Abstract
Background.
Individuals with hoarding disorder (HD) demonstrate exaggerated subjective distress and hyper-activation of cingulate and insular cortex regions when discarding personal possessions. No prior studies have sought to determine whether this subjective distress is associated with specific profiles of abnormal brain function in individuals with HD.
Methods.
We used Multimodal Canonical Correlation Analysis+joint Independent Component Analysis (“mCCA+jICA”) to test whether five hoarding-relevant domains of subjective distress when deciding to discard possessions (anxiety, sadness, monetary value, importance, and sentimental attachment) are associated with fMRI-measured whole-brain functional connectivity in 72 participants with HD and 44 healthy controls (HCs).
Results.
Three extracted components differed between HD participants and HCs, each of which depicted an abnormal profile of functional connectivity during discarding decisions and a specific distress response profile. One component pair showed a relationship between anxiety ratings during discarding decisions and connectivity among pallidum, perirhinal ectorhinal cortex, and dorsolateral prefrontal cortex (dlPFC). Another component was comprised of sadness ratings during discarding decisions and connectivity in pallidum, nucleus accumbens, amygdala, and dlPFC. The third pair linked HD brain connectivity in several dlPFC regions with perceived importance ratings during discarding decisions.
Conclusions.
The findings indicate that HD patients’ subjective intensity of anxiety, sadness, and perceived possession importance is related to abnormal functional connectivity in key frontal and emotional processing brain regions. Findings are discussed in terms of emerging neurobiological models of HD.
Keywords: Hoarding Disorder (HD), Independent Component Analysis (ICA), Canonical Correlation Analysis, fMRI, Functional Connectivity, Discarding
Introduction
Hoarding disorder (HD) is characterized by difficulty discarding possessions, resulting in excessive clutter in the home that precludes the normal use of living spaces (1). Neuroimaging studies indicate that patients with HD have characteristic functional abnormalities in cingulate and insular cortex regions elicited during symptom provocation tasks (2, 3). Specifically, when patients with HD make decisions to discard possessions (either real or imagined personal possessions), they exhibit relatively greater activation of anterior cingulate cortex (ACC) and insula. In contrast, personally-irrelevant emotionally neutral decisions result in activity levels in these brain regions that are generally blunted compared to non-patient controls or patients with obsessive-compulsive disorder (OCD; 2, 3). Furthermore, neural activity in ACC and insula correlated with self-reported hoarding severity in these studies (2, 3). Although other HD brain activation abnormalities have been reported, they have yet to be clearly and reliably linked to specific neural circuit- or neurocognition-based impairments in HD. Some of these not-yet-replicated HD abnormalities include lateral prefrontal cortex, posterior cingulate gyrus, inferior temporal cortex, and several parietal lobe regions (2–5). There also is initial evidence that HD patients have abnormally low network functional connectivity within frontoparietal cognitive control neural systems and disrupted default mode network integrity (6). Although findings have begun to converge across HD studies on cingulo-opercular regions within the brain’s salience network as one clear circuit-based HD abnormality, it is not yet clear what role these other brain function abnormalities play in HD.
One question that has not yet been extensively addressed involves the possibility that some HD brain function abnormalities might reflect HD patients’ emotional state during symptom provocation. Cognitive-behavioral theories of HD posit that maladaptive beliefs about, and emotional attachment to, possessions contribute to emotional distress (7). Consistent with these theories, HD patients report significant subjective distress when discarding personal possessions (2, 8). It would be useful to learn whether any of the functional brain abnormalities identified in prior fMRI studies reflect this hoarding-related distress. One relevant neuroimaging study conducted in non-HD participants demonstrated that hoarding-relevant symptom provocation activated ventral prefrontal cortex and amygdala regions, suggesting that these regions may be specifically associated with hoarding-related distress (9). Other studies in HD and OCD participants have linked subjective distress ratings (anxiety and sadness) and neural activity during hoarding symptom provocation in left precentral gyrus, right middle and inferior frontal gyri, and anterior ventromedial prefrontal cortex (2, 4, 10). However, these were post hoc analyses of primary study group differences that leave questions as to the full scope of possible HD distress/brain dysfunction relationships. To our knowledge, all prior studies that have shed light on relationships between hoarding-related distress and neural activity have focused on brain activation data, not functional connectivity. Given the emerging understanding of the importance of distributed brain network models to understanding psychopathology, it will be especially informative to determine how hoarding-related distress might be associated with abnormalities in functional connectivity.
The purpose of the present study was to learn whether hoarding-related emotional distress is associated with specific profiles of abnormal functional connectivity during a symptom provocation fMRI challenge. Specifically, we examined relationships between subjective distress during item discarding/acquiring decisions and functional connectivity across the entire brain in patients with HD and in healthy controls (HCs). We re-examined fMRI data from a recent study which compared brain activation between HD participants and HCs during both discarding and acquiring decisions (3). We used a multivariate technique that combines independent component analysis with canonical correlation analysis (CCA+jICA; 11) to examine the associations between functional connectivity measurements extracted from fMRI timeseries data and visual analogue scale (VAS) ratings across five domains relevant to HD (fear/anxiety, sadness/regret, monetary value, importance/usefulness, and sentimental attachment). In contrast to less sophisticated, univariate techniques (e.g., simple linear correlation with VAS ratings), mCCA+jICA can identify and represent complex patterns of data relationships using a relatively small handful of components. This facilitates interpretation and mitigates the Type I error rate control burden that usually would be insurmountable when examining such high-dimensional brain function data for these types of relationships, making it ideally suited to assess how VAS ratings and connectivity measurements systematically covary across HD patients and HCs.
By design, the mCCA+jICA approach will find systematic relationships between brain connectivity and VAS ratings. As such, we expected the technique would successfully isolate meaningful associations between functional connectivity in specific brain regions and separate VAS ratings. Of these, we focused only on relationships found by mCCA+jICA that depicted abnormalities in HD, while relationships between distress and connectivity found not to differ between HD and HC were of secondary interest. We also were most interested in connectivity-VAS rating associations during discarding decisions. Our prior fMRI studies have tended to observe a greater extent and severity of HD brain activity abnormalities in discarding relative to acquiring decisions, possibly because acquiring decisions may elicit less subjective distress in HD patients. Based on prior HD research (9), we hypothesized that VAS anxiety ratings would be associated with metrics that quantified overall brain inter-connectedness for the amygdala. Consistent with previous studies (2, 10), we also hypothesized that sadness/regret ratings would be associated with connectivity strength in the precentral gyrus and middle and inferior frontal gyri. Such associations would build upon these prior studies by linking specific forms of distress in HD to the role each of these different regions play within distributed brain networks.
Methods and Materials
Participants
Participants were 72 right-handed adults (mean age 55.0 years, 86% female) with a primary diagnosis of HD of at least moderate severity who enrolled in a waitlist-controlled trial of group cognitive-behavioral therapy (CBT; Clinicaltrials.gov identifier NCT01956344) for HD (“HD group”). An age- and sex-equivalent healthy control group of 44 right-handed adults (mean age 54.0 years, 77% female) also participated (“HC group”). See Table 1 for participant characteristics. Participants were enrolled between 10/22/13–11/27/17 and have been previously described in other studies (3, 12). The HD sample reported here differs slightly from the overall available sample examined in Stevens et al. (3) due to VAS and fMRI data availability (see Supplement for details). The HD and HC groups differed on HD symptom severity (more severe in HD) and race (more racial diversity based on chi-square analyses in the HC group), but not on ethnicity or other demographic variables (see Table 1). Full study inclusion/exclusion criteria are detailed in the Supplement.
Table 1.
Sample Characteristics
| HD (n = 72) | HC (n = 44) | Comparison | |
|---|---|---|---|
|
| |||
| Variable | t or X2 (p) | ||
| Age, M (SD) | 55.26 (8.74) | 53.97 (7.12) | 0.82 (.414) |
| Female sex, n (%) | 62 (86.1) | 34 (77.3) | 1.495 (.221) |
| Race, n (%) | 10.182 (.006) | ||
| White | 69 (95.8) | 34 (77.3) | |
| Black | 2 (2.8) | 9 (20.5) | |
| Asian | 1 (1.4) | 1 (2.3) | |
| Hispanic Ethnicity, n (%) | 2 (2.8) | 3 (6.8) | 1.081 (.298) |
| Comorbid diagnoses | --- | --- | |
| OCD | 10 (13.9) | ||
| Anxiety disorder | 25 (34.7) | ||
| Depressive disorder | 39 (54.2) | ||
| ADHD | 12 (16.7) | ||
| Psychiatric medications | --- | --- | |
| Antidepressants | 13 (18.1) | ||
| Benzodiazepines | 7 (9.7) | ||
| Stimulants | 5 (6.9) | ||
| SI-R Saving, M (SD) | 20.06 (3.71) | 3.59 (2.85) | 25.23 (< .001) |
| SI-R Clutter, M (SD) | 25.89 (5.79) | 2.52 (3.17) | 28.04 (< .001) |
| SI-R Acquiring, M (SD) | 15.51 (5.51) | 2.75 (2.16) | 17.57 (< .001) |
| SI-R Total, M (SD) | 61.46 (10.95) | 11.36 (5.84) | 32.07 (<.001) |
| Discarding Task VAS, M (SD) | |||
| Fear/anxiety | 23.71 (22.51) | 2.09 (6.49) | 7.65 (< .001) |
| Sadness/regret | 15.74 (18.79) | 1.16 (5.27) | 6.20 (< .001) |
| Monetary value | 31.65 (17.50) | 27.77 (20.10) | 1.10 (.276) |
| Importance/usefulness | 38.03 (20.67) | 26.36 (19.95) | 2.99 (.003) |
| Sentimental value | 23.06 (22.39) | 11.00 (15.87) | 3.12 (.002) |
| Acquiring Task VAS, M (SD) | |||
| Fear/anxiety | 17.85 (19.32) | 3.64 (8.24) | 5.48 (< .001) |
| Sadness/regret | 9.81 (18.50) | 0 (0) | 4.50 (< .001) |
| Monetary value | 31.99 (18.74) | 25.11 (18.90) | 1.99 (.049) |
| Importance/usefulness | 38.40 (21.19) | 28.11 (23.25) | 2.45 (.016) |
| Sentimental value | 15.75 (17.32) | 6.14 (14.05) | 3.26 (.002) |
Note. HD = Hoarding disorder group. HC = Healthy control group. OCD = Obsessive-compulsive disorder. ADHD = Attention-deficit/hyperactivity disorder. SI-R = Saving Inventory-Revised. VAS = Visual analogue scale.
Study Procedure Overview
All study procedures were approved by the hospital’s Institutional Review Board. On the first day of the study, the informed consent form was reviewed with participants in detail, after which written informed consent was obtained. Participants then completed the intake interview. If eligible for the study, HD group participants returned to the laboratory approximately one week later for the MRI session, during which they completed discarding and acquiring fMRI symptom provocation decision-making tasks (see description, below). Immediately after each of these two tasks, they provided their VAS ratings. They also completed self-report measures of HD severity during this visit. The HC group completed the interview, fMRI tasks, VAS ratings, and self-report measures on the same day. The entire fMRI session took approximately 90 minutes, and participants received $50 for completing the session.
Measures
Diagnostic assessments.
We used the Diagnostic Interview for Anxiety, Mood, and Obsessive-Compulsive and Related Neuropsychiatric Disorders (DIAMOND; 13), a structured diagnostic interview based on the DSM-5, to assess participants’ diagnoses. Because moderate HD severity was required for patient inclusion, we assessed global illness severity using a modified version of the Clinical Global Impression (CGI) scale (14) (15). Interviewers were licensed psychologists or psychology postdoctoral fellows under supervision.
Self-report measures.
Self-reported HD severity was assessed with the Saving Inventory-Revised (SI-R; 16), which contains three subscales that assess the primary symptoms of HD (acquiring, difficulty discarding, and clutter). Items are rated on a 5-point scale with higher scores indicating greater HD severity. The SI-R has demonstrated adequate internal consistency in previous studies (16) and showed excellent internal consistency in this sample (total score, α = 0.98; subscales, all αs ≥ .95).
Visual analogue scales (VAS).
Participants were asked the following questions immediately after the discarding and acquiring fMRI tasks, making a total of 10 VAS ratings (5 from each task) available for analysis. The questions were presented on the computer screen in the MRI scanner using a visual analogue scale of 0–100, where 0 = Not at all and 100 = Extremely:
Fear/anxiety. How much fear or anxiety did you experience?
Sadness/regret. How much sadness or regret did you experience?
Monetary value. How much monetary value did these items seem to have overall?
Importance/usefulness. How much importance or usefulness did these items seem to have overall?
Sentimental attachment. How much sentimental value did these items seem to have overall?
fMRI Paradigms.
The discarding and acquiring tasks were based on the tasks described in Preston et al. (17). The tasks are detailed in prior reports (3, 18) and in the Supplement. Briefly, in the discarding task, participants were presented with pictures of household items and were asked to decide either to keep or discard them as part of spring cleaning. In the acquiring task, participants saw pictures of items that could be acquired for free and were asked to decide whether or not to acquire them. In a control condition, participants viewed pictures and were asked to decide if the object was once alive (e.g., a wooden spoon) or never alive (e.g., a sponge). We have shown that these simulated acquiring and discarding decisions provoke excessive “saving” and “acquiring” behaviors in HD, and are useful for probing HD-related brain dysfunction (18).
MRI Data Collection and Preparation
Details of the Siemens Skyra 3T scanner sequences and the Human Connectome Project data processing approach (19) were reserved for the study Supplement to focus here on how the functional connectivity data were generated and prepared for mCCA+jICA analyses. To quantify functional connectivity, vertices or voxels within 387 separate brain regions were averaged for each timepoint in each participant’s fMRI task timeseries. These regions included HCP’s 360 cortical parcels (20), HCP’s standard subcortical regions, and 11 discrete cerebellar regions that could be reliably mapped across participants from a published cerebellar parcellation (21). R software (22) was used to create Pearson cross-correlation matrices (adjacency matrices) among these brain regions for each participant’s parcellated timeseries data. Each correlation value (i.e., edge) quantified the strength of functional connectivity between two separate regions. These edge values underwent Fisher Z transformation, then were flattened into a single row of 74,691 edge values.
Data Fusion With mCCA+jICA
Two-group (HD and HC), two-feature (functional connectivity and VAS ratings) multimodal Canonical Correlation Analysis+joint Independent Component Analysis (mCCA+jICA) (11) was conducted using the Fusion ICA Toolbox (FIT; available at http://trendscenter.org/software/fit/). mCCA+jICA is an approach to “fuse” different data features together. It is highly reliable, robust to measurement noise, more likely than several other fusion methods to uncover hidden relationships between or within modalities (23–25), and has been used successfully to discover disorder biomarkers and novel insights into disease (26–36). The methodology is described in detail in Sui et al. (34). The technical details of its application in the current study are described in the Supplement. Briefly, mCCA+jICA identifies jointly covarying information between two features within a multivariate context. Here, we sought to discover the correspondence of different patterns of fMRI-measured functional connectivity while participants made decisions to acquire or discard items with their VAS-rated emotional state immediately after they finished all trials for each fMRI task. The focus of mCCA+jICA is on identifying how relationships between data features differ between study groups, not finding group differences in the raw data themselves. So mCCA+jICA results are best used to link complex profiles of information to find associations that otherwise might be obscured by conventional analysis approaches which often have insurmountable statistical power requirements. When mCCA+jICA is executed, it typically is constrained to identify a manageable handful of associated patterns. Here, the mCCA+jICA analysis sought 8 joint components, which was judged likely to capture at least a handful of unique relationships between features given that there were only 10 VAS datapoints for each participant. After mCCA+jICA finds linked component pairs, a data back-reconstruction process expresses the contribution of each datapoint as z scores. More extreme z scores represent higher positive or negative expression within the relationship depicted in each component pair. For example, a VAS component with only one or two high z scores would be driven by participants’ responses on those specific VAS questions. Furthermore, those VAS responses would be linked to whichever specific brain connectivity data features had high z scores in the other feature.
Component Visualization.
Because the two features had different dimensionality, we aggregated mCCA+jICA results across participants in different ways to best visualize the relative importance of specific datapoints within each component pair. For the VAS ratings, it was straightforward to see which VAS questions loaded onto which components simply by depicting the sample’s mean z scores. Interpretation of which VAS questions are most important to each component pair should follow commonly-used thresholds (e.g., z >2.0, or two standard deviations above the mean). However, there were 74,691 edges in the functional connectivity adjacency matrices. So for the connectivity feature, it was useful to find a way to summarize these edge z weights in a way that focused on the overall connectivity of specific brain regions. To do this, we used a common graph theoretic algorithm in R software (centrality_degree) to estimate a “degree” value for each of the 387 parcels for each participant. Each degree value represented that brain region’s overall weighted importance to that component pair. For example, if the amygdala had numerous edges with high z weights for one component pair, but its z scores were close to zero for another pair, the degree metric would be high in the first, and low in the second. As such, we could identify which handful of brain regions had connectivity values important to each component pair. We then evaluated a bootstrapped estimate of each degree value’s probability of being at the 95th percentile of the observed distribution for each region to focus only on regions whose values were sufficiently different from its average across participants that were unlikely to have occurred by chance. This was calculated using the OutlierDetection package in R software (37). To ensure a conservative estimate of statistical significance, the intersection of three different bootstrapping methods was used [depth (38), dispersion (39), and k-nearest neighbor (40), each calculated using a 95th percentile threshold].
Testing mCCA+jICA Components for Group Differences.
mCCA+jICA also produces mixing coefficients for each participant. These coefficients represent how much each participant expressed the relationship depicted in each component. There is one mixing coefficient for each participant for the VAS feature and connectivity feature for each of the 8 component pairs. These were used in two-sample t test random effects analyses to test how the HD and HC groups differed. We were interested primarily in fused components where both the connectivity component and VAS rating component differed between groups. This would represent associations between HD brain network integration linked to a specific VAS rating profile that could be deemed abnormal in HD relative to HC. One-sample t tests also were done separately for each study group to evaluate whether the coefficients significantly differed from zero for either HD, HC, or both groups. The latter can be useful to provide additional interpretive context, as not every component or fused relationship between features necessarily will be strongly expressed in both groups. For instance, the presence of a brain connectivity profile in HC that is absent in HD can be straightforwardly interpreted as an HD deficiency. Lastly, we conducted Pearson correlations between SI-R total scores, functional connectivity, and VAS ratings for each component to learn if any features already determined to be abnormal in HD also were associated with the severity of HD symptomatology. We applied Bonferroni corrections for testing group differences between the 8 component pairs (α .05/8 = .00625).
Results
Table 2 summarizes the results of all mCCA+jICA analyses of the discarding task fMRI data and VAS ratings. Pearson correlations between connectivity and VAS ratings across components ranged from r = 0.26 to 0.57, which were all statistically significant after Bonferroni correction. This indicates mCCA+jICA was successful in linking VAS ratings with connectivity features. Next, we present paired components of a priori interest that showed HD vs. HC group differences in both functional connectivity and VAS ratings. Again, because our interest was on abnormal HD discarding-related VAS/connectivity results, acquiring fMRI task component pairs and details for any discarding task component pairs that did not differ between HD and HC are described in the study Supplement.
Table 2.
Summary of hypothesis-testing on mCCA+jICA mixing coefficients for the discarding task fMRI connectivity data and VAS ratings. Component pairs are sorted in descending order of the Pearson r correlation strength between connectivity and VAS features. Subsequent columns list the significance p values of one-sample, two-sample, and post hoc correlation tests on the coefficient data.
| Component Pair | r | Connectivity Feature | VAS Rating Feature | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HC p | HD p | HC vs HD p | HD SI-R Total p | HCp | HD p | HC vs HD p | HD SI-R Total p | ||
| 1 | 0.574* | 0.0023* | ns | 0.0006* | 0.01 | <0.0001* | 0.0016* | 0.0001* | 0.0001* |
| 2 | 0.569* | ns | <0.0001* | 0.0001* | 0.0352 | 0.0483 | <0.0001* | <0.0001* | 0.0382 |
| 3 | 0.543* | ns | 0.0247 | 0.0377 | 0.0278 | ns | 0.0043* | 0.0177 | ns |
| 4 | 0.466* | <0.0001* | ns | <0.0001* | 0.0326 | 0.013 | <0.0001* | <0.0001* | 0.0007* |
| 5 | 0.434* | <0.0001* | ns | 0.0059* | 0.015 | <0.0001* | <0.0001* | 0.0003* | ns |
| 6 | 0.420* | 0.0045* | ns | 0.0106 | ns | <0.0001* | <0.0001* | 0.0033* | ns |
| 7 | 0.349* | <0.0001* | <0.0001* | 0.0238 | ns | <0.0001* | <0.0001* | 0.0132 | ns |
| 8 | 0.260* | ns | 0.0073 | ns | ns | <0.0001* | <0.0001* | ns | ns |
Survives Bonferroni correction for multiple comparisons (p < .00625)
HC = Healthy control group. HD = Hoarding disorder group. VAS = Visual analogue scale.
Group Difference Results.
Component pairs 1, 2, and 4 differed between HD and HC groups on both connectivity and VAS rating features. These met our criteria of a priori interest as they reflect HD vs. HC group differences in functional connectivity and VAS ratings for these component pairs. Component pairs 1 and 4 depict a profile of brain connectivity that was abnormally absent in HD, while Component pair 2 characterizes HD connectivity that was abnormally expressed (i.e., not seen in HC). Data re-analysis to ensure these differences were unrelated to HD versus HC racial/ethnic differences confirmed the results (see Supplement).
Component Pair Description.
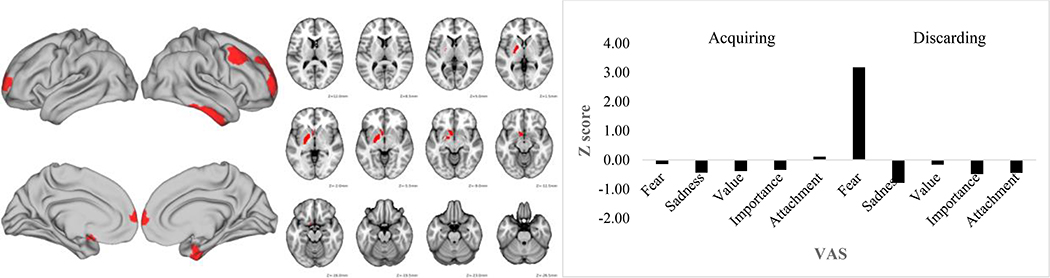
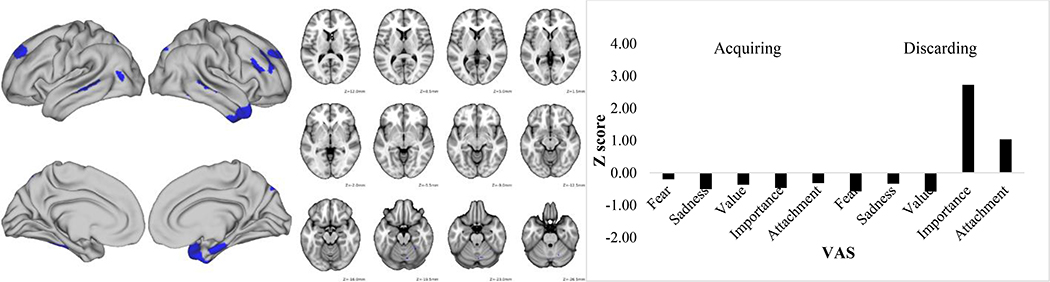
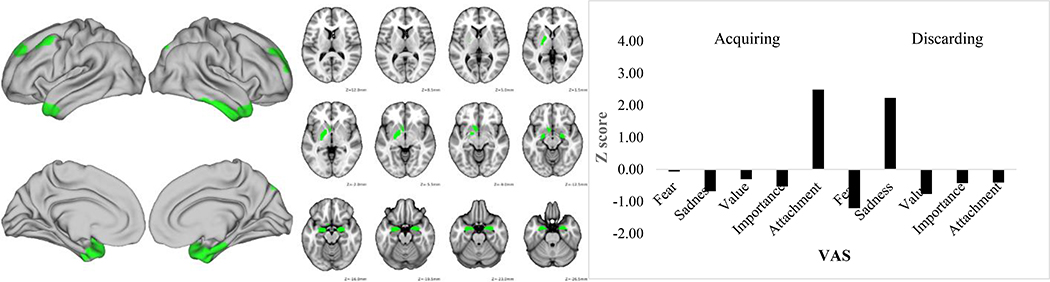
Figures 1–3 depict the functional connectivity fMRI data and the VAS scores for these three component pairs of interest. Table 3 lists the specific brain parcels in the fMRI feature that survived the bootstrapping test for their statistical significance. Figure 1 shows that component pair 1 was driven primarily by high fear/anxiety VAS scores. As seen in Table 3 and Figure 1, this component was comprised of connectivity in largely right hemisphere-lateralized dorsolateral, orbitofrontal, and frontopolar brain regions along with left nucleus accumbens and pallidum, lateral temporal cortex, and medial temporal cortex. Component pair 2 was characterized by high sadness/regret after the discarding task, but also was linked to sentimental attachment VAS ratings after completing the acquiring task. This profile of VAS ratings was linked to connectivity of bilateral amygdala, left accumbens and pallidum, bilateral medial temporal lobe, and bilateral dorsolateral prefrontal cortex (Table 3 and Figure 2). Finally, component pair 4 was comprised of high VAS ratings about the importance/usefulness of items discarded and connectivity strength in several lateral prefrontal cortex and medial and lateral temporal cortex parcels (Table 3 and Figure 3).
Figure 1.
Discarding task Component 1 functional connectivity map (left) and Visual Analogue Scale standardized score (right). VAS = Visual analogue scale.
Figure 3.
Discarding task Component 4 functional connectivity map (left) and Visual Analogue Scale standardized score (right). VAS = Visual analogue scale.
Table 3.
List of Human Connectome Project brain parcels that were identified by bootstrapping analysis as having significantly strong connectivity representation for each pair of fused components from the mCCA+jICA analysis. Each parcel is describing using a brief parcel abbreviation and name along with anatomical region and super-region categories as described in Glasser et al. (20). The weighted degree value for each parcel is also listed.
| Component | Hemisp here | Abbreviation | Name | Region | SuperRegion | Weighted Degree |
|---|---|---|---|---|---|---|
| Discard 1 | Left | Pallidum | Subcortical | Subcortical | 673 | |
| Discard 1 | Left | PI | Para-Insular Area | Insular and Frontal Opercular Cortex | Insula/Frontal Operculum | 581 |
| Discard 1 | Left | pOFC | posterior OFC Complex | Orbital and Polar Frontal Cortex | Frontal | 557 |
| Discard 1 | Right | TGv | Area TG Ventral | Lateral Temporal Cortex | Temporal | 548 |
| Discard 1 | Right | 10d | Area 10d | Orbital and Polar Frontal Cortex | Frontal | 510 |
| Discard 1 | Left | Accumbens | Subcortical | Subcortical | 505 | |
| Discard 1 | Right | TE2a | Area TE2 anterior | Lateral Temporal Cortex | Temporal | 494 |
| Discard 1 | Right | 9a | Area 9 anterior | DorsoLateral Prefrontal Cortex | Frontal | 489 |
| Discard 1 | Right | a10p | Area anterior 10p | Orbital and Polar Frontal Cortex | Frontal | 480 |
| Discard 1 | Right | p10p | Area posterior 10p | Orbital and Polar Frontal Cortex | Frontal | 479 |
| Discard 1 | Right | 8Av | Area 8Av | DorsoLateral Prefrontal Cortex | Frontal | 478 |
| Discard 1 | Right | PeEc | Perirhinal Ectorhinal Cortex | Medial Temporal Cortex | Temporal | 477 |
| Discard 1 | Right | 8C | Area 8C | DorsoLateral Prefrontal Cortex | Frontal | 469 |
| Discard 1 | Right | 9p | Area 9 Posterior | DorsoLateral Prefrontal Cortex | Frontal | 460 |
| Discard 1 | Left | p10p | Area posterior 10p | Orbital and Polar Frontal Cortex | Frontal | 459 |
| Discard 1 | Left | 10d | Area 10d | Orbital and Polar Frontal Cortex | Frontal | 457 |
| Discard 2 | Right | TGv | Area TG Ventral | Lateral Temporal Cortex | Temporal | 830 |
| Discard 2 | Left | Pallidum | Subcortical | Subcortical | 636 | |
| Discard 2 | Left | PI | Para-Insular Area | Insular and Frontal Opercular Cortex | Insula/Frontal Operculum | 583 |
| Discard 2 | Right | 9a | Area 9 anterior | DorsoLateral Prefrontal Cortex | Frontal | 567 |
| Discard 2 | Right | PeEc | Perirhinal Ectorhinal Cortex | Medial Temporal Cortex | Temporal | 549 |
| Discard 2 | Right | EC | Entorhinal Cortex | Medial Temporal Cortex | Temporal | 535 |
| Discard 2 | Left | Accumbens | Subcortical | Subcortical | 532 | |
| Discard 2 | Left | PeEc | Perirhinal Ectorhinal Cortex | Medial Temporal Cortex | Temporal | 526 |
| Discard 2 | Right | TGd | Area TG dorsal | Lateral Temporal Cortex | Temporal | 507 |
| Discard 2 | Right | Amygdala | Subcortical | Subcortical | 488 | |
| Discard 2 | Right | TE2a | Area TE2 anterior | Lateral Temporal Cortex | Temporal | 475 |
| Discard 2 | Right | V6A | Area V6A | Dorsal Stream Visual Cortex | Visual | 471 |
| Discard 2 | Left | TGd | Area TG dorsal | Lateral Temporal Cortex | Temporal | 466 |
| Discard 2 | Left | 9p | Area 9 Posterior | DorsoLateral Prefrontal Cortex | Frontal | 457 |
| Discard 2 | Right | 9p | Area 9 Posterior | DorsoLateral Prefrontal Cortex | Frontal | 457 |
| Discard 2 | Left | Amygdala | Subcortical | Subcortical | 448 | |
| Discard 2 | Left | 8Av | Area 8Av | DorsoLateral Prefrontal Cortex | Frontal | 437 |
| Discard 4 | Right | V6A | Area V6A | Dorsal Stream Visual Cortex | Visual | 631 |
| Discard 4 | Right | TGv | Area TG Ventral | Lateral Temporal Cortex | Temporal | 611 |
| Discard 4 | Right | Cerebellum 3 | Cerebellum | Cerebellum | 589 | |
| Discard 4 | Right | EC | Entorhinal Cortex | Medial Temporal Cortex | Temporal | 580 |
| Discard 4 | Right | TGd | Area TG dorsal | Lateral Temporal Cortex | Temporal | 544 |
| Discard 4 | Left | 7Pl | Lateral Area 7P | Superior Parietal Cortex | Parietal | 513 |
| Discard 4 | Right | TE2p | Area TE2 posterior | Lateral Temporal Cortex | Temporal | 500 |
| Discard 4 | Right | 9p | Area 9 Posterior | DorsoLateral Prefrontal Cortex | Frontal | 480 |
| Discard 4 | Right | 46 | Area 46 | DorsoLateral Prefrontal Cortex | Frontal | 480 |
| Discard 4 | Right | IFSp | Area IFSp | Inferior Frontal Cortex | Frontal | 477 |
| Discard 4 | Left | TE2p | Area TE2 posterior | Lateral Temporal Cortex | Temporal | 462 |
| Discard 4 | Left | STSvp | Area STSv posterior | Auditory Association Cortex | Auditory | 458 |
| Discard 4 | Left | MT | Middle Temporal Area | MT+ Complex and Neighboring Visual Areas | Visual | 447 |
| Discard 4 | Left | 9p | Area 9 Posterior | DorsoLateral Prefrontal Cortex | Frontal | 439 |
| Discard 4 | Right | STSvp | Area STSv posterior | Auditory Association Cortex | Auditory | 436 |
Figure 2.
Discarding task Component 2 functional connectivity map (left) and Visual Analogue Scale standardized score (right). VAS = Visual analogue scale.
Correlations with Hoarding Symptom Severity.
Table 2 also reports Pearson correlations between SI-R total scores and connectivity features and VAS ratings for the discarding task fMRI data. SI-R scores were significantly correlated with the VAS loading scores for all three of these component pairs, surviving corrections for multiple comparisons for component pairs 1 and 4. There also were significant associations between SI-R scores and the brain connectivity features for all three component pairs on which we focused (component pairs 1, 2, and 4), but none of these correlations survived Bonferroni correction. These results are evidence not only that the subjective distress features here predict self-reported HD severity, but also their associated abnormal HD connectivity profiles do as well, albeit not as robustly.
Discussion
The present study revealed several abnormal brain activity profiles which were linked to different types of subjective distress during discarding decisions in HD. Fear/anxiety VAS ratings were associated with connectivity in largely right hemisphere-lateralized dorsolateral, orbitofrontal, and frontopolar brain regions along with left nucleus accumbens and pallidum, lateral temporal cortex, and medial temporal cortex. Importance/usefulness ratings were associated with connectivity strength in similar regions. However, it should be noted that while the specific VAS rating profiles for fear/anxiety and importance/usefulness were found in both the HC and HD groups, the connectivity features associated with these ratings were only found in HCs. Thus, HD participants appear to lack patterns of normal connectivity. Based on the regions found in these two component pairs, one can speculate that HD patients might not have normal inter-regional communication in many different functional contexts related to emotional reaction and expression [e.g., fear generalization or stimulus discrimination (amygdala and medial temporal lobe; (41–43)], which should be explored in future studies. It should be noted that these frontal regions, particularly dlPFC, have been implicated in cognitive control/emotion regulation processes [for a meta-analysis, see (44)], suggesting that patients with HD may have emotion regulation deficits, particularly during discarding decisions. Previous studies have indeed reported greater self-reported emotion dysregulation in HD patients relative to control groups (45–47).
Sadness, regret, and sentimental attachment ratings during discarding were associated with connectivity of bilateral amygdala, left accumbens and pallidum, bilateral medial temporal lobe, and bilateral dorsolateral prefrontal cortex. Some of these brain regions match those known to be engaged by sadness in prior studies (48). More interestingly, they closely resemble the “medial amygdala network” delineated by Bickart et al. (49) as brain regions having strong functional connectivity to the central and medial nuclei of the amygdala. Indeed, most of the key nodes in the amygdala sub-network overlap with parcels identified in this component pair feature (e.g., bilateral amygdala, nucleus accumbens). The anatomical connections of this centromedial amygdala sub-region position it as a major output node of the limbic system (50), with strong inter-connectivity to ventromedial striatum and medial temporal lobe regions (51). Interestingly, HC participants did not express this sadness-centromedial amygdala sub-region relationship at all, suggesting that it is an abnormal emotional expression-network connectivity association seen in HD patients. Alternatively, because HCs generally expressed very low levels of sadness when discarding, another possible explanation is that this is a normal relationship that is irrelevant to HCs in this fMRI task context. Given the absence of these associations in the HC group, these findings may again point to potential emotion regulation deficits in HD.
We were surprised that the ACC did not appear in any component. The ACC has been identified several times in prior HD neuroimaging research, emerging as perhaps the most important area of interest in our own previous fMRI studies (2, 3). Since the salience network is reliably engaged in this task (3) and when detecting the emotional significance of stimuli and generating emotional responses, we would have expected to see ACC brain function linked to HD subjective distress. But these results indicate that the cingulate’s connectivity with other regions is unrelated to HD patients’ emotional reactions or any automatic attempts to regulate their feelings when discarding possessions.
Limitations.
Limitations of the current study include the use of a sample with limited racial/ethnic diversity, making it challenging to generalize the findings to individuals from minority groups. Second, participants rated their negative emotions after the discarding and acquiring tasks, not after each individual item. More frequent ratings might allow for a more nuanced understanding of changes in negative affect when making decisions about specific types of items (e.g., items of greater vs. lesser value). Third, participants were shown a standardized set of stimuli (not an individualized set based on specific hoarding concerns). It is possible that some items were irrelevant to individual participants and therefore easier to discard. Fourth, we did not include a clinical control group, so we cannot be certain that the functional connectivity deficits we observed in this study are specific to patients with HD. Fifth, our power analysis indicated that we were adequately powered to detect medium-to-large effects with our sample size and Bonferroni corrections. Thus we may have been unable to detect potentially interesting effects of smaller magnitudes. Sixth, we did not control for medication use in the analyses. Only a minority of HD group participants (22%) were taking psychiatric medications, so there were insufficient numbers of any single medication to make a statistical control approach viable. We also did not control for comorbid diagnoses in the analyses. Last, we did not use an analysis method that was able to differentiate connectivity related to the control task within the fMRI paradigms we used. So it remains possible that some of the functional connectivity-VAS findings might be not be specific to discarding or acquiring. While is seems unlikely that the control task would significantly influence HD patients affective state, future research should keep the possibility in reserve.
Supplementary Material
Acknowledgments and Disclosures
This work was supported by the National Institute of Mental Health (Grant R01 MH101163, awarded to the two last authors).
All authors report no biomedical financial interests or potential conflict of interest.
The authors wish to thank Katrina Aberizk, Amber Billingsley, Akanksha Das, Marla Genova, Benjamin Katz, Krishna Pancholi, James Ransom, and Kathryn Young for their assistance with data collection; Drs. Elizabeth Davis, Christina Gilliam, Scott Hannan, Kristen Springer, and Blaise Worden for serving as study therapists or independent evaluators; and Drs. Jonathan Abramowitz, John Goethe, and Vincent Calhoun for serving as the data and safety monitoring board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: Author. [Google Scholar]
- 2.Tolin DF, Stevens MC, Villavicencio AL, Norberg MM, Calhoun VD, Frost RO, et al. (2012): Neural mechanisms of decision making in hoarding disorder. Arch Gen Psychiatry. 69:832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens MC, Levy HC, Hallion LS, Wootton BM, Tolin DF (2020): Functional neuroimaging test of an emerging neurobiological model of hoarding disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 5:68–75. [DOI] [PubMed] [Google Scholar]
- 4.An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. (2009): To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Molecular Psychiatry. 14:318–331. [DOI] [PubMed] [Google Scholar]
- 5.Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, et al. (2004): Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 161:1038–1048. [DOI] [PubMed] [Google Scholar]
- 6.Levy HC, Stevens MC, Glahn DC, Pancholi K, Tolin DF (2019): Distinct resting state functional connectivity abnormalities in hoarding disorder and major depressive disorder. J Psychiatr Res. 113:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steketee G, Frost RO, Kyrios M (2003): Cognitive aspects of compulsive hoarding. Cognitive Therapy and Research. 27:463–479. [Google Scholar]
- 8.Frost RO, Ong C, Steketee G, Tolin DF (2016): Behavioral and emotional consequences of thought listing versus cognitive restructuring during discarding decisions in hoarding disorder. Behav Res Ther. 85:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, et al. (2003): Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biol Psychiatry. 53:482–493. [DOI] [PubMed] [Google Scholar]
- 10.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML (2004): Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 61:564–576. [DOI] [PubMed] [Google Scholar]
- 11.Sui J, Adali T, Pearlson G, Yang H, Sponheim SR, White T, et al. (2010): A CCA+ICA based model for multi-task brain imaging data fusion and its application to schizophrenia. Neuroimage. 51:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolin DF, Wootton BM, Levy HC, Hallion LS, Worden BL, Diefenbach GJ, et al. (2019): Efficacy and mediators of a group cognitive-behavioral therapy for hoarding disorder: A randomized trial. J Consult Clin Psychol. 87:590–602. [DOI] [PubMed] [Google Scholar]
- 13.Tolin DF, Gilliam C, Wootton BM, Bowe W, Bragdon LB, Davis E, et al. (2018): Psychometric properties of a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive-compulsive and related disorders. Assessment. 25:3–13. [DOI] [PubMed] [Google Scholar]
- 14.Guy W (1976): Assessment manual for psychopharmacology. Washington, DC: U.S. Government Printing Office. [Google Scholar]
- 15.Tolin DF, Gilliam CM, Davis E, Springer K, Levy HC, Frost RO, et al. (2018): Psychometric properties of the Hoarding Rating Scale-Interview. Journal of Obsessive-Compulsive and Related Disorders. 16:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost RO, Steketee G, Grisham J (2004): Measurement of compulsive hoarding: Saving Inventory-Revised. Behaviour Research and Therapy. 42:1163–1182. [DOI] [PubMed] [Google Scholar]
- 17.Preston SD, Muroff JR, Wengrovitz SM (2009): Investigating the mechanisms of hoarding from an experimental perspective. Depress Anxiety. 26:425–437. [DOI] [PubMed] [Google Scholar]
- 18.Levy HC, Stevens MC, Tolin DF (2019): Validation of a behavioral measure of acquiring and discarding in hoarding disorder. Journal of Psychopathology and Behavioral Assessment. 41:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. (2013): The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. (2016): A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team (2013): R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 23.Calhoun VD, Liu J, Adali T (2009): A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 45:S163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calhoun VD, Sui J (2016): Multimodal fusion of brain imaging data: A key to finding the missing link(s) in complex mental illness. Biol Psychiatry Cogn Neurosci Neuroimaging. 1:230–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui J, Adali T, Yu Q, Chen J, Calhoun VD (2012): A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods. 204:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correa NM, Eichele T, Adali T, Li YO, Calhoun VD (2010): Multi-set canonical correlation analysis for the fusion of concurrent single trial ERP and functional MRI. Neuroimage. 50:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffis JC, Nenert R, Allendorfer JB, Szaflarski JP (2017): Linking left hemispheric tissue preservation to fMRI language task activation in chronic stroke patients. Cortex. 96:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harms MP, Wang L, Csernansky JG, Barch DM (2013): Structure-function relationship of working memory activity with hippocampal and prefrontal cortex volumes. Brain Struct Funct. 218:173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SG, Jung WH, Kim SN, Jang JH, Kwon JS (2015): Alterations of Gray and White Matter Networks in Patients with Obsessive-Compulsive Disorder: A Multimodal Fusion Analysis of Structural MRI and DTI Using mCCA+jICA. PLoS One. 10:e0127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerman-Sinkoff DB, Sui J, Rachakonda S, Kandala S, Calhoun VD, Barch DM (2017): Multimodal neural correlates of cognitive control in the Human Connectome Project. Neuroimage. 163:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangalathu-Arumana J, Liebenthal E, Beardsley SA (2018): Optimizing Within-Subject Experimental Designs for jICA of Multi-Channel ERP and fMRI. Front Neurosci. 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang X, Chen K, Yao L, Hu B, Wu X, Ye Q, et al. (2015): Simultaneous changes in gray matter volume and white matter fractional anisotropy in Alzheimer’s disease revealed by multimodal CCA and joint ICA. Neuroscience. 301:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soh P, Narayanan B, Khadka S, Calhoun VD, Keshavan MS, Tamminga CA, et al. (2015): Joint Coupling of Awake EEG Frequency Activity and MRI Gray Matter Volumes in the Psychosis Dimension: A BSNIP Study. Front Psychiatry. 6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J, Pearlson G, Caprihan A, Adali T, Kiehl KA, Liu J, et al. (2011): Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 57:839–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, et al. (2015): In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry. 78:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sui J, Qi S, van Erp TGM, Bustillo J, Jiang R, Lin D, et al. (2018): Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat Commun. 9:3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiwari V, Kashikar A (2019): OutlierDetection: Outlier Detection R package version 0.1.1..
- 38.Johnson T, Kwok I, Ng R (1998): Fast Computation of 2Dimensional Depth Contours. Proc Int Conf on Knowledge Discovery and Data Mining (KDD). New York, pp 224–228. [Google Scholar]
- 39.Jin W, Tung A, Han J (2001): Mining top-n local outliers in large databases. ACM SIGKDD Int Conf on Knowledge Discovery and Data Mining San Francisco, CA, pp 293–298. [Google Scholar]
- 40.Hautamaki V, Karkkainen I, Franti P (2004): Outlier detection using k-nearest neighbour graph. Proc IEEE Int Conf on Pattern Recognition (ICPR). Cambridge, UK. [Google Scholar]
- 41.LeDoux JE (2012): Evolution of human emotion: a view through fear. Prog Brain Res. 195:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corodimas KP, LeDoux JE (1995): Disruptive Effects of Posttraining Perirhinal Cortex Lesions on Conditioned Fear: Contributions of Contextual Cues. Behav Neurosci. 109:613–619. [DOI] [PubMed] [Google Scholar]
- 43.Sangha S, Diehl MM, Bergstrom HC, Drew MR (2020): Know safety, no fear. Neuroscience and biobehavioral reviews. 108:218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. (2014): Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez de la Cruz L, Landau D, Iervolino AC, Santo S, Pertusa A, Singh S, et al. (2013): Experiential avoidance and emotion regulation difficulties in hoarding disorder. J Anxiety Disord. 27:204–209. [DOI] [PubMed] [Google Scholar]
- 46.Tolin DF, Levy HC, Wootton BM, Hallion LS, Stevens MC (2018): Hoarding disorder and difficulties in emotion regulation. J Obsessive Compuls Relat Disord. 16:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worden B, Levy HC, Das A, Katz BW, Stevens M, Tolin D (2019): Perceived emotion regulation and distress tolerance in patients with hoarding disorder. Journal of Obsessive-Compulsive and Related Disorders. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arias JA, Williams C, Raghvani R, Aghajani M, Baez S, Belzung C, et al. (2020): The neuroscience of sadness: A multidisciplinary synthesis and collaborative review. Neuroscience and biobehavioral reviews. 111:199–228. [DOI] [PubMed] [Google Scholar]
- 49.Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC (2012): Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 32:14729–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pape HC, Pare D (2010): Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 90:419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.