Highlights
-
•
This is the first time to study and find out that sinomenine hydrochloride and iodine-131 synergic enhance the apoptosis and regulate DNA repair and cell cycle checkpoint on papillary thyroid carcinoma cells.
-
•
This is the first time to study and find out that sinomenine hydrochloride increased the radiosensitivity of papillary thyroid carcinoma cells and normal thyroid cells.
-
•
This is the first time to study and find out that sinomenine hydrochloride could be a potential therapeutic radiosensitizer in papillary thyroid carcinoma radiotherapy after total thyroidectomy .
Keywords: Sinomenine hydrochloride, Iodine-131, Papillary thyroid carcinoma cell, Radiosensitivity, Apoptosis
Abstract
Radioiodine (131I) therapy is an important treatment for thyroid carcinoma. The response to radiotherapy sometimes limited by the development of radioresistance. Sinomenine hydrochloride(SH), was reported as a prospective radiosensitizer. This study was aim to evaluate synergic radiosensitization of SH and 131I on papillary thyroid carcinoma (PTC). We evaluated HTori-3, BCPAP and TPC-1 cells, the cell viability was evaluated by MTT. The experiment was divided into 4 groups: control group, SH (0.8 mM) group, I (131I 14.8 MBq/ml) group and ISH (SH 0.8 mM plus 131I 14.8 MBq/ml) group. Flow cytometry was used to investigate cell cycle phases and cell apoptosis. RT-PCR and western blotting were performed to determine the molecular changes. Compared to control group, SH significantly increased apoptosis and enhanced radiosensitivity of HTori-3 and PTC cells were related to the ratio of Bcl-2 to Bax protein downregulation and Fas, p21, p-ATM, p-Chk1, p-Chk2 and p53 protein expression upregulation in the ISH group (P < 0.05). Our results indicate that synergic radiosensitization of SH and iodine-131 on PTC cells and SH could be a potential therapeutic radiosensitizer in PTC radio therapy after total thyroidectomy.
Introduction
In recent years, the incidence of thyroid cancer has increased rapidly worldwide. Papillary thyroid cancer (PTC) constitutes approximately 80% of all thyroid cancer cases [1], [2], [3]. RAI is one of the most important therapy of PTC. Although most PTC cases have a quite favorable prognosis after standard therapeutic approaches, including surgery, selective radioactive iodine (RAI) therapy, and thyroid stimulating hormone (TSH) suppressive therapy, there are still some patients who are gradually insensitive to iodine and have a poor therapeutic effect [4]. Patients may fail it when the cancer has lost radioiodine avidity; a primary cause for PTC related morbidity and mortality. There are many studies on the mechanism of radiation resistance of PTC cells at present, but it will take a long time to convert into clinical radiosensitizer [5], [6], [7], [8]. Therefore, it is urgent to find a radiosensitizer, which can be used in clinic as soon as possible.
The alkaloid sinomenine is extracted from the Chinese medical plant Sinomenium acutum. Within the past 30 years, the therapeutic efficacy and lower side effects of sinomenine in patients with rheumatoid arthritis (RA) have been confirmed in open clinical trials [9,10]. Moreover, Sinomenine hydrochloride (SH) has already been effectively used in rheumatoid arthritis during clinical practice [11]. Recently, several studies have demonstrated that SH not only has antineoplastic effects against various types of cancer, including lung cancer, gastric adenocarcinoma, breast cancer, but also it has been found that SH appears to be a prospective radiosensitizer in cervical cancer and esophageal squamous cell carcinoma therapy [12], [13], [14], [15], [16]. Zhang et al. found that SH sensitized human cervical cancer cell line cells to ionizing radiation (IR) by promoting accumulation of IR‑induced DNA double‑strand breaks (DSBs) and by interfering with the DNA damage checkpoint activation [15]. Fu et al. found that SH could improve the sensitivity of radiation in esophageal squamous cell carcinoma cells by inducing G2/M phase arrest, promoting radiation‑induced apoptosis and inhibiting DSB‑repair pathways [16]. However, its effect on thyroid cancer is unclear till now.
The radioresistance of thyroid cells were related with different molecular. Depending on molecular investigations, it is believed that mutations in RAS (rat sarcoma), BRAF (B-Raf proto-oncogene) and RET (rearranged during transfection)/PTC rearrangement, which take part in mitogen activated protein kinases (MAPK) pathway, cause an aberrant activation and lead to the development of PTC [17]. Several studies reveal that the most widespread molecular damage in thyroid cancer is caused by BRAF mutation (29–83%) [18]. With this in mind, in the present study we determined the synergic radiosensitization of sinomenine hydrochloride and radioiodine of both in the papillary thyroid cells and normal thyroid cells and whether the different radioresistance caused by a BRAF mutation or not. Meanwhile, we aimed to clarify the molecular mechanisms underpinning these effects in the human thyroid cancer cell line. Thus, in this study, we used V600E mutation (BRAFV600E: 8505C, BCPAP, SW-1736) or the cells of BRAF WT (BRAFWT: U-Hth-74, TPC-1) thyroid cancer cell lines, thereby representing the most common genetic alterations found in PTC, meanwhile the HTori-3 was taken as normal thyroid cells for contrast.
Materials and methods
Cell cultures and preparation of SH
The HTori-3, BCPAP and TPC-1 cell line were provided by Professor Peng Hou (Endocrinology Laboratory, The First Affiliated Hospital of Xi'an Jiaotong University College of Medicine, Xi'an, China). The HTori-3 and TPC-1 cells were cultured in DEME/F12 medium (Hyclone, Logan, UT, USA) and supplemented with 10% Certified FBS (04–001–1ACS, Biological Industries, Israel) and 1% penicillin/streptomycin (Hyclone) in a 5% CO2 humidified atmosphere at 37°C. The BCPAP cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA), 1% sodium pyruvate (Gibco, No.11360070), 1% nonessential amino acids (Gibco, No.11140050) and supplemented with 10% certified FBS (04–001–1ACS, Biological Industries, Israel) and 1% penicillin/streptomycin (Hyclone) in a 5% CO2 humidified atmosphere at 37°C.
SH (B21440 Shanghai Yuanye Biotechnology Co., Ltd. China) was dissolved in phosphate‑buffered saline (PBS) to a concentration of 100 mM, and stored at ‑20°C for up to 4 weeks.
Methylthiazoltetrazolium (MTT) assay
The cells were seeded at 1.5 × 104/ml in 96-well plates for 24 h, 48 h, 72 h. SH solutions were prepared with the same culture medium as HTori-3, BCPAP and TPC-1 cells with final gradient concentrations of 0.2, 0.4, 0.8, 1 and 2 mM, while identical volumes of PBS were added to the control wells. After the cells were incubated for 24, 48 and 72 h, 20μl 3- (4, 5‑dimethylthiazol‑2‑y1) ‑2, 5‑diphenytetrazolium bromide was added to each well and the cell cultures were incubated for an additional 4 h. The medium was discarded and the formazan crystals were solubilized in 150μl/well DMSO. The colored solution was quantified by a spectrophotometer at an absorbance of 490 nm. The inhibition rate of the cells was then calculated. The absorbance was directly associated with the viable cell number. The experiment was performed at least in triplicate.
The cells were seeded at 1.5 × 104/ml in 96-well plates for 12 h, 24 h, and 48 h. Radio-I solutions were prepared with the same culture medium as HTori-3, BCPAP and TPC-1 cells with final gradient concentrations of 7.4, 14.8 and 29.6 MBq/ml, while identical volumes of PBS were added to the control wells. After the cells were incubated for 12, 24 and 48 h, 20μl 3- (4, 5‑dimethylthiazol‑2‑y1) ‑2, 5‑diphenytetrazolium bromide was added to each well and the cell cultures were incubated for an additional 4 h. The medium was discarded and the formazan crystals were solubilized in 150 μl/well DMSO. The colored solution was quantified by a spectrophotometer at an absorbance of 490 nm. The inhibition rate of the cells was then calculated. The absorbance was directly associated with the viable cell number. The experiment was performed at least in triplicate.
Cell apoptosis and cell cycle assay
A cell apoptosis assay was performed using an Annexin V-FITC/PI Apoptosis Detection Kit (Shanghai Qihai Futai Biotechnology Co., Ltd. China). The HTori-3, BCPAP and TPC-1 cells were seeded at 5 × 104/ml in 6‑well plates for 24 h and then divided into 4 groups: control group, SH (0.8 mM) group, I-131 (iodine‑131 14.8 MBq/ml)) group, ISH (SH 0.8 mM plus iodine‑131 14.8 MBq/ml) group. The follow Cells were treated with SH for 24 h, and then treated with iodine‑131. The density plots show cell populations (live, early apoptosis, necrosis, and late apoptosis or dead cells) according to their fluorescence characteristics.
Cell cycle of apoptosis was quantitated using the Cell Cycle and Apoptosis Analysis Kit (C1052, Shanghai Biyuntian Biotechnology Co., Ltd., China) following the manufacturer's instructions. Cells were fixed with 70% ethanol (2 h, 4°C), and stained with propidium iodide and RNase A (30 min, 37°C) for cell cycle analysis.
Western blotting
The cells were lysed with RIPA lysis buffer (Shanghai Biyuntian Biotechnology Co., Ltd., China) and the protein concentrations were quantified using a BCA kit (Shanghai Biyuntian Biotechnology Co., Ltd., China). Total protein (~150 μg) was denatured in loading buffer at 100°C for 8 min and electrophoresed using 10% sodium dodecyl sulfate‑polyacrylamide gel electrophoresis (SDS‑PAGE). Lysates containing 150 μg proteins were subjected to SDS‑PAGE, followed by transfer of proteins to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% non‑fat milk in Tris‑buffered saline with 0.05% Tween‑20 at pH 7.5 for 1 h. And then, the membranes were incubated at 4°C with the following primary antibodies: The Bcl-2 antibody (Abcam ab196495 1:2000), Bax antibody (Abcam ab32503 1:2000), p53 antibody (Abcam ab241566 1:1000), phosphor-ATM S1981 antibody (Abcam ab81292 1:10,000), p21 antibody (Proteintech Group Inc. China,10,335–1-AP, 1:1000), GADPH antibody (Proteintech Group Inc. China, 60,004–1-Ig 1:10,000), phosphor-Chk2 Thr68 antibody (Cell Signaling Technology. no. 2661 1:1000), phosphor-Chk1 Ser345 antibody (Cell Signaling Technology. no. 2348 1:1000) and Fas antibody (Cell Signaling Technology no. 4233 1:1000), and then incubated with secondary anti‑rabbit or ‑mouse immunoglobulin (Proteintech Group Inc. China) for 1 h at 37°C. The membranes were washed 3 times with Tris‑buffered saline with 0.05% Tween‑20 and once with Tris‑buffered saline.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The RNA was extracted from cells with TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's instructions. The concentration of total RNA was determined using a spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) and the 260/280 absorbance ratio, used to investigate the sample purity, was between 1.8 and 2.0, cDNA synthesis was performed using 500 ng total RNA in a reaction volume of 10μl. The thermal cycling conditions were as follows: 15 min at 37°C and 5 s at 85°C. Copy number quantification was performed with RT-qPCR using GADPH as the internal reference. The primer sequences of Bcl‑2, Bax, p53, p21, ATM, Fas and GADPH are shown in Table I. Following reverse transcription, the conditions were as follows: 95°C for 30 s, 40 cycles of 5 s at 95°C and 30 s at 60°C for annealing and extensions were run on a Roche LightCycler96 Real-time Fluorescence Quantitative PCR Instrument (Roche Diagnostics GmbH Sandhofer Straße 116 68,305 Mannheim, Germany). PrimeScript™ RT Master Mix(RR036A) and the SYBR® Premix Ex Taq™ II (No. RR820A) were purchased from Takara Biotechnology, Co., Ltd. (Dalian, China) and used for real‑time PCR. All real‑time PCR experiments were performed in triplicate.
Table I.
Primers sequences of Bcl‑2, Bax, P53, P21, ATM, Fas and GADPH.
| Gene | Forward | Reverse | Length (bp) |
|---|---|---|---|
| Bcl-2 | CTTTGAGTTCGGTGGGGTCA | GGGCCGTACAGTTCCACAAA | 162 |
| Bax | CAGAGGCGGGGGATGATTG | TGTCCAGCCCATGATGGTTC | 198 |
| P53 | CCTGGATTGGCCAGACTGC | TTTTCAGGAAGTAGTTTCCATAGGT | 121 |
| P21 | TGTCCGTCAGAACCCATGC | AAAGTCGAAGTTCCATCGCTC | 139 |
| ATM | TGTGACTTTTCAGGGGATTTG | ATAGGAATCAGGGCTTTTGGA | 150 |
| Fas | ACTGTGACCCTTGCACCAAA | AGACAAAGCCACCCCAAGTT | 112 |
| GADPH | CACTAGGCGCTCACTGTTCT | GCGCCCAATACGACCAAATC | 105 |
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X protein; P53: the tumor suppressor gene; P21: cyclin dependent kinase inhibitor; ATM, Ataxia telangiectasia mutated kinase; Fas: tumor necrosis factor receptor.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Differences between the control and treatment groups were determined by Student's t‑test and considered to be significant at P < 0.05.
Results
SH inhibited thyroid cell proliferation
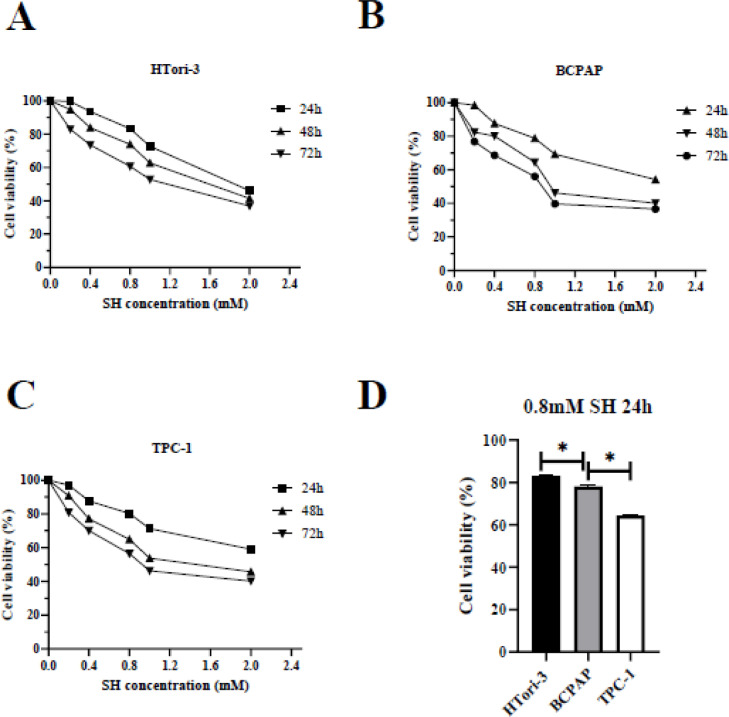
To address this question, first, we tested the effect of SH on HTori-3, BCPAP and TPC-1 cells by performing MTT‑based cell viability analysis (Fig. 1). The cell viability inhibition rate was calculated at 24, 48 and 72 h after SH treatment and as shown in Fig. 1A, B and C, cellular survival was significantly inhibited by SH at various concentrations. When at 24 h, concentrations of 1.0 and 2 mM induced severe cell death (40 to 60%), while 0.2, 0.4 and 0.8 mM SH exhibited a similar and relatively moderate effect on cell survival compared with higher concentrations (Fig. 1A, B and C). A higher 35.89% inhibition of cell growth was observed at a concentration of 0.8 mM in TPC-1 cells after 24 h compared with 21.76% inhibition that observed in BCPAP cells, and 16.82% inhibition, which observed in HTori-3 cells (Fig. 1D).
Fig. 1.
Sinomenine inhibits the viability of HTori-3, BCPAP and TPC-1 cells in vitro. (A) HTori-3, (B) BCPAP and (C)TPC-1 cells were incubated with SH at the indicated concentrations for various time-points. An MTT assay was performed 24 h after treatment. (D) The cell viability of cell growth among three cells in the condition of SH(0.8 mM) treated after 24 h (*P<0.05). The data are presented as the mean ± standard deviation of three independent experiments.SH, sinomenine hydrochloride.
Given that 1 mM was an already established concentration in previously published studies for a number of carcinoma cell lines [11,12], thus safe concentration of SH (0.8 mM) was selected for the subsequent experiments.
Iodine‑131 inhibits cell growth in a time‑ and dose‑dependent manner
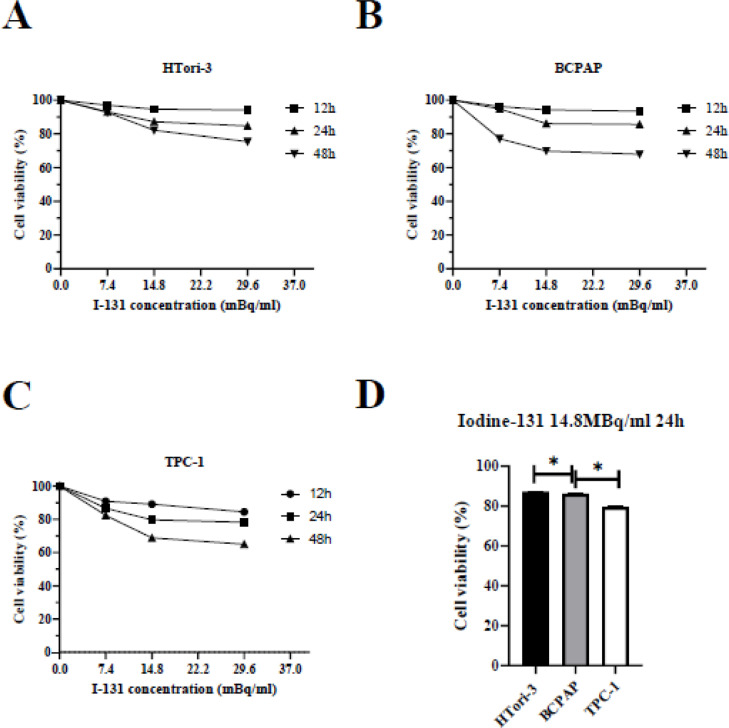
In order to investigate the effect of iodine‑131 on HTori-3, BCPAP and TPC-1 cells, thyroid cells were incubated with various concentrations of iodine‑131 (7.4, 14.8 or 29.6 MBq/ml) for designated time periods (12, 24 and 48 h) and the cell viability was determined using an MTT assay. The results (Fig. 2) revealed that iodine‑131 produced a significant inhibition of cell growth compared with the control group. A higher 20.3% inhibition of cell growth was observed at a dose of 14.8MBq/ml in TPC-1 cells after 24 h compared with 13.9% inhibition, which observed in BCPAP cells, and 12.8% inhibition which observed in HTori-3 cells (Fig. 2D). And a select dose of iodine‑131 (14.8 MBq/ml) treated for 24 h was selected for the following experiments.
Fig. 2.
Iodine-131 inhibits the viability of HTori-3, BCPAP and TPC-1 cells in vitro. (A) HTori-3, (B) BCPAP and (C)TPC-1 cells were incubated with iodine-131 at the indicated concentrations for various time-points. An MTT assay was performed 24 h after treatment. (D) The cell viability of cell growth among three cells in the condition of iodine-131(14.8MBq/ml) treated after 24 h (*P < 0.05). The data are presented as the mean ± standard deviation of three independent experiments.
SH could enhance the rate of apoptosis in thyroid cells treated with iodine-131
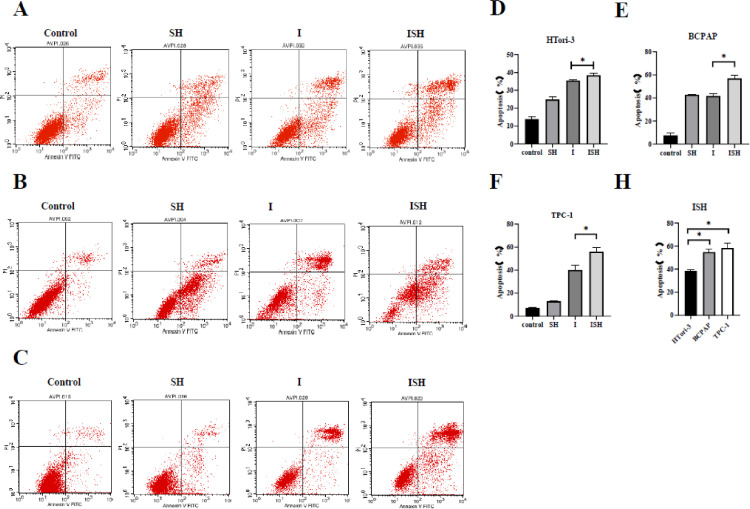
Annexin V-FITC and PI dual staining were used to measure whether iodine-131 and SH could induce apoptosis in HTori-3, BCPAP and TPC-1 cells. We next conducted flow cytometric analysis to test apoptosis in HTori-3, BCPAP and TPC-1 cells incubated with four groups, and observed significantly increased apoptosis in group ISH compared with the other groups (Fig. 3A, B and C).
Fig. 3.
SH could enhance the rate of apoptosis in thyroid cells treated with iodine-131. (A and D) HTori-3, (B and E) BCPAP and (C and F) TPC-1 cells apoptosis analysis by flow cytometry. Quantitation of apoptotic cells in D, E and F (*P < 0.05). (H) Compare the apoptosis rate of ISH group among three cell lines(*P < 0.05). The data are presented as the mean ± standard deviation of three independent experiments. SH, sinomenine hydrochloride; I, iodine-131; ISH, co-treatment of iodine-131 and SH.
In comparison with the control group, SH group exhibited a slight increase in apoptosis level while I-131 group and ISH group both induced a moderate level of apoptosis, moreover, there was a significant difference found between I-131 group and ISH group in HTori-3 cells, BCPAP cells and TPC-1 cells (P < 0.05) (Fig. 3D, E and F). (Table II)
Table II.
The rate of apoptosis in thyroid cells treated with SH and iodine-131.
| Control | SH | I | ISH | |
|---|---|---|---|---|
| HTori-3 | 13.93% ± 0.92% | 24.89% ± 1.09% | 35.47% ± 0.47% | 38.51% ± 0.83% |
| BCPAP | 7.69% ± 4.11% | 42.78% ± 0.89% | 44.32% ± 3.7% | 56.98% ± 5.41% |
| TPC-1 | 7.11% ± 1.09% | 13.01% ± 1.01% | 40.07% ± 8.86% | 56.01% ± 7.34% |
SH, sinomenine hydrochloride; I, iodine-131; ISH, co-treatment of iodine‑131 and SH.
When contrasting apoptosis of ISH group among three cell lines, a higher 58.39% apoptosis in TPC-1 cells and a higher 54.88% apoptosis in BCPAP cells were observed when compared with a 38.51% apoptosis rate of HTori-3 cells(P < 0.05). (Fig. 3H).
The effect of SH plus iodine-131 on cell cycles in HTori-3, BCPAP and TPC-1 cells
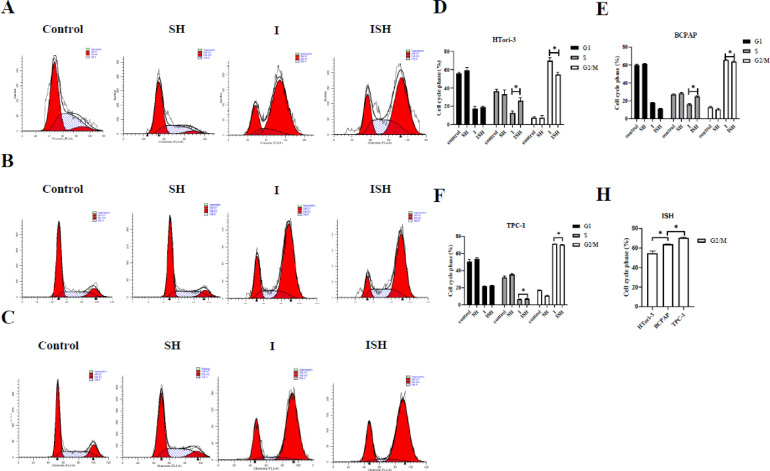
To identify whether SH can affect iodine-131 induced cell cycle distribution, PI staining in HTori-3, BCPAP and TPC-1 cells were evaluated (Fig. 4A, B and C).
Fig. 4.
Cell cycle changes induced by SH and I-131 in HTori-3, BCPAP and TPC-1 cells. (A and D) HTori-3, (B and E) BCPAP and (C and F) TPC-1 cells were pretreated with SH and/or exposed to iodine-131(14.8MBq/ml), and then analyzed using flow cytometry. The distribution of cell cycle phases is revealed in the histogram in D, E and F (*P < 0.05). The data are presented as the mean ± standard deviation of three independent experiments. (H) Compare the distribution of cell cycle phases of ISH group among three cell lines(*P < 0.05). SH, sinomenine hydrochloride; I, iodine-131; ISH, co-treatment of iodine-131 and SH; PI, propidium iodide.
Cell cycle analysis demonstrated that SH combined with iodine-131 arrested HTori-3, BCPAP and TPC-1 cells in the G2/M phase. Among three kinds of cells, the number of cells in G2/M phase was lower in the ISH group than it in the I-131 group (P < 0.05), meanwhile the number of cells in S phase was significantly higher in the ISH group than it in I-131 group in HTori-3, BCPAP and TPC-1 cells (P < 0.05) (Fig. 4D, E and F).
When contrasting the cells in the G2/M phase of group ISH among three cell lines, the number in G2/M phase of HTori-3, BCPAP and TPC-1 cell lines were increasing difference in proper order showed in Fig. 4H (P < 0.05). Notably the sensitive effect of SH in TPC-1 cells is more pronounced than the other cell lines (P < 0.05).
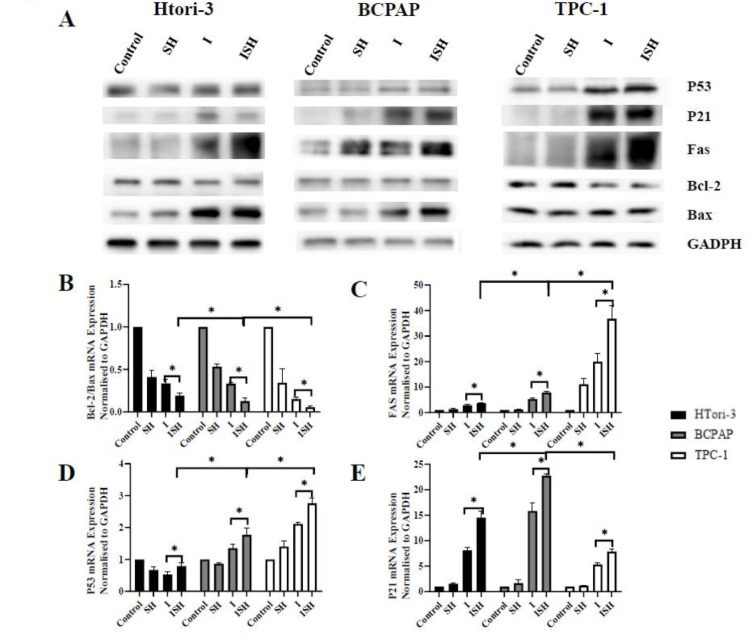
SH regulated the protein expression of Bcl-2, Bax, p21, p53, and Fas in HTori-3, BCPAP and TPC-1 cells
To understand the molecular mechanisms underlying the SH and iodine-131 induction of apoptosis and regulation of cell cycle, the protein expression of Bcl-2, Bax, Fas, p53 and p21 were examined by western blot analysis in HTori-3, BCPAP and TPC-1 cells(Fig. 5A).
Fig. 5.
Activation of apoptosis and other molecules after SH and iodine-131. HTori-3 BCPAP and TPC-1 cells were treated with or with 0.8 mM SH along, RAI along or SH plus RAI 14.8 MBq/ml. (A)The protein expression levels of p53, p21, Fas, Bcl-2, and Bax were determined by western blot analysis, GAPDH was probed as a loading control. The mRNA expression levels of (B)ratio of Bcl-2 to Bax, (C)Fas, (D) p53, and (E) p21 were determined by reverse transcription-quantitative polymerase chain reaction. The data are presented as the mean ± standard deviation of four independent experiments(*P<0.05). SH, sinomenine hydrochloride; I, iodine-131; ISH, co-treatment of iodine-131 and SH.
In HTori-3 cells, the level of Bax and Fas, were highly upregulated in ISH group when compared with control group, while the expression of Bcl-2 was down-regulated. In BCPAP cells, the level of Bax, p21, p53 and Fas, were highly upregulated in ISH group when compared with control groups, while the expression of Bcl-2 was almost no change. In TPC-1 cells, the level of p21, p53 and Fas, were highly upregulated in the ISH group when compared with control group, while the expression of Bcl-2 was down-regulated, meanwhile the level of Bax was almost no change.
Furthermore, we evaluated the expression levels of Bcl‑2, p21, p53, Bax and Fas were determined using RT‑qPCR in HTori-3, BCPAP and TPC-1 cells, and we evaluated the ratio of Bcl-2 to Bax. The mRNA expression of Fas, p53 and p21 were found to be transcriptionally increased in ISH group when compared with I-131 group (P < 0.05) (Fig. 5C, D and E), while the ratio of Bcl-2 to Bax mRNA expression decreased following added SH treatment (P < 0.05) (Fig. 5B), which were observed in all Htori-3, BCPAP and TPC-1 cells.
When analyzing these genes specifically in ISH group, we figured out that Fas and p53 mRNA expression were transcriptionally increased in the order of HTori-3, BCPAP and TPC-1 cells determined by RT‑qPCR (Fig. 5C and D), but p21 mRNA expression in BCPAP was higher than it in HTori-3 and TPC-1 cells (Fig. 5E).
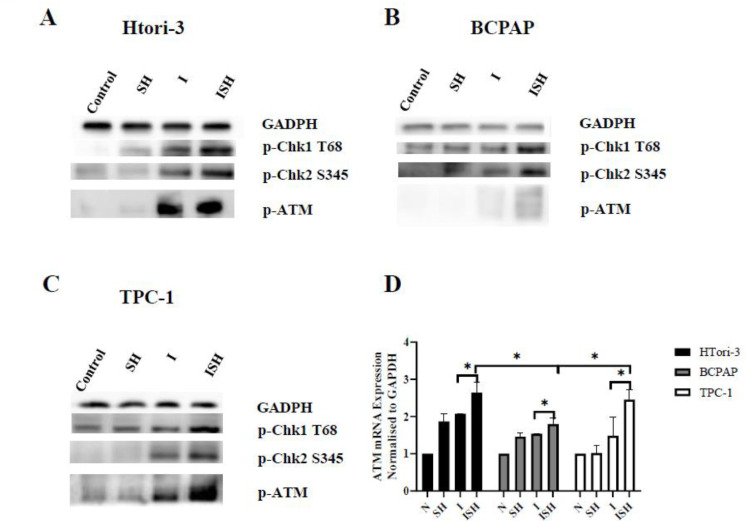
SH enhances expression of p-ATM and the iodine-131‑induced activation of Chk1 and Chk2
In HTori-3, BCPAP and TPC-1 cells, the level of p-ATM, p-Chk1 and p-Chk2 were highly upregulated in ISH group when compared with I-131 group, meanwhile the ATM mRNA expression transcriptionally increased determined by RT‑qPCR. (Fig. 6). When analysis the ATM mRNA expression of ISH group, it was lowest in BCPAP than the other two cell lines (HTori-3 and TPC-1 cells) determined by RT‑qPCR (P < 0.05) (Fig. 6D). From our result show in Fig. 5 and Fig. 6, we found that followed by treated with SH and iodine-131, the radiation induced an up regulation of p-ATM, p-Chk1 and p-Chk2 in the same time. In addition, an up regulation of p53 and p21 caused by uptrend of ATM also made effort on cell cycle in PTC cells.
Fig. 6.
SH enhances expression of p-ATM and the iodine-131-induced activation of Chk1 and Chk2. SH and iodine-131 regulate the expression of p-Chk1(T68) p-Chk2(S345) and p-ATM in (A)HTori-3, (B)BCPAP and (C)TPC-1 cells. The protein expression levels of p-Chk1(T68) p-Chk2(S345) and p-ATM were determined by western blot analysis, Gapdh was probed as a loading control. The mRNA expression levels of (D) ATM mRNA were determined by reverse transcription-quantitative polymerase chain reaction. And normalized using GAPDH as an internal control (*P < 0.05). SH, sinomenine hydrochloride; I, iodine-131; ISH, co-treatment of iodine-131 and SH.
Discussion
Radioactive iodine-131 is a cornerstone in the routine adjuvant management in patients with high-risk differentiated thyroid cancer (DTC), although it is sometimes limited by the development of radioresistance [4]. Thus, it is of great importance to identify a way to promote the radiosensitivity of PTC and improve tumor control. Several studies provide evidence that SH is a promising radiosensitizer both in cervical cancer and in esophageal squamous cell for improving the therapeutic efficacy of radiation [19,20]. In the present study, we investigated the increase effects of SH combined with iodine-131 treatment on thyroid cells (HTori-3, BCPAP and TPC-1 cells) in vitro. The results of present study revealed that synergic radiosensitization of SH and iodine-131 of SH and iodine-131 on HTori-3, BCPAP and TPC-1 cells was related to apoptosis and DNA damage repair.
Apoptosis plays a vital role in all kinds of diseases, and it is considered the primary process of cell death following radiotherapy [21]. It is activated intrinsic (mitochondrial death pathway which is dominated by the Bcl‑2 family of proteins) and extrinsic pathways (death receptor pathway which is dominated by the cell surface receptor, Fas (CD95/Apo‑1)). The Bcl-2 family that is divided into proapoptotic proteins, including Bax, and anti-apoptotic proteins, including Bcl-2, regulates the former pathway [22]. There is increasing evidence that the whether cell undergo apoptosis depends on the ratio of anti-and pro‑apoptotic members, especially the ratio of Bcl‑2 to Bax [23,24]. During extrinsic pathways, Fas is known to be critical in the process of cell apoptosis induced by radiation damage and other DNA damage [25,26]. p21 appears could protect from cell death through various mechanisms, including growth arrest and cytoplasmic effect [27]. p21 could activate by p53, In p53-dependent apoptotic signaling pathway, p21 is indirectly involved in apoptosis through cell cycle arrest, which means it is still unclear whether a physiologic complex of p53/p21/Bcl-w regulates apoptosis [28]. SH has been demonstrated to sensitize radiation in various cancer cells. As for ESCC cells, SH enhanced radiosensitivity was related to Bcl‑2 down-regulation and Bax protein expression up-regulation [16]. Our results demonstrated that iodine-131 and SH could synergistically increase the fraction of apoptotic cells. To investigate the mechanisms underpinning this synergistic effect, we performed Western blot and RT-qPCR analysis. In this present study, co-treatment of PTC cells and normal thyroid cells resulted in decreasing the ratio of Bcl‑2 to Bax and significantly increased Fas expression levels, indicating that the Bcl‑2, Bax and Fas expression levels are involved in signal transmission in the process of cell apoptosis induced by iodine-131 radiation and promoted by SH efforts. In addition, the apoptosis induced by iodine‑131 may occur through the death receptor pathway and the mitochondrial pathway and enhanced by SH in PTC cells and normal thyroid cells. Interestingly, we found that SH played a more significant role in PTC cells compared with normal thyroid cells under the same conditions. Therefore, we can imagine that SH may also be used in Graves' disease iodine-131 treatment, which needs more study in future research.
Following irradiation, the DNA damage checkpoint responses and DNA repair machinery are important in cellular response to radiation [29]. To maintain genome stability, organisms have evolved a complex network of DNA damage response (DDR) mechanisms, including DNA repair, DNA damage checkpoint mechanisms and programmed cell death. Double‑strand break sensor (DSB) induces activation of the DNA damage checkpoint, resulting in cell cycle arrest to allow enough time for DSB repair and prevent mitosis in the presence of a broken chromosome [30]. DDR is a kinase‑based signal transduction pathway that involves multiple DSB sensor proteins such as DNA repair proteins, transducer proteins such as ATM and ATR, mediator proteins such as 53BP1 and BRCA1, and effectors such as Chk1 and Chk2, which protect and maintain genome stability [31,32]. ATM is a serine/threonine kinase that regulates cell cycle checkpoints and DNA repair [33]. Activation of ATM by auto phosphorylation on Ser1981 occurs in response to expose DNA double stranded breaks. ATM kinase regulates a number of proteins involved in cell cycle checkpoint control, apoptosis, and DNA repair [34]. As many studies reported, upon cell DNA damage caused by radiation, ATR and ATM phosphorylate Chk1 and Chk2 at Serine 345 (S345) and Threonine 68 (T68), respectively, facilitating G1/S, intra‑S and G2/M cell cycle checkpoint activation [35], [36], [37], [38]. SH could cause DNA damage accumulation and interfered with cell‑cycle checkpoint activation in Hela cells and ESCC cells [15,16]. Similarly, in our study, we demonstrated that the co-treatment of SH and iodine-131 could inhibit DSB‑repair pathways and cause a rise of ATM, p-Chk1 and p-Chk2 protein expression in both PTC cells and normal thyroid cells. During DDR after radiation, there are still many other DSB sensor proteins, which may require further study.
The sensitivity of cells to radiation is related to the cell cycle phase. It has been reported that cells in the specific cell cycle phases exhibit different degrees of radiosensitivity. In general, cells are most sensitive to irradiation in the G2 and M phases [39], [40], [41]. Many studies have demonstrated that many radiosensitizing agents, such as SH for cervical cancer and esophageal squamous cell carcinoma and valproic acid for thyroid cancer cells increase the cells in the G2/M cell cycle, which enhances the radiosensitivity of many malignant tumors [16,42]. However, we found that there were not reveal any cell cycle disturbance by SH alone, but a significantly decreased iodine-131 induced G2/M arrest and increased S‑phase arrest by co-treatment SH and iodine-131, which was same as Zhang et al. found in Hela cells [11]. This special event may due to the elevated phosphorylation level of Chk1 S345, which is important for DNA damage‑mediated S‑phase checkpoint activation [43].
When DNA damage occurs in cells, such as radiation, elevated levels of p53 always induce a variety of downstream events, including cell cycle arrest, apoptosis and DNA repair or differentiation [44,45]. The results of the present study (Fig. 4) indicated that treatment of PTC cells with both SH and iodine‑131 resulted in significantly increased p53 and p21 expression levels, indicating that the p53 and p21 expression levels are involved in signal transmission in the process of cell apoptosis. Considering the expression of ATM, it can be indicated that DNA damage caused by iodine-131 activates stimulator modification ATM in thyroid cells, then only up-regulate the protein expression of p53 in PTC cells that may cause a transcriptional stimulator modification of p21. Notably the protein expression of p21 in ISH group was lower compared with I-131 group in HTori-3, meanwhile the mRNA expression of p21 was higher which meant the modification and translation of p21 protein in HTori-3 may affect by some other factors, which needs a further study in the future.
Ryan R. et al. demonstrated that BRAFV600E directly promotes radiation resistance through heightened nuclear DNA DSB repair [46]. Our findings join this viewpoint by observing that ATM expression was lower in BCPAP cells compared with TPC-1 cells. Interestingly, both the ratio of Bcl-2 to Bax in BCPAP cells and TPC-1 cells decreased follow by SH treatment added compared with iodine-131 alone. However, Bax protein expression increased, meantime, there was no significant difference in Bcl-2 protein expression in SH and iodine treatment compared with iodine alone in BCPAP cells. The trend of Bax and Bcl-2 protein expression was just on the contrary in TPC-1 cells. These differences may associate with BRAF V600E mutation. It can suggest that BCPAP cells keep a lower radiosensitivity of iodine-131 than TPC-1. BRAF V600E alone is strongly associated with the loss of RAI avidity in recurrent PTC, showing a robust predictive value for failure of RAI treatment of PTC [47], [48], [49]. In clinical studies, radioactive iodine(RAI) treatment were more likely be taken in PTC patients follow by BRAF V600E mutation compared with patients with no BRAF V600E mutation [50]. For this situation, the clinic doctors could pay more attention in RAI treatment when facing PTC patients with BRAF V600E mutation.
Overall, the current study demonstrated that synergic radiosensitization of SH and iodine-131 on PTC cells in vitro, which might be supported by the apoptosis, DNA repair and cell cycle checkpoint regulation. Our findings provide evidence that SH appeared to be a novel and promising radiosensitizer for iodine-131 therapy of PTC treatment in clinical. Further investigation of synergic radiosensitization of SH and 131I in vivo is required in order to enable its direct application in clinical nuclear medicine.
Author contributions statement
Aomei Zhao: Conceptualization, Validation, Writing - Original Draft; Jing Zhang: Validation; Yan Liu: Methodolog, Data Curation;Xi Jia: Methodology, Writing- Reviewing and Editing; Xueni Lu: Resources; Qi Wang: Resources; Ting Ji: Resources; Lulu Yang: Resources; Jianjun Xue: Writing- Reviewing and Editing; Rui Gao: Methodology, Writing- Reviewing and Editing; Yan Yu: Data Curation, Formal analysis; Aimin Yang: Conceptualization, Funding acquisition, Writing- Reviewing and Editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors of the present study would like to thank the staff of the Nuclear Medicine Department of The First Affiliated Hospital of Xi'an Jiaotong University College of Medicine (Xi'an, China) for their support. The project was supported by a grant from the National Natural Science Foundation of China (no. 81871389).
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (no. 81871389).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101172.
Appendix. Supplementary materials
References
- 1.Mao Y., Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr. Relat. Cancer. 2016;23:313–322. doi: 10.1530/ERC-15-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu X., Yao J., Tian W. Microarray technology to investigate genes associated with papillary thyroid carcinoma. Mol. Med. Rep. 2015;11:3729–3733. doi: 10.3892/mmr.2015.3180. [DOI] [PubMed] [Google Scholar]
- 3.Raposo L., Morais S., Oliveira M., Marques A., José Bento M., Lunet N. Trends in thyroid cancer incidence and mortality in Portugal. Eur. J. Cancer Prevent. 2017;26:135–143. doi: 10.1097/CEJ.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 4.Haugen B., Alexander E., Bible K., Doherty G., Mandel S., Nikiforov Y., Pacini F., Randolph G., Sawka A., Schlumberger M., Schuff K., Sherman S., Sosa J., Steward D., Tuttle R., Wartofsky L. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hongwei Gao, Peirong Bai, Lin Xiao, Mengjia Shen, Qiuxiao Yu, Yuanyuan Lei, Wenting Huang, Xiang Lin, Xinyi Zheng, Tao Wei, Jiang Yong., Feng Ye, Hong Bu. Mediator complex subunit 16 is down-regulated in papillary thyroid cancer, leading to increased transforming growth factor-β signaling and radioiodine resistance. J. Biol. Chem. 2020;295(31):10726–10740. doi: 10.1074/jbc.RA119.012404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukun Chen, Shuting Yin, Zhiping Feng, Chao Liu, Juan Lv, Yuanjiao Chen, Ruoxia Shen, Jiaping Wang, Zhiyong Deng. Knockdown of circ_NEK6 decreased I resistance of differentiated thyroid carcinoma via regulating miR-370-3p/MYH9 Axis. Technol. Cancer Res.Treat. 2021;20 doi: 10.1177/15330338211004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan Robb, Linlin Yang, Changxian Shen, Wolfe Adam R., Amy Webb, Xiaoli Zhang, Marall Vedaie, Motoyasu Saji, Sissy Jhiang, Ringel Matthew D., Williams Terence M. Inhibiting BRAF oncogene-mediated radioresistance effectively radiosensitizes BRAF-mutant thyroid cancer cells by constraining DNA double-strand break repair. Clin. Cancer Res. 2019;25(15):4749–4760. doi: 10.1158/1078-0432.CCR-18-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen-Wei. Zou, Ting. Liu, Yong. Li, Peng. Chen, Xin. Peng, Charlie. Ma, Wen-Jie. Zhang, Pin-Dong. Li. Melatonin suppresses thyroid cancer growth and overcomes radioresistance via inhibition of p65 phosphorylation and induction of ROS. Redox Biol. 2018;16:226–236. doi: 10.1016/j.redox.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., Zhang Y., Zhu W., Ma C., Ruan J., Long H., Wang Y. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front. Immunol. 2018;9:2228. doi: 10.3389/fimmu.2018.02228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang R., Pan H., Wu J., Zhou H., Li Z., Qiu P., Zhou Y., Chen X., Xie Z., Xiao Y., Huang Q., Liu L. Comparison of combination therapy with methotrexate and sinomenine or leflunomide for active rheumatoid arthritis: a randomized controlled clinical trial. Phytomedicine. 2019;57:403–410. doi: 10.1016/j.phymed.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Gao L., Zhong B., Wang Y. Mechanism underlying antitumor effects of sinomenine. Chin. J. Integr. Med. 2019;25:873–878. doi: 10.1007/s11655-019-3151-2. [DOI] [PubMed] [Google Scholar]
- 12.Jiang T., Zhou L., Zhang W., Qu D., Xu X., Yang Y., Li S. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol. Med. Rep. 2010;3:51–56. doi: 10.3892/mmr_00000217. [DOI] [PubMed] [Google Scholar]
- 13.Lv Y., Li C., Li S., Hao Z. Sinomenine inhibits proliferation of SGC-7901 gastric adenocarcinoma cells via suppression of cyclooxygenase-2 expression. Oncol. Lett. 2011;2:741–745. doi: 10.3892/ol.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X., Li P., Liu C., Ren Y., Tang X., Wang K., He J. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–13574. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D., Dong Y., Zhao Y., Zhou C., Qian Y., Hegde M., Wang H., Han S. Sinomenine hydrochloride sensitizes cervical cancer cells to ionizing radiation by impairing DNA damage response. Oncol. Rep. 2018;40:2886–2895. doi: 10.3892/or.2018.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S., Jin L., Gong T., Pan S., Zheng S., Zhang X., Yang T., Sun Y., Wang Y., Guo J., Hui B., Zhang X. Effect of sinomenine hydrochloride on radiosensitivity of esophageal squamous cell carcinoma cells. Oncol. Rep. 2018;39:1601–1608. doi: 10.3892/or.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdullah M., Junit S., Ng K., Jayapalan J., Karikalan B., Hashim O. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int. J. Med. Sci. 2019;16:450–460. doi: 10.7150/ijms.29935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D., Zhang Y., Xu H., Zhang X. The role of BRAF in the pathogenesis of thyroid carcinoma. Front. Biosci. (Landmark Ed) 2015;20:1068–1078. doi: 10.2741/4359. [DOI] [PubMed] [Google Scholar]
- 19.Friesen C., Lubatschofski A., Kotzerke J., Buchmann I., Reske S., Debatin K. Beta-irradiation used for systemic radioimmunotherapy induces apoptosis and activates apoptosis pathways in leukaemia cells. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1251–1261. doi: 10.1007/s00259-003-1216-z. [DOI] [PubMed] [Google Scholar]
- 20.Sawka A., Thephamongkhol K., Brouwers M., Thabane L., Browman G., Gerstein H. Clinical review 170: a systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2004;89:3668–3676. doi: 10.1210/jc.2003-031167. [DOI] [PubMed] [Google Scholar]
- 21.Takasawa R., Nakamura H., Mori T., Tanuma S. Differential apoptotic pathways in human keratinocyte HaCaT cells exposed to UVB and UVC. Apoptosis. 2005 Oct;10(5):1121–1130. doi: 10.1007/s10495-005-0901-8. [DOI] [PubMed] [Google Scholar]
- 22.Brunelle J., Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell. Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L., Chen J., Yang J., Pan T., Zhang S., Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Naseri M., Mahdavi M., Davoodi J., Tackallou S., Goudarzvand M., Neishabouri S. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015;15:55. doi: 10.1186/s12935-015-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villa-Morales M., Fernández-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:85–101. doi: 10.1517/14728222.2011.628937. [DOI] [PubMed] [Google Scholar]
- 26.Yasmeen Saeed, Abdul Rehman, Bingjie Xie, Jin Xu, Ma Hong, Qing Hong, Yulin Deng. Astroglial U87 cells protect neuronal SH-SY5Y cells from indirect effect of radiation by reducing DNA damage and inhibiting fas mediated apoptotic pathway in coculture system. Neurochem. Res. 2015;40(8):1644–1654. doi: 10.1007/s11064-015-1642-x. [DOI] [PubMed] [Google Scholar]
- 27.El-Deiry W. p21(WAF1) mediates cell-cycle inhibition, relevant to cancer suppression and therapy. Cancer Res. 2016;76:5189–5191. doi: 10.1158/0008-5472.CAN-16-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez Borrero L., Sikder R., Lulla A., Gokare P., Del Valle P., Tian X., Zhang S., Abbosh P., El-Deiry W. Bcl-2 protein targeting by the p53/p21 complex-letter. Cancer Res. 2018;78:2770–2771. doi: 10.1158/0008-5472.CAN-17-2556. [DOI] [PubMed] [Google Scholar]
- 29.Pranatharthi A., Thomas P., Udayashankar A., Bhavani C., Suresh S., Krishna S., Thatte J., Srikantia N., Ross C., Srivastava S. RhoC regulates radioresistance via crosstalk of ROCK2 with the DNA repair machinery in cervical cancer. J. Exp. Clin. Cancer Res. 2019;38:392. doi: 10.1186/s13046-019-1385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo C., Leu Y., Wang T., Tseng W., Feng C., Wang S., Chen C. A novel DNA repair inhibitor, diallyl disulfide (DADS), impairs DNA resection during DNA double-strand break repair by reducing Sae2 and Exo1 levels. DNA Repair (Amst.) 2019;82 doi: 10.1016/j.dnarep.2019.102690. [DOI] [PubMed] [Google Scholar]
- 31.Polo S., Jackson S. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman A., Monteiro A. Phosphatases in the cellular response to DNA damage. Cell Commun. Signal. 2010;8:27. doi: 10.1186/1478-811X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J., Paull T. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 34.Tang X., Hui Z., Cui X., Garg R., Kastan M., Xu B. A novel ATM-dependent pathway regulates protein phosphatase 1 in response to DNA damage. Mol. Cell. Biol. 2008;28:2559–2566. doi: 10.1128/MCB.01711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson K., Stern D. NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway. Cell Cycle. 2008;7:3584–3594. doi: 10.4161/cc.7.22.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlessi L., Buscemi G., Fontanella E., Delia D. A protein phosphatase feedback mechanism regulates the basal phosphorylation of Chk2 kinase in the absence of DNA damage. Biochim. Biophys. Acta. 2010;1803:1213–1223. doi: 10.1016/j.bbamcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Choi K., Kim J., Lim S., Choi Y., Kim Y., Kang S., Park T., Lim I. TIS21(/BTG2/PC3) accelerates the repair of DNA double strand breaks by enhancing Mre11 methylation and blocking damage signal transfer to the Chk2(T68)-p53(S20) pathway. DNA Repair (Amst.) 2012;11:965–975. doi: 10.1016/j.dnarep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Gamper A., Rofougaran R., Watkins S., Greenberger J., Beumer J., Bakkenist C. ATR kinase activation in G1 phase facilitates the repair of ionizing radiation-induced DNA damage. Nucleic Acids Res. 2013;41:10334–10344. doi: 10.1093/nar/gkt833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y., Liang H., Zhang Q., Li S., Li X., Huo X., Yang Q., Li W., Gu J., Hua Q., Lu P., Miao Z. Radiosensitivity enhancement by arsenic trioxide in conjunction with hyperthermia in the EC-1 esophageal carcinoma cell line. Asian Pac. J. Cancer Prev. 2012;13:1693–1697. doi: 10.7314/apjcp.2012.13.4.1693. [DOI] [PubMed] [Google Scholar]
- 40.Qian X., Tan C., Yang B., Wang F., Ge Y., Guan Z., Cai J. Astaxanthin increases radiosensitivity in esophageal squamous cell carcinoma through inducing apoptosis and G2/M arrest. Dis. Esophagus. 2017;30:1–7. doi: 10.1093/dote/dox027. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W., Gao R., Yu Y., Guo K., Hou P., Yu M., Liu Y., Yang A. Iodine-131 induces apoptosis in HTori-3 human thyrocyte cell line and G2/M phase arrest in a p53-independent pathway. Mol. Med. Rep. 2015;11:3148–3154. doi: 10.3892/mmr.2014.3096. [DOI] [PubMed] [Google Scholar]
- 42.Perona M., Thomasz L., Rossich L., Rodriguez C., Pisarev M., Rosemblit C., Cremaschi G., Dagrosa M., Juvenal G. Radiosensitivity enhancement of human thyroid carcinoma cells by the inhibitors of histone deacetylase sodium butyrate and valproic acid. Mol. Cell. Endocrinol. 2018;478:141–150. doi: 10.1016/j.mce.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Sørensen C., Syljuåsen R., Falck J., Schroeder T., Rönnstrand L., Khanna K., Zhou B., Bartek J., Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 44.Ma J., Li Y., Wu M., Li X. Oxidative stress-mediated p53/p21 pathway may be involved in microcystin-LR-induced cytotoxicity in HepG2 cells. Chemosphere. 2018;194:773–783. doi: 10.1016/j.chemosphere.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 45.Ma J., Chen X., Xin G., Li X. Chronic exposure to the ionic liquid [Cmim]Br induces inflammation in silver carp spleen: involvement of oxidative stress-mediated p38MAPK/NF-κB signalling and microRNAs. Fish Shellfish Immunol. 2019;84:627–638. doi: 10.1016/j.fsi.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 46.Robb R., Yang L., Shen C., Wolfe A., Webb A., Zhang X., Vedaie M., Saji M., Jhiang S., Ringel M., Williams T. Inhibiting BRAF oncogene-mediated radioresistance effectively radiosensitizes BRAF-mutant thyroid cancer cells by constraining DNA double-strand break repair. Clin. Cancer Res. 2019;25:4749–4760. doi: 10.1158/1078-0432.CCR-18-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Liu R., Shen X., Zhu G., Li B., Xing M. BRAF the genetic duet of V600E and promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer, journal of nuclear medicine: official publication. Soc. Nuclear Med. 2020;61:177–182. doi: 10.2967/jnumed.119.227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing M., Haugen B., Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celik M., Bulbul B., Ayturk S., Durmus Y., Gurkan H., Can N., Tastekin E., Ustun F., Sezer A., Guldiken S. The relation between BRAFV600E mutation and clinicopathological characteristics of papillary thyroid cancer, Medicinski glasnik. 2020;17:30–34. doi: 10.17392/1086-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.