system caused by Brownian motion.[26] The mass and fractal dimension of the aggregates in turn determine the critical shear stress under which they dissociate.[26,73] Thus the critical shear stress can be fine-tuned by altering the surface characteristics of the particles and physical properties of the aggregates. Additional experiments have supported that macromolecules adsorbed onto the surface of NPs have an effect on NP aggregation.[74,75] For example, it was found that quantum dots coated with various polymers formed aggregates of differential hydrodynamic diameters, with PEG-COOH inducing the smallest aggregates, PEG-NH2 the next smallest and PEG the largest, due to varying electrostatic and steric repulsive forces between the NPs in each system.[74] Santander-Ortega et al. also found that poly(lactic-co-glycolic acid) (PLGA) NPs coated with polypropylene oxide and polyethylene oxide tri-block copolymers prevented the aggregation of PLGA NPs.[75] Finally, altering the valency, or number of repeating units, of cross-linker groups between gold NPs affected the aggregate size.[76] Pentaerythritol tetrakis(3-mercaptopropionate) cross-linkers with two repeating groups formed the largest gold NPAs, while those with four formed the second largest and three formed the smallest. Overall, changing the size of the cross-linkers and altering the type of adsorbed polymer groups on NPs influence the aggregate size and therefore the critical shear-rate for aggregate dispersion. A more comprehensive study by Papa et al. found that multiple NP properties affected the breakability of NPAs when under the application of mechanical ultrasound energy. More specifically, it was found that NPs composed of higher weight PLGA, higher percentages of leucine (used to hold the NPAs together) and larger NP size (from 200 to 400 nm) increased breakability of the NPAs under ultrasound application.[77]
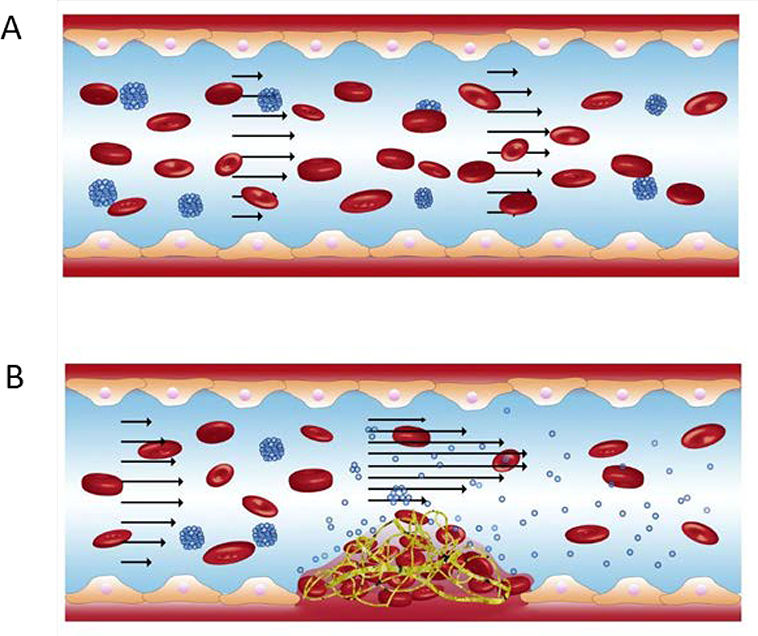
Such material parameters can be varied to produce NPAs with specific shear sensitivity, and reversibly aggregating systems have been developed. PLGA NPs coated with recombinant tissue-type plasminogen activator (r-tPA), for example, have been shown to form microscale aggregates through hydrophobic interactions and disaggregate into individual NPs under local shear stress greater than 100 dyn/cm2 (Figure 2).[14] Dispersed NPs experience less drag force and therefore bind more easily to the vascular wall, enabling localized drug delivery. This DDS has enhanced the safety of thrombolytic therapies by reducing the systemic drug dose required via its local targeting mechanism. In vivo study in mice further supported this DDS’ ability to reduce pulmonary embolism. [14] Additionally, in a 2015 experiment, r-tPA coated NPA treatment was combined with temporary endovascular bypass (TEB) to concentrate r-tPA at complete carotid occlusion sites, as TEB greatly increases local shear stress.[78] Performed in an in vivo rabbit model, results indicated the effectiveness this treatment. However, further study is needed to determine the minimally effective dose, toxicology and interaction of the NPAs with other components in blood.
Figure 2.
Nanoparticle aggregates (NPAs) dissociate at high shear stenosis sites to deliver antithrombotic drugs. (A) NPAs flow through a blood vessel under healthy, physiological shear flow conditions after intravenous injection. (B) At the thrombus site, the blood vessel narrows, producing a stenotic site and increasing local shear stress. This high shear stress region induces NP disaggregation, such that individual NP coated with tissue plasminogen activator (r-tPA) can amass around thrombus and dissolve it. Modified from [14].
NPs may also be delivered to drug target sites via cell carriers. Such carriers include macrophages and stem cells, which have been shown to effectively deliver NPs after cell release[79], and red blood cells (RBCs), which can carry shear-sensitive NPs on their surface.[80,81] In the case of the shear-sensitive RBC DDS, NPs are attached to the cells via electrostatic attraction. This protects NPs from systemic clearance, as RBCs naturally possess long-circulation capacity, and also permits the shear-induced detachment of NPs under high shear. Therefore, in this DDS system, NPs aggregate on the surface of a cell carrier rather than with each other. One recent study in this field was published by Chen et al.[80] Thiolated poly-L-lysine was hybridized with heparin, one of the most potent antithrombotic drugs, forming cross-linked NPs (cNPs) which could then be adsorbed onto RBCs. A higher shear (10 Pa compared to 1 Pa) condition led to a significantly greater detachment of NPs (~100% compared to ~30%, respectively) from RBCs after 48-h exposure to shear. In the same study, the biomimetic RBC-cNPs were also evaluated as a potential DDS to treat thrombosis in a mouse model. The therapeutic effect of heparin in the RBC-cNP DDS (measured by plasma anti-factor Xa activity in blood plasma) was significantly higher, more stable and longer compared to that of free heparin. A different RBC-NP DDS has also shown therapeutic promise in cancer treatment.[81] In this DDS, biodegradable NPs attached to RBCs were designed to deliver the chemotherapy drug doxorubicin for lung metastasis treatment in mice. High shear (~6 Pa) applied in vitro caused NPs to dissociate from both human and mouse RBCs to a significantly greater extent than when under low shear (~1 Pa). Additionally, 20 minutes after administration of the RBC-bound NPs, there was a 16.6-fold increase in the doxorubicin delivered to mouse lungs with melanoma metastasis as compared to that delivered by free NPs. The therapeutic efficacy of this drug delivery platform was confirmed in an early-stage lung metastasis model in mice, where the RBC-coupled NPs had a 100- to 300-fold increased antimetastatic effect compared to that produced by free NPs or free doxorubicin.
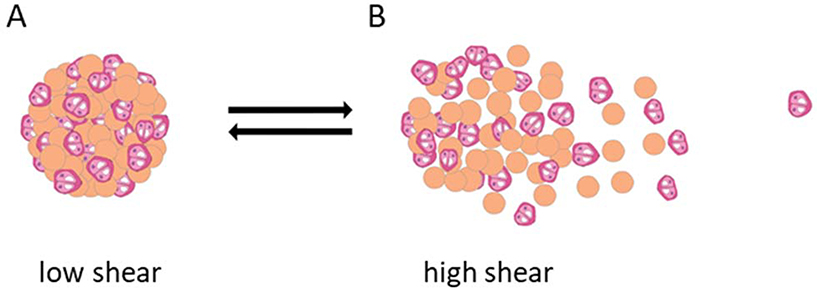
Less clinically developed shear-sensitive DDSs include NP aggregates from natural polymers. In one study, microspheres of algae polysaccharide alginate modified with peptides containing the arginine-glycine-aspartic acid (RGD) sequence were created.[82] This material is favorable due to the biocompatibility and low toxicity of alginate. The RGD ligand has an affinity for primary chondrocyte cell receptors and enables the formation of microsphere-cell aggregates, which can then be delivered at sites of degraded cartilage. The microsphere-cell spheres are a shear-thinning material that disaggregates upon injection and re-aggregates when there is low or no shear stress (Figure 3). Ultimately, the RGD-cell receptor interaction determines the shear-sensitivity and reversible aggregation of the DDS, as microspheres lacking the RGD modification could neither form nor reform aggregates before and after an applied shear of 75 Pa. Although these microspheres are larger than the NPs discussed throughout this review, the reversible aggregation mechanism enabled by ligand-receptor association is translatable to NPs. Furthermore, in vivo mice studies confirmed that aggregates of RGD-modified microsphere and cells were successful in regenerating the cartilage tissue with more uniformity, lacunae and collagen fibers six weeks after injection than that generated by aggregates of non-RGD microspheres and cells. Collectively, recent research supports that both synthetic and natural polymeric particle aggregates can successfully perform in vivo as pathology-targeting, shear-responsive DDSs whose shear sensitivity is controlled by particle size, hydrophobic interactions and ligand-receptor affinity. Still, the efficacy of injectable natural polymer aggregates for the applications of tissue regeneration and cardiovascular disease treatment in human clinical trials remain to be seen.
Figure 3.
Shear-reversible microsphere-chondrocyte aggregates. Chondrocytes and peptide-modified alginate microspheres form aggregates due to binding between chondrocyte integrin receptors and microsphere peptide ligands. During injectable delivery, aggregates can dissociate at high shear (B) and then re-associate (A) at drug delivery sites possessing low shear stress, such as sites of cartilage damage. Modified from [72].
4. Discussion
Shear-activated nanocarriers have seen significant advances in targeted drug delivery applications such as cardiovascular disease treatment over the past few decades. Improvement of these DDSs in recent years has largely benefited from the rapid development of characterization methods and engineering tools such as computer simulations. Deformable NPs and NPAs that respond to shear stress remain to be the two major pathways to construct shear-triggered DDSs as discussed above. NPs that respond to shear stress by deformation allow site-specific drug delivery without producing byproducts or having any compositional changes. Within the design of deformable NPs, lenticular liposomes have gained the most research interest compared to traditional spherical liposomes, NGs and micelle-composited shear-thinning hydrogels. Up to this point, no in vivo human study has been reported on any of the shear-controlled deformable NP systems, indicating there are still challenges on the reliability and biocompatibility of these materials before clinical applications. Most related work reported for lenticular liposomes or nanogels remains in the phase of in vitro tests, using imaging molecules dye or antibodies as payloads.[31,58] There are, however, in vivo animal tests on spherical liposome and micellar hydrogel DDSs where thrombus formation are inhibited in both cases.[39,71] More generally, many publications have nevertheless proven their potential as drug carriers. The balance between stability and flexibility is vital for further applications such as shear-regulated DDSs.[82–85] On the other hand, NPAs and microparticle aggregates have been shown to disperse at high-shear flow in blood vessels for payload delivery and reversibly aggregate as a shear-thinning material for tissue engineering applications. While various NP parameters can be modified to affect shear-sensitive properties, thus far, PLGA NP clusters whose hydrophobic interactions can be overcome by high shear have been the most successful model for shear-sensitive NPA DDSs. The therapeutic efficacy of such DDSs, including the reduction of pulmonary embolisms[14] and carotid occlusions[78], the inhibition of lung metastasis[81] and the regeneration of cartilage tissue[72], have been demonstrated in animal models. They have proven to have significant advantages over other DDSs by reducing unwanted systemic bleeding and effective dose[14] and by increasing therapeutic efficacy, stability and duration[77]. Still, the translatability and safety of such particle aggregate systems into human populations remains to be seen.
The mechanical properties of shear-sensitive nanomaterial DDSs greatly control their drug release efficiency. Such properties include many parameters and can be characterized by multiple methods.[86] In the field of shear-responsive DDSs, bulk rheology has been widely used to investigate the viscoelastic properties of hydrogels,[71,87] which are further used to infer mechanical properties of nanogels. However, such extrapolation may not be accurate given the large surface-to-volume ratio of NPs. Recently, Myerson et al. reported the mechanical characterization of shear deformable nanogels using quartz crystal acoustical measurements.[58,88] They found shear moduli of 67.88 kPa and 8.47 MPa for nanogels and polystyrene particles, respectively. Particle tracking microrheology (PTM) is another tool that provides information about the rheology of NPs under flow. It measures mechanical parameters using a lower magnitude of force than bulk rheology methods, and thus holds great potential in characterizing nano-scaled DDSs.[89–91] Despite these reports, more systematic research on the mechanical behavior of shear-sensitive NPs such as liposomes and polymeric particles is still needed.
Aside from the nanocarrier formulation and its payload release under shear flow, other aspects should also be examined, such as the NP circulation time affected by shear flow. Circulation time of NPs has long been known to depend on the particle size, surface property and particle shape.[92] Recently, NP behavior under shear flow has been studied for the purpose of developing nanodrug carriers. Physiological shear could affect circulation time of NPs by influencing their distribution, aggregation and binding probability. It has been shown that NP binding and biodistribution can be largely altered by local shear rate that depends on the blood vessel geometry.[93–95] Furthermore, shear may affect their circulation time by modulating cellular uptake of the nanocarriers, eroding the NP and destabilizing them with pathological high shear at, for example, tumor sites.[96,97] More advanced NP design has been investigated to overcome these limitations including the insertion of a PEGylated core into lipid shells and NP attachment to red blood cells.[80,81,98]]
It should also be noted that shear environment can vary significantly in different blood vessels, depending on the vascular size and geometry, which can further affect NP distribution and the drug release of shear sensitive DDSs.[99] Under physiological shear flow, it has been reported that wall shear stress goes up to 9.55 Pa at the smallest human conjunctival capillaries and down to 0.28 Pa at post capillary venules with larger diameters.[100] At these shear rates, most shear sensitive DDSs should have minimum drug release. On the other hand, shear flow associated with pathological vessel conditions have significant effects on the drug release of shear sensitive DDSs. One example is a blood vessel with stenosis. The work by Korin et al. examined the release of fluorescent NPs from shear-activated NPAs under different levels of shear flow for drug release in obstructed blood vessels. The NP concentration increased 8- to 12-fold when the shear increased from a physiological level of 0.1 or 1 Pa to a pathological level of 10 or 100 Pa.[14]
Materials to be further investigated include single molecule shear-responsive materials and nanocrystals. While the reviewed research has explored the shear responsiveness of bulk polymers and molecular assemblies, limited research has been conducted on single molecule constructs whose conformation and function are altered by flow environments. The shear responsiveness of single molecules often involves different force scales than those of nanovesicles, NPs and NPAs, making the molecules more advantageous in specific flow environments and shear rates than other DDSs. Characterizing the biophysical and chemical properties of endogenous shear-sensitive single biomolecules such as various proteins and DNA may provide design insights for synthetic, flow-responsive single molecular drugs.[101,102] One such molecule is the clotting protein VWF, whose flow-sensing macrostructures and domains respond to high-shear at bleeding sites and thereby induce platelet aggregation. Additionally, nanocrystals, or nano-sized crystals that are composed entirely of the active drug and possess no carrier molecules, have yet to be explored for shear-sensitive NPA DDSs. Nanocrystals are advantageous due to their increased bioavailability, especially for poorly soluble drugs. Their surfaces can be stabilized with polymers and/or surfactants, which influence the aggregation properties and the shear sensitivity of such materials. Preliminary research confirming the shear sensitivity of cellulose nanocrystal aggregates indicates a starting point for this novel DDS.[103]
5. Conclusion
Shear-sensitive nanocarriers have shown unique advantages in targeted drug delivery especially for cardiovascular disease treatments. Deformable NPs and dissociable NPAs are the major materials applied in this field. With the advances of chemical synthesis and drug delivery characterization methods, recent research indicates more opportunities and great potential of shear-activated nanomedicine. Remaining challenges include the precise shear control of release processes, stability of the nanocarriers under high shear and biocompatibility of the materials, which need to be resolved in the future for further clinical translation of these shear-controlled DDSs.
Table 2.
A summary of major shear-sensitive nanoparticle aggregate and related drug delivery systems.
| Drug carrier | Free nanoparticle aggregates | Red blood cell-bound nanoparticles | Microsphere aggregates | |
| Payload | Recombinant tissue-type plasminogen activator (r-tPA)[14,78] | Heparin[80] | Doxorubicin[81] | Chondrocytes[72] |
| Drug target | Thrombosis | Thrombosis | Lung metastasis | Damaged cartilage |
| Drug development phase | Reduction of pulmonary embolism in mice[14] and carotid occlusion in rabbits[78] | Antithrombotic activity in mice | Inhibition of lung metastasis growth in mice | Regeneration of cartilage tissue in mice |
| Carrier materials | Poly(lactic-co-glycolic acid) (PLGA) | Thiolated poly-L-lysine (PLL-SH) | Poly(lactic-co-glycolic acid) (PLGA) | Peptide modified alginate |
| Carrier size | Individual: 180 nm Aggregates: 3.8 μm |
Individual: 170 nm NPs aggregate on red blood cells |
Individual: 136 nm NPs aggregate on red blood cells |
Individual: 15.3 μm |
| Shear range of release | > 100 dyne/cm2 | ~10 Pa | ~6 Pa | Disaggregation between 0–75 Pa |
Acknowledgments:
This work was supported in part by NSF grant 2004475 (to X.C. and X.F.Z.) and NIH grant HL152348 (to X.F.Z.).
Biography
Yi Wang received her Bachelor’s degree in Polymer Science and Engineering at Beijing University of Chemical Technology in 2016. She recently gradudated with a PhD degree from the Department of Materials Science and Engineering at Lehigh University under the supervision of Drs. Xuanhong Cheng and Frank Zhang. Her research focuses on the shear-induced extensional behavior of single molecule Von Willebrand Factor (VWF) and the development of mechanical force-triggered nanotherapeutics.

Avani Pisapati is currently a Research Associate in Dr. Frank Zhang’s lab of the Bioengineering Department at Lehigh University. At the moment, she uses single-molecule techniques to study the biomechanical properties and binding affinities of various proteins. Proteins she has investigated include the clotting factor Von Willebrand factor (VWF) and the prototoxin lynx, a modulator of nicotinic acetylcholine receptors in the brain. She received her B.A. from Middlebury College in 2016.

X. Frank Zhang is a core faculty member in the Department of Bioengineering at Lehigh University, PA, USA. He received his Ph.D. in Physiology and Biophysics from University of Miami School of Medicine, FL, USA, and completed his postdoctoral training at Harvard Medical School, MA, USA. He is an expert in single-molecule and single-cell detection techniques. His research focuses on the structure and function of cell adhesion molecules, mechanotransduction in the vasculature, coagulation, and virus-host cell interactions.

Xuanhong Cheng has joint appointents in the Department of Bioengineering and Department of Mateirals Science and Engineering at Lehigh University, PA, USA. She received her Ph.D. in Bioengineering from University of Washingtin, Seattle, WA, USA, and completed her postdoctoral training at Massachusetts General Hospital, MA, USA. Her research focuses on the development of microfludic systems and biosensors as diagnostic devices and for fundamental studies.

Footnotes
Conflict of Interests:
The authors declare no competing interests – financial or otherwise.
Contributor Information
Yi Wang, Department of Materials Science and Engineering, Lehigh University, Bethlehem, PA, 18015, United States.
Avani V. Pisapati, Department of Bioengineering, Lehigh University, Bethlehem, PA, 18015, United States
X. Frank Zhang, Department of Bioengineering, Lehigh University, Bethlehem, PA, 18015, United States.
Xuanhong Cheng, Department of Materials Science and Engineering, Lehigh University, Bethlehem, PA, 18015, United States; Department of Bioengineering, Lehigh University, Bethlehem, PA, 18015, United States.
References
- 1.Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S,Shin HS, J Nanobiotechnology 2018, 16,71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF,Chan WCW, Nature Reviews Materials 2016, 1. [Google Scholar]
- 3.Servant A, Methven L, Williams RP,Kostarelos K, Adv Healthc Mater 2013, 2,806. [DOI] [PubMed] [Google Scholar]
- 4.Porta F, Lamers GE, Morrhayim J, Chatzopoulou A, Schaaf M, den Dulk H, Backendorf C, Zink JI,Kros A, Adv Healthc Mater 2013, 2,281. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Chan HF,Leong KW, Adv Drug Deliv Rev 2013, 65,104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang CY, Pan DY, Luo K, She WC, Guo CH, Yang Y,Gu ZW, Adv Healthc Mater 2014, 3,1299. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yu J, Bomba HN, Zhu Y,Gu Z, Chem Rev 2016, 116,12536. [DOI] [PubMed] [Google Scholar]
- 8.Gao ST, Tang GS, Hua DW, Xiong RH, Han JQ, Jiang SH, Zhang QL,Huang CB, Journal of Materials Chemistry B 2019, 7,709. [DOI] [PubMed] [Google Scholar]
- 9.Wilczewska AZ, Niemirowicz K, Markiewicz KH,Car H, Pharmacological Reports 2012, 64,1020. [DOI] [PubMed] [Google Scholar]
- 10.Streeter VL, Wylie EB, Fluid Mechanics. McGraw-Hill, New York, NY: 1985 [Google Scholar]
- 11.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL, Blood 1996, 88,525. [PubMed] [Google Scholar]
- 12.Ballermann BJ, Dardik A, Eng E, Liu A, Kidney International 1998, 54,S100. [DOI] [PubMed] [Google Scholar]
- 13.Saxer T, Zumbuehl A,Muller B, Cardiovascular Research 2013, 99,328. [DOI] [PubMed] [Google Scholar]
- 14.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Coskun AU, Feldman CL, Wagner DD,Ingber DE, Science 2012, 337,738. [DOI] [PubMed] [Google Scholar]
- 15.Paszkowiak JJ,Dardik A, Vasc Endovascular Surg 2003, 37,47. [DOI] [PubMed] [Google Scholar]
- 16.Casa LDC, Deaton DH, Ku DN. J Vasc Surg 2015, 61,1068. [DOI] [PubMed] [Google Scholar]
- 17.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ, Blood 2016, 108,1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stalker TJ, Welsh JD, Brass LF, Curr Opin Hematol 2014, 21,410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuenter A, Selwaness M, Arias Lorza AMA, Schuurbiers JCH, Speelman L, Cibiş M, van der Lugt A, de Bruijne M, van der Steen AFW, Franco OH, Vernooij MW, Wentzel JJ, Atherosclerosis 2016, 251,348. [DOI] [PubMed] [Google Scholar]
- 20.Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, Mannini L, Atherosclerosis 2011, 214,249. [DOI] [PubMed] [Google Scholar]
- 21.Heo KS, Fujiwara K, Abe J, Mol Cells 2014, 37,435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korin N, Gounis MJ, Wakhloo AK, Ingber DE, JAMA Neurol 2015, 72,119. [DOI] [PubMed] [Google Scholar]
- 23.Epshtein M,Korin N, J Biomech 2017, 50,217. [DOI] [PubMed] [Google Scholar]
- 24.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M,Nejati-Koshki K, Nanoscale Res Lett 2013, 8,102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurhidayah D, Maruf A, Zhang X, Liao Z, Wu W, Wang G, Nanomedicine 2019, 14,3105. [DOI] [PubMed] [Google Scholar]
- 26.Xia Y, Shi CY, Xiong W, Hou XL, Fang JG,Wang WQ, Curr Pharm Des 2016, 22,5855. [DOI] [PubMed] [Google Scholar]
- 27.Sutton JT, Haworth KJ, Pyne-Geithman G,Holland CK, Expert Opin Drug Deliv 2013, 10,573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu S,Ding X, Semin Thromb Hemost 2019, 46, 587. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y, Zhang X, Shen H, He Q, Wu Z, Liao W,Yuan M, Front Bioeng Biotechnol 2019, 7,489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Kaplan JA, Colson YL, Grinstaff MW, Adv Drug Deliv Rev 2017, 108,68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holme MN, Fedotenko IA, Abegg D, Althaus J, Babel L, Favarger F, Reiter R, Tanasescu R, Zaffalon PL, Ziegler A, Muller B, Saxer T,Zumbuehl A, Nat Nanotechnol 2012, 7,536. [DOI] [PubMed] [Google Scholar]
- 32.Gabizon A, Goren D, Cohen R,Barenholz Y, J Control Release 1998, 53,275. [DOI] [PubMed] [Google Scholar]
- 33.Gao W, Hu CM, Fang RH,Zhang L, J Mater Chem B 2013, 1, 6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang T, Cho Y, Park C, Kim SD, Oh E, Cui JH, Cao QR,Lee BJ, International Journal of Pharmaceutics 2016, 510,42. [DOI] [PubMed] [Google Scholar]
- 35.Papademetriou I, Vedula E, Charest J,Porter T, PLoS One 2018, 13,e0205158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.York-Duran MJ, Ek PK, Godoy-Gallardo M,Hosta-Rigau L, Colloids and Surfaces B-Biointerfaces 2018, 171,427. [DOI] [PubMed] [Google Scholar]
- 37.Brochard-Wyart F, de Gennes PG,Sandre O, Physica A 2000, 278,32. [Google Scholar]
- 38.Bernard AL, Guedeau-Boudeville MA, Marchi-Artzner V, Gulik-Krzywicki T, di Meglio JM,Jullien L, J Colloid Interface Sci 2005, 287,298. [DOI] [PubMed] [Google Scholar]
- 39.Molloy CP, Yao Y, Kammoun H, Bonnard T, Hoefer T, Alt K, Tovar-Lopez F, Rosengarten G, Ramsland PA, van der Meer AD, van den Berg A, Murphy AJ, Hagemeyer CE, Peter K,Westein E, J Thromb Haemost 2017, 15,972. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi H, Phys Rev E Stat Nonlin Soft Matter Phys 2003, 67,041901. [DOI] [PubMed] [Google Scholar]
- 41.Dubois M, Demé B, Gulik-Krzywicki T, Dedieu JC, Vautrin C, Désert S, Perez E, Zemb T, Nature 2001, 411,672. [DOI] [PubMed] [Google Scholar]
- 42.Dubois M, Lizunov V, Meister A, Gulik-Krzywicki T, Verbavatz JM, Perez E, Zimmerberg J, Zemb T, Proc Natl Acad Sci U S A 2004, 101,15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta CM,Bali A, Biochimica Et Biophysica Acta 1981, 663,506. [DOI] [PubMed] [Google Scholar]
- 44.Sunamoto J, Goto M, Iwamoto K, Kondo H,Sato T, Biochimica Et Biophysica Acta 1990, 1024,209. [DOI] [PubMed] [Google Scholar]
- 45.Fedotenko IA, Zaffalon PL, Favarger F,Zumbuehl A, Tetrahedron Letters 2010, 51,5382. [Google Scholar]
- 46.Buscema M, Matviykiv S, Meszaros T, Gerganova G, Weinberger A, Mettal U, Mueller D, Neuhaus F, Stalder E, Ishikawa T, Urbanics R, Saxer T, Pfohl T, Szebeni J, Zumbuehl A,Muller B, J Control Release 2017, 264,14. [DOI] [PubMed] [Google Scholar]
- 47.Neuhaus F, Mueller D, Tanasescu R, Balog S, Ishikawa T, Brezesinski G,Zumbuehl A, Angew Chem Int Ed Engl 2017, 56,6515. [DOI] [PubMed] [Google Scholar]
- 48.Fedotenko LA, Stefaniu C, Brezesinski G,Zumbuehl A, Langmuir 2013, 29,9428. [DOI] [PubMed] [Google Scholar]
- 49.Weinberger A, Tanasescu R, Stefaniu C, Fedotenko LA, Favarger F, Ishikawa T, Brezesinski G, Marques CM,Zumbuehl A, Langmuir 2015, 31,1879. [DOI] [PubMed] [Google Scholar]
- 50.Buscema M, Deyhle H, Pfohl T, Zumbuehl A,Muller B, Materials Today Bio 2019, 1, 10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuhaus F, Mueller D, Tanasescu R, Balog S, Ishikawa T, Brezesinski G,Zumbuehl A, Langmuir 2018, 34,3215. [DOI] [PubMed] [Google Scholar]
- 52.Matviykiv S, Prec. Nanomed. 2018, 1, 43. [Google Scholar]
- 53.Arjmand S, Pardakhty A, Forootanfar H,Khazaeli P, Med Hypotheses 2018, 121,137. [DOI] [PubMed] [Google Scholar]
- 54.Vinogradov SV, Bronich TK,Kabanov AV, Adv Drug Deliv Rev 2002, 54,135. [DOI] [PubMed] [Google Scholar]
- 55.Oh JK, Drumright R, Siegwart DJ,Matyjaszewski K, Progress in Polymer Science 2008, 33,448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kabanov AV,Vinogradov SV, Angewandte Chemie-International Edition 2009, 48,5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckmann DM, Composto RJ, Tsourkas A,Muzykantov VR, J Mater Chem B 2014, 2,8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myerson JW, Braender B, McPherson O, Glassman PM, Kiseleva RY, Shuvaev VV, Marcos-Contreras O, Grady ME, Lee HS, Greineder CF, Stan RV, Composto RJ, Eckmann DM,Muzykantov VR, Adv Mater 2018, 30,e1802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo P, Liu D, Subramanyam K, Wang B, Yang J, Huang J, Auguste DT,Moses MA, Nat Commun 2018, 9,130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uman S, Dhand A,Burdick JA, Journal of Applied Polymer Science 2020, 137, 48668. [Google Scholar]
- 61.Liao XS, Yang XS, Deng H, Hao YT, Mao LZ, Zhang RJ, Liao WZ,Yuan MM, Frontiers in Bioengineering and Biotechnology 2020, 8, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qureshi D, Nayak SK, Maji S, Anis A, Kim D,Pal K, European Polymer Journal 2019, 120, 109220. [Google Scholar]
- 63.Bhujbal SS, Darade SB,Dharmadhikari SS, International Journal of Pharmaceutical Sciences and Research 2020, 11,1007. [Google Scholar]
- 64.Gaharwar AK, Peppas NA,Khademhosseini A, Biotechnol Bioeng 2014, 111,441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei L, Cai C, Lin J,Chen T, Biomaterials 2009, 30,2606. [DOI] [PubMed] [Google Scholar]
- 66.Liu M, Song X, Wen YT, Zhu JL,Li J, Acs Applied Materials & Interfaces 2017, 9,35673. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Xu G, Wang C, Li C,Yao P, Int J Pharm 2017, 530,53. [DOI] [PubMed] [Google Scholar]
- 68.Niu YL, Yuan XY, Zhao YH, Zhang WY,Ren LX, Macromolecular Chemistry and Physics 2017, 218, 1600540. [Google Scholar]
- 69.Najafi M, Asadi H, van den Dikkenberg J, van Steenbergen MJ, Fens MH, Hennink WE, Vermonden T, Biomacromolecules 2020, 21, 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghoorchian A, Simon JR, Bharti B, Han W, Zhao XH, Chilkoti A,Lopez GP, Advanced Functional Materials 2015, 25,3122. [Google Scholar]
- 71.Yao SY, Shen ML, Li SJ, Wu XD, Zhang MM, Ma LN,Li YP, Colloids Surf B Biointerfaces 2020, 186,110718. [DOI] [PubMed] [Google Scholar]
- 72.Woo E, Park H,Lee KY, Macromolecular Bioscience 2014, 14,740. [DOI] [PubMed] [Google Scholar]
- 73.Harshe YM, Lattuada M, Langmuir 2012, 28, 283. [DOI] [PubMed] [Google Scholar]
- 74.Jiang JK, Oberdorster G, Biswas P, Journal of Nanoparticle Research 2009, 11,77. [Google Scholar]
- 75.Santander-Ortega MJ, Jodar-Reyes AB, Csaba N, Bastos-Gonzalez D, Ortega-Vinuesa JL, Journal of Colloid and Interface Science 2006, 302,522. [DOI] [PubMed] [Google Scholar]
- 76.Liu AT, Berlin JM, Langmuir 2017, 33,14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papa AL, Korin N, Kanapathipillai M, Mammoto A, Mammoto T, Jiang A, Mannix R, Uzun O, Johnson C, Bhatta D, Cuneo G, Ingber DE, Biomaterials 2017, 139,187. [DOI] [PubMed] [Google Scholar]
- 78.Marosfoi MG, Korin N, Gounis MJ, Uzun O, Vedantham S, Langan ET, Papa A, Brooks OW, Johnson C, Puri AS, Bhatta D, Kanapathipillai M, Bronstein BR, Chueh J, Ingber DE, Wakhloo AK, Stroke 2015, 46, 3507. [DOI] [PubMed] [Google Scholar]
- 79.McMillan J, Batrakova E, Gendelman HE, Prog Mol Biol Transl Sci 2011, 104,563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen C, Li S, Liu K, Ma G, Yan X, Small 2016, 12,4719. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Z, Ukidve A, Gao Y, Kim J, Mitragotri S S, Sci Adv 2019, 5,eaax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Na JH, Lee SY, Lee S, Koo H, Min KH, Jeong SY, Yuk SH, Kim K,Kwon IC, J Control Release 2012, 163,2. [DOI] [PubMed] [Google Scholar]
- 83.Chen HD, Pan H, Li PP, Wang H, Wang X, Pan WS,Yuan Y, Colloids and Surfaces B-Biointerfaces 2016, 143,455. [DOI] [PubMed] [Google Scholar]
- 84.Fish MB, Fromen CA, Lopez-Cazares G, Golinski AW, Scott TF, Adili R, Holinstat M,Eniola-Adefeso O, Biomaterials 2017, 124,169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tosato MG, Maya Giron JV, Martin AA, Krishna Tippavajhala V, Fernandez M de Mele Lorenzo,Dicelio L, Mater Sci Eng C Mater Biol Appl 2018, 90,356. [DOI] [PubMed] [Google Scholar]
- 86.Guo D, Xie G, Luo J, J Phys D Appl Phys 2013, 47,013001. [Google Scholar]
- 87.Sun Han Chang R, Lee JC, Pedron S, Harley BAC, Rogers SA, Biomacromolecules 2019, 20,2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Voinova MV, Rodahl M, Jonson M, Kasemo B, Phys Scr 1999, 59,391. [Google Scholar]
- 89.Ensign LM, Schneider C, Suk JS, Cone R, Hanes J, Adv Mater 2012, 24,3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu N, Schultz KM, Soft Matter 2020, 16,6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crocker JC, Hoffman BD, Methods Cell Biol 2007, 83,141. [DOI] [PubMed] [Google Scholar]
- 92.Duan X, Li Y, Small 2013, 9,1521. [DOI] [PubMed] [Google Scholar]
- 93.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S, J Control Release, 2010, 146,196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y, Microfluid Nanofluidics 2013, 14,77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng B, Liu Y, Zhou Y, Yang L, Zhang G, Liu Y, Nanoscale Res Lett 2015, 10,235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang T, Park C, Choi JS, Cui JH, Lee BJ, J Drug Deliv Sci Technol 2016, 31,130. [Google Scholar]
- 97.Takeda K, Yamasaki Y, Dirisala A, Ikeda S, Tockary TA, Toh K, Osada K, Kataoka K, Biomaterials 2017, 126,31. [DOI] [PubMed] [Google Scholar]
- 98.Shen Z, Ye H, Kröger M, Li Y, Phys Chem Chem Phys 2017, 19,13294. [DOI] [PubMed] [Google Scholar]
- 99.Gomez-Garcia MJ, Doiron AL, Steele RRM, Labouta HI, Vafadar B, Shepherd RD, Gates ID, Cramb DT, Childs SJ, Rinker KD, Nanoscale 2018, 10,15249. [DOI] [PubMed] [Google Scholar]
- 100.Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ, Biorheology 2007, 44,375. [PubMed] [Google Scholar]
- 101.Zhang XH, Halvorsen K, Zhang CZ, Wong WP,Springer TA, Science 2009, 324,1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith DE, Babcock HP,Chu S, Science 1999, 283,1724. [DOI] [PubMed] [Google Scholar]
- 103.Xu C, Wei Z, Gao H, Bai Y, Liu H, Yang H, Lai Y,Yang L, Adv Sci (Weinh) 2017, 4,1600410. [DOI] [PMC free article] [PubMed] [Google Scholar]