Abstract
Introduction
Adoption of robotic surgery in pediatrics has been slow. Robotic surgery within spatially-constrained workspaces in children makes traditional platforms less translatable. Da Vinci's newest single port (SP) robotic platform provides narrow, and deep access, making pediatric robotic surgery more feasible.
Case presentation
A five-year old female presented with hepatosplenomegaly due to hemolytic anemia from pyruvate kinase deficiency (PKD). When she progressed to requiring monthly transfusions, a splenectomy was performed to avoid the complications associated with frequent blood transfusions. The robotic approach was used to remove the intact spleen because traditional minimally invasive surgery can result in post-operative splenosis.
Discussion
The patient successfully underwent single-port, robotic splenectomy - the first known splenectomy in a child using this approach. Furthermore, during the operation an accessory spleen was encountered in the omentum and was also successfully removed robotically. The patient tolerated the procedure well.
Conclusion
This case demonstrates that the SP robot can be used for splenectomy to eliminate the risk of splenosis and achieve a superior cosmetic result.
Keywords: Case report, Splenectomy, Robotic, Single port, Pediatric, da Vinci
Highlights
-
•
Child with Pyruvate Kinase Deficiency (PKD) refractory to medical management.
-
•
First known single port robotic splenectomy in a child.
-
•
Robotic approach yields decreased postoperative pain, improved cosmetic results, and eliminates the chance of splenosis.
1. Introduction [[1], [2], [3], [4], [5], [6]]
Widespread adoption of robotic surgery in pediatrics has been slow because minimal access surgery within spatially-constrained workspaces in children makes traditional platforms less translatable. Da Vinci's single port (SP) robotic platform appears to make pediatric robotic surgery more feasible.
SP surgery can be performed with a single incision. We used a Pfannenstiel incision, well concealed in the lower abdominal crease, for removal of the intact spleen, eliminating the concern for splenosis. This approach also minimized the scarring and incisional pain associated with multiple points of entry used during laparoscopic surgery.
The SP consists of a flexible 3-D camera and three flexible, interchangeable instruments, all of which pass through a 2.5 cm cannula. The instruments have mid instrument flexion and can be manipulated immediately beyond the end of the cannula. Once deployed the telescope and instruments are functional within a 7 cm diameter space. This technology provides narrow, deep access [1] making it highly applicable to pediatric surgery.
A full array of interchangeable instruments are available for tissue manipulation, suturing, and cauterization. Notably, the SP allows the telescope to move in a full 180° arc without moving instruments relative to the tissue field, improving visualization in areas that previously were difficult to access. Finally, the robot can rotate in a complete 360° circle without changing ports, allowing the surgeon to access the complete abdomen.
Our patient suffered from Pyruvate kinase deficiency (PKD), a rare hemolytic anemia. Hepatosplenomegaly is due to extramedullary hematopoiesis and trapped cells leading to engorgement. Patients present with anemia, jaundice, hepatosplenomegaly and possibly cholelithiasis. The primary treatment is blood transfusion [2]. Splenectomy for hypersplenism is reserved for patients who require chronic transfusions. When splenectomy is required, the standard of care is laparoscopic splenectomy [3]. However, splenosis is a risk with this approach. The advent of the SP system made a robotic approach more feasible, safer and provided an opportunity for the standard of care to be advanced.
2. Patient information and clinical findings
This patient was a five years-old Romani female who suffered from hemolytic anemia due to PKD. Splenectomy was delayed because this patient's mutation was associated with decreasing transfusion requirements with age. Transfusion requirements, however, progressively increased. The patient suffered from hepatosplenomegaly, transfusion iron overload, erythrocyte alloimmunization, multiple admissions for fever of unknown origin and chronic cholestatic jaundice. After extended discussions the family agreed to splenectomy by Dr. Thom Lobe.
On preoperative examination the patient was active with normal affect. She had mild scleral icterus, the liver was noted 2–3 cm below the costal margin and the spleen 11 cm below the costal margin. The skin was jaundiced in the face, trunk and abdomen. Her blood-work 4 days prior to operation revealed hemoglobin = 8.1, total bilirubin = 3.5 and ferritin = 3046 NG/ML (ref range 5–116 NG/ML).
3. Timeline
-
•
Born 1/2016 at 38 WGA, shortly after diagnosed with PKD.
-
•
4/2016 Right upper extremity midline venous catheter placed for frequent transfusions.
-
•
10/2017 MRI heart/liver demonstrated splenomegaly to 12 cm and transfusion-associated iron overload.
-
•
3/2018 Patient prescribed iron chelator.
-
•
2/2019 MRI abdomen demonstrated splenomegaly (14.6 cm).
-
•
7/2019–8/2020 Admitted multiple times for fever and empiric antibiotics.
-
•
1/2021 - Received pre-splenectomy pneumococcal & meningococcal vaccines (Hib administered in 2016).
-
•
2/28/2021 SP robotic splenectomy, patient discharged four days later taking acetaminophen for pain and prophylactic penicillin.
-
•
3/28/2021 Patient was tolerating diet, having regular bowel movements, and not requiring pain medications.
4. Diagnostic assessment
-
•
Physical exam at birth revealed hepatosplenomegaly, laboratory values showed hyperbilirubinemia, anemia, and thrombocytopenia.
-
•
Genetic testing revealed homozygous PKLR mutational deletion of exon 11, an autosomal recessive inheritance seen in Romani families causing PKD.
-
•
Postoperative pathology reported a 16.9 × 8.3 × 6.7 cm spleen weighing 361 g consisting of benign splenic tissue with congestion and extramedullary hematopoiesis and confirmed the presence of an accessory spleen.
5. Intervention
The patient was admitted the night prior to the operation for RBC transfusion and IV fluid administration. Overnight IV fluids were administered to prevent sludging secondary to hypersplenism in the setting of NPO.
Under general anesthesia, Foley catheter was placed and the patient was prepped and draped and prophylactic cefazolin was administered.
 Preoperative marking for Pfannenstiel incision and palpable caudal extent of spleen. |
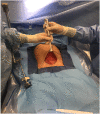 Skin flap raised in preparation for single port. |
A curvilinear incision was made in the lower abdominal crease and the flap was raised cephalad to the level of the umbilicus. Midway between the incision and the umbilicus, underneath the flap, the single-port was inserted in the midline, and the abdomen was insufflated.
The port was used to dock the robot, and the short gastric vessels were divided with cautery. A laparoscopic Kittner was placed as a retractor through an accessory 5 mm cannula, inserted in the midline just cephalad to the port. Attention was then turned posteriorly where the spleen was retracted anterolaterally, exposing the hilum. Hilar tissue was divided with cautery until the splenic vessels were encountered.
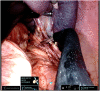 Clipping of splenic vessels. |
 Ex-vivo spleen removed completely intact, measuring 16.9 cm. |
The splenic vein and artery were divided between clips, and the rest of the hilum was divided to completely free the spleen. Prior to undocking the robot, a small accessory spleen was seen in the omentum and removed. The incision was opened in the midline from the umbilicus to pubis to insert a hand and extract the intact spleen.
Once the spleen was removed, the abdomen was closed. Estimated blood loss was 20 ml. A caudal regional block was performed for postoperative analgesia. The length of the surgery was 2 h and 44 min. Work has been reported in line with the SCARE 2020 criteria [6] and has been registered at ClinicalTrials.gov (NCT04899557).
6. Follow-up and outcomes
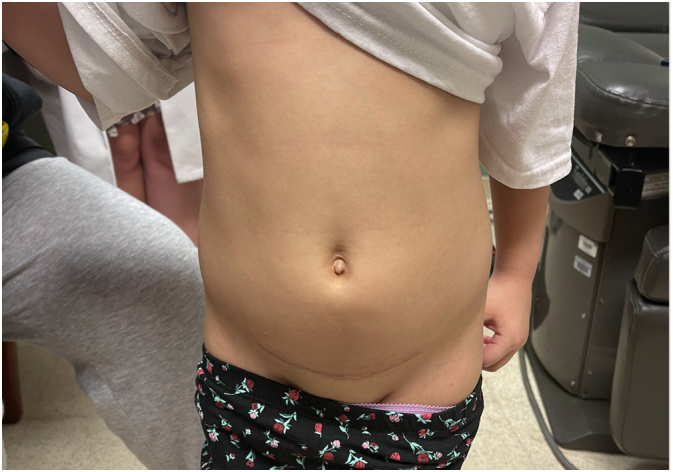
One month follow up photograph of hidden incision in abdominal crease.
The patient tolerated the procedure well and was seen in clinic one month after surgery. She did not require additional analgesics but took benadryl for a rash that developed around the incision. She had not required blood transfusion since before surgery.
7. Discussion
Partial splenectomy, open splenectomy, and laparoscopic splenectomy are all alternatives to robotic splenectomy. Although robotic surgery can be more costly than traditional approaches, the increased functionality gives the experienced robotic surgeon far more tools to safely perform an operation. The robotic approach has the same relative and absolute contraindications as any laparoscopic surgery: inability to tolerate pneumoperitoneum, uncorrectable coagulopathy, abdominal compartment syndrome, abdominal wall infection, and previous extensive abdominal surgery [4].
When the splenic capsule is violated there is opportunity for tiny fragments of splenic tissue to seed the abdomen and the risk of splenosis after laparoscopic splenectomy is well documented. Splenosis can cause abdominal pain, adhesions and the need for additional surgery [5]. Remaining splenic tissue can also defeat the benefits of splenectomy for patients suffering from hemolytic disease. Given this concern the family followed our recommendation for a robotic approach.
8. Conclusion
In this case, the SP robotic splenectomy was safe and effective. The curvilinear incision in the abdominal crease provided cosmetic improvement over multiple abdominal trocar sites, and the patient reported less pain than usual with a prompt return to full activity. The cost, length of procedure and long-term complications must all be considered, but this operation sets an exciting precedent for SP robotic surgery and pushes the needle forward for what is possible in children's robotic surgery.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
University of Illinois at Chicago Department of Surgery.
Ethical approval
Research studies involving patients require ethical approval. Please state whether approval or exemption has been given, name the relevant ethics committee and the state the reference number for their judgement. Please give a statement regarding ethnical approval that will be included in the publication of your article, if the study is exempt from ethnical approval in your institution please state this.
Approval given by the University of Illinois at Chicago Institutional Review Board. The research protocol number is 2014-0396.
Informed consent(s)
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
a) Waiver of Informed Consent granted under [45 CFR 46.116(d)]
Assent(s):
a) Waiver of Child Assent granted [45 CFR 46.116(d)] for the retrospective phase of
research.
Parental Permission(s):
b) Waiver of Parental Permission granted [45 CFR 46.116 (d)] for the retrospective chart
review phase.
HIPAA Authorization(s):
a) Waiver of Authorization granted under [45 CFR 164.512(i)]
Author contribution
Greg Klazura, original draft, project administration.
Thomas Sims, review & editing.
Marko Rojnica, review & editing.
Nathaniel Koo, review & editing.
Thom Lobe, review and editing.
Registration of research studies
https://clinicaltrials.gov/ct2/show/NCT04899557.
https://clinicaltrials.gov/ct2/show/NCT04899557?id=04899557&draw=2&rank=1.
Guarantor
Greg Klazura, MD, Thom Lobe, MD.
Declaration of competing interest
No conflicts of interest.
Contributor Information
Greg Klazura, Email: greg.klazura@gmail.com.
Thomas Sims, Email: tlsims@uic.edu.
Marko Rojnica, Email: mrojnica@uic.edu.
Nathaniel Koo, Email: nkoo2@uic.edu.
Thom Lobe, Email: tlobe@uic.edu.
References
- 1.Intuitive.com www.intuitive.com/en-us/healthcare-professionals/surgeons
- 2.Karakousis, George, and Douglas Fraker. Greenfield's Surgery: Scientific Principles & Practice, by Michael W. Mulholland et al., Wolters Kluwer, 2017, pp. 1276–1276.
- 3.Prchal Josef. Pyruvate kinase deficiency. In: Mahoney Donald, Tirnauer Jennifer., editors. UpToDate. 29 May 2020. www.uptodate.com/contents/pyruvate-kinase-deficiency?search=pyruvate+kinase+deficiency [Google Scholar]
- 4.Guidelines for Diagnostic Laparoscopy - a SAGES Publication. SAGES; 5 Jan. 2021. www.sages.org/publications/guidelines/guidelines-for-diagnostic-laparoscopy [Google Scholar]
- 5.Sato Masahito. A case of splenosis after laparoscopic splenectomy. Pediatr. Surg. Int. 2007;23(10):1019–1021. doi: 10.1007/s00383-007-1989-4. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]


