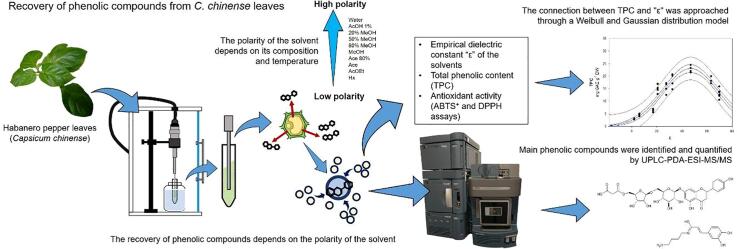
Graphical abstract
Abbreviations: UPLC, Ultra-high performance liquid chromatography; PDA, Photodiode detector array; MS, mass spectrometry; ε, dielectric constant; CE, Conventional extraction; UAE, Ultrasound-Assisted extraction; TPC, Total phenolic content; GAE, Gallic acid equivalent; Eq CA, Caffeic acid equivalents; Eq L, Luteolin equivalents; Eq A, Apigenin equivalent; Eq Q, Quercetin equivalents; Dry wt, Dry weight; HT, Proton transfer; SET, Simple electron transfer; Hx, Hexane; AcOEt, Ethyl acetate; Ace, Acetone; 80% Ace, 80% Acetone; MeOH, Methanol; 80% MeOH, 80% Methanol; 50% MeOH, 50% Methanol; 20% MeOH, 20% Methanol; 1% AcOH, Acetic acid; W, Water
Keywords: Capsicum chinense leaves, Phenolic compounds, Solvent polarity, Dielectric constant, Mass spectrometry, UAE
Highlights
-
•
Ultrasound is a fast method for phenolic compounds recovery from C. chinense leaves.
-
•
Solvent polarity has a strong effect on the recovery of phenolic compounds.
-
•
Dielectric constant was propose to predict the most appropriate solvent for phenolic extraction.
-
•
Antioxidant activity correlates with total phenolic content of C. chinense leaves extracts.
-
•
Identification of phenolic compounds was performed by UPLC-PDA-ESI-MS/MS.
Abstract
Phenolic compounds are secondary metabolites involved in plant adaptation processes. The development of extraction procedures, quantification, and identification of this compounds in habanero pepper (Capsicum chinense) leaves can provide information about their accumulation and possible biological function. The main objective of this work was to study the effect of the UAE method and the polarity of different extraction solvents on the recovery of phenolic compounds from C. chinense leaves. Quantification of the total phenolic content (TPC), antioxidant activity (AA) by ABTS+ and DPPH radical inhibition methods, and the relation between the dielectric constant (ε) as polarity parameter of the solvents and TPC using Weibull and Gaussian distribution models was analyzed. The major phenolic compounds in C. chinense leaves extracts were identified and quantified by UPLC-PDA-ESI-MS/MS. The highest recovery of TPC (24.39 ± 2.41 mg GAE g−1 dry wt) was obtained using MeOH (50%) by UAE method. Correlations between TPC and AA of 0.89 and 0.91 were found for both radical inhibition methods (ABTS+ and DPPH). The Weibull and Gaussian models showed high regression values (0.93 to 0.95) suggesting that the highest phenolic compounds recovery is obtained using solvents with “ε” values between 35 and 52 by UAE. The major compounds were identified as N-caffeoyl putrescine, apigenin, luteolin and diosmetin derivatives. The models presented are proposed as a useful tool to predict the appropriate solvent composition for the extraction of phenolic compounds from C. chinense leaves by UAE based on the “ε” of the solvents for future metabolomic studies.
1. Introduction
The habanero pepper (Capsicum chinense Jacq.) is a representative crop of the Mexican Yucatan Peninsula with a great cultural and commercial importance [15]. As this region is recognized since 2010 by the protection designation of origin (PDO) as the center of habanero pepper production and domestication, the measurements of regulated parameters are necessary to guarantee its quality [22], [41]. Plant growth can be affected by different factors such as soil moisture, nutrient deficiency, exposure to UV-B radiation, temperature, and pathogen attack that can significantly reduce the quality and productivity of the habanero pepper crops [8], [10], [17], [32], [39]. The production of phenolic compounds during plant growth is one of the main triggered responses to different biotic and abiotic stress factors and strongly influences the plant adaptation processes and interactions with the environment [4], [14], [47]. It has been found the presence of chlorogenic acid and flavonoids (apigenin and luteolin) in C. annuum leaves as a response to oxidative stress cause by UV-B radiation [27]. Phenol polyamides have been observed in C. annuum fruits produced in response to Colletotrichum gloeosporioides infection suggesting its effect as a physical barrier [39].
On the other hand, different traditional methods and solvents have been evaluated for phenolic compound extraction from Capsicum fruits, such as Soxhlet and maceration methods [7]. Nevertheless, low recovery of phenolic compounds has been obtained. Nowadays green technologies such as Ultrasound assisted extraction (UAE) for phytochemical compounds recovery has been evaluated for Capsicum fruits. Dias et al. [18] reported higher recovery of total phenolic content (TPC) by UAE than Soxhlet extraction on C. baccatum fruits, and a significant effect of the extraction solvent is also mentioned. It has been reported that UAE promotes cell wall decomposition and allows mass transfer of the solutes into the solvent preventing the degradation of phenolic compounds producing an increment on TPC recovery with respect to other methods [31]. At the same time, the recovery of phenolic compounds is strongly correlated with the biological activity of the extracts such as antioxidant activity and it is also affected by the polarity of the solvent used during extraction [29]. Based on the properties of the solvents, the dielectric constant (ε) has been proposed as a good polarity parameter considering solvent temperature and composition [24] that can help to predict the performance and profile of extracted phenolic compounds [6], [37]. Additionally, thermodynamic models of solubility of phenolic compounds based on experimental results and empirical thermodynamic models such as the conductor-like screening model for realistic solvation (COSMO-RS) have been reported [12], [19].
Extraction protocols with green technologies in combination with analytical tools such as liquid chromatography (LC) coupled to mass spectrometry (MS) have allowed the identification of phenolic compounds related to the complex metabolic responses caused by changes or alterations in various plant species [25] including the Capsicum genus [27], [39]. Nevertheless, the majority of the studies have focused on the characterization of bioactive compounds in Capsicum fruits [11]. Phenolic characterization from other organs such as the leaves of C. chinense can provide useful information for the understanding of possible metabolic responses of the crop to different environmental conditions, and it could facilitate the development of engineering strategies for crop production and protection. Furthermore, solvent selection is a key factor for the application of phenolic compound extraction protocols that will be targeted to metabolomics studies. Therefore, the main objective of this work was to study the effect of UAE method and the polarity of the solvents according to its “ε” on the total phenolic content (TPC) and its antioxidant activity in extracts of habanero pepper leaves (C. chinense). Additionally, the relation between the “ε” of the solvent and the TPC with the Weibull and Gaussian distribution models was analyzed. Ultra-high-performance liquid chromatography (UPLC) coupled with a photodiode array detector (PDA) and a mass spectrometer was used to obtain the profile of phenolic compounds and their content in the extracts using different solvents.
2. Materials and methods
2.1. Chemicals
The reagents, Folin-Ciocalteu (2 N), gallic acid monohydrate (≥98.0%), formic acid (≥95.0%), acetonitrile (≥99.9%), acetic acid (AcOH; ≥99.7%) and ethyl acetate (AcOEt; ≥99.9%); and analytical standards, caffeic acid (≥98.0%), luteolin (≥99.7%), apigenin (≥95.0%) and quercetin (≥95.0%) were purchased from SIGMA-Aldrich (St. Louis, MO, USA). Sodium carbonate (Na2CO3), hydrochloric acid (≥36.5%), methanol (MeOH; ≥99.90%) and acetone (Ace; ≥ 99.60%) were purchased from Avantor J. T. Baker (Radnor, PA, USA), n-hexane (Hx; ≥95%) were purchased from Fermont (Monterrey, NL, MX) and ultrapure water (W) was obtained through a Milli-Q water filtration system (Millipore, Bedford, MA, USA.
2.2. Vegetal material and sample pretreatment
C. chinense seedlings of the Chichén Itzá variety with 45 days of post-germination were obtained from a local producer in the community of Suma, Yucatán (Mexico) in March 2018.
The leaves of the seedlings were carefully separated with scissors and frozen in liquid nitrogen. They were then cold pulverized in a mill (KRUPS, Model GX41000, Mexico) to a powder with a particle size of < 500 μm. Finally, the samples were freeze dried using a freeze dryer (FreeZone 6 Liter Benchtop, Labconco, USA) to obtain a moisture percentage of 10% determined by a thermobalance (OHAUS, MB45, USA).
2.3. Empirical determination of dielectric constant (ε) of the solvents
The “ε” of pure solvents at different temperatures reported by Akerlof (1932) were used to calculate the empirical “ε” of binary solvent mixtures according to equation developed by Jouyban et al. [24]. This is an empirical model to determine the contribution of solvent composition and temperature on the “ε” and has been compared with experimental data. (Eq. 1):
| (1) |
Where:
εm, T: Dielectric constant of the mixture at the temperature evaluated.
εi, T: Dielectric constant of the pure solvent at the temperature evaluated.
ϕi: Proportion of each solvent in the mixture
Aj: Model constant to calculate dielectric constant of the solvent mixture at different temperatures [24].
2.4. Phenolic compounds recovery by Ultrasound-Assisted extraction (UAE) and Conventional extraction (CE)
A 2 × 10 factorial design was performed to evaluate the effect of solvent and extraction method on the recovery of phenolic compounds. Factor A (extraction methods) were defined as: Conventional Extraction (CE) and Ultrasound-Assisted Extraction (UAE), and Factor B (different solvents or concentrations of the solvents) were defined as: hexane (Hx), ethyl acetate (AcOEt), acetone (Ace), methanol (MeOH), 80% acetone (80% Ace), 80% methanol (80% MeOH), 50% methanol (50% MeOH), 20% methanol (20% MeOH), 1% acetic acid (1% AcOH) and water (W). For all treatments, four replicates were performed. See Additional information (Table S1) for experimental design table.
For the extraction, the lyophilized powder (200 mg) was placed in 15 mL conical tubes (FalconTM) with 10 mL of solvent. CE was carried out in a hot water bath using a heating plate (Thermo Scientific, Model No.: SP131325Q, China) at 50 °C (323.15 K), with magnetic stirring for 4 h. The volume of solvent remained without change during extraction due to the conical tube was previously sealed. (scheme of the CE method is shown in Figure S1, Supplementary material). UAE methodology was performed using the same weight-volume ratio (1:50 g/mL) and ultrasonic extraction conditions reported by Covarrubias-Cardenas et al. (2018). The extraction was carried out using a 3 mm (1/8″) high intensity probe (operating volume range: 0.25 to 10 mL) coupled to an ultrasound processor (GEX130PB, Sonics and Materials Inc., Newtown, USA) at 80% amplitude for 15 min. In addition to the reported method, an ice bath was used to minimize solvent losses during extraction by the effect of the increment of the temperature during sonication. The temperature was found in a range of 20 to 50 °C (293.15 to 323.15 K) (scheme of the UAE method is shown in Figure S2, supplementary material). For all treatments, the extracts were centrifuged (Centrific 225, Fisher Scientific, USA) at 6,500 rpm for 15 min and supernatant was graduated in a 10 mL volumetric flask. The samples were stored in amber vials at – 40° C until further analysis.
2.5. Determination of total phenolic content and antioxidant activity
TPC determination was performed by the Folin-Ciocalteu method according to the modifications used by Covarrubias-Cárdenas et al. [16]. The TPC of samples was expressed in mg equivalents of gallic acid (GAE) g−1 of dry weight (dry wt).
The antioxidant activity was determined by the ABTS and DPPH radical assays. ABTS was performed according to described by Alonso-Carrillo et al. [5] and DPPH radical assay according to described by Covarrubias-Cárdenas et al. [16]. The antioxidant activity of samples was expressed in µEq of Trolox g−1 dry wt.
2.6. Weibull and Gaussian distribution model adjustment of TPC and dielectric constant
TPC was correlated with dielectric constant of the solvents using a Weibull (Eq. 2) and Gaussian distribution (Eq. 3) model equation:
| (2) |
| (3) |
Where:
a: Represents the maximum peak or the highest TPC of the curve centered with respect to the parameter “X0”.
b: Represents the distribution coefficient or standard deviation related to the width of the Weibull and Gaussian bell.
X: Value of the dielectric constant of the pure solvent or solvent mixture.
X0: Value of the dielectric constant in which the “a” or higher TPC value is obtained in the curve.
2.7. Chromatographic analysis by UPLC-PDA-ESI-MS/MS
Phenolic compounds were identified from the 50% MeOH extract obtained by the UAE and its hydrolyzed extract. Chromatographic profiles were obtained using a Waters Acquity H Class UPLC (Milford, MA, USA) with a quaternary pump (UPQSM), an automatic injector (UPPDALTC) and a PDA λ photodiode array detector (UPPDALTC). The chromatographic separation was carried out with a Waters Acquity UPLC BEH C18 column, 1.7 μm, 100 × 2.1 mm ID (Milford, MA, USA) using a mobile phase of 0.1% formic acid in ultrapure water (A) and 0.1% formic acid in acetonitrile (B), with the conditions reported by Covarrubias-Cárdenas et al. [16]. The PDA reading λ was performed in a range of 190 to 400 nm. The analytical response absorbance was taken at 290 nm. The quantification of phenolic compounds identified in the samples was expressed as µmol Eq of luteolin, apigenin, quercetin, and caffeic acid g−1 dry wt according to their similarity with λ of the analytical standards used.
For the mass spectrometry (MS/MS) analysis, a Waters Xevo TQ-S micro instrument was used. Conditions were used as reported by Covarrubias-Cárdenas et al. [16]. The collision energy used was 10 eV for scanning in negative ion mode and 3 eV in positive ion mode. The mass spectra were recorded in full scan mode in a range of 50 m/z to 700 m/z. The MassLynx V4.1 software (Waters, Milford, MA, USA) was used for data acquisition and processing. The tentative identification was assigned by comparing fingerprint and MS data of the compounds detected with the reported in the literature and in public European Mass Bank database (https://massbank.eu/MassBank/index.html) and ReSpect for phytochemicals (http://spectra.psc.riken.jp/menta.cgi/respect/index).
2.8. Analysis of phenolic compounds in aglycone form by acid hydrolysis
Phenolic compounds in plants are commonly found in glycosylated forms [26]. The analysis of the phenolic compounds in their aglycone form provides complementary information such as the structure of the flavonoid-based skeleton, which supports the identification of their possible substitutes. To release the substituents, present in the glycosylated flavonoids from the UAE extract obtained by the 50% MeOH concentration was subjected to acid hydrolysis. The extract (2 mL) was treated with 2 N HCl (1 mL) at 90 °C for 1 h according to the modified methodology of Bae et al., (2012b). Subsequently, the extracts were neutralized using 2 N NaOH/MeOH solution and centrifuged at 6,500 rpm for 15 min using a refrigerated centrifuge (Centrific 225, Fisher Scientific, USA) (schematic of hydrolysis shown in Figure S3, Supplementary material). The supernatant was concentrated in a rotary evaporator (BUCHI, Model: R-215, Switzerland) at 50 °C and 250 mbar vacuum. Finally, samples were filtered using acrodiscs with 0.2 μm membranes (Millex - FG, PTFE 0.2) and stored at – 40 °C until further analysis. The chromatographic analysis of the hydrolyzed extract was performed according to the conditions already detailed above.
2.9. Statistical analysis
The results of all treatments were expressed as the mean ± standard deviation. The factorial design was analyzed as a multifactorial ANOVA (p > 0.05) and to determine the effect of each factor on the analysis of variance components (AVC). The correlation between TPC and Antioxidant Activity (ABTS+ and DPPH assay) was analyzed by a linear model to obtain the Pearson correlation coefficient. To perform both analyses was used the software Statgraphics Centurion Version XVI (Manugistic Inc., Rockville MD, USA). The Weibull and Gaussian distribution model were generated using the Sigma Plot software (Systat Software Inc., USA).
3. Results and discussions
3.1. Empirical determination of the dielectric constant “ε” of solvents
The “ε” is a macroscopic physical parameter and a relative measure of polarity related to the molecular interaction between the solvent and solutes. For binary mixtures, the “ε” value is calculated by additive function according to the concentration of each component. However, this property is also affected by changes in temperature and solvent composition. In this sense, an increase of temperature decreases the “ε” value of the solvents due to the weakening of the intermolecular interactions, an opposite effect is reported when the water is presented showing a non-ideal behavior due to higher intermolecular interactions among solvent components [24]. Herein the effect of temperature and solvent composition were considered to calculate the “ε” values by the Jouyban equation described in the previous section. The temperature used for the calculation of the “ε” was 323.15 °K for CE as process temperature remains constant during extraction (Table 1). In the case of the UAE, averages of temperatures from 293.15 to 323.15 K were considered for the “ε” calculation according to the different solvents used, as cavitation produced by ultrasonic waves generates a wide range of temperature (Table 1). Based on the numerical values obtained for the pure and the binary mixtures of solvents, the order from the lowest to the highest polarity values expressed as “ε” was: Hx < AcOEt < Ace < 80% Ace < MeOH < 80% MeOH < 50% MeOH < 20% MeOH < 1% AcOH < W. “ε” values from the different solvents were considered to correlated solvent polarity with phenolic compounds recovery.
Table 1.
Total phenolic content (TPC) and antioxidant activity of C. chinense leaves extracts obtained by UAE and CE using different pure solvents and aqueous binary mixtures.
| Solvent | Polarity |
Phenolic content |
Antioxidant Activity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UAE |
CE |
TPC (mg GAE g−1 dry wt) |
DPPH (µEq Trolox g−1 dry wt) |
ABTS+ (µEq Trolox g−1 dry wt) |
||||||
| T (K)A | ε | T (K) | ε | UAE | CE | UAE | CE | UAE | CE | |
| W | 323.15 | 76.73 | 323.15 | 69.98 | 5.75 ± 1.85 cd | 11.93 ± 0.94c | 19.74 ± 2.36c | 18.16 ± 1.04e | 41.97 ± 0.37c | 41.61 ± 0.46d |
| 1% AcOH | 313.15 | 73.12C | 323.15 | 69.85C | 8.91 ± 3.06c | 14.19 ± 1.11c | 22.13 ± 1.58bc | 26.60 ± 1.09d | 44.90 ± 1.06bc | 50.70 ± 0.74c |
| 20% MeOH | 313.15 | 66.51 | 323.15 | 63.31 | 15.32 ± 1.56b | 18.31 ± 1.16b | 23.08 ± 2.04bc | 22.75 ± 1.60d | 46.86 ± 1.92bc | 42.82 ± 1.97d |
| 50% MeOH | 313.15 | 52.09 | 323.15 | 46.99 | 24.39 ± 2.41a | 22.77 ± 1.34a | 28.46 ± 1.87ab | 32.21 ± 0.01bc | 64.06 ± 2.88a | 67.39 ± 0.52ab |
| 80% MeOH | 303.15 | 35.91 | 323.15 | 32.39 | 21.21 ± 1.70a | 18.14 ± 2.79b | 32.66 ± 1.35a | 36.27 ± 1.84ab | 61.42 ± 0.10a | 71.61 ± 1.17a |
| 80% Ace | 303.15 | 22.45 | 323.15 | 20.62 | 16.44 ± 0.55b | 14.85 ± 1.69bc | 33.03 ± 2.43a | 37.48 ± 1.87a | 63.29 ± 4.31a | 61.88 ± 0.51b |
| MeOH | 303.15 | 30.68 | 323.15 | 27.44 | 15.82 ± 0.70b | 15.31 ± 2.11bc | 23.90 ± 1.21bc | 31.14 ± 0.46c | 51.43 ± 1.05b | 54.19 ± 3.04c |
| Ace | 303.15 | 18.67 | 323.15 | 16.98 | 3.68 ± 0.73de | 6.30 ± 0.61d | 7.03 ± 1.09d | 7.90 ± 0.85f | 19.11 ± 0.12d | 24.08 ± 1.04e |
| AcOEtB | 293.15 | 6.02 | 323.15 | 6.02 | 3.26 ± 0.55de | 4.40 ± 0.46d | 2.49 ± 1.06de | 0.00 ± 0.00 g | 13.33 ± 3.55de | 21.94 ± 4.22e |
| HxB | 293.15 | 1.89 | 323.15 | 1.89 | 1.72 ± 0.19e | 2.92 ± 0.20d | 0.00 ± 0.00e | 0.00 ± 0.00 g | 4.89 ± 0.79e | 13.57 ± 0.59f |
Different letters by column indicate significant differences (p < 0.05).
The average temperature during UAE was used to calculate the dielectric constant.
Low polarity solvents dielectric constants were not calculated for different temperatures as they are poorly affected.
Temperature effect was the only consideration for dielectric constant for the low acid solution as the Jouyban equation did not was applied for this solution.
3.2. Total phenolic content in C. Chinense leaves extracts obtained by Ultrasound Assisted extraction (UAE) and Conventional extraction (CE) with different solvents.
The TPC values obtained with the different solvents and both extraction methods (UAE and CE) were in a range of 1.70 to 24.40 mg GAE g−1 dry wt (Table 1). A higher TPC was observed using 50% MeOH, 24.39 ± 2.41 and 22.77 ± 1.34 mg GAE g−1 dry wt for UAE and CE method, respectively. A better TPC extraction by UAE can be explained by the rupture of the cell wall and cell vesicles that contain phenolic compounds produced by cavitation and allowing solvent penetration favoring solvation process [31]. In general, solvents of low polarity (Hx, AcOET and Ace) showed low response for TPC recovey (1.72 to 6.30 mg GAE g−1 dry wt), while solvents of higher polarity (MeOH, MeOH 80%, MeOH 50%, MeOH 20% and Ace 80%) are more effective for the recovery of phenolic compounds (14.85 to 24.39 mg GAE g−1 dry wt). However, some exceptions were observed using solvents such as water and 1% AcOH, where low response for TPC was observed (5.75 to 14.19 mg GAE g−1 dry wt) independently of the extraction method used. These observations are summarized through the multivariate Analysis of Variance (ANOVA) that indicated a significant effect of both factors (solvent and extraction method) on the TPC. The percentage of the contribution of these factors was estimated through a variance components analysis (VCA) that showed a higher contribution (88.44%) of the extraction solvent than the extraction method (7.32%). The differences in the response for TPC among treatments using different extraction solvents are more evident than those observed between extraction methods. However, some advantages of the UAE method over CE should be considered, i.e., a high response for TPC is obtained in a short extraction time (15 min) and cavitation produced during the UAE improves the mass transfer of solutes in the solvent due to cell wall disruption and microstreaming effect.
In the studies conducted by Dias et al. [18] and Bae et al. (2012a), a clear contribution of the extraction solvent in the recovery of phenolic compounds is showed. Dias et al. [18] reported that a higher TPC response is obtained from C. baccatum fruit extracts using MeOH as solvent and Soxhlet extraction method (4.93 mg GAE g−1 of raw material), while lower polarity solvents such as Ace, AcOEt and Hx showed a low response for TPC. Phenolic compounds are generally polar; therefore, they can be recovered with solvents of high polarity. However, in the study conducted by Bae et al. (2012a) a different behavior of the effect of the solvent on the TPC response was observed. They reported a TPC between 24.80 and 68.90 mg of catechin equivalents (CAE) g−1 dry wt in extracts of C. annuum fruit varieties obtained by Soxhlet extraction using different solvents; and the highest TPC was obtained using AcOEt, while higher polarity solvents such as Ace, MeOH and 80% MeOH showed a lower response for TPC. The results reported by Bae et al. (2012a) may be due to the presence of capsaicinoids, which are predominantly hydrophobic and mainly found in the fruits of Capsicum species that are not synthesized in other tissues such as leaves and stems [21]. Additionally, other factors could be related to the different responses for TPC, i.e., the profile and content of bioactive compounds in the different species, varieties and tissues of the genus Capsicum, stage of growth and development, agricultural practices, exposure to biotic and abiotic stresses, processes of harvesting, pretreatment of plant material, method and conditions for the extraction of phenolic compounds and overestimation of phenolic compounds by the Folin-Ciocalteu method caused by capsaicinoids, sterols and carotenoids contained in the extracts.
Focusing on the method and conditions for the extraction of phenolic compounds different parameters can be improved, i. e., Dias et al. [18] evaluated the effect of solvent, temperature and ultrasonic intensity on TPC and capsaicinoids content in extracts of C. baccatum fruits. Additionally, they presented a mathematical model that correlates the different parameters evaluated with the recovery of bioactive compounds. Nevertheless, the effect of the UAE method and solvent selection for the recovery of phenolic compounds from habanero pepper leaves is presented herein for the first time.
3.3. Antioxidant activity of C. Chinense leaves extracts.
The antioxidant activity evaluated by the ABTS+ assay was in the range of 4.89 to 71.61 µEq Trolox g−1 dry wt, while for the DPPH assay it was in the range of 0.00 to 37.48 µEq Trolox g−1 dry wt (Table 1). The multivariate ANOVA determined that the method and solvent had a significant effect on the antioxidant activity of the extracts, however, as expected the solvent has a greater contribution on antioxidant activity by both assays (ABTS+ and DPPH and assay). The highest values of antioxidant activity were obtained using 50% MeOH, 80% MeOH and 80% Ace as extraction solvents. Commonly, extracts obtained with high polarity solvents show higher antioxidant activity, the polar phase of the extract contributes to the inhibition of ABTS+ and DPPH radicals through simple electron transfer (SET) and proton transfer (HT) [43]. Both methods are reliable for the measurement of antioxidant activity, however, the ABTS+ assay has advantages over DPPH assay, i.e., the ABTS+ radical is soluble in aqueous and organic solvents; therefore, the antioxidant activity of both hydrophilic and lipophilic compounds can be evaluated. Moreover, results obtained through the DPPH are difficult to be interpreted when the compounds in the extracts have UV spectra that overlap the DPPH at 515 nm (i.e., carotenes and xanthophylls) [36], [43].
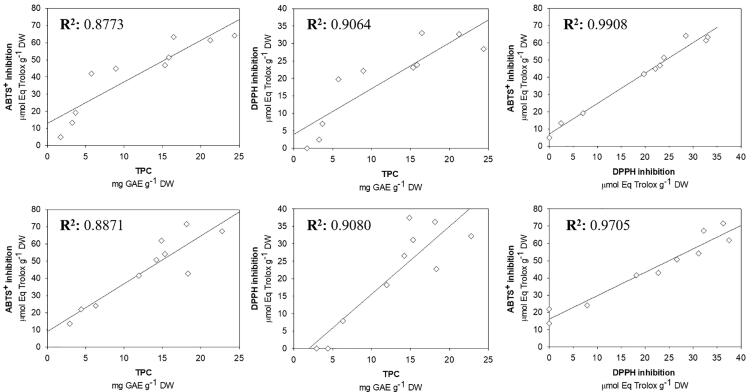
Both antioxidant activity assays showed a high correlation with the TPC (Fig. 1). The TPC and antioxidant activity by ABTS+ assay showed a Pearson correlation coefficient of 0.8773 and 0.8871 for extracts obtained by CE and UAE, respectively. A similar correlation was observed between the TPC and antioxidant activity by DPPH assay (0.9064 and 0.9080, for CE and UAE, respectively). Finally, the correlation coefficients between ABTS+ and DPPH assay were 0.9908 and 0.9705 by CE and UAE, respectively. The high correlation between the antioxidant activity measured by ABTS+ and DPPH assay could be attributed to the fact that both are methods of antioxidant capacity mainly classified as SET mechanism. Although, these radicals can also be inhibited by HT [43]. Antioxidant activity is commonly correlated with TPC, this has been shown by some studies on the extraction of bioactive compounds from fruits of different Capsicum species [7], [18], [29], however, in pepper fruits, the antioxidant activity could be attributed to other secondary metabolites such as capsaicinoids, carotenoids, and organic acids, therefore, the multiple linear correlations are more appropriate to explain antioxidant activity in pepper fruits, similar to presented by Sora et al. [45] to chemometric studies applications. A high linear correlation between TPC and antioxidant activity suggests that the principal antioxidant compounds present in C. chinense leaves correspond to phenolic compounds.
Fig. 1.
Pearson correlation matrix between total phenolic content (TPC) and antioxidant activity (ABTS+ and DPPH assays) in C. chinense leaves extracts obtained by UAE and CE.
3.4. Weibull and Gaussian distribution model for the correlation of solvent dielectric constant and total phenolic content
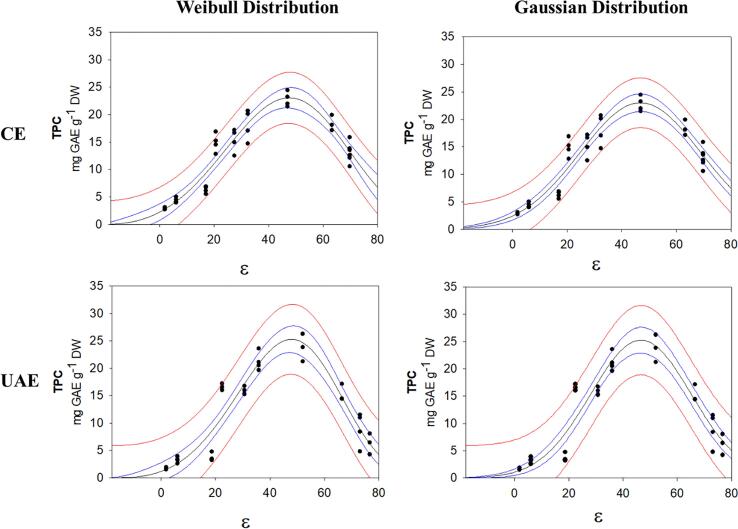
For the recovery of phenolic compounds from C. chinense leaves by UAE and CE, the TPC increased as the solvent polarity increased, however, a low TPC response was observed when the higher polarity solvents (20% MeOH, 1% AcOH and W) were used. Oreopoulou et al. [37] reported changes in TPC as a function of the composition of the extraction solvent, this phenomenon is described in more detail by Catena et al. [12], they reported similar behavior in the extraction of phenolic compounds and anthocyanins from rice (Oryza sativa L. 'Violet Nori'), an increase in the recovery of phenolic compounds and anthocyanins as a function of solvent composition was observed; a higher concentration of ethanol in the hydroalcoholic solutions improved the recovery of phenolic compounds, however, at very high ethanol concentration (70 to 100% EtOH v/v) a lower response for TPC was observed. This phenomenon is attributed to a change of polarity of the solvent, therefore, by modifying the composition of the solvent it is possible to increase and/or decrease the solvent polarity to improve the recovery of phenolic compounds. This behavior can be correlated with an asymmetric or normal distribution model such as the Weibull (Eq. 2) and Gaussian (Eq. 3) distribution models, respectively. These models were proposed to predict the best condition for obtaining the highest phenolic content and to describe the recovery of phenolic compounds as a function of solvent polarity during UAE extractions.
The graphical models that show the correlation of the TPC with the “ε” are presented in Fig. 2. The equation constants (“a”, “b”, “c” and “X0”), the R and R2 of the models are presented in Table 2. Both distribution models showed a high correlation (R: 0.93 to 0.95). The coefficient “a” represents the maximum value of TPC that can be obtained according to the models, similar values of 25.20 and 23.07 mg of GAE g−1 dry wt, were obtained by UAE and CE, respectively, for the Weibull and the Gaussian distribution models. The values of coefficient “b”, related to the width of the bell behavior of the models, were 18.59 and 22.11 for UAE and CE methods respectively, for the Gaussian distribution model, while for the Weibull distribution model were 72.82 (UAE) and 76.97 (CE). This coefficient was lower by UAE than CE in both models and low values indicate reduced a narrow spectrum of polarity values. The X0 (“ε”) values to the Weibull distribution model were 47.93 and 47.57 for UAE and CE, respectively, while for the Gaussian distribution model values were 46.57 (UAE) and 46.85 (CE). The coefficient values (X0) were similar between the Weibull and Gaussian distribution model. This coefficient represents the “ε” of the solvent that provides the highest TPC response according to the models, 50% MeOH had a similar “ε” (46.99 to 52.09) and was the solvent that showed the highest capacity for the recovery of phenolic compounds by UAE. Finally, all correlations presented a Normal distribution due to the models passed normality test of Shapiro-Wilk.
Fig. 2.
The Weibull and Gaussian distribution models to the correlation between TPC and the “ε” of the solvent and solvent mixtures evaluated by CE and UAE.
Table 2.
Equation constants, Pearson correlation, determination coefficients, and Normality Test (Shapiro-Wilk) of Gaussian and Weibull distribution model: Total phenolic content (TPC) vs dielectric constants (ε) as polarity parameter.
| Model | Extraction method | Equation constants B |
R | R2 | SD | Normality Test (Shapiro-Wilk) A | Constant Variance Test | |||
|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | X0 | |||||||
| Gaussian Distribution | UAE | 25.26 (1.18) | 18.59 (0.83) | – | 46.57 (0.77) | 0.93 | 0.87 | 2.90 | 0.3777 | 0.2761 |
| CE | 23.01 (0.73) | 22.11 (0.87) | – | 46.85 (0.66) | 0.95 | 0.90 | 2.08 | 0.6036 | 0.1000 | |
| Weibull Distribution | UAE | 25.29 (1.20) | 72.82 (16.43) | 4.15 (0.99) | 47.93 (1.28) | 0.94 | 0.85 | 2.91 | 0.2970 | 0.2225 |
| CE | 23.07 (0.92) | 76.97 (14.01) | 3.73(0.82) | 47.57 (1.09) | 0.95 | 0.90 | 2.11 | 0.5337 | 0.1417 | |
Normality test (Shapiro-Wilk) significance level to p-value > 0.05.
Equation constant (SD: standard deviation).
The determination of the relation between solvent polarity and the recovery of phenolic compounds using mathematical models is useful for understanding and improving extraction procedures. Oreopoulou et al. [37] applied a kinetic model for the recovery of phenolic compounds from oregano (Origanum vulgare) controlling as main parameters, particle size, time, and extraction solvents. Dong et al. [19] evaluated the empirical model COSMO-RS and an experimental model to determine the solubility of daidzein in different solvents and discussed the importance of solvent polarity and hydrogen bonding in the dissolution of these molecules and their application to extraction and purification methods. Álvarez et al. [6] developed a semi-empirical model for the recovery of grape pomace compounds based on the dielectric properties of the solvents. In summary, through the understanding of the processes of solubilization and solvation of phenolic compounds using mathematical models it is possible to obtain information to improve extraction methodologies and propose purification strategies.
The results suggest that the “ε” can be considered as a suitable polarity parameter to evaluate the effect of a pure solvent or binary mixture on the extraction of phenolic compounds from C. chinense leaves and it can be used as criteria for selection of the suitable solvent in phenolic extraction protocols. However, to have a complete understanding of the solvent effect on the extraction procedure it is necessary to evaluate the effect that it produces on the profile and content of each phenolic compound extracted.
3.5. Phenolic compounds identification by UPLC-PDA-ESI-MS/MS
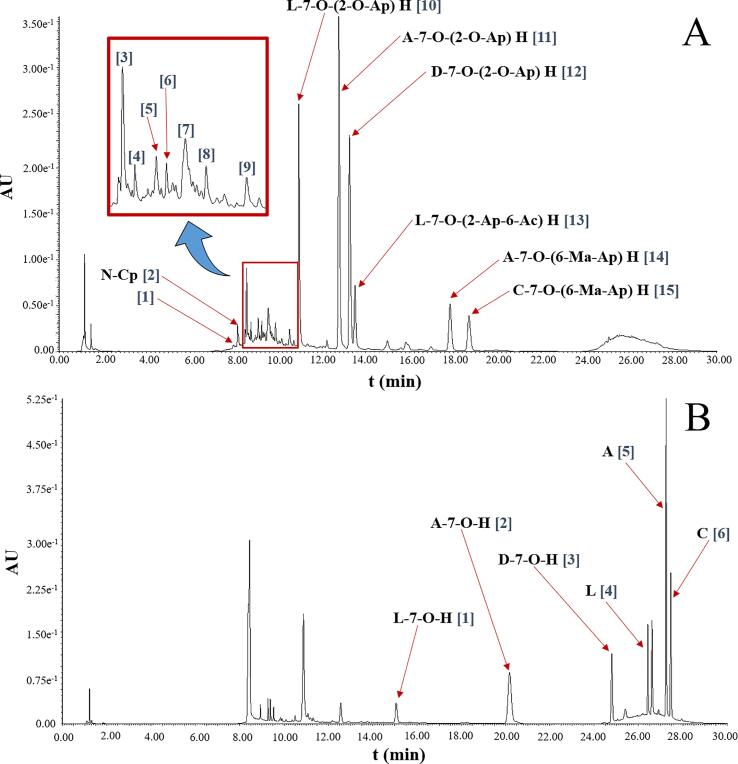
The chromatogram in Fig. 3 shows the compounds identified by UPLC-PDA-ESI-MS/MS in an extract of C. chinense leaves obtained by UAE using 50% methanol and in the hydrolyzed extract. A total of 15 phenolic compounds and 6 flavonoid compounds were identified, respectively (Table 3).
Fig. 3.
Chromatogram of phenolic compounds identified in C. chinense leaves extract obtained by UPLC-PDA analysis (A: 50% MeOH by UAE; B: hydrolyzed extract obtained from A; Compounds was showed according to Table 3).
Table 3.
Phenolic compounds identified in C. chinense leaves by UPLC-PDA-ESI-MS/MS in the extract obtained using 50% MeOH by UAE and hydrolyzed extract.
| Number | TR | PDA UV bands (nm) | Experimental Accurate Mass |
Molecular Formula | Fragments (m/z) |
Tentative identification | ||
|---|---|---|---|---|---|---|---|---|
| [M–H] - | [M + H] + | Ion negative | Ion positive | |||||
| 1 | 7.941 | 194, 213, 293, 317 | 249.0850 | 251.1308 | C13H18N2O3 | 249 (1 0 0) | 251 (95) 234 (21) 163 (1 0 0) 72 (80) | N-caffeoyl putrescine (Isomer I) |
| 2 | 8.130 | 194, 213, 293, 317 | 249.0850 | 251.1308 | C13H18N2O3 | 249 (1 0 0) | 251 (92) 234 (21) 163 (1 0 0) 72 (61) | N-caffeoyl putrescine (Isomer II) |
| 3 | 8.550 | 197, 218, 278 | 315.0823 | 227.1421 | C13H16O9 | 315 (1 0 0) 203 (54) | 227 (18) 188 (1 0 0) 146 (91) 118 (28) 100 (17) | Protocatechuic acidhexoside |
| 4 | 8.740 | 212, 299, 325 | 353.0936 | C16H18O9 | 353 (1 0 0)215 (13) 293 (19) 191 (82) | 5-Caffeolquinic acid | ||
| 5 | 9.080 | 209, 284 | 215.0827 | 217.1303 | C12H12N2O2 | 215 (1 0 0) | 217 (47) 200 (95) 88 (1 0 0) | Unknown |
| 6 | 9.235 | 205, 270, 297, 338 | 593.1991 | 595.2100 | C27H30O15 | 593 (72) 431 (9) 302 (9) 177 (1 0 0) | 595 (7) 471 (1 0 0) 144 (57) 100 (31) | Unknown |
| 7 | 9.540 | 195, 213, 312 | 329.0902 | C14H18O9 | 329 (55) 265 (62) 177 (1 0 0) | Vanillic Acid-4-β-D-glucoside | ||
| 8 | 9.860 | 272, 320 | 597.2781 | C27H34O15 | 387 (1 0 0) 597 (9) | Unknown | ||
| 9 | 10.485 | 210, 306 | 337.1141 | C16H18O8 | 337 (1 0 0) 191 (89) | Coumaroylquinic acid | ||
| 10 | 10.904 | 205, 254, 348 | 579.1603 | 581.1707 | C26H28O15 | 579 (1 0 0) | 581 (1 0 0) 287 (18) | Luteolin-7-O-(2-O-apiosyl) hexoside |
| 11 | 12.730 | 199, 266, 336 | 563.1839 | 565.1846 | C26H28O14 | 563 (1 0 0) | 565 (1 0 0) 433 (9) 271 (29) | Apigenin-7-O-(2-O-apiosyl) hexoside |
| 12 | 13.212 | 206, 251, 266, 347 | 593.1651 | 595.2073 | C27H30O15 | 593 (1 0 0) | 595 (1 0 0) 387 (25) 301 (14) | Diosmetin-7-O-(2-O-apiosyl) hexoside |
| 13 | 13.466 | 206, 253, 348 | 665.1592 | 667.1755 | C26H34O20 | 621 (1 0 0) 665 (57) | 667 (1 0 0) | Luteolin-7-O-(2-apiosyl-6-malonyl) hexoside |
| 14 | 17.761 | 208, 266, 336 | 649.1833 | 651.1900 | C29H30O17 | 649 (9) 635 (32) 605 (1 0 0) | 651 (1 0 0) | Apigenin-7-O-(6-malonyl-apiosyl) hexoside |
| 15 | 18.603 | 210, 251, 266, 347 | 679.1975 | 681.1900 | C23H40O20 | 679 (12) 635 (1 0 0) | 681 (1 0 0) | Chysoeriol-7-O-(6 malonyl-apiosyl) hexoside |
| 1* | 15.148 | 207, 253, 347 | 447.1658 | 449.1168 | C21H20O11 | 447 (1 0 0) 285 (90) 284 (80) | 449 (1 0 0) 287 (61) | Luteolin-7-O-hexoside |
| 2* | 20.271 | 206, 266, 336 | 431.1199 | 433.1222 | C21H20O10 | 431 (88) 268 (1 0 0) | 433 (1 0 0) 271 (79) | Apigenin-7-O-hexoside |
| 3* | 24.880 | 205, 251, 265, 347 | 461.1377 | 463.1222 | C22H22O11 | 461 (1 0 0) 297 (6) 283 (32) 255 (22) | 463 (1 0 0) 453 (30) 301 (44) | Diosmetin-7-O-hexoside |
| 4* | 26.505 | 210, 252, 267, 347 | 285.061 | 287.0468 | C15H10O6 | 285 (68) 133 (1 0 0) | 287 (1 0 0) | Luteolin |
| 5* | 27.359 | 214, 267, 336 | 269.0797 | 271.0555 | C15H10O5 | 269 (27) 117 (1 0 0) | 271 (1 0 0) 246 (34) | Apigenin |
| 6* | 27.529 | 210, 247, 267, 346 | 299.0699 | 301.0551 | C16H12O6 | 299 (1 0 0) 284 (49) 227 (46) 151 (30) 107 (35) | 301 (1 0 0) | Chrysoeriol |
Identified in the hydrolyzed extract.
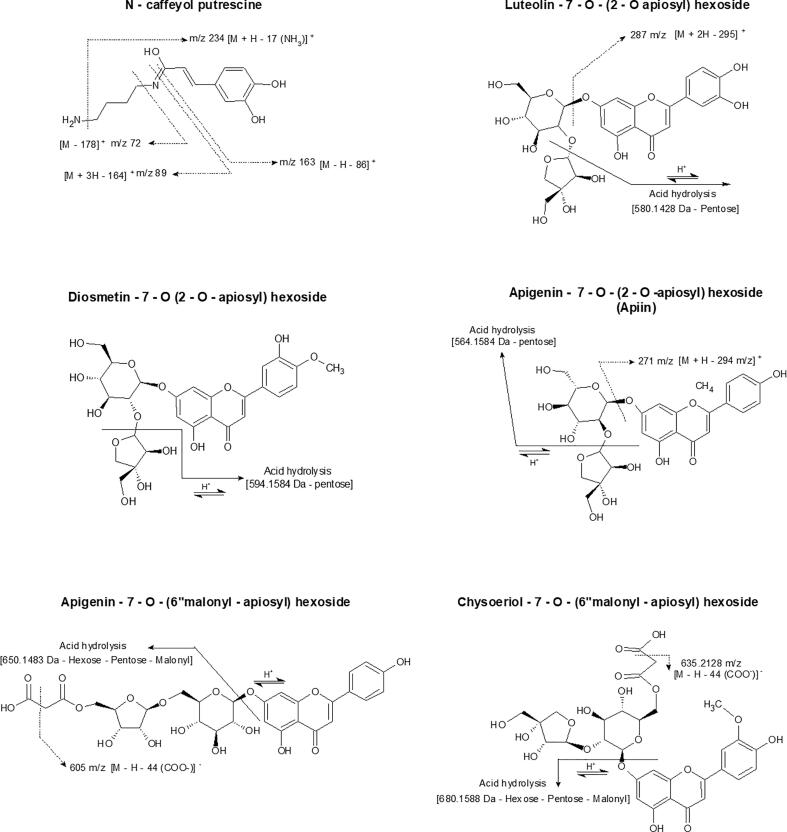
Compounds 1 (Isomer I; RT: 7.941 min; λmax: 194, 213, 293, 317 nm) and 2 (Isomer II; RT: 8.130 min; λmax: 194, 213, 293, 317 nm) were identified as N-caffeoyl putrescine (N-Cp). A molecular ion at m/z 251 ([M + H]+) was found in positive ion mode. The fragments at m/z 234 ([M – H – 17 (NH3 loss)]+), m/z 163 ([M – H – 86 (putrescine loss)]+), and m/z 89 (putrescine) ([M + 3H – 164 (loss of caffeoyl)]+) were identified. Additionally, it is suggested that the identified fragment at m/z 72 ([M – 178]+) represent the loss of an NH3 group from putrescine (Fig. 4). This compound has been reported in Nicotiana tabacum L. [9], [10]. Furthermore, Park et al. [39] have also identified this compound in C. annuum fruits in response to the anthracnose infection produced by Colletotrichum gloeosporioides.
Fig. 4.
Structure of principal phenolic compounds found in C. chinense leaves by UPLC-PDA-ESI-MS/MS.
Compound 3 (RT: 9.550 min; λmax: 197, 218, 278 nm) was tentatively identified as protocatechuic acid hexoside (PAH). The molecular ion at m/z 315 ([M – H]-) and a fragment at m/z 203 in negative ion mode were found. In positive ion mode, the fragment at m/z 227 ([M + H − 90 (3 · HCHO)]+) was detected, which suggests that correspond to the loss of three aldehyde groups, which is usual in glycosylated compounds. This compound has been reported by Vallverdú-Queralt et al. [46] in tomato sub-products by HPLC-ESI-QTOF and by Moco et al. [33] as aglycone in tomato fruits (Solanum Lycopersicum).
Compound 4 (RT: 8.740 min; λmax: 212, 299, 325 nm) was identified as 5-caffeoylquinic acid (5-CQA) with a molecular ion at m/z 353 ([M – H]-) and a fragment at m/z 191 that corresponds to the quinic acid ([M – H – 162]-) which is a characteristic fragment of this compound. It has been identified in tomatoes [33], cherry tomatoes [44], tomato-based by-products [46], and in C. annuum var. Lemeška and Lakošnička [35]. Mikulic-Petkovsek et al. [32] have reported the presence of this compound in response to the infection produced by Colletotrichum coccodes.
Compound 5 (RT: 9.080 min; λmax: 209, 284 nm), 6 (RT: 9.235 min; λmax: 205, 270, 297, 338 nm) and 8 (RT: 9.860; λmax: 272, 320 nm) were no identified. However, according to its UV spectrum is suggested that compounds 5 and 8 correspond to phenolic acids, while compound 6 correspond to a flavonoid.
Compound 7 (RT: 9.540; λmax: 195, 213, 312 nm) presented a molecular ion at m/z 329 ([M - H]-). This compound was identified as vanillic acid-4-β-D-glucoside (VAG) and is reported by Vallverdú-Queralt et al., [46] in tomato-based by-products, and in C. annuum fruits by Morales-Soto et al. [34]. In addition, has also been detected in several vegetables sources such as artichoke [3], cucumber [2] and araceae leaves [1].
Compound 9 (Isomer II, RT: 10.485 min, λmax = 210, 306 nm) showed molecular ion at m/z 337 in negative ion mode. It was identified as coumaroylquinic acid (CQA). The fragment at m/z 191 was also detected, this corresponds to quinic acid ([M – H – 146]- or [192 (quinic acid) – H]-) and is a fragment commonly reported for CQA [3], [35], [40], [42], [44], [46].
Compound 10 (RT: 10.904 min; λmax: 205, 254, 348 nm) showed molecular ions at m/z 579 ([M – H]-) and m/z 581 ([M + H]+) in negative and positive ion mode, respectively. It was identified as luteolin-7-O-(2-O-apiosyl) hexoside (L-7-(2-Ap) H) [30], [32], [39]). The fragment at m/z 287 in positive ion mode corresponding to the protonated luteolin molecule ([M + 2H – 295]+) was also detected. In the hydrolyzed extract, the molecular ions at m/z 447 ([M – H]-) and m/z 449 ([M + H]+) were found in negative and positive ion mode, respectively. It was identified as luteolin-7-O-hexoside (L-7-H) [1], [34], [35], [39] and is suggested that its presence is derived from to the loss of pentose belonging to the L-7-(2-Ap) H by acid hydrolysis. The fragments at m/z 285 ([M – 163 (hexoside)]-), m/z 284 ([M – H – 163 (hexoside)]-), and m/z 287 were identified ([M + H – 163 (hexoside)]+) to correspond to the luteolin after the loss of a hexose during ionization (Fig. 4).
Compounds 11 (RT: 12.730 min; λmax: 199, 266, 336 nm) was identified as apigenin-7-O-(2-O-apiosyl) hexoside (A-7-(2-Ap) H) with a molecular ions at m/z 563 and m/z 565 in negative ion mode and positive ion mode, respectively [35]. The fragment at m/z 433 was also found in positive ion mode that may correspond to the loss of a pentose ([M + 2H – 133]+) and the fragment at m/z 271 to the protonated apigenin molecule (M + H – 294]+) after the loss of both sugars. Additionally, the molecular ions at m/z 431 ([M – H]-) and m/z 433 ([M + H]+) were found in the hydrolyzed extract (Table 3). This compound was identified as apigenin-7-O-hexoside (A-7-H) [1], [34], [39] after the loss of a pentose as a result of acid hydrolysis. The fragments at m/z 268 ([M – H – 163]-) and m/z 271 ([M + 2H – 163]+) are suggested to correspond to the apigenin molecule after the loss of both sugars after ionization (Fig. 4).
Compound 12 (RT: 13.212 min; λmax: 206, 251, 266, 347 nm) showed molecular ions at m/z 593 ([M – H]-) and m/z 595 ([M + H]+) in negative and positive ion mode, respectively. It was identified as diosmetin-7-O-(2-O-apiosyl) hexoside (D-7-(2-Ap) H) [2]. In the hydrolyzed extract, the molecular ions at m/z 461 ([M – H]-) and m/z 463 ([M + H]+) in negative and positive ion mode were observed, respectively (Table 4). It is suggested its identification as diosmetin-7-O-hexoside (D-7-H) [44]). It is also suggested that is the result of the release of the pentose from 2-O-glucosidic bond of D-7-(2-Ap) H after acid hydrolysis. For this same compound were identified the fragment at m/z 446 corresponding to the loss of a methyl group ([M – H – 15 (CH3]-), fragment m/z 297 possible attributed to the loss of the hexose of the 7-O-glycosidic bond ([M – 2H – 163 (glycosyl)]-), fragment at m/z 283 possible represented the loss of a hexose ([M – 179 (hexoside)]-) and fragment at m/z 255 attributed to the rupture of the “C” ring of the flavonoid skeleton of the diosmetin and the loss of an OH - group ([M + H – 192]-). In the positive ion mode, the fragment at m/z 301 ([M + 2H – 163]+) corresponded to the protonated diosmetin (Fig. 3) was detected.
Table 4.
Quantitation of phenolic compounds presents in C. chinense leaves extracts obtained by maceration (CE) and UAE using different pure solvents and aqueous binary mixtures.
| CE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound* | Solvent |
|||||||||
| W | 1% AcOH | 20% MeOH | 50% MeOH | 80% MeOH | 80% Ace | MeOH | Ace | AcOEt | Hx | |
| N-C pA | NQ | NQ | 0.58 ± 0.01b | 0.85 ± 0.06a | 0.82 ± 0.00a | NQ | 0.89 ± 0.04a | NQ | NQ | NA |
| L-7-(2-Ap) HB | NQ | NQ | NQ | 5.05 ± 0.16c | 6.93 ± 0.16b | 8.69 ± 0.12a | 7.34 ± 0.05b | 1.73 ± 0.35d | 0.49 ± 0.00e | NA |
| A-7-(2-Ap) HC | NQ | NQ | NQ | 8.33 ± 0.36d | 11.24 ± 0.28c | 14.23 ± 0.03a | 12.41 ± 0.11b | 4.11 ± 0.78e | 1.06 ± 0.00f | NA |
| D-7-(2-Ap) HD | NQ | NQ | NQ | 13.21 ± 0.21c | 15.92 ± 0.43b | 18.81 ± 1.07a | 17.24 ± 0.11ab | 6.66 ± 0.24d | 1.59 ± 0.03e | NA |
| A-7-(6-Ma-Ap) HC | NQ | NQ | NQ | 0.55 ± 0.05c | 1.23 ± 0.01b | 1.91 ± 0.02a | 1.17 ± 0.03b | 0.26 ± 0.05d | 0.08 ± 0.00e | NA |
| C-7-(6-Ma-Ap) HD | NQ | NQ | NQ | 1.48 ± 0.23d | 2.75 ± 0.02b | 3.91 ± 0.03a | 2.39 ± 0.06c | 0.83 ± 0.12e | 0.36 ± 0.00f | NA |
| TOTAL | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.58 ± 0.01f | 29.48 ± 0.04c | 38.89 ± 0.86b | 47.55 ± 1.26a | 41.43 ± 0.26b | 13.59 ± 1.54d | 3.57 ± 0.04e | NA |
| UAE | ||||||||||
| Compound* | Solvent | |||||||||
| W | 1% AcOH | 20% MeOH | 50% MeOH | 80% MeOH | 80% Ace | MeOH | Ace | AcOEt | Hx | |
| N-C pA | 0.10 ± 0.00d | 0.39 ± 0.00bc | NQ | 0.90 ± 0.08a | 0.83 ± 0.22a | 0.22 ± 0.02c | 0.43 ± 0.02b | NQ | NQ | NA |
| L-7-(2-Ap) HB | NQ | NQ | NQ | 7.25 ± 0.33b | 7.20 ± 0.08b | 8.01 ± 0.01a | 6.18 ± 0.05c | 1.33 ± 0.09d | 0.29 ± 0.04e | NA |
| A-7-(2-Ap) HC | NQ | NQ | NQ | 11.53 ± 0.64b | 11.79 ± 0.18ab | 12.74 ± 0.31a | 10.44 ± 0.01c | 2.58 ± 0.19d | 0.47 ± 0.18e | NA |
| D-7-(2-Ap) HD | NQ | NQ | NQ | 16.73 ± 0.89b | 16.50 ± 0.23b | 18.43 ± 0.64a | 14.90 ± 0.19c | 3.19 ± 0.31d | 0.83 ± 0.29e | NA |
| A-7-(6-Ma-Ap) HC | NQ | NQ | NQ | 1.21 ± 0.17c | 1.63 ± 0.02b | 1.89 ± 0.02a | 1.13 ± 0.07c | 0.23 ± 0.02d | 0.07 ± 0.02d | NA |
| C-7-(6-Ma-Ap) HD | NQ | NQ | NQ | 2.89 ± 0.33b | 3.70 ± 0.01a | 4.05 ± 0.04a | 2.63 ± 0.31b | 0.67 ± 0.02c | 0.40 ± 0.05c | NA |
| TOTAL | 0.10 ± 0.00 g | 0.39 ± 0.00f | 0.00 ± 0.00 | 40.51 ± 2.43b | 41.65 ± 0.71ab | 45.34 ± 0.41a | 35.71 ± 0.64c | 7.89 ± 0.63d | 1.94 ± 0.55e | NA |
Quantified as µmol Eq of caffeic acid g−1 dry wt; B Quantified as µmol Eq of luteolin g−1 dry wt; C Quantified as µmol Eq of apigenin g−1 dry wt; D Quantified as µmol Eq of quercetin g−1 dry wt.
N-C p: N-Caffeyol putrescine; L-7-(2-Ap) H: Luteolin-7-O-(2-O-apiosyl) hexoside; A-7-(2-Ap) H: Apigenin-7-O-(2-O-apiosyl) hexoside; D-7-(2-Ap) H: Diosmetin-7-O-(2-O-apiosyl) hexoside; A-7-(6-Ma-Ap) H: Apigenin-7-O-(6-malonyl-apiosyl) hexoside; C-7-(6-Ma-Ap) H: Chrysoeriol-7-O-(6-malonyl-apiosyl) hexoside. NQ: Non-quantifiable; NA: Analysis of non-polar solvent hexane is not compatible with reverse phase chromatography.
Compound 13 (RT = 13.212 min; λmax: 206, 253, 266, 348) showed molecular ions at m/z 665 ([M – H]-) and m/z 667 ([M + H]+) in negative and positive ion mode, respectively. It was identified as luteolin-7-O-(2-apiosyl-6-malonyl) hexoside (L-7-(2-Ap-Ma) H) [2], [23], [30], [32], [35]). For this compound, the fragment at m/z 621 was detected in negative ion mode corresponding to the loss of a carboxyl ion group ([M – H – 44 (COO–]-). The molecular ions at m/z 285 ([M – H]-) and m/z 287 ([M + H]+) in negative and positive ion mode, respectively, were found in the hydrolyzed extract that allows identifying the compound as luteolin (Table 3) after the loss of its substituents by acid hydrolysis.
Compounds 14 (RT: 17.761; λmax: 208, 266, 336 nm) showed molecular ions at m/z 649 ([M – H]-) and m/z 651 ([M + H] +) in negative and positive ion mode, respectively. It was identified as apigenin-7-O-(6-malonyl-apiosyl) hexoside (A-7-(6-Ma-Ap) H) [28]. For this compound, the fragment at m/z 605 correspondings to the loss of a carboxyl group ([M – H – COO -] -) was also observed. The molecular ion at m/z 269 ([M – H] -) and m/z 271 ([M + H] +) were detected in the hydrolyzed extract corresponding to the apigenin compound after the loss of all its substituents by acid hydrolysis. The fragments at m/z 246 were also found in positive ion mode that possibly corresponds to the loss of a C2H2 group ([M + 2H – 26 (C2H2)] +) and fragment at m/z 117 in negative ion mode suggested the rupture of the “C” ring of the flavonoid skeleton ([M – H – 152] -) (Table 4).
Compound 15 (RT: 18.603 min; λmax: 210, 251, 266, 347 nm) showed molecular ions at m/z 679 ([M – H] -) and m/z 681 ([M + H] +) in negative and positive ion mode, respectively. It was identified as chrysoeriol-7-O-(6-malonyl-apiosyl) hexoside (C-7-(6-Ma-Ap) H) [28]). The fragment at m/z 635 was also detected and its suggested that it corresponding to the loss of a carboxyl ion ([M – H – 44 (COO -]-) after ionization (Fig. 4). The molecular ions at m/z 299 ([M – H]-) and m/z 301 ([M + H]+) fragments were detected in the hydrolyzed extract in negative and positive ion mode, respectively. It was identified as chrysoeriol, the aglycone form of C-7-(6-Ma-Ap) H (Table 3). The fragment at m/z 284 corresponding to the loss of a methyl group ([M – H – 15 (CH3)]-), fragment at m/z 151 representing the “C” ring breakage of the flavonoid skeleton ([M – 149]-) and fragment at m/z 107 representing a product of the “C” ring breaking were also observed.
3.6. Quantification of identified phenolic compounds from C. Chinense leaves extracts.
The best response for TPC was obtained using 50% MeOH as solvent, which can be selected as the best option to achieve the major recovery of phenolic compounds in C. chinense leaves. However, the spectrophotometric method for the determination of TPC is not specific, therefore, to a better understanding of the interactions of each identified compound with the solvent used for its extraction the quantification of each compound obtained by the different solvents is showed in table 4. The highest content of N-caffeoyl putrescine was 0.85 ± 0.06 and 0.90 ± 0.08 µmol Eq CA g−1 dry wt by CE and UAE respectively, using 50% MeOH as solvent, while the highest general content of identified flavonoids was obtained using 80% Ace independently of the extraction method employed. The flavonoids with the highest concentration were: L-7-(2-Ap) H, A-7-(2-Ap) H and D-7-(2-Ap) H containing 8.69 ± 0.12 µmol Eq luteolin g−1 dry wt, 14.23 + 0.03 µmol Eq of apigenin g−1 dry wt and 18.81 ± 1.07 µmol Eq of quercetin g−1 dry wt, respectively. In both cases, for phenolic acid (N-Cp) and flavonoids it was observed a similar behavior to that described for the correlation between TPC and “ε” of the evaluated solvents, an increase in the individual content of each phenolic compound as a function of polarity (ɛ), followed by a maximum recovery point and a decrease as the polarity of the solvent increases. However, the ideal values of “ε” for the recovery of each phenolic compound is different from each other. In this sense, for the recovery of N-Cp the values are between 46.99 and 52.09, while for flavonoids are between 20.62 and 22.45 that are obtained with solvents of MeOH at 50% and Ace at 80% respectively. The results also show similarities in the recovery of phenolic compounds between both methods, the UAE and CE. However, the advantages offered by the UAE such as the mechanism and time of extraction must be considered. For example, the results reported by Pacheco et al. [38] show that through UAE a higher recovery of rosmarinic acid can be achieved from Cordia dodecandra fruits than through extraction by maceration.
Regarding the biological activity of these compounds, Park et al. [39] observed the accumulation of chlorogenic acid and N-caffeoyl putrescine in C. annuum fruits at the local site of C. coccodes infection. The latter compound was reported as a “de novo” synthesis in C. annuum and is classified as a possible phytoalexin. The proposed function is that its accumulation is related to the formation of mechanical barriers to prevent the progression of the infection. The accumulation of flavonoids glycosylated is observed in healthy leaves due to antiradical activity. León-Chan et al. [27] reported that in C. annuum leaves flavonoids as apigenin-7-O-glucoside (A-7-G) and luteolin-7-O-glucoside (L-7-G) are related to defending mechanism against UV-B radiation and low temperatures. Du et al. [20] reported that the accumulation of flavonoids as apigenin and luteolin in sorghum are related to inhibition of spore germination of C. sublineolum, while Mikulic-Petkovsek et al. [32] reported that accumulation of flavonoids is observed in tissues compromised to infection produced by C. coccodes. Secondly, glycosylation to flavonoids is an important step to their accumulation in different tissues. This process is realized by UDP-glycosyltransferases (UGT) and allow stored flavonoids in cellular vacuoles contributing to plant homeostasis and resistance mechanisms against abiotic and biotic stresses [26].
4. Conclusions
The results indicated a higher recovery of phenolic compounds with MeOH at 50% according to the response of TPC, besides an important correlation between TPC and antioxidant activity (ABTS+, DPPH) was found. TPC showed relation with the “ε” that is a polarity parameter related to the molecular interaction between solvents and solutes. This relation was analyzed by the Weibull and Gaussian models presenting high regression values (0.93 to 0.95), suggesting that the highest phenolic compounds recovery is obtained using solvents with “ε” values between 35 and 52 by UAE. The chromatographic analysis showed that there are important differences in the recovery of specific phenolic compounds, while MeOH at 50% is adequate for a better recovery of phenolic acids (N-Cp), the use of 80% Ace turned out to be more favorable for the recovery of flavonoids. Finally, this study provides a key criterion for the selection of the extraction solvent to be used in UAE method for TPC recovery of C. chinense leaves that could be useful for the study of metabolic changes of different groups of phenolic compounds, which are one of the main mechanisms triggered by plants to interact with their environment.
Funding
This research was financed by the CONACyT through the project (Grant 035/2015) “Cambios Metabólicos y el Sistema de Transducción de Señales Asociados a la Interacción de Capsicum chinense con Pythium sp.”
CRediT authorship contribution statement
Emanuel Herrera-Pool: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - original draft. Ana Luisa Ramos-Díaz: Conceptualization, Resources, Writing - review & editing, Funding acquisition. Manuel Alejandro Lizardi-Jiménez: Validation, Writing - review & editing. Soledad Pech-Cohuo: Methodology, Writing - review & editing, Supervision. Teresa Ayora-Talavera: Conceptualization, Resources, Writing - review & editing, Supervision. Juan C. Cuevas-Bernardino: Writing - review & editing, Supervision. Ulises García-Cruz: Writing - review & editing, Supervision. Neith Pacheco: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors acknowledge CONACyT for the grant of Emanuel Herrera-Pool and for the equipment used at the laboratory acquired by the project Cátedras CONACYT 1039: “Desarrollo de laboratorio y pruebas de chile habanero y productos hortofrutícolas”, To the Scholarship of the Postdoctoral Program for Indigenous Mexican Women in Science, Technology, Engineering, and Mathematics, of CONACYT—Center for Research and Higher Studies in Social Anthropology (CIESAS, in Spanish acronym)—International Development Research Center-Canada (IDRC) CEAR2019-06.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105658.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Abu-Reidah I.M., Ali-Shtayeh M.S., Jamous R.M., Arráez-Román D., Segura-Carretero A. Comprehensive metabolite profiling of Arum palaestinum (Araceae) leaves by using liquid chromatography–tandem mass spectrometry. Food Res. Int. 2015;70:74–86. doi: 10.1016/j.foodres.2015.01.023. [DOI] [Google Scholar]
- 2.Abu-Reidah I.M., Arráez-Román D., Quirantes-Piné R., Fernández-Arroyo S., Segura-Carretero A., Fernández-Gutiérrez A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012;46:108–117. doi: 10.1016/j.foodres.2011.11.026. [DOI] [Google Scholar]
- 3.Abu-Reidah I.M., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013;141:2269–2277. doi: 10.1016/j.foodchem.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 4.Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Carrillo N., Aguilar-Santamaría M.d.L.Á., Vernon-Carter E.J., Jiménez-Alvarado R., Cruz-Sosa F., Román-Guerrero A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017;103:213–221. [Google Scholar]
- 6.Álvarez A., Fayos-Fernández J., Monzó-Cabrera J., Cocero M.J., Mato R.B. Measurement and correlation of the dielectric properties of a grape pomace extraction media. Effect of temperature and composition. J. Food Eng. 2017;197:98–106. doi: 10.1016/j.jfoodeng.2016.11.009. [DOI] [Google Scholar]
- 7.Bae H., Jayaprakasha G.K., Jifon J., Patil B.S. Variation of antioxidant activity and the levels of bioactive compounds in lipophilic and hydrophilic extracts from hot pepper (Capsicum spp.) cultivars. Food Chem. 2012;134:1912–1918. doi: 10.1016/j.foodchem.2012.03.108. [DOI] [PubMed] [Google Scholar]
- 8.Baker C.J., Smith J.M., Yarberry A.J., Rice C. Induction of apoplast phenolics in pepper (Capsicum annuum) leaves in response to pathogenic bacteria. Physiol. Mol. Plant Pathol. 2020;109 doi: 10.1016/j.pmpp.2019.101453. [DOI] [Google Scholar]
- 9.Baumert A., Mock H.-P., Schmidt J., Herbers K., Sonnewald U., Strack D. Patterns of phenylpropanoids in non-inoculated and potato virus Y-inoculated leaves of transgenic tobacco plants expressing yeast-derived invertase. Phytochemistry. 2001;56:535–541. doi: 10.1016/S0031-9422(00)00422-2. [DOI] [PubMed] [Google Scholar]
- 10.Camacho-Cristóbal J.J., Lunar L., Lafont F., Baumert A., González-Fontes A. Boron deficiency causes accumulation of chlorogenic acid and caffeoyl polyamine conjugates in tobacco leaves. J. Plant Physiol. 2004;161:879–881. doi: 10.1016/j.jplph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho Lemos V., Reimer J.J., Wormit A. Color for life: biosynthesis and distribution of phenolic compounds in pepper (Capsicum annuum) Agric. 2019 doi: 10.3390/agriculture9040081. [DOI] [Google Scholar]
- 12.Catena S., Rakotomanomana N., Zunin P., Boggia R., Turrini F., Chemat F. Solubility study and intensification of extraction of phenolic and anthocyanin compounds from Oryza sativa L. ‘Violet Nori’. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105231. [DOI] [PubMed] [Google Scholar]
- 14.Cheynier V., Comte G., Davies K.M., Lattanzio V., Martens S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Cisneros-Pineda O., Torres-Tapia L.W., Gutiérrez-Pacheco L.C., Contreras-Martín F., González-Estrada T., Peraza-Sánchez S.R. Capsaicinoids quantification in chili peppers cultivated in the state of Yucatan. Mexico. Food Chem. 2007;104:1755–1760. doi: 10.1016/j.foodchem.2006.10.076. [DOI] [Google Scholar]
- 16.Covarrubias-Cárdenas A., Martínez-Castillo J., Medina-Torres N., Ayora-Talavera T., Espinosa-Andrews H., García-Cruz N., Pacheco N. Antioxidant capacity and UPLC-PDA ESI-MS phenolic profile of stevia rebaudiana dry powder extracts obtained by ultrasound assisted extraction. Agron. 2018;8(9):170. doi: 10.3390/agronomy8090170. [DOI] [Google Scholar]
- 17.Das S., Teja K.C., Duary B., Agrawal P.K., Bhattacharya S.S. Impact of nutrient management, soil type and location on the accumulation of capsaicin in Capsicum chinense (Jacq.): One of the hottest chili in the world. Sci. Hortic. (Amsterdam) 2016;213:354–366. doi: 10.1016/j.scienta.2016.10.041. [DOI] [Google Scholar]
- 18.Dias A.L.B., Arroio Sergio C.S., Santos P., Barbero G.F., Rezende C.A., Martínez J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017;198:36–44. doi: 10.1016/j.jfoodeng.2016.11.020. [DOI] [Google Scholar]
- 19.Dong X., Cao Y., Lin H., Yao Y., Guo Y., Wang T., Wu S., Wu Z. Solubilities of formononetin and daidzein in organic solvents: effect of molecular structure and interaction on solvation process. J. Mol. Liq. 2017;231:542–554. doi: 10.1016/j.molliq.2017.02.051. [DOI] [Google Scholar]
- 20.Du Y., Chu H., Wang M., Chu I.K., Lo C. Identification of flavone phytoalexins and a pathogen-inducible flavone synthase II gene (SbFNSII) in sorghum. J. Exp. Bot. 2010;61:983–994. doi: 10.1093/jxb/erp364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estrada B., Bernal M.A., Díaz J., Pomar F., Merino F. Capsaicinoids in vegetative organs of Capsicum annuum L. in relation to fruiting. J. Agric. Food Chem. 2002;50(5):1188–1191. doi: 10.1021/jf011270j. [DOI] [PubMed] [Google Scholar]
- 22.Fabela-Morón M.F., Cuevas-Bernardino J.C., Ayora-Talavera T., Pacheco N. Trends in capsaicinoids extraction from habanero chili pepper (Capsicum Chinense Jacq.): recent advanced techniques. Food Rev. Int. 2020;36(2):105–134. doi: 10.1080/87559129.2019.1630635. [DOI] [Google Scholar]
- 23.Guclu G., Keser D., Kelebek H., Keskin M., Emre Sekerli Y., Soysal Y., Selli S. Impact of production and drying methods on the volatile and phenolic characteristics of fresh and powdered sweet red peppers. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128129. [DOI] [PubMed] [Google Scholar]
- 24.Jouyban A., Soltanpour S., Chan H.-K. A simple relationship between dielectric constant of mixed solvents with solvent composition and temperature. Int. J. Pharm. 2004;269:353–360. doi: 10.1016/j.ijpharm.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Kumar B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs) J. Pharm. Anal. 2017;7:349–364. doi: 10.1016/j.jpha.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Roy J., Huss B., Creach A., Hawkins S., Neutelings G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.León-Chan R.G., López-Meyer M., Osuna-Enciso T., Sañudo-Barajas J.A., Heredia J.B., León-Félix J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017;139:143–151. doi: 10.1016/j.envexpbot.2017.05.006. [DOI] [Google Scholar]
- 28.Lin L.-Z., Lu S., Harnly J.M. Detection and quantification of glycosylated flavonoid malonates in celery, chinese celery, and celery seed by LC-DAD-ESI/MS. J. Agric. Food Chem. 2007;55(4):1321–1326. doi: 10.1021/jf0624796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loizzo M.R., Pugliese A., Bonesi M., Menichini F., Tundis R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: a comparison between fresh and processed peppers. LWT - Food Sci. Technol. 2015;64:623–631. doi: 10.1016/j.lwt.2015.06.042. [DOI] [Google Scholar]
- 30.Marín A., Ferreres F., Tomás-Barberán F.A., Gil M.I. Characterization and quantitation of antioxidant constituents of sweet pepper (Capsicum annuum L.) J. Agric. Food Chem. 2004;52(12):3861–3869. doi: 10.1021/jf0497915. [DOI] [PubMed] [Google Scholar]
- 31.Medina-Torres N., Ayora-Talavera T., Espinosa-Andrews H., Sánchez-Contreras A., Pacheco N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agron. 2017;7(3):47. doi: 10.3390/agronomy7030047. [DOI] [Google Scholar]
- 32.Mikulic-Petkovsek M., Schmitzer V., Jakopic J., Cunja V., Veberic R., Munda A., Stampar F. Phenolic compounds as defence response of pepper fruits to Colletotrichum coccodes. Physiol. Mol. Plant Pathol. 2013;84:138–145. doi: 10.1016/j.pmpp.2013.09.003. [DOI] [Google Scholar]
- 33.S. Moco, R.J. Bino, O. Vorst, H.A. Verhoeven, J. de Groot, T.A. van Beek, J. Vervoort, C.H.R. de Vos. A Liquid Chromatography-Mass Spectrometry-Based Metabolome Database for Tomato. Plant Physiol. 141 2006 1205 LP – 1218. https://doi.org/10.1104/pp.106.078428. [DOI] [PMC free article] [PubMed]
- 34.Morales-Soto A., Gómez-Caravaca A.M., García-Salas P., Segura-Carretero A., Fernández-Gutiérrez A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013;51:977–984. doi: 10.1016/j.foodres.2013.02.022. [DOI] [Google Scholar]
- 35.Mudrić S.Ž., Gašić U.M., Dramićanin A.M., Ćirić I.Ž., Milojković-Opsenica D.M., Popović-Đorđević J.B., Momirović N.M., Tešić Ž.L. The polyphenolics and carbohydrates as indicators of botanical and geographical origin of Serbian autochthonous clones of red spice paprika. Food Chem. 2017;217:705–715. doi: 10.1016/j.foodchem.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Olszowy M., Dawidowicz A.L. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chem. Pap. 2018;72(2):393–400. doi: 10.1007/s11696-017-0288-3. [DOI] [Google Scholar]
- 37.Oreopoulou A., Goussias G., Tsimogiannis D., Oreopoulou V. Hydro-alcoholic extraction kinetics of phenolics from oregano: optimization of the extraction parameters. Food Bioprod. Process. 2020;123:378–389. doi: 10.1016/j.fbp.2020.07.017. [DOI] [Google Scholar]
- 38.N. Pacheco, G.K. Méndez-Campos, I.E. Herrera-Pool, C.J. Alvarado-López, A. Ramos-Díaz, T. Ayora-Talavera, S.U. Talcott, J.C. Cuevas-Bernardino. Physicochemical composition, phytochemical analysis and biological activity of ciricote (Cordia dodecandra A. D.C.) fruit from Yucatán. Nat. Prod. Res. 2020 1–5. https://doi.org/10.1080/14786419.2020.1774763. [DOI] [PubMed]
- 39.Park S., Jeong W.Y., Lee J.H., Kim Y.-H., Jeong S.W., Kim G.-S., Bae D.W., Lim C.-S., Jin J.S., Lee S.J., Shin S.C. Determination of polyphenol levels variation in Capsicum annuum L. cv. Chelsea (yellow bell pepper) infected by anthracnose (Colletotrichum gloeosporioides) using liquid chromatography–tandem mass spectrometry. Food Chem. 2012;130:981–985. doi: 10.1016/j.foodchem.2011.08.026. [DOI] [Google Scholar]
- 40.Pavlović A.V., Papetti A., Zagorac D.Č.D., Gašić U.M., Mišić D.M., Tešić Ž.L., Natić M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016;87:304–314. doi: 10.1016/j.indcrop.2016.04.052. [DOI] [Google Scholar]
- 41.Pérez-Ambrocio A., Guerrero-Beltrán J.A., Aparicio-Fernández X., Ávila-Sosa R., Hernández-Carranza P., Cid-Pérez S., Ochoa-Velasco C.E. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biol. Technol. 2018;135:19–26. doi: 10.1016/j.postharvbio.2017.08.023. [DOI] [Google Scholar]
- 42.A. Plazonić, F. Bucar, Ž. Maleš, A. Mornar, B. Nigović, N. Kujundžić. Identification and Quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Mol. 2009. https://doi.org/10.3390/molecules14072466. [DOI] [PMC free article] [PubMed]
- 43.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Rodríguez E., Ruiz J.M., Ferreres F., Moreno D.A. Phenolic profiles of cherry tomatoes as influenced by hydric stress and rootstock technique. Food Chem. 2012;134:775–782. doi: 10.1016/j.foodchem.2012.02.180. [DOI] [PubMed] [Google Scholar]
- 45.Sora G.T.S., Haminiuk C.W.I., da Silva M.V., Zielinski A.A.F., Gonçalves G.A., Bracht A., Peralta R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: an application of chemometrics. J. Food Sci. Technol. 2015;52(12):8086–8094. doi: 10.1007/s13197-015-1935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallverdú-Queralt A., Jáuregui O., Di Lecce G., Andrés-Lacueva C., Lamuela-Raventós R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC–ESI-QTOF) Food Chem. 2011;129:877–883. doi: 10.1016/j.foodchem.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 47.Verma N., Shukla S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants. 2015;2:105–113. doi: 10.1016/j.jarmap.2015.09.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.