Abstract
We evaluated outcomes of treatment with 5-fluorouracil (5-FU), doxorubicin, and streptozocin (FAS) in well-differentiated pancreatic neuroendocrine tumors (PanNETs) and its impact on subsequent therapy (everolimus or temozolomide). Advanced PanNET patients treated at our center from 1992 to 2013 were retrospectively reviewed. Patients received bolus 5-FU (400 mg/m2), streptozocin (400 mg/m2) (both IV, days 1–5) and doxorubicin (40 mg/m2 IV, day 1) every 28 days. Overall response rate (ORR) was assessed using RECIST version 1.1. Of 243 eligible patients, 220 were evaluable for ORR, progression-free survival (PFS), and toxicity. Most (≥90%) had metastatic, nonfunctional PanNETs; 14% had prior therapy. ORR to FAS was 41% (95% confidence interval [CI]: 36–48%). Median follow-up was 61 months. Median PFS was 20 (95% CI: 15–23) months; median overall survival (OS) was 63 (95% CI: 60–71) months. Cox regression analyses suggested improvement with first-line vs subsequent lines of FAS therapy. Main adverse events ≥ grade 3 were neutropenia (10%) and nausea/vomiting (5.5%). Dose reductions were required in 32% of patients. Post-FAS everolimus (n=108; 68% second line) had a median PFS of 10 (95% CI: 8–14) months. Post-FAS temozolomide (n=60; 53% ≥ fourth line) had an ORR of 13% and median PFS of 5.2 (95% CI: 4–12) months. In this largest reported cohort of PanNETs treated with chemotherapy, FAS demonstrated activity without significant safety concerns. FAS did not appear to affect subsequent PFS with everolimus; this sequence is being evaluated prospectively. Responses were noted with subsequent temozolomide-based regimens although PFS was possibly limited by line of therapy.
Keywords: Neuroendocrine tumors, gastro-enteropancreatic neuroendocrine tumors, pancreatic neoplasms, streptozocin, everolimus, temozolomide
Introduction
Neuroendocrine tumors that originate from the pancreatic islet cells represent a rare and heterogeneous group of tumors with diverse clinical presentations and behavior patterns [1, 2]. Although well-differentiated pancreatic NETs (PanNETs) are considered to be indolent, they still have high potential to metastasize [1, 2] ([3]. The treatment of choice for localized tumors therefore remains potentially curative surgery. However, surgical approaches are not always feasible in patients with advanced PanNET; these patients are often treated with systemic therapies. Clinical benefit has been demonstrated with somatostatin analogues, peptide receptor radionuclide therapy and targeted agents, including everolimus or sunitinib. Unlike most other well-differentiated neuroendocrine tumors, PanNETs are also relatively chemosensitive. The most commonly used cytotoxic regimens include alkylating agents (temozolomide, streptozocin, dacarbazine) as single agents or in combination with antimetabolites such as 5-fluorouracil (5-FU) or capecitabine. Streptozocin was the first drug approved for patients with advanced PanNET, based on historical randomized trials; however, these trials were limited by size and the lack of modern diagnostic and tumor measurement methodologies, making definitive conclusions regarding activity difficult [4, 5]. The combination FAS (5-fluorouracil, doxorubicin [Adriamycin], and streptozocin) has been used for decades. In our group’s previous retrospective study of 84 patients with advanced PanNETs treated with FAS, current standard imaging and response criteria showed an overall response rate (ORR) of 39%, median progression-free survival (PFS) of 18 months and median overall survival (OS) of 37 months [6]. Other retrospective studies suggested activity for temozolomide, an orally active analog of dacarbazine, in PanNETs, with ORRs of up to 54% [7]. Based on these data, the phase II E2211 study randomized advanced PanNET patients to temozolomide with or without capecitabine. This study showed improvement in the primary endpoint of PFS (22.7 vs 14.4 months; HR = 0.58; p = 0.023) and a trend towards improvement in ORR (33% vs 28%; p = 0.47) [8] with the addition of capecitabine. Prespecified on-protocol treatment was up to 13 cycles due to concern regarding long-term hematological and other toxicities.
The majority of trials leading to FDA approval of drugs for PanNETs have included patients with PanNETs with and without prior therapy, and hence whether the initial use of a cytotoxic regimen would affect subsequent activity of targeted agents such as everolimus is not firmly established. Our post-FAS cohort allowed focused on patients that had prior FAS. In addition, whether temozolomide has activity after prior therapy with streptozocin remains to be defined. In this study, we updated our experience with FAS regarding ORR, PFS and OS as well as its impact on subsequent therapies.
The objectives of this study were to a) provide updated ORR, PFS, OS and adverse event data for FAS therapy, b) evaluate PFS in patients treated with everolimus-based therapy after FAS and c) evaluate ORR and PFS in patients treated with temozolomide-based therapy after FAS. The ORR of everolimus in PanNETs has been well defined in large, prospective studies [9] [10] and hence was not included as an objective in the current study.
Methods
Patient population
We conducted a single-institution retrospective analysis of patients with locally advanced unresectable or metastatic well-differentiated PanNET who received FAS. The study was conducted under a protocol approved by The University of Texas MD Anderson Cancer Center Institutional Review Board, with requirement for informed consent waived given the retrospective nature of the study. Our NET database was reviewed from June 1992 to September 2013 (> 20-year timeframe). We included in the study patients seen at MD Anderson who a) were histopathologically confirmed to have locally advanced or metastatic PanNET that was defined as well differentiated based on morphology and proliferative indices (Ki-67, mitotic index) when reported, b) were treated with FAS and c) had measurable disease on radiographic imaging at the start of FAS. Patients who received FAS prior to consultation at MD Anderson were excluded from the study, and those who lacked radiographic follow-up imaging at our institution were excluded from efficacy endpoints (except OS). We collected the following data elements from the patients’ medical records: demographics (age, sex), tumor type [functioning vs nonfunctioning hormone secretion], tumor status (locally advanced vs metastatic disease), prior treatment, primary tumor or metastatic disease resection if performed and treatment after FAS. Other collection points were radiographic imaging and dates of treatment start, disease progression, death, and last follow-up.
Efficacy endpoints and toxicity
Chemotherapy consisted of bolus 5-FU (400 mg/m2 IV) on days 1–5, streptozocin (400 mg/m2 IV) on days 1–5 and doxorubicin (40 mg/m2 IV) on day 1 only. Each cycle was repeated every 28 days. Blood counts and biochemical studies were obtained before each course in all patients. Patients were typically re-evaluated with imaging every 8 weeks after initiation of therapy by CT or MRI. Somatostatin receptor scintigraphy and other imaging studies such as bone scans and tumor markers were obtained as needed. Cardiac function was evaluated by echocardiogram after the sixth or seventh course of chemotherapy to aid with doxorubicin dosing, which was reduced or discontinued if the left ventricular ejection fraction dropped by ≥ 15%. Additionally, doxorubicin was discontinued during this time period in some cases to keep below a lifetime cumulative doxorubicin dose (< 300 mg/m2) dependent on physician. Therapy was continued until disease progression, unacceptable toxicity or patient intolerance. Toxicity data reported were any grade 3 or 4 adverse events, along with any adverse events that led to dose reductions, dose omissions, delays in treatment, extension of the chemotherapy cycle, growth factor addition or complete chemotherapy discontinuation. Common Terminology Criteria for Adverse Effects (CTCAE) version 4.0 was utilized to determine adverse effect grades retrospectively when applicable [11].
The primary objective was to determine ORR for patients who received FAS therapy. The original radiographic imaging studies were re-evaluated independently by two medical oncologists (ML, AD) for treatment response criteria. Response to treatment was determined using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [12]. Complete response (CR) was defined as disappearance of all lesions. Partial response (PR) was defined as at least a 30% reduction in the sum of the longest diameters of target lesions. Progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameters of target lesions, and stable disease was defined as disease that showed neither CR, PR nor PD. The secondary objectives were to determine PFS, OS and toxicity for patients who received FAS. PFS was defined as the time from FAS start date to progression date or last follow-up. OS was defined as the time from FAS start date to the date of death or last follow-up.
Among the patients who received FAS, two cohorts of patients who subsequently received everolimus-based therapy or temozolomide-based therapy were identified. ORR on imaging studies (for the temozolomide-based therapy cohort) and PFS (for both cohorts) were reported.
Statistical analyses
Continuous variables were reported using median and range. Categorical data was described using frequencies and percentages. The Kaplan-Meier method was used to estimate PFS and OS. The Cox proportional hazards model was used for comparing effects of prognostic variables on survival. All differences were considered statistically significant if two-sided p was < 0.05. Statistical analysis was conducted utilizing R 3.4.3, IBM SPSS 25 and Microsoft Excel 2016 software.
Results
Patient characteristics
We identified 243 patients (137 male; 106 female) with a median age of 56 years (range: 15–76 years). Demographic and clinical characteristics are included in Table 1. The majority of patients, 223 (92%), had metastatic disease. Functioning tumors were noted in 25 (10%) patients: the types included insulinoma (n = 9), glucagonoma (n = 5), gastrinoma (n = 5), vasoactive intestinal polypeptide-secreting tumor (VIPoma) (n = 4) and adrenocorticotropic hormone-secreting tumor (ACTHoma) (n = 2). Twelve (5%) patients had multiple endocrine neoplasia type 1 syndrome. Eighty-six percent of patients received FAS as first-line treatment; the remaining 14% of patients had received therapy prior to FAS, of which the majority (65%) had received a somatostatin analogue. All 243 patients were included for OS analysis, and data on 220 patients were available for ORR, PFS and toxicity review.
Table 1:
Demographics and clinical characteristics of all 243 patients treated with FAS
| Characteristic | N=243 N (%) |
|---|---|
| Age, years | |
| Median | 56 |
| Interquartile range | 47–63 |
| Sex | |
| Male | 137 (56) |
| Female | 106 (44) |
| Tumor type | |
| Nonfunctioning | 218 (90) |
| Functioning | 25 (10) |
| Genetic syndrome | |
| Sporadic | 231 (95) |
| Multiple endocrine neoplasia type 1 | 12 (5) |
| Tumor status | |
| Locally advanced | 20 (8) |
| Metastatic | 223 (92) |
| FAS line of therapy | |
| First | 208 (86) |
| Second or greater | 35 (14) |
| Resection of the primary tumor | |
| No | 191 (79) |
| Yes | 52 (21) |
| First post-FAS scan result, % (95% CI) | |
| Complete or partial response | 41 % (95% CI: 36–48%) |
Toxicity
Grade 3 or 4 adverse events seen with FAS are reported in Table 2. Dose reductions were required in 70 patients (32%) of the 220 patients, with a median of 1 dose modification per patient (range: 0–5). Of the 220 patients, 16 (7%) were started at lower doses due to comorbidities, and in 35 patients (16%) doxorubicin was omitted because the patients had reached a close to maximum doxorubicin lifetime dose. Another 7 patients (3.2%) required dose reductions due to cardiac toxicity related to doxorubicin. The most common grade 3 and 4 adverse events were hematologic (12%) and gastrointestinal (14%). Growth factor support was added in 40 patients (18%) of cases. Only 11 patients (5%) required chemotherapy to be discontinued due to toxicity.
Table 2:
Grade 3 or 4 adverse events for the 220 evaluable patients treated with FAS
| Adverse event | N=220 N (%) |
|---|---|
| Hematologic | |
| Neutropenia | 22 (10) |
| Thrombocytopenia | 3 (1.4) |
| Anemia | 2 (0.9) |
| Gastrointestinal | |
| Nausea/vomiting | 12 (5.5) |
| Oral Mucositis | 8 (3.6) |
| Diarrhea | 11 (5) |
| Fatigue | 6 (2.7) |
| Blood glucose | |
| Hypoglycemia | 2 (0.9) |
| Hyperglycemia | 5 (2.3) |
| Anorexia | 4 (1.8) |
| Cardiac | 3 (1.4) |
| Fever | 3 (1.4) |
| Elevated serum creatinine | 2 (0.9) |
| Edema | 2 (0.9) |
| Hepatic | |
| Elevated liver enzymes | 1 (0.5) |
| Hand-foot syndrome | 1 (0.5) |
| Pancreatitis | 1 (0.5) |
FAS therapy efficacy endpoints
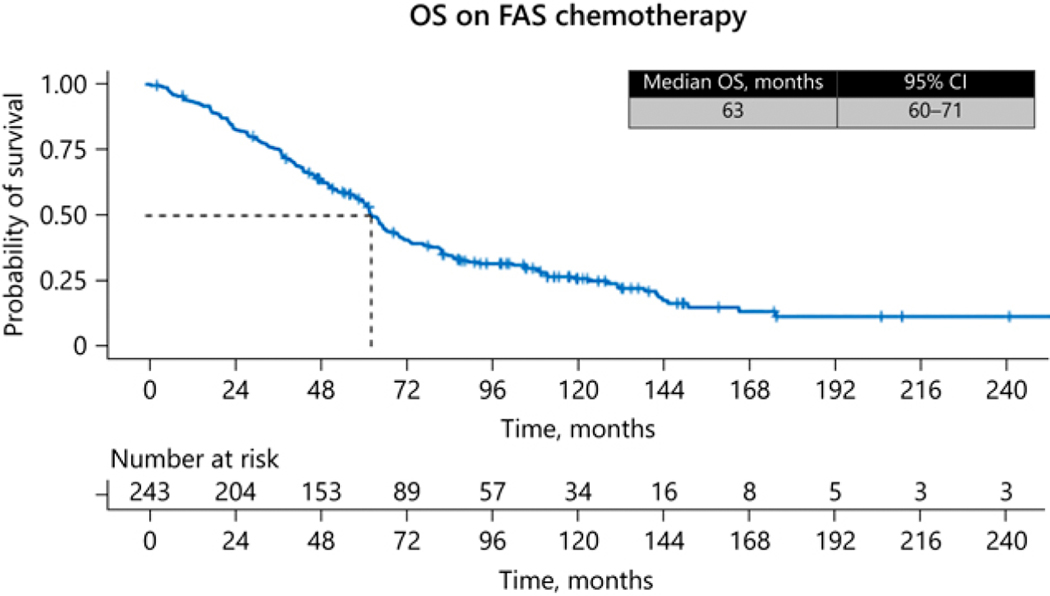
In the 220 patients evaluable for response, the median time on therapy with FAS was 5.5 months, and 86% had disease control, with 41% having a CR/PR (95% CI: 36–48%), including 3 patients with CR, and an additional 45% having stable disease (95% CI: 38–51%). After a median follow-up of 61 months, median PFS was 20 months (95% CI: 15–23 months) (Fig. 1) and the median OS was 63 months (95% CI: 60–71 months) (Fig. 2). Univariate Cox regression analysis for PFS revealed only line of therapy (first line vs later lines) as a significant covariate (Table 3). Univariate Cox regression analysis for OS suggested age (≤ 55 years), line of therapy (first line) and functional status (functional tumors) to be significant covariates, and multivariate regression confirmed age and line of therapy as being significant (Table 4).
Figure 1.
PFS on FAX chemotherapy.
Figure 2.
OS on FAX chemotherapy.
Table 3:
Cox proportional hazards analysis of progression-free survival for the 220 evaluable patients treated with FAS
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age (>55 vs ≤55 years) | 0.86 | 0.63–1.16 | 0.307 |
| Sex (male vs female) | 0.99 | 0.72–1.34 | 0.932 |
| Race (white vs other) | 2.6 | 0.95–2.72 | 0.078 |
| Stage (metastatic vs locally advanced) | 0.74 | 0.42–1.31 | 0.30 |
| Functional status (no vs yes) | 1.47 | 0.86–2.50 | 0.15 |
| Resected primary (yes vs no) | 0.897 | 0.61–1.31 | 0.57 |
| Line of therapy (≥2nd line vs 1st line) | 2.106 | 1.29–3.16 | 0.002 |
| Chromogranin A (normal vs elevated) | 0.799 | 0.57–1.14 | 0.20 |
HR: hazard ratio; CI: confidence interval
Table 4:
Cox proportional hazards analysis of overall survival for all 243 patients treated with FAS
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Age (>55 vs ≤55 years) | 1.44 | 1.06–1.94 | 0.018 | 1.49 | 1.1–2.03 | 0.01 |
| Sex (male vs female) | 0.91 | 0.68–1.23 | 0.58 | NS | ||
| Race (white vs other) | 1.37 | 0.83–2.27 | 0.22 | NS | ||
| Stage (metastatic vs locally advanced) | 0.74 | 0.42–1.31 | 0.30 | NS | ||
| Functional status (no vs yes) | 1.78 | 1.04–3.01 | 0.034 | 1.29 | 0.74–2.23 | 0.36 |
| Resected primary (yes vs no) | 0.81 | 0.57–1.15 | 0.25 | NS | ||
| Line of therapy (≥2nd line vs 1st line) | 1.82 | 1.21–2.74 | 0.004 | 1.65 | 1.08–2.52 | 0.02 |
| Chromogranin A (normal vs elevated) | 0.82 | 0.57–1.18 | 0.29 | NS | ||
HR: hazard ratio; CI: confidence interval
Subsequent therapies
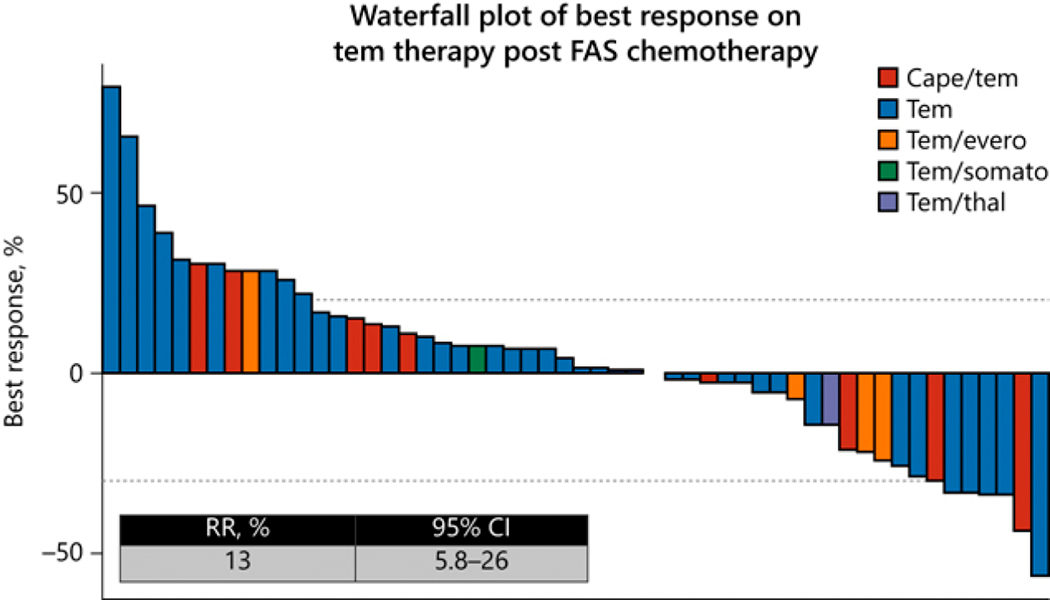
The majority of patients (76%) went on to receive further treatment after progression on FAS with a median of 2 lines of therapy (range: 0–6 treatments) consisting of locoregional therapy (such as liver-directed therapy) and/or systemic therapies. Subsequent everolimus-based therapies were administered in 108 patients, of which 68% received it as second-line therapy (Table 1). The median PFS in this cohort was 10 months (95% CI: 8–14 months; Fig. 3) and a disease-control (response + stable disease) of 70%. Temozolomide-based therapies were administered after FAS in 60 patients as salvage therapies, with 53% receiving it as fourth line or beyond (Table 1); their ORR was 13% (Fig. 4) and median PFS was 5.2 months (95% CI: 4–12 months; Fig. 5). The majority of patients (70%) received single-agent temozolomide; the others received it in combination with capecitabine (17%). The remaining eight patients (13%) received in various combination with everolimus, somatostatin analogue or thalidomide (Table 1).
Figure 3.
PFS on everolimus therapy.
Figure 4.
Waterfall plot of best response on tem therapy post FAX chemotherapy.
Figure 5.
PFS on tem-based treatment post FAS.
Discussion
We report the largest cohort of PanNET patients treated with cytotoxic chemotherapy to date. Our review reinforced historical data with this regimen showing therapeutic activity without new safety concerns. Disease control was achieved in most patients (86%). The median PFS and ORR of patients treated with FAS in our cohort were 20 months (95% CI: 15–23 months) and 41% (95% CI: 36–48%), consistent with prior reports for this regimen [6, 13]. Along with revealing findings consistent with historical data, we explored multiple analyses in areas unclear in well-differentiated PanNET treatment.
In the past decade, targeted therapies focused on the mammalian target of rapamycin (mTOR) pathway and vascular-endothelial growth factor (VEGF) have been major discoveries in the PanNET treatment landscape [10, 14, 15]. RADIANT 3, a randomized placebo-controlled trial, paved the way for FDA approval of everolimus, an mTOR inhibitor, for advanced well-differentiated PanNETs [10, 15]. RADIANT 3 showed a median PFS of 11 months (95% CI: 8.4– 13.9 months), with the primary response being disease stability (5% partial responses; 73% stable disease) [15]. A randomized phase III placebo-controlled trial of sunitinib, a tyrosine-kinase inhibitor targeting VEGFR1, amongst other targets, showed similar outcomes in advanced well-differentiated PanNETs: median PFS of 11.4 months (95% CI: 7.4–19.8 months) and a response rate of 9.3%, with 63% stable disease [14]. Only 40 patients received sunitinib in our subsequent-therapy setting; therefore, an analysis of outcomes with sunitinib was not conducted. However, 108 patients started everolimus subsequently as a second-line or downstream treatment after FAS. This cohort showed a median PFS of 10 months (95% CI: 8–14 months) and disease-control rate of 70%, aligning with the results previously seen in the RADIANT 3 trial [15]. Overall, these data suggest that prior therapy with FAS may not reduce the efficacy of everolimus. The question of whether cytotoxic chemotherapy such as FAS should be used before or after everolimus therapy has been raised. Intriguingly, in our study population, differences in survival were noted based on line of therapy with FAS. For OS, uni- and multivariate Cox regression analyses suggested better outcomes in patients who received FAS as first-line therapy as compared to subsequent lines of therapy. Conceivably, this outcome could be related to the longer time that may have elapsed since diagnosis in patients receiving FAS beyond first-line therapy and thus being at a later stage in their disease trajectory. Alternatively, there may be a beneficial effect of FAS chemotherapy in the first-line setting related to reducing tumor burden in terms of radiographic responses and thereby allowing surgical resection in patients previously deemed unsuitable for surgical resection, or related to delaying risk of tumor-related decline in performance status or organ function. Perhaps even more striking than the improvement in OS was the improvement in PFS when FAS was used in first- vs later lines of therapy. One potential explanation could be related to the previously described clonal evolution and grade progression associated with therapy that may reduce benefit from FAS therapy [16]. Together, these data suggest that cytotoxic chemotherapy may be best used prior to everolimus therapy, at least in patients with higher tumor burden, but are far from conclusive given the limitations of a single-center, retrospective study, as discussed below. The ongoing randomized phase III SEQTOR study that is evaluating the efficacy and safety of everolimus followed by streptozocin, 5-FU chemotherapy upon progression vs the reverse sequence [17] may help address this question.
In a smaller cohort of patients, we also evaluated the activity of temozolomide after prior FAS therapy. Temozolomide given in various combinations and schedules has been studied retrospectively and prospectively in advanced PanNET [18–20]. Responses reported from these studies have ranged from 30% to upwards of 70%. In our cohort with post-FAS treatment with a temozolomide-based regimen, some activity was seen, with a 13% response rate and a median PFS of 5.2 months despite previous exposure to an alkylating agent. However, the applicability of this finding is limited by the line of therapy in which temozolomide was used (primarily for refractory disease) and should therefore be further investigated. A small retrospective study of 28 patients evaluated the sequencing of alkylating agents (streptozocin followed by dacarbazine vs dacarbazine followed by streptozocin) in patients with pancreatic (n = 25) and lung NETs (n = 3) showed second-line response rates of 33% and 38% for streptozocin and dacarbazine, respectively, in the full patient group [21]. These data together suggest that there may be a role for re-introduction of alkylating agents after prior use. Based on the ECOG E2211 trial, capecitabine/temozolomide has become the cytotoxic regimen of choice, especially given its convenience of oral over IV chemotherapy and the concerns for toxicity associated with streptozocin and doxorubicin [8]. However, as demonstrated in our study, the FAS regimen is well tolerated, with only a minority of patients needing to stop therapy related to toxicity. The expanding role of temozolomide in this malignancy does present an overlap with regimens like FAS. However, given that temozolomide is typically not used beyond 12–13 cycles because of concerns for cumulative hematological toxicity, whether streptozocin-based regimens may have activity subsequently needs to be evaluated in prospective studies.
Our study is not without drawbacks, and thus these findings should be interpreted within the context of their limitations. Firstly, given that this is a retrospective study, confounding by indication, i.e. factors affecting therapy choices, cannot be reliably discerned and may have had an impact on outcomes. Secondly, since all the patients were identified from a tertiary referral center, there may be a referral bias, limiting the external validity of the findings. Thirdly, in spite of our best efforts, all known prognostic factors that may have affected outcomes could not be accounted for; for instance, the evolving patterns of classification of NETs resulted in significant inconsistency in reporting of grade, Ki-67 and mitotic index, and thus these factors were excluded from the current analyses. Lastly, our study was a retrospective review over a long period of time in which practice changes occurred during this time.
Nevertheless, our study further supports the activity of FAS in the management of PanNETs and raises intriguing questions regarding sequencing of therapies, some of which are being addressed by ongoing trials.
Acknowledgements:
Sunita Patterson with Scientific Publications Services, Research Medical Library, MD Anderson Cancer Center, Julia Grosch with Department of Gastrointestinal Medical Oncology, MD Anderson Cancer Center and the University Hospital Heidelberg, Germany
Funding Sources
This work was supported by the National Institutes of Health/National Cancer Institute (award number P30CA016672; used the Clinical Trials Office).
Footnotes
Statement of Ethics
The study was conducted under a protocol approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. Reference Number LAB03-0913. IRB approval date 12/31/2003.
Conflict of Interest Statement
No authors have any relevant disclosures to this manuscript.
References:
- 1.Gao L, Natov NS, Daly KP, Masud F, Chaundhry S, Sterling MJ, et al. , An update on the management of pancreatic neuroendocrine tumors. Anticancer Drugs, 2018. 29(7): p. 597–612. [DOI] [PubMed] [Google Scholar]
- 2.Perri G, Prakash LR, and Katz MHG, Pancreatic neuroendocrine tumors. Curr Opin Gastroenterol, 2019. 35(5): p. 468–477. [DOI] [PubMed] [Google Scholar]
- 3.Network, N.C.C. Neuroendocrine and Adrenal Tumors. 2019; Available from: www.nccn.org
- 4.Moertel CG, Hanley JA, and Johnson LA, Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med, 1980. 303(21): p. 1189–94. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D, Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med, 1992. 326(8): p. 519–23. [DOI] [PubMed] [Google Scholar]
- 6.Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, et al. , Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol, 2004. 22(23): p. 4762–71. [DOI] [PubMed] [Google Scholar]
- 7.Cives M, Ghayouri M, Morse B, Brelsford M, Black M, Rizzo A, et al. , Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer, 2016. 23(9): p. 759–67. [DOI] [PubMed] [Google Scholar]
- 8.Kunz PL, Catalano PJ, Nimeiri H, Fisher GA, Longacre TA, Suarez CJ, et al. , A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). Journal of Clinical Oncology, 2018. 36(15_suppl): p. 4004–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulke MH, Ruszniewski P, Van Cutsem E, Lombard-Bohas C, Valle JW, De Herder WW, et al. , A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol, 2017. 28(6): p. 1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E. et al. , Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet, 2016. 387(10022): p. 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diagnosis, N.C.I.D.o.C.T.a. Common Terminology Criteria for Adverse Events (CTCAE) version 4. 6.4.2019]; Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 13.Clewemar Antonodimitrakis P, Sundin A, Wassberg C, Granberg D, Skogseid B, Eriksson B, Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology, 2016. 103(3–4): p. 345–53. [DOI] [PubMed] [Google Scholar]
- 14.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. , Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med, 2011. 364(6): p. 501–13. [DOI] [PubMed] [Google Scholar]
- 15.Yao JC, Shah MH, Ito T. Lombard-Bohas C, Wolin EM, Van Cutsem E, et al. , Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med, 2011. 364(6): p. 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raj N, Shah R. Stadler Z,Mukherjee S, Chou J, Untch B, et al. , Real-Time Genomic Characterization of Metastatic Pancreatic Neuroendocrine Tumors Has Prognostic Implications and Identifies Potential Germline Actionability. JCO Precis Oncol, 2018. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinicaltrials.gov, Randomized Open Label Study to Compare the Efficacy and Safety of Everolimus Followed by Chemotherapy with Streptozocin–Fluorouracil upon Progression or the Reverse Sequence, in Advanced Progressive Pancreatic NETs: NCT02246127. [Google Scholar]
- 18.Chan JA, Blaszkowsky L, Stuart K, Zhu AX, Allen J, Wadlow R, et al. , A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer, 2013. 119(17): p. 3212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, et al. , Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol, 2012. 30(24): p. 2963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine RL, Gulati AP, Krantz BA, Moss RA, Schreibman S, Tsushima DA, et al. , Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol, 2013. 71(3): p. 663–70. [DOI] [PubMed] [Google Scholar]
- 21.Krug S, Boch M, Rexin P, Gress TM, Michl P. Rinke, A., Impact of Therapy Sequence with Alkylating Agents and MGMT Status in Patients with Advanced Neuroendocrine Tumors. Anticancer Res, 2017. 37(5): p. 2491–2500. [DOI] [PubMed] [Google Scholar]