Abstract
Keloids are fibroproliferative disorders characterized by high recurrence rates, with few factors known to influence the same. We conducted a study to determine whether keloids histology influences recurrence. This was a prospective longitudinal study to determine whether histopathological parameters of keloid influence recurrence. Patients with keloids managed by surgical excision were followed up at Kenyatta National Hospital between August 2018 and July 2020. The excised keloids were processed for histology using Hematoxylin, /Eosin, Masson, and Trichrome stains. The slides were analyzed for inflammatory cells, fibroblasts and capillary density using the hot spot technique and correlated to keloid recurrence.
Postoperative follow-up was for a minimum of one year.
A total of 90 patients with 104 keloids were recuited in the study. Overall keloid recurrence rate was 28.6 percent. There was a correlation between the absolute count of more than 50 per HPF of lymphocytes, fibroblasts, and macrophages with recurrence of the disease. The sensitivity and specificity for the above parameters were ;lymphocytes 48 and 81 %, macrophages 57 and 83 %, Mast cells 32and 33 % and fibroblasts at 41 and 91% respectively. There was no correlation between mast cells and vascularity status with recurrence. Routine histology should, therefore, be performed to determine these parameters. Close monitoring and second-line therapy should be considered for patients with elevated macrophages and or lymphocytes so as to reduce the risk of recurrence.
Keywords: Keloids, Histology, Recurrence
Introduction
Keloid is a benign skin disorder characterized by excess deposition of collagen in the dermal layer presenting with disfigurement, pain, and pruritus(1,2). The disease has many modalities of treatment broadly classified as surgical and nonsurgical (3,4). All modalities are associated with high recurrence rates (4). Few factors have been shown to influence the recurrence of the disease. Keloid histology has been shown to be heterogeneous with varying amounts of inflammatory and fibroblast cell densities (5,6). Other histological variants include vascularity and collagen deposition densities (7). The majority of studies on keloid histology and its role on recurrence have focused mainly on the status of the keloids margins and not on the morphology or cellular composition of the keloid perse (8,9). To the best of our knowledge, whether keloid cellular and extracellular composition have a prognostic role in the outcome of the disease and whether it can influence decisions on which treatment options to use, has not been documented in the literature. This study was, therefore, conducted to establish whether keloid histological features could influence treatment outcomes.
Patients and Methods
This study was approved by the Kenyatta National Hospital /University of Nairobi Ethics and Research Committee. Patients with keloids managed by surgical excision who consented to the study were recruited. History and physical examination were performed. Pain and pruritus scores were assessed using the visual analog scale. Anatomical locations of keloids were noted for all patients. Surgical excision was done under local or general anesthesia. Surgery was performed by a consultant plastic surgeon with more than five years of surgical experience. Wounds were closed in two layers with polyglycolic acid 3/0 for dermal stitch and nylon 3/0 for transcutaneous suture. All wounds were subjected to post-excision superficial radiation therapy of 15 Gy within 24 hours of surgery. The excised keloid was processed for histology using Hematoxylin, /Eosin, Masson, and Trichrome stains. Depended variables analyzed per slide were absolute counts of lymphocyte, mast cells, macrophages, fibroblasts, and capillary density per high power magnification of 400 (HPF). All slides were reported by an experienced dermatopathologist. On each slide, two cell counts were taken using a hot spot technique with one at the periphery and the other at the center. An average of the two cell counts was calculated to represent the particular slide. The same was repeated for vascularity by measuring capillary density per HPF. Collected data was summarized into three groups. Patients were followed up for one year to determine keloid recurrence. Recurrence was defined as keloids that had grown beyond the excision margins or had pruritus or pain greater than the pre-excision levels requiring further medical intervention. The analysis was performed using SPSS version 20 software. Fischers exact test were performed to compare any significance at 95 percent confidence levels. And mutlivariant analysis done using the Anova Technique.
Results
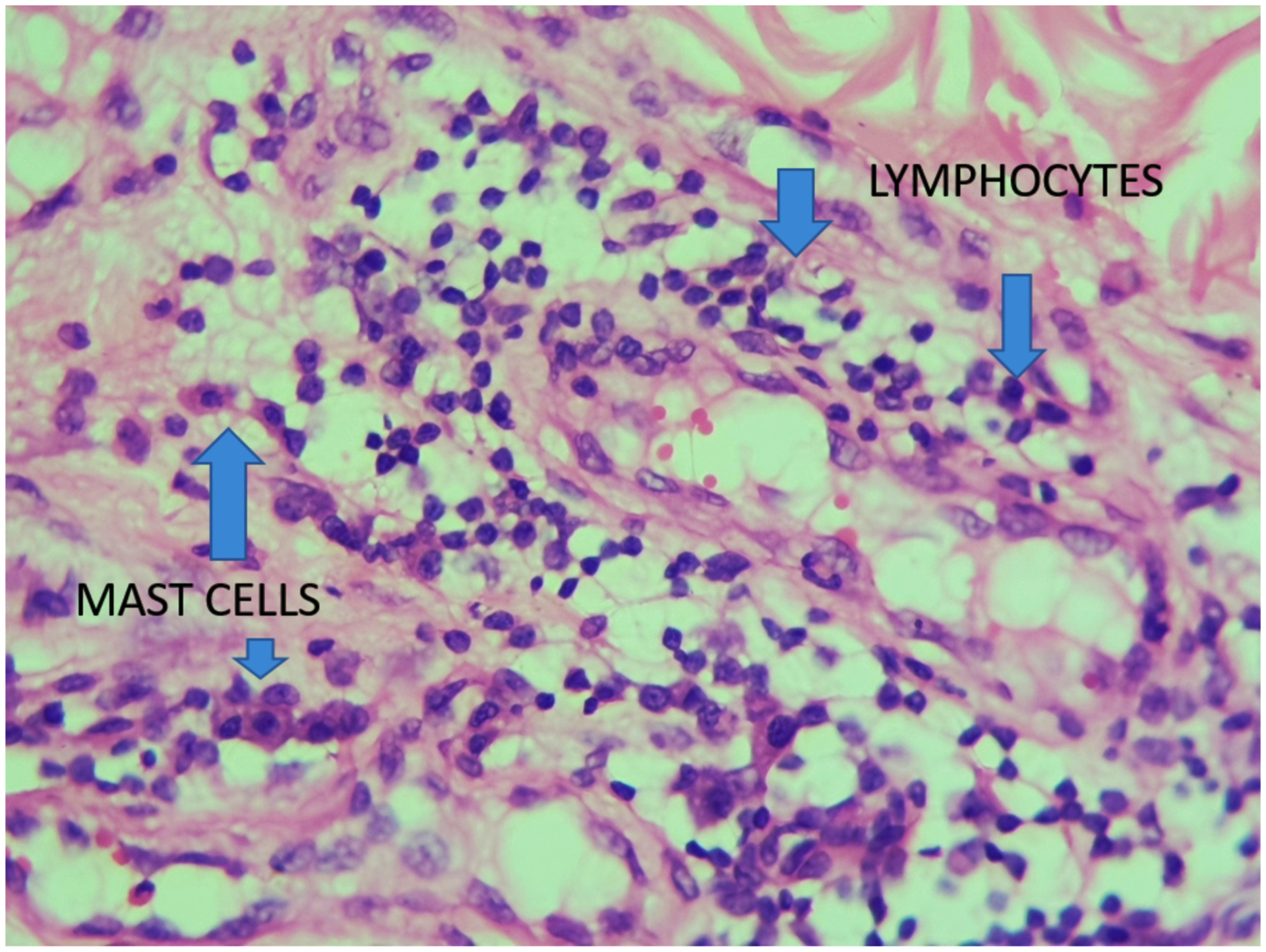
A total of 96 consecutive patients with 113 keloids were recruited for the study. Six patients were lost to follow-up, leaving 90 patients with 104 keloids. The range of age of patients was 15 to 65 years, with a mean age of 29.6. The male to female ratio was 1:2. Forty-seven percent of the keloids were on the ears, followed by the cheek with 14.4 % and abdomen with 12.5 %. All keloid specimens contain inflammatory cells (Figures 2–4). About 60 percent of the slides studied had macrophages less than 50 /HPF, and 4 % had more than 100/HPF. Thirty-seven percent of the specimens had mast cells more than 100/HPF, with only 17 % had less than 50/HPF. Fifty-two percent of specimens have lymphocytes count less than 50/HPF, with 27 % of specimens having fibroblasts more than 100/HPF (Table 1, figure 5).
FIGURE 2.
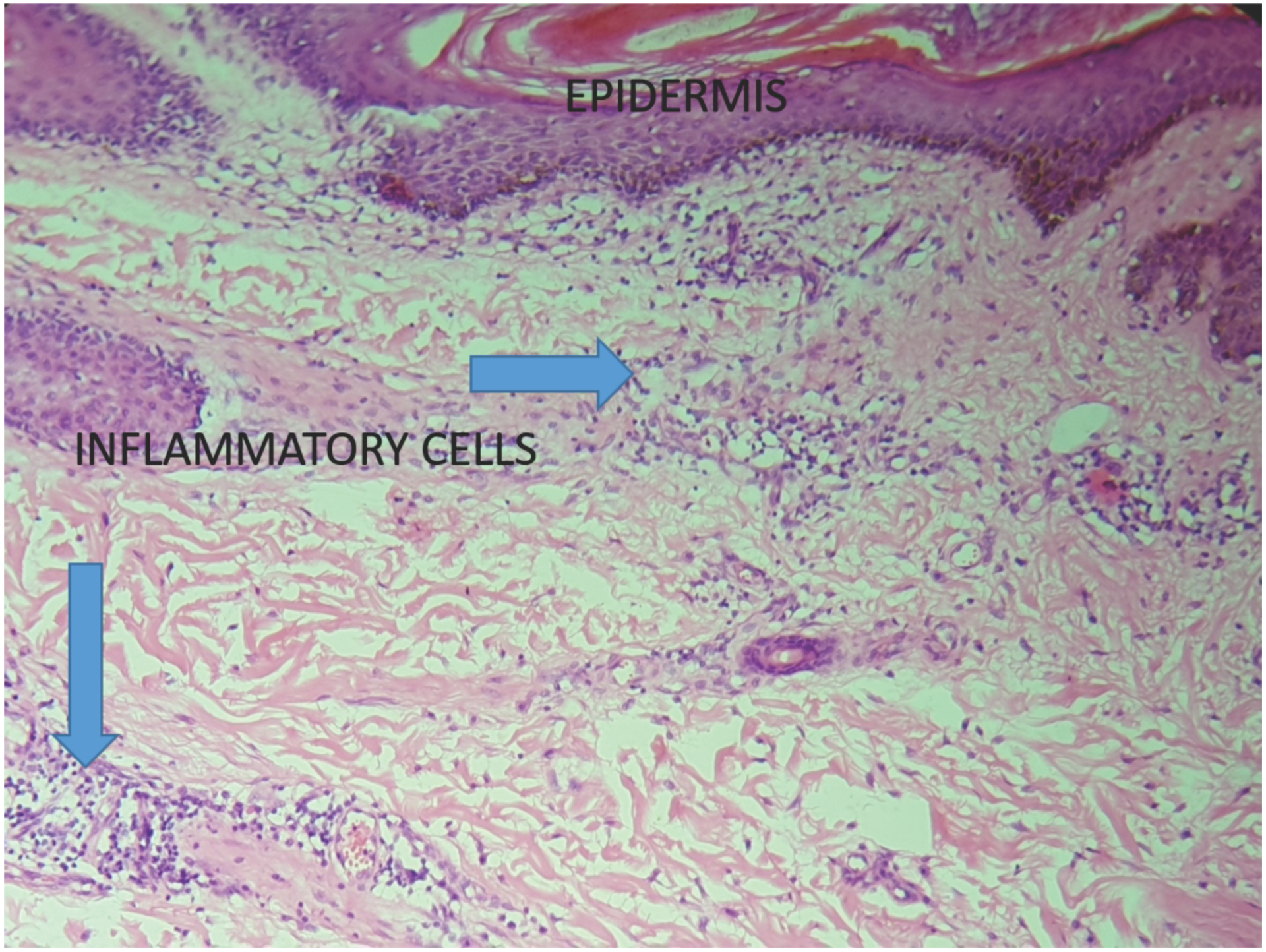
Keloids specimen with a abundance of inflammatory cells at HPF magnification.
Figure 4,
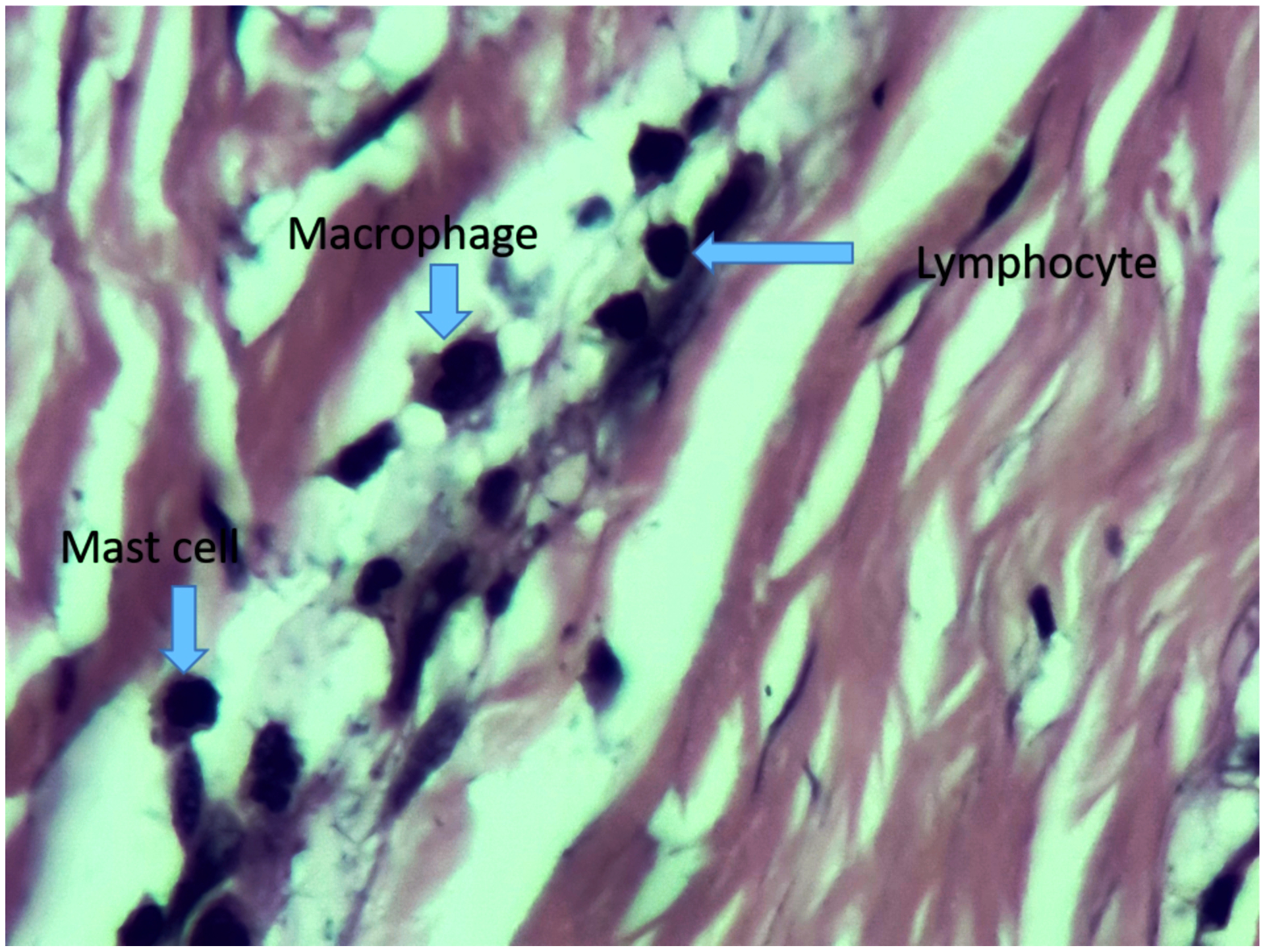
Demonstrates mast cells, lymphocte and macrophage in the same slide at HPF
Table 1.
Anatomical location of the keloids. Forty-seven percent of the keloids are located on the ears
| Anatomical location | Frequency | Percentage |
|---|---|---|
| Ears | 49 | 47 |
| Abdomen | 13 | 12.5 |
| Chest | 10 | 9.6 |
| Cheek | 15 | 14.4 |
| Neck | 3 | 2.8 |
| Upper limb | 3 | 2.8 |
| Back | 10 | 9.6 |
| Scalp | 1 | 1 |
| Total | 104 | 100% |
Figure 5,
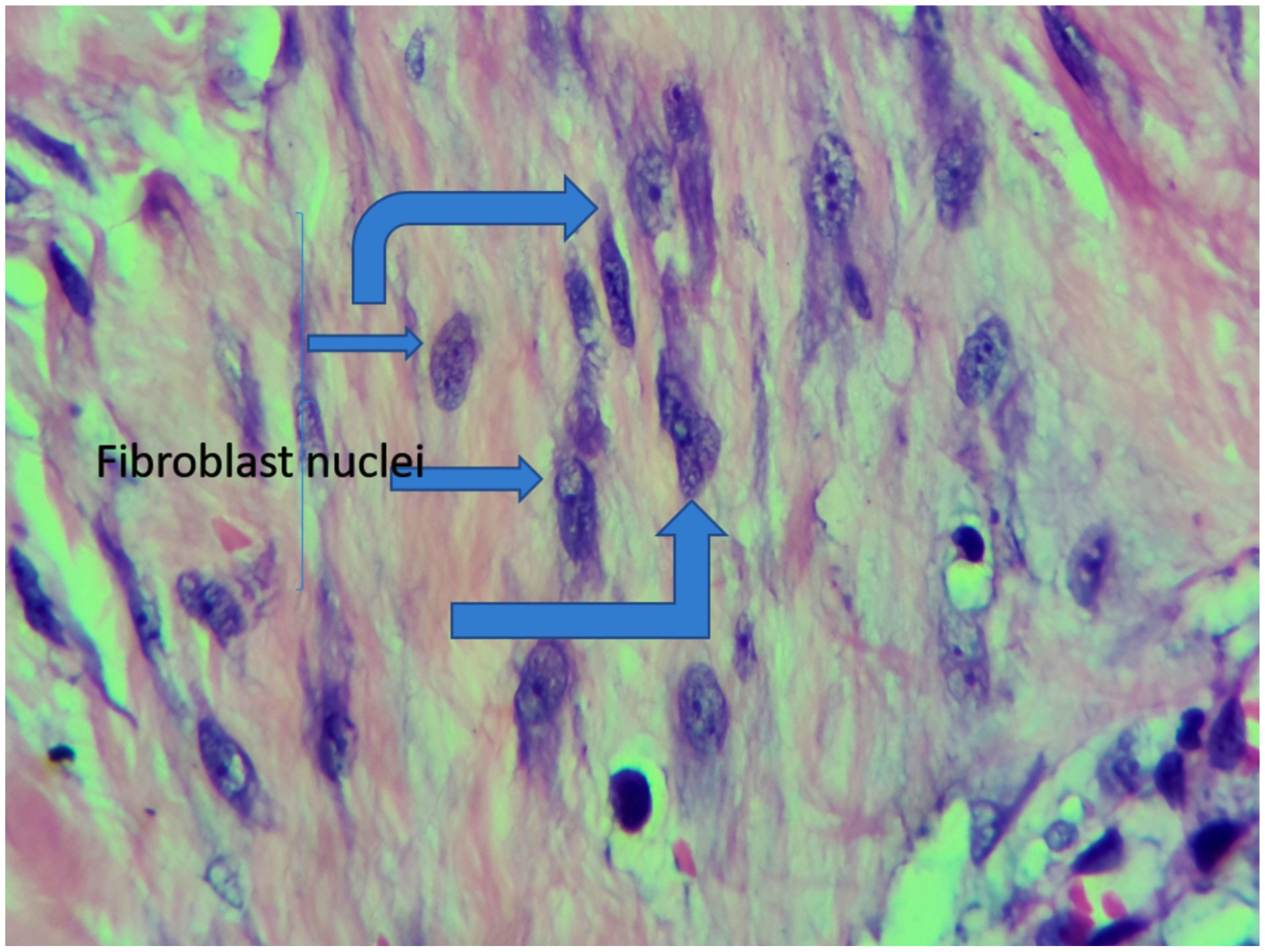
Abundance of fibroblasts and dense collagen fibres in a keloid specimen
Sixty-seven percent of keloids that recurred had lymphocytes count more than 50 per HPF compared to 37 percent of keloids that did not recur (p-value 0.0064). Seventy-one percent of keloids that recurred had macrophages count more than 50 /HPF compared to 26 % of keloids that did not recur (p-value 0.05). Eighty-two percent of keloids that recurred had a mast cell count of more than 50 per HPF compared to 83 % of the keloids that did not recur (p-value 0.128). Ninety-four percent of keloids that recurred had fibroblast count more than 50/HPF compared to 66 % of keloids that did not recur (p-value 0.005). Seventy-one percent of keloids that recurred had capillary density greater than 5/HPF compared to 70.5 % of keloids that recurred (p-value > 0.05). The sensitivity and specificity of lymphocytes count of more than 50/HPF in the recurrence of keloids were 48% and 81%, respectively. The macrophage counts of more than 50/HPF had a sensitivity of 57 % and a specificity of 83 % with mast cell sensitivity of less than 32 % and specificity of 33%. Fibroblasts count of more than 50/HPF have a sensitivity determining the recurrence of keloids, and a count of less than 50/HPF has a sensitivity of 41 percent with a specificity of 91% (Table 3).
Table 3A.
Comparison of histological parameters assessment in patients who have keloid recurrence and those without recurrence
| Parameter Assessed | Count/10 HPF | No recurrence N=70 | Recurrence N=34 | P-value | Senstivity % | Specificity % |
|---|---|---|---|---|---|---|
| Lymphocytes | 0. 00064 | 48 | 81 | |||
| <50 | 44 | 10 | ||||
| 50–100 | 18 | 22 | ||||
| >100 | 8 | 2 | ||||
| Macrophages | 0.000066 | 57 | 83 | |||
| <50 | 52 | 10 | ||||
| 50–100 | 16 | 22 | ||||
| >100 | 2 | 2 | ||||
| Mast cells | 0.128742 | 32 | 33 | |||
| <50 | 12 | 6 | ||||
| 50–100 | 28 | 20 | ||||
| >100 | 30 | 8 | ||||
| Fibroblasts | 0.007191 | 41 | 91 | |||
| <50 | 24 | 2 | ||||
| 50–100 | 28 | 20 | ||||
| >100 | 18 | 12 | ||||
| Capillary density /hpf |
0.1767 | |||||
| <5 | 20 | 10 | ||||
| 5–10 | 44 | 12 |
Discussion
Our study analyzed inflammatory cells, fibroblasts, and vascularity of keloids as variables that could influence the recurrence of keloids. All patients underwent the same surgical procedures with post-excision superficial radiation therapy. The surgeries were performed in a standardized method by an experienced plastic surgeon. All wounds were closed with similar suture materials. All patients have received the same prophylactic antibiotics and analgesia regime. Postoperative management included post-excision superficial radiation therapy for all patients. All specimens were analyzed by a dermatopathologist with a special interest in keloids pathology. The minimum follow-up duration was one year.
Important findings in this study were that keloids in our patient’s cohort had an elevated inflammatory cell population of macrophages, lymphocytes, and mast cells; thus, it was strongly suggested that they might have a critical role in keloid pathogenesis as demonstrated by a number of other studies (6,10–12). Regarding the inflammatory cell count, mast cells were the most elevated with a count of more than 50 per HPF in at least 87 percent of the slides analyzed. Equally raised were fibroblasts with at least 75 percent of slides with more than 50 per HPF. Macrophages were the least elevated, with more than 60 percent of slides with counts less than 50 per HPF. These findings were consistent with several studies that have demonstrated elevated inflammatory cells in many keloid specimens (6,10–12). Ali et al., in a study in Kenya, demonstrated high inflammatory cell counts in most keloid specimens (6). Bagabir R.et al. demonstrated not only an increase in T-lymphocytes but also a high CD4: CD8 ratio. In addition, aggregation of lymphoid tissue in the keloid, referred to as the keloid-associated lymphoid tissues, were observed in at least 15% of the histological specimens (11). Their study did not, however, correlate the histological findings with disease recurrence. Similar findings were noted by Boyce et al., who noticed that the keloid specimens have a higher concentration of lymphocytes and macrophages than that of the normal skin (10). T Beer et al. found a high absolute count of mast cells not only in keloids but also in the normal scars and hypertrophic scars (12).
Analysis between keloids cell count and recurrence of the disease revealed a positive correlation between the absolute cell count of lymphocytes, macrophages, and fibroblasts with recurrence. Mast cells and vascularity of the keloids did not have any correlation with keloid recurrence. Of all variables assessed in our study, elevated macrophages of more than 50 per HPF had the highest sensitivity and specificity of 57 and 83 %, respectively, in determining keloid recurrence. These could be a pointer to the influence of macrophages on the pathogenesis and severity of the keloid disease. Lymphocytes count of more than 50 per HPF had a sensitivity of 48 % and a specificity of 81 percent, with mast cell count being the least sensitive and specific in determining keloid recurrence. Though morphological studies of the macrophages were not conducted in this study, previous papers had demonstrated macrophage Type 2 (M2) to be the predominant macrophage in keloids compared to Type 1 (M1) (14). M2 sub-type is predominantly anti-inflammatory. They have also been shown to have a strong angiogenetic potential compared to M1, a factor that could be critical in keloid formation (15). They produce platelet-derived growth factor and fibroblast growth factors that stimulate the proliferation of fibroblasts and stimulate collagen synthesis that is critical in keloid formation (14,15). In addition, they are thought to stimulate lymphocytes activities in keloids, which could be responsible for chronic inflammation that is now thought to be an essential component of keloid disease (7 16,17).
The significance of keloid histology in the management and recurrence of keloids was previously thought to be of little importance (18,19). Park TH et al. on investigating the role of clinical-pathological factors of keloids and recurrence found out that there was no correlation between keloid histological features and recurrence of the disease (18). Their study, unlike this study, analyzed factors such as lymphocytic infiltration in the upper dermis, peri-vascular lymphocytic infiltration in keloid, presence of an epidermal cyst, presence of foamy histocytes and foreign body reaction. They did not consider the quantitative numbers of inflammatory cells or fibroblasts as a confounding factor to keloid recurrence as done in this study. Gulamhuseinwala et al. on analysis of excised suspected keloid histology specimens concluded that with good clinical judgment, there was no need for histological analysis of the keloid specimen, whether for diagnosis or to assist with guiding treatment (19).
Surprising findings in our study was the insignificance of absolute mast cells counts in keloid recurrence. Mast cell count was found to have the least sensitivity and specificity in determining the recurrence of keloids among the inflammatory cells. Mast cells had previously been thought to be critical in keloid formation. They were thought to be responsible for pain and pruritus in patients with keloid (7,19). A number of studies had demonstrated not only high mast cell count in keloid patients but also in normal skin and hypertrophic scar casting doubt to whether the absolute mast cell numbers have any relevance in keloid scarring and recurrence (7,20). In our study, more than 85 percent of patients had an absolute mast cell count of more than 50 per HPF. There was no statistical significance on the absolute number of counts and recurrence of the disease. Amandale et al., in their study, demonstrated activated mast cells that were tryptase positive to be elevated in keloids than normal skin, probably implying that it was not the absolute number of mast cells that could be critical in keloid formation and recurrence but the activated mast cells (21).
Conclusion
Keloid histology, as demonstrated in this study, has a role in the management of keloids. The histological variants such as lymphocytes, macrophages, and fibroblasts could influence the recurrence of the disease. Routine analyses of keloid specimens for these variants should thus be encouraged since they may have a bearing on predicting keloid recurrence and could thus influence the management of the patient. Patients with keloid with elevated levels of either macrophages or lymphocytes in histology specimens should be monitored closely for recurrence.
Figure 1.
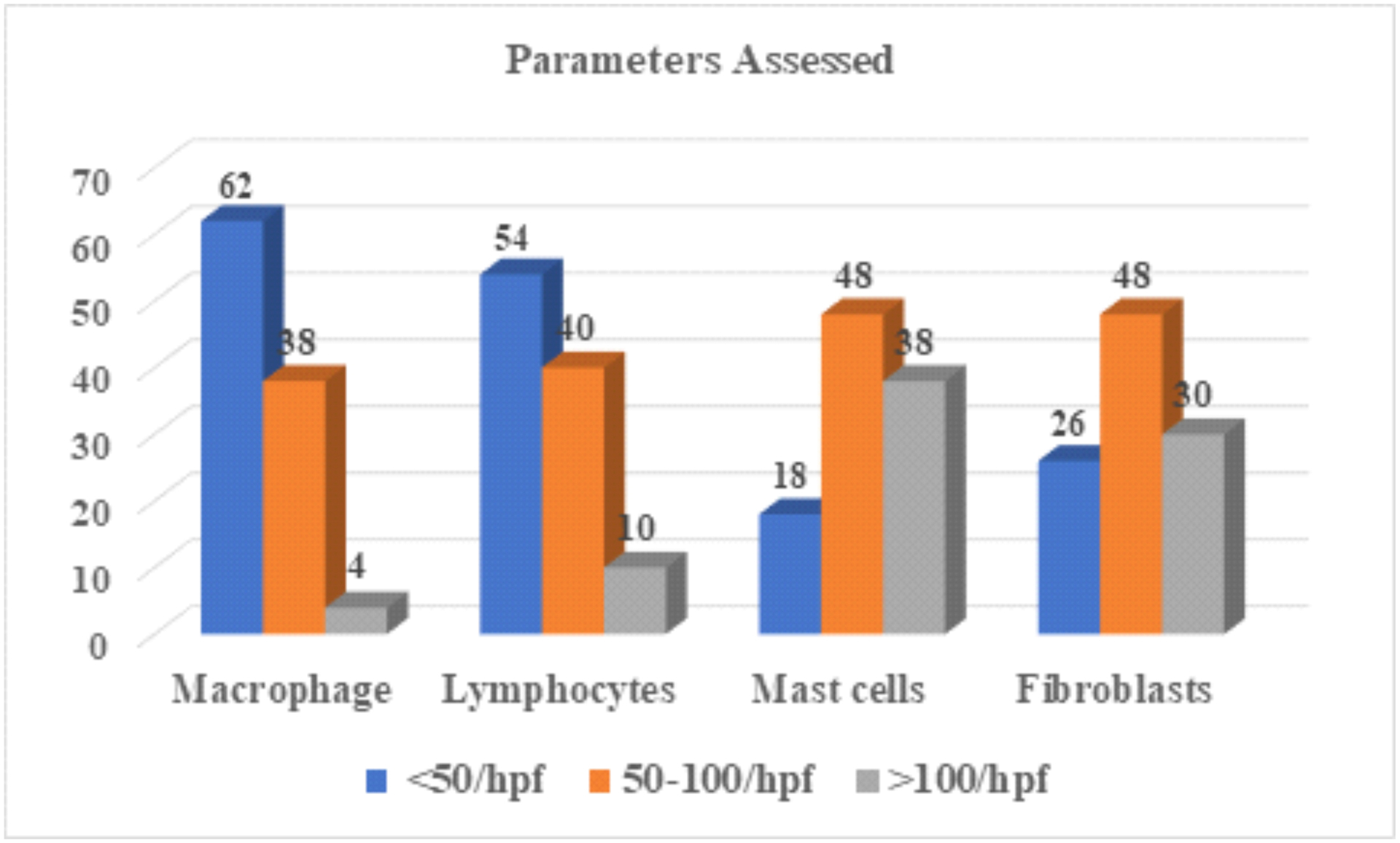
Mean cell counts of lymphocytes, fibroblast,mast cells and macrophages. Major aity of slides had mast cells and macrophages count more than 50 per HPF.
Figure 3,
Thick collagen bundles with inflammatory cells and fibroblasts
Table 2A.
Assessment of histological parameters in each keloid. Mast cells have more than 75 percent of slides with a count of more than 50 per HPF
| Parameters | <50/hpf | 50–100/hpf | >100/hpf | P-value |
|---|---|---|---|---|
| Macrophage | 62 | 38 | 4 | 0.000 |
| Lymphocytes | 54 | 40 | 10 | 0.000 |
| Mast cells | 18 | 48 | 38 | 0.001 |
| Fibroblasts | 26 | 48 | 30 | 0.019 |
| Parameters | <5/hpf | 5–10/hpf | >10 | |
| Capillary | 30 | 56 | 18 | 0.000 |
Table 2B.
Anova test for Variance
| Parameters | Sum of squares | Df | Mean squares | F | P-value |
|---|---|---|---|---|---|
| Between groups | 1773.33 | 2 | 886.67 | 4.375 | 0.374 |
| Within groups | 2432 | 12 | 202.67 | ||
| Total | 4205.33 | 14 |
Table 3B,
Anova test for Variance
| Parameters | Sum of squares | df | Mean squares | F | P-value |
|---|---|---|---|---|---|
| Between groups | 1235.57 | 1 | 1235.57 | 9.537 | 0.005 |
| Within groups | 3368.29 | 26 | 129.55 | ||
| Total | 4603.86 | 27 |
Footnotes
Conflicts of Interest / Sources of Funding: none declared
References
- 1.Brissett A, Sherris DA Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg 2001; 17:263–272. [DOI] [PubMed] [Google Scholar]
- 2.Alexander,Petra P Hypertrophic scars and keloids-A review of their pathophysiology and therapeutic management, Dermatologic Surgery. March2009, 35 (2) 171–181 [DOI] [PubMed] [Google Scholar]
- 3.Davis SA,Feldman SR,McMichael AJrvey. Management of keloids in the United States, 1990–2009: An analysis of the National Ambulatory Medical Care Survey. Dermatol Surg. 2013; 39(7):988–94(ISSN: 1524–4725) [DOI] [PubMed] [Google Scholar]
- 4.Nast A, Eming S, Fluhr J, et al. German S2k guidelines for the therapy of pathological scars (hypertrophic scars and keloids). J Dtsch Dermatol Ges.. 2012October;10(10):747–62. [DOI] [PubMed] [Google Scholar]
- 5.Chike-Obi CJ, Cole PD, Brissett AE. Keloids: pathogenesis, clinical features, and management. Semin Plast Surg. 2009;23(3):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6..Ali MM, Karanja FW, Nang’ole FW, Opot EON, Mulehane KO, Mbithi DZ Determination of the prevalence, clinical characteristics and histopathological features of keloids in patients managed at the Kenyatta National Hospital. East Afri. Med. J 2019; 96(1); 2220–2229 [Google Scholar]
- 7.jumper N, Raus R, A Bayat Functional histopathology of Keloid disease Histo Histopahtology (2015) 1033–1057 [DOI] [PubMed] [Google Scholar]
- 8.Tan Kian Tjon; Shah Nigam Pritchard, Susan A. The influence of surgical margins on keloid recurrence Annals of Plastic Surgery: 2010;64; 1 - p 55–58 [DOI] [PubMed] [Google Scholar]
- 9.Goutos I Intralesional excision as a surgical strategy to manage keloid scars: what’s the evidence?. Scars Burn Heal. 2019;5:2059513119867297. Published 2019 Aug 27. doi: 10.1177/2059513119867297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce DE, Ciampolini J, Ruge F, Murison MS and Harding KG Inflammatory-cell subpopulations in keloid scars. Br. J. Plast. Surg 2001; 54, 511–516 [DOI] [PubMed] [Google Scholar]
- 11.Bagabir R, Byers RJ, Chaudhry IH, Muller W, Paus R and Bayat A Site-specific immune-pheno-typing of keloid disease demonstrates immune regulation and the presence of lymphoid aggregates. Br. J. Dermatol 2012; 167, 1053–1066 [DOI] [PubMed] [Google Scholar]
- 12.Beer T, Baldwin H, West L, Gallagher P, and Wright D Mast cells in pathological and surgical scars Br J Ophthalmol. 1998June; 82(6): 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13..Li Xuechuan, Wang Yu, Yuan Bo Yang HUizhong Qiao Liang Status of M1 and M2 type macrophages in Keloids. Int J. Clin Exp Pathology, 10 (11) (2017), pp. 11098–11105 [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Xiangwen, Gu Shuchen, Huang Xin, The role of macrophages in the formation of hypertrophic scars and keloids burns & Trauma,2020, 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jetten Nadine, Verbruggen Sanne, Gijbels Marion J. Anti-inflammatory M2, but not pro- inflammatory M1 macrophages promote angiogenesis in vivo Angiogenesis, 2014; 17, pages109–118 [DOI] [PubMed] [Google Scholar]
- 16..Jin Qi 1, Gui Lai 1, Niu Feng 1, Yu Bing Macrophages in Keloid Are Potent at Promoting the Differentiation and Function of Regulatory T Ce Exp Cell Res 2018;15;362(2):472–476. [DOI] [PubMed] [Google Scholar]
- 17..Nangole Ferdinand W. aAgak George W. bKeloid patho-physiology: fibroblast or inflammatory disorders? JPRAS Open: Vol 22, December2019, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park Tae Hwan 1, Lee Boram, Park Ji Hae Do Histopathologic Parameters Affect the Rate of Recurrence in Auricular Keloid Patients? J Craniofac Surg 2015;26(7):e571–3. [DOI] [PubMed] [Google Scholar]
- 19.Nadim Gulamhuseinwala; Simon Mackey; Peter Meagher; More Should Excised Keloid Scars Be Sent for Routine Histologic Analysis? Annals of Plastic Surgery. 2008; 60(2):186–187. [DOI] [PubMed] [Google Scholar]
- 20..Wilgus Traci A. * and Wulff Brian C., The Importance of Mast Cells in Dermal Scarring v Wound Care (New Rochelle). 2014. April 1; 3(4): 356–365. doi: 10.1089/wound.2013.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammendola M, Zuccalà V, Patruno R, Russo E, Luposella M, Amorosi A, VescioSammarco G GTryptase-positive mast cells and angiogenesis in keloids: a new possible post-surgical target for prevention, Updates Surg, 2013; 65 (1);, pp. 53–57 [DOI] [PubMed] [Google Scholar]


