Abstract
Although ketamine, a multimodal dissociative anesthetic, is frequently used for analgesia and treatment-resistant major depression, molecular mechanisms of ketamine remain unclear. Specifically, differences in the effects of ketamine on neuroplasticity-related proteins in the brains of males and females need further investigation. In the current study, adult male and female Sprague-Dawley rats with an indwelling jugular venous catheter received an intravenous ketamine infusion (0, 10, or 40 mg/kg, 2-h), starting with a 2 mg/kg bolus for ketamine groups. Spontaneous locomotor activity was monitored by infrared photobeams during the infusion. Two hours after the infusion, brain tissue was dissected to obtain the medial prefrontal cortex (mPFC), hippocampus including the CA1, CA3, and dentate gyrus, and amygdala followed by Western blot analyses of a transcription factor (c-Fos), brain-derived neurotrophic factor (BDNF), and phosphorylated extracellular signal-regulated kinase (pERK). The 10 mg/kg ketamine infusion suppressed locomotor activity in male and female rats while the 40 mg/kg infusion stimulated activity only in female rats. In the mPFC, 10 mg/kg ketamine reduced pERK levels in male rats while 40 mg/kg ketamine increased c-Fos levels in male and female rats. Female rats in proestrus/estrus phases showed greater ketamine-induced c-Fos elevation as compared to those in diestrus phase. In the amygdala, 10 and 40 mg/kg ketamine increased c-Fos levels in female, but not male, rats. In the hippocampus, 10 mg/kg ketamine reduced BDNF levels in male, but not female, rats. Taken together, the current data suggest that subanesthetic doses of intravenous ketamine infusions produce differences in neuroplasticity-related proteins in the brains of male and female rats.
Keywords: Intravenous ketamine; Estrous cycle, Brain-derived neurotrophic factor; C-Fos; Extracellular signal-regulated kinase; Sex differences
1. Introduction
Initially derived from phencyclidine in the 1960s, ketamine, a non-competitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist, has recently acquired popularity because it is used for multiple clinical applications. Most commonly administered intravenously (IV), health care professionals use ketamine to provide rapid sedation and/or anesthesia without compromising hemodynamic stability and spontaneous respiration (Mankowitz et al., 2018). In addition, subanesthetic ketamine provides effective analgesia when compared to opioids, such as morphine, for both chronic (Niesters et al., 2014) and acute pain (Schwenk et al., 2018), potentially reducing the reliance on opioids for pain management. Furthermore, ketamine at subanesthetic doses has shown validity as a rapid-acting antidepressant in patients considered to be treatment-resistant (Berman et al., 2000). The growing clinical utilization of ketamine has led many preclinical researchers to explore ketamine’s mechanism of action. However, many preclinical studies have ignored sex as a biological variable, resulting in a lack of studies investigating potential sex-related differences in the effects of ketamine.
Clinical studies have demonstrated that males and females can exhibit different responses to the same drug treatments (Fletcher et al., 1994, Harris et al., 1995, Loikas et al., 2013, Loikas et al., 2011, Stock et al., 2008, Van der Heyden et al., 2009). These sex-related differences are important for the optimization of drug treatment for patient care. A few preclinical findings have confirmed differences between male and female rodents when given ketamine. In animal behavioral models, such as the forced swim test and novelty suppressed feeding, female rodents were more sensitive to a low dose (2.5 mg/kg, IP) of ketamine injection when compared to males (Carrier and Kabbaj, 2013, Saland et al., 2016). Moreover, female mice showed greater sensitivity to antidepressant effects of ketamine when compared to males based on the forced swim test (Franceschelli et al., 2015). Additionally, female rats have higher levels of ketamine and its primary metabolite, norketamine, in both the mPFC and hippocampus following a low dose of ketamine (2.5 mg/kg, IP), indicating slower metabolism of ketamine in female rats (Saland and Kabbaj, 2018). Moreover, hydroxynorketamine (HNK), a secondary ketamine metabolite, was found at higher levels in female mice compared to males after a low dose of IP ketamine (10 mg/kg) (Zanos et al., 2016). These sex differences may be due to interactions between circulating gonadal hormones, such as estrogen and progesterone, and ketamine, as gonadal hormones can impact sensitivity to ketamine (Saland et al., 2016).
Other preclinical studies have demonstrated that ketamine can stimulate multiple signaling pathways and proteins involved in synaptic plasticity, such as brain-derived neurotrophic factor (BDNF) and extracellular signal-regulated kinase (ERK) (Autry et al., 2011, Li et al., 2010). Furthermore, the immediate early gene c-Fos is used as an index of neuronal activity (Herdegen et al., 1995) and has been implicated in the processes of learning and memory (Gallo et al., 2018). Using male rats, a previous study of ours investigated effects of IV ketamine infusion on c-Fos, BDNF, and pERK levels in brain regions implicated in neuroplasticity (Zhang et al., 2019). There were dose-dependent effects of ketamine (10 and 40 mg/kg) on c-Fos and pERK levels in the mPFC and amygdala of male rats. However, investigations detailing the effect of IV ketamine on these proteins in female rodents are sparse. A few studies reported a greater increase in BNDF and pERK levels after ketamine administration (2.5 mg/kg and 3 mg/kg) in female rodents compared to male rodents (Dossat et al., 2018, Saland et al., 2016), indicating potential sex-related differences in ketamine effects on BDNF and pERK levels in rodents.
It is important to note that most preclinical studies utilize an intraperitoneal (IP) injection to deliver ketamine. However, in clinical settings, ketamine is commonly administered through an IV route. This discrepancy is often overlooked in preclinical studies, but the route of administration may affect the bioavailability of ketamine, which can be as high as 93% with intramuscular administration or as low as 20% with oral administration (Mion and Villevieille, 2013). Following an IP injection, blood plasma drug levels of ketamine rapidly peak and decline secondary to drug distribution and do not maintain a steady-state drug concentration over time (Palenicek et al., 2011). In contrast, a continuous IV ketamine infusion causes elevated plasma drug levels and is maintained over the duration of the infusion (Radford et al., 2017). Consequently, this allows for a longer sustained blockade of NMDA receptors in the brain and this difference in routes of administration may provide limitations in translating preclinical results to clinical practice. Therefore, we utilized intravenous infusion rather than bolus injection of ketamine in the current study to model clinical situations. For antidepressant effects in rodents, a low-dose IV ketamine infusion (1.47 mg/kg, 40 min) was used (Wright et al., 2019). In the current study, higher-dose IV ketamine infusions (10 and 40 mg/kg, 2-h) were tested because these doses produce strong analgesia with various behavioral effects, as reported previously (Radford et al., 2017, Radford et al., 2020).
In order to address sex as a biological variable, we included male and female adult rats in the current study. Furthermore, we took into consideration the possibility of an interaction between circulating gonadal hormones and ketamine effects on brain protein levels in female rats. We hypothesized that female rats may be more sensitive to the effects of IV ketamine on neuroplasticity-related protein levels and that gonadal hormones may contribute to differences between male and female rats.
2. Experimental procedures
2.1. Animals
Adult male and female Sprague-Dawley (SD) rats (9 weeks old upon arrival) were purchased from Envigo Laboratories (Dublin, VA). Animals were acclimated and handled regularly for one week before the experiment. A catheter (Polyurethane; Instech, Plymouth Meeting, PA, USA) was implanted in the jugular vein of the animal under isoflurane anesthesia as described previously (Radford et al., 2020, Radford et al., 2020). Animals were given a minimum of one week to recover from the surgery and were housed individually in a climate-controlled environment with food and water available ad libitum. Male and female rats were housed in separate rooms with a 12-h reversed light/dark cycle (lights off at 0600 h and on at 1800 h), and all procedures were conducted during the dark cycle when animals were active. The catheter was flushed twice a week with 0.1 mL of sterile heparin and glycerol solution to maintain catheter patency. Animal use and procedures were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and Use Committee at the Uniformed Services University (Bethesda, MD).
2.2. Estrous cycle monitoring in female rats
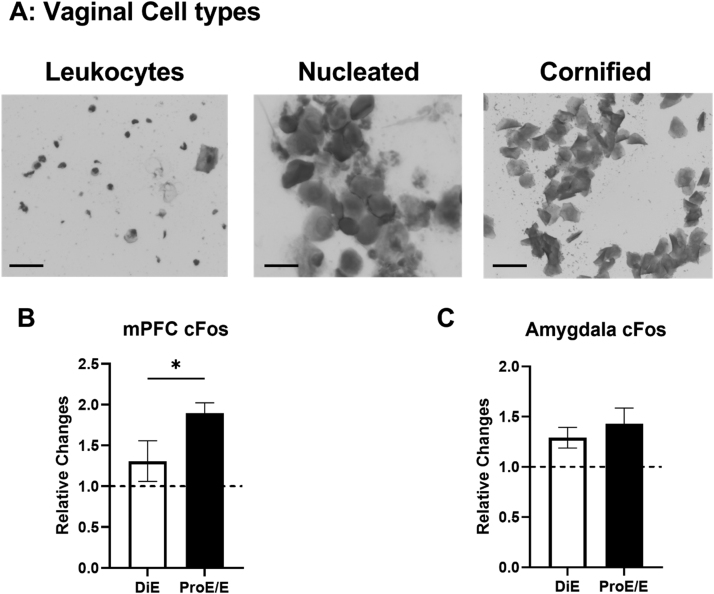
The estrous cycles of the female rats were monitored on a daily basis using a vaginal cytology method before (two weeks as a baseline) and on the testing day. Vaginal cells such as leukocytes, nucleated epithelial cells, and cornified epithelial cells were collected using vaginal lavage, spread onto microscope slides (Fisher Scientific, Waltham, MA), and dried. Subsequently, the cells were stained with 0.01% Crystal violet solution (Sigma-Aldrich, St. Louis, MO) to enhance morphology of cells. Based on the predominant vaginal cell type on the testing day, female rats were classified into the diestrus group (leukocytes) or the proestrus/estrus group (nucleated and/or cornified epithelial cells). Similar dichotomous grouping strategy was used for estrous cycle analysis in previous studies (D'Souza and Sadananda, 2017, LeFevre and McClintock, 1988, Pare and Redei, 1993, Radford et al., 2020).
2.3. IV Ketamine Infusion
Animals were randomized into three groups: saline, 10 mg/kg ketamine, and 40 mg/kg ketamine. The saline control group received a saline IV bolus followed by a 2-h saline infusion (1 mL/h). The ketamine groups received a 2 mg/kg IV ketamine bolus followed by a 2-h ketamine infusion (10 mg/kg or 40 mg/kg). All doses were delivered in a 1 mL/kg volume. Racemic (±) ketamine hydrochloride (100 mg/mL) (Mylan Institutional LLC, Rockford, IL) was diluted in 0.9% sterile saline and administered to each rat placed in an infusion chamber (Med Associates Inc., St. Albans, VT). Each chamber was equipped with an infusion pump (Harvard Pump 11 Elite, Holliston, MA) using a 5 mL Hamilton glass syringe connected to a fluid swivel (Instech, Plymouth Meeting, PA) by a polyurethane tubing encased in a metal spring-wire tether (Instech, Plymouth Meeting, PA). The spring-wire tether was attached to the vascular access button on the back of the animal. Each tethered rat had free mobility in the chamber during the 2-h infusion period, and each infusion chamber was equipped with two infrared photobeams. The number of photobeam breaks was recorded by a computer for quantification of spontaneous locomotor activity during the infusion period.
2.4. Western Blot experiment
Animals were returned to their home cages for 2 h following IV ketamine infusion. At the end of the 2 h, they were euthanized, and the bilateral medial prefrontal cortex (mPFC), amygdala, and dorsal hippocampus were rapidly dissected out using a brain matrix chilled in wet ice as described previously (Zhang et al., 2019). All tissue samples were immediately frozen on dry ice and stored at −80 °C. Brain tissue samples were homogenized in RIPA buffer containing 0.22% Beta glycerophosphate, 10% Tergitol-NP40, 0.18% Sodium orthovanadate, 5% Sodium deoxycholate, 0.38% EGTA, 1% SDS, 6.1% Tris, 0.29% EDTA, 8.8% Sodium chloride, and 1.12% Sodium pyrophosphate decahydrate. The protein concentration was quantified using a BCA protein assay (ThermoFisher Scientific, Waltham, MA) and an equal amount of protein samples were loaded and separated on an SDS-polyacrylamide gel (NuPage 4–12% Bis-Tris gel, ThermoFisher, Waltham, MA). Following electrophoresis, proteins were transferred to a nitrocellulose membrane and blocked for 1 h in 5% milk TBS-T (TBS and 0.1% Tween 20). After the blocking, membranes were incubated with a primary antibody at 4 °C overnight. The following primary antibodies were used: BDNF (1:2000 Santa Cruz, Dallas, TX), c-Fos (1:1000 Biolegend, San Diego, CA), pERK (1:2000 Biolegend, San Diego, CA), and beta-actin (1:200,000 Abcam, Cambridge, United Kingdom). Next, the membranes were washed in TBS-T and incubated with HRP-conjugated secondary antibody for 1 h. Protein bands were detected using the ECL plus method (BioRad, Hercules, CA) with ChemiDoc equipment (BioRad, Hercules, CA). After the target protein quantification, the membranes were stripped for 15 min using Western Blot Stripping Buffer (ThermoFisher, Carlsbad, CA) and re-probed for a reference protein (β-actin) to adjust for the variations in sample loading in the gel. Each protein band was normalized with the sum of the same protein bands in the same blot to reduce variability between the blots based on the previous study (Degasperi et al., 2014). After the sum normalization, each target protein such as c-Fos, pERK1, pERK2, and BDNF was further normalized with the reference protein (β-actin) of the same sample.
2.5. Data analyses
The intensities of the protein bands were quantified by using the Image Lab software (BioRad, Hercules, CA). All data are presented as mean ± standard error of the mean (SEM) and were analyzed using GraphPad Prism (GraphPad Software Version 8.0). A two-way analysis of variance (ANOVA) with sex and ketamine as factors and Tukey’s post-hoc tests were used to compare group differences in protein levels. For the estrous cycle data, an independent samples t-test was used to compare diestrus and proestrus/estrus groups. The accepted level of significance was p < 0.05.
3. Results
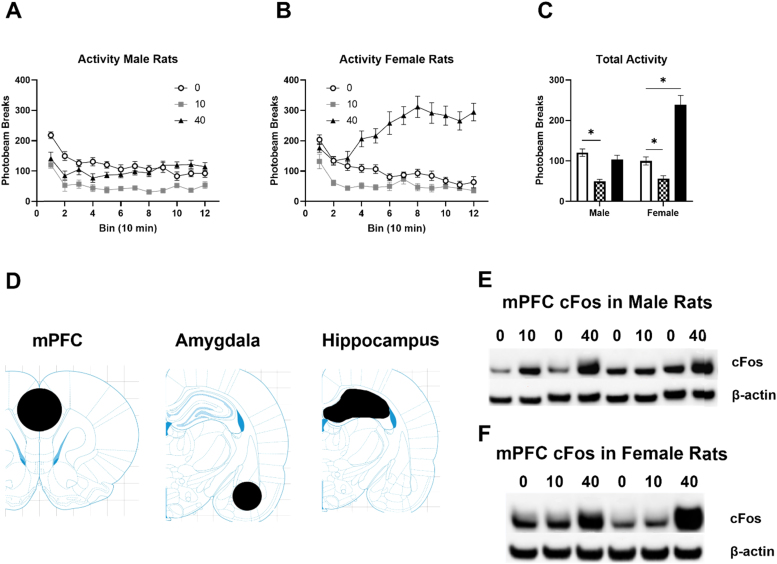
The IV ketamine infusion produced dose-dependent effects on locomotor activity in male and female rats. The 10 mg/kg ketamine infusion reduced locomotor activity in both male and female rats (Fig. 1A). However, the 40 mg/kg ketamine infusion stimulated locomotor activity only in female rats (Fig. 1A). A two-way ANOVA on locomotor activity indicated significant main effects of ketamine F (2, 57) = 42.81, p < 0.0001 and sex F (1, 57) = 16.51, p = 0.0001, and an interaction between ketamine and sex F (2, 57) = 23.91, p < 0.0001. A detailed time course during the 2-h infusion indicated that the 10 mg/kg ketamine infusion consistently suppressed activity in male rats compared to the saline control group (Fig. 1B). On the contrary, the 40 mg/kg ketamine infusion stimulated activity from 40 min to 120 min in female rats (Fig. 1C). This indicates that the 10 mg/kg ketamine infusion produces sedative effects in both male and female rats, while the 40 mg/kg ketamine infusion produces locomotor stimulation in female rats. The three brain regions analyzed in the current study are depicted in Fig. 1D. Fig. 1E and F show example Western blot images of c-Fos in the mPFC of male and female rats, respectively.
Fig. 1.
Effects of IV ketamine infusion on spontaneous locomotor activity in male and female rats. A: Total locomotor activity of male and female rats during the infusion. B: A time course of ketamine-induced locomotor activity in male rats. C: A time course of ketamine-induced locomotor activity in female rats. Saline (white), 10 mg/kg (checkered), and 40 mg/kg (black). D: Brain regions dissected for Western blot analysis. Bregma: mPFC (2.52 mm), Amygdala (−3.24 mm), Hippocampus (−3.6 mm) E: Representative blot images of c-Fos (top) and beta-actin (bottom) in the mPFC of male rats. 0: Saline, 10: 10 mg/kg ketamine, 40: 40 mg/kg ketamine F: Representative blot images of c-Fos (top) and beta-actin (bottom) in the mPFC of female rats. 0: Saline, 10: 10 mg/kg ketamine, 40: 40 mg/kg ketamine. Data are shown as mean ± SEM (*p < 0.05 compared to saline controls).
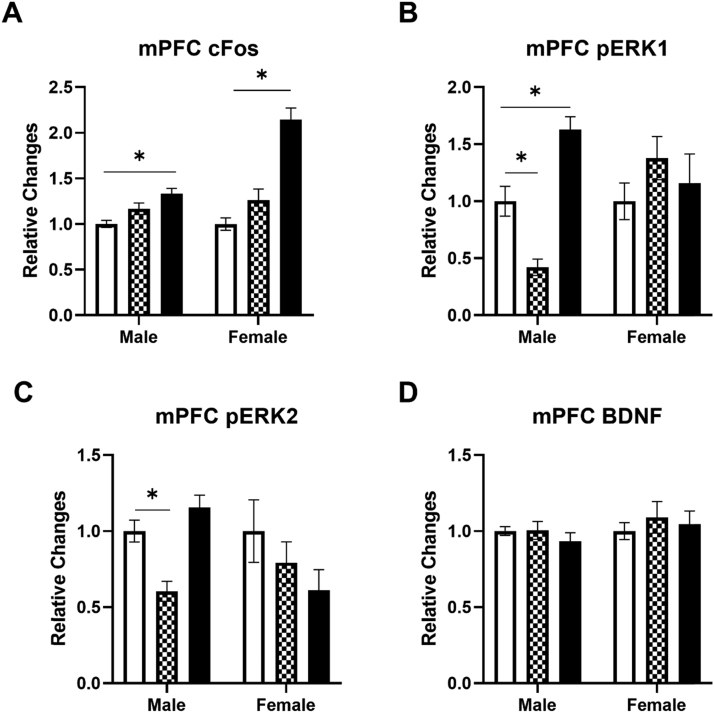
The c-Fos, pERK1, pERK2, and BDNF levels were determined in the mPFC of male and female rats following IV ketamine infusion (Fig. 2). The 40 mg/kg ketamine infusion significantly increased c-Fos levels in the mPFC of both male and female rats (Fig. 2A). A two-way ANOVA indicated significant main effects of ketamine F (2, 59) = 45.28, p < 0.0001 and sex F (1, 59) = 20.51, p < 0.0001, and an interaction F (2, 59) = 14.97, p < 0.0001 on c-Fos levels in the mPFC. Post hoc tests revealed that the 40 mg/kg ketamine infusion increased c-Fos levels in both male and female rats (p < 0.05). There was a significant difference between males and females in c-Fos elevation following the 40 mg/kg ketamine infusion. Ketamine also dose-dependently altered pERK1 levels in male rats (Fig. 2B). A two-way ANOVA indicated significant main effects of ketamine F (2, 55) = 4.196, p = 0.02 and an interaction between ketamine and sex F (2, 55) = 8.506, p = 0.0006 on pERK1 levels in the mPFC. Post hoc tests revealed that 10 mg/kg reduced, but 40 mg/kg increased, pERK1 levels in the mPFC of male rats. Similar to pERK1 levels, 10 mg/kg ketamine reduced pERK2 levels in male rats (Fig. 2C). A two-way ANOVA indicated that there was a significant interaction between ketamine and sex F (2, 57) = 4.099, p = 0.021 on pERK2 levels in the mPFC. In contrast, ketamine did not alter BDNF levels in the mPFC of either male or female rats (Fig. 2D). These data indicate that a ketamine infusion produces dose-dependent and sex-specific effects on c-Fos and pERK levels in the mPFC of male and female rats.
Fig. 2.
The effects of ketamine infusion (0, 10, and 40 mg/kg, 2-h) on protein levels in the mPFC of male and female rats. A: Ketamine (40 mg/kg) increased c-Fos levels in male and female rats. B: Ketamine (10 mg/kg) reduced pERK1 while 40 mg/kg ketamine increased pERK1 levels in male rats. C: Ketamine (10 mg/kg) reduced pERK2 levels in male rats. D: Ketamine did not alter BDNF levels in either male or female rats. Saline group (white), 10 mg/kg (checkered), and 40 mg/kg (black). Male rats: Saline (n = 18), 10 mg/kg (n = 8), 40 mg/kg (n = 8–10); Female rats: Saline (n = 10), 10 mg/kg (n = 9–10), 40 mg/kg (n = 7–9). Data shown as mean ± SEM (*p < 0.05 compared to saline controls).
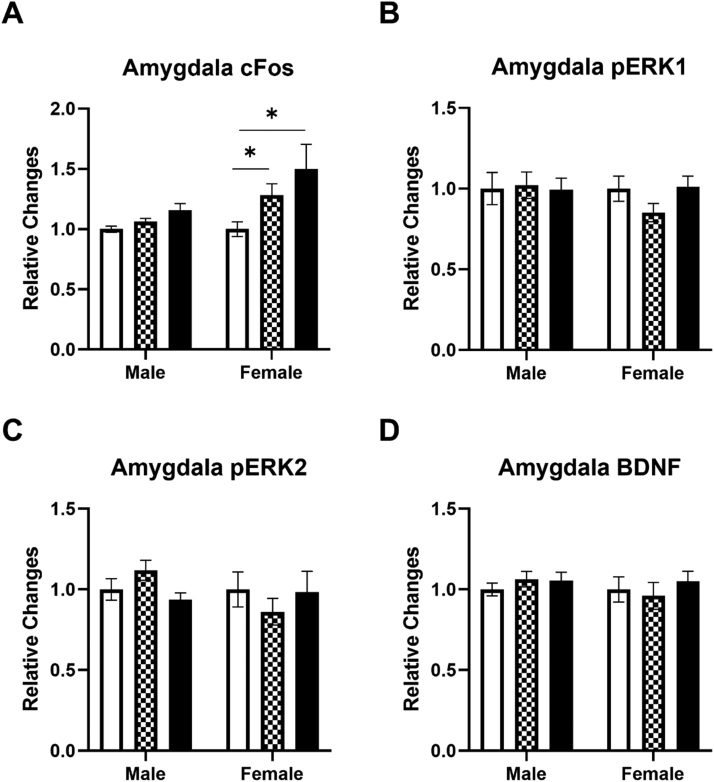
The effects of a ketamine infusion on c-Fos, pERK1, pERK2 and BDNF levels in the amygdala of male and female rats were also determined (Fig. 3). Ketamine significantly increased c-Fos levels in the amygdala of female rats (Fig. 3A). A two-way ANOVA indicated significant main effects of ketamine F (2, 58) = 9.311, p = 0.0003 and sex F (1, 58) = 8.400, p = 0.0053 on c-Fos levels in the amygdala. Post hoc tests revealed that 10 mg/kg and 40 mg/kg ketamine doses were significantly different from the saline control group. Contrary to the mPFC, the ketamine infusion did not alter either pERK1 (Fig. 3B) or pERK2 (Fig. 3C) levels in the amygdala of either male or female rats. Similar to the mPFC, ketamine did not alter BDNF levels in the amygdala of either male or female rats (Fig. 3D). These results indicate that an IV ketamine infusion produces sex-related differences in c-Fos levels in the amygdala of rats.
Fig. 3.
The effects of ketamine infusion (0, 10, and 40 mg/kg, 2-hr) on protein levels in the amygdala of male and female rats. A: Both 10 mg/kg and 40 mg/kg ketamine increased c-Fos levels in female rats. B: Ketamine did not alter pERK1 levels in either male or female rats. C: Ketamine did not alter pERK2 levels in either male or female rats. D: Ketamine did not alter BDNF levels in either male or female rats. Saline group (white), 10 mg/kg (checkered), and 40 mg/kg (black). Male rats: Saline (n = 18), 10 mg/kg (n = 8), 40 mg/kg (n = 10); Female rats: Saline (n = 11), 10 mg/kg (n = 10), 40 mg/kg (n = 7–9). Data shown as mean ± SEM (*p < 0.05 compared to saline controls).
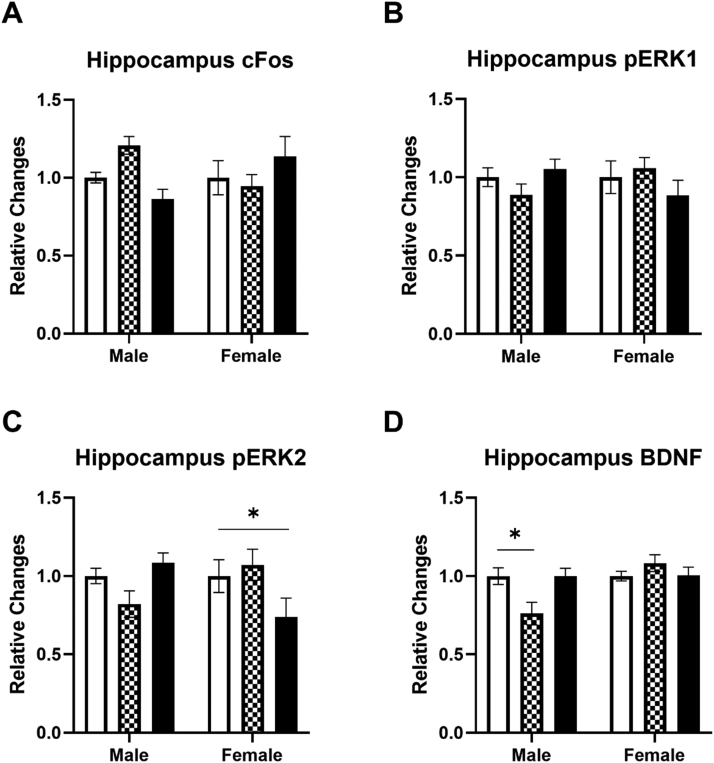
Next, the c-Fos, pERK1, pERK2, and BDNF levels in the hippocampus were determined following an IV ketamine infusion in male and female rats (Fig. 4). In contrast with the mPFC and amygdala, the ketamine infusion failed to increase c-Fos levels in the hippocampus of both male and female rats (Fig. 4A). The ketamine infusion also did not alter pERK1 levels in the hippocampus of either male or female rats (Fig. 4B). However, the 40 mg/kg ketamine infusion significantly reduced pERK2 levels in the hippocampus of female rats (Fig. 4C). A two-way ANOVA on pERK2 levels indicated a significant interaction between ketamine and sex F (2, 60) = 5.391, p = 0.007. Contrary to the mPFC and amygdala, the 10 mg/kg ketamine infusion reduced BDNF levels in the hippocampus of male rats (Fig. 4D). A two-way ANOVA on BDNF levels indicated a significant main effect of sex F (1, 59) = 5.605, p = 0.021 and an interaction between ketamine and sex F (2, 59) = 4.971, p = 0.01 in the hippocampus of male rats.
Fig. 4.
The effects of ketamine infusion (0, 10, and 40 mg/kg, 2-hr) on protein levels in the hippocampus of male and female rats. A: Ketamine did not alter c-Fos levels in either male or female rats. B: Ketamine did not alter pERK1 levels in either male or female rats. C: Ketamine (40 mg/kg) reduced pERK2 levels in female rats. D: Ketamine (10 mg/kg) reduced BDNF levels in male rats. Saline (white), 10 mg/kg (checkered), and 40 mg/kg (black). Male rats: Saline (n = 18), 10 mg/kg (n = 8), 40 mg/kg (n = 10); Female rats: Saline (n = 11), 10 mg/kg (n = 10), 40 mg/kg (n = 9). Data shown as mean ± SEM (*p < 0.05 compared to saline controls).
Because female rats showed elevated c-Fos levels in the mPFC and amygdala following an IV ketamine infusion, potential effects of the estrous cycle on c-Fos levels were examined in female rats. Fig. 5A depicts representative images of vaginal cell types including leukocytes (diestrus phase), nucleated epithelial cells (proestrus phase), and cornified epithelial cells (estrus phase) in female rats. Based on the majority of cell types, animals that received a ketamine infusion (10 and 40 mg/kg) were classified into the diestrus group (low estrogen levels) or proestrus/estrus group (high estrogen levels). Ketamine-induced c-Fos elevation in the mPFC was greater in female rats in the proestrus/estrus phases as compared to those in diestrus phase (Fig. 5B) (t = 2.338, df = 17, p = 0.03). However, ketamine-induced c-Fos elevation in the amygdala was not different between females in diestrus phase and proestrus/estrus phases (Fig. 5C) (t = 0.656, df = 15, p = 0.52). This suggests that circulating estrogen levels may produce differential effects on c-Fos expression in the mPFC of female rats following IV ketamine infusion.
Fig. 5.
The effects of ketamine infusion (0, 10 and 40 mg/kg, 2-h) on c-Fos levels in the mPFC (B) and amygdala (C) of female rats with diestrus phase or proestrus/estrus phases. A: Representative images of different cell types including leukocytes (diestrus), nucleated epithelial cells (proestrus), and cornified epithelial cells (estrus) with the vaginal cytology method. Scale bar: 30 µm. B: Ketamine-induced c-Fos elevation was greater in female rats in the proestrus phase as compared to females in the diestrus phase. C: Ketamine-induced elevation of c-Fos was not significantly different between females in the diestrus and proestrus/estrus phases. Diestrus (DiE, n = 7), Proestrus/Estrus (ProE/E, n = 10–12). Data shown as mean ± SEM. (*p < 0.05 compared to saline controls).
4. Discussion
Comparative and systematic evaluations of differences in ketamine effects on behaviors and brain mechanisms between males and females are sparse. The goal of the current investigation was to determine sex-related differences in the effects of ketamine on locomotor activity and neuroplasticity-related protein levels in the brain using a clinically relevant route of administration. Our results indicate that IV ketamine produced dose-dependent and region-specific effects on those measures in male and female rats. The 10 mg/kg ketamine infusion over 2 h significantly reduced locomotor activity both in male and female rats, indicating sedative effects without severe dissociation. However, the 40 mg/kg ketamine infusion stimulated locomotor activity only in female rats. In addition, the IV ketamine infusion produced sex-related differences in c-Fos and pERK levels in the PFC and amygdala of male and female rats. Female rats were more sensitive to ketamine-induced c-Fos elevation in these regions compared to male rats. Female rats with high estrogen levels (proestrus/estrus phases) exhibited greater elevation of c-Fos compared to the ones with low estrogen levels (diestrus phase). Taken together, the current findings suggest that IV ketamine produces sex-related differences in spontaneous locomotor activity and neuroplasticity-related proteins in multiple brain regions of male and female rats.
The IV ketamine infusion produced sex-specific effects on spontaneous locomotor activity in female rats. Specifically, the 10 mg/kg ketamine infusion suppressed activity whereas the 40 mg/kg ketamine infusion stimulated spontaneous activity in female rats. This is surprising given the male rats did not show stimulation during the 40 mg/kg ketamine infusion. Previous studies reported differences in dissociative stereotypy between male and female rodents following ketamine administration. A previous study found that female rats exhibited greater levels of ataxia than male rats following 5 mg/kg IV bolus ketamine (Radford et al., 2020). However, other behaviors such as horizontal activity and rearing during the 10-minute period following ketamine bolus administration were not significantly different between male and female rats in that study. The discrepancy between these findings may be due to the duration of ketamine administration (bolus vs. infusion) and doses (5 mg/kg bolus vs. 40 mg/kg infusion). It has been well-documented that pharmacokinetic and pharmacodynamic properties of the same drug are different depending on the route and duration of administration (Palenicek et al., 2011). Nevertheless, the current findings agree with previous studies that reported female rats are more sensitive to locomotor stimulant effects of ketamine and other NMDA receptor antagonists when compared to male rats (Honack and Loscher, 1993, McDougall et al., 2019, Nabeshima et al., 1984, Wilson et al., 2007). It is important to note that the time course of locomotor-stimulating effects of ketamine are dependent on the route of administration. For example, in the current study, IV ketamine infusion (40 mg/kg over 2 h) produced locomotor stimulation at 40 min which lasted until the end of the infusion in female rats. However, a higher-dose 80 mg/kg ketamine IP injection produced a slower time course as hyperactivity started at 90 min and lasted until 270 min in female rats (McDougall et al., 2019). These results indicate that adult male and female rats may have drastic differences in locomotor responses to higher doses of ketamine, which warrants a future investigation to examine to how this difference occurs.
Subanesthetic doses of IV ketamine produced dose-dependent, brain region-specific, and sex-specific effects on c-Fos levels in rats. The 40 mg/kg ketamine infusion significantly increased c-Fos levels in the mPFC of male and female rats. However, the ketamine infusion elevated c-Fos levels only in the amygdala of female rats. Interestingly, c-Fos levels in the dorsal hippocampus were not altered following ketamine infusions in either male or female rats. These findings support previous studies that reported elevated c-Fos levels in the mPFC (Girgenti et al., 2017) and amygdala of male rats following ketamine administration (Zhang et al., 2019). The c-Fos levels were increased in the mPFC of male rats when measured two days after a 10 mg/kg IP ketamine injection (Girgenti et al., 2017). Increased c-Fos levels in the mPFC and amygdala following ketamine infusion in the current study demonstrate ketamine’s possible role in brain function relevant to learning and memory as both regions are critical in the acquisition and retrieval of fear memory and extinction (Quirk and Mueller, 2008). Interestingly, female rats showed a greater elevation of c-Fos levels in the mPFC and amygdala following ketamine infusion compared to male rats. These results indicate that females may have a heightened sensitivity to the effects of ketamine on fear memory. Furthermore, female rats may exhibit prolonged c-Fos expression as compared to male rats. A study confirmed female rats to show greater c-Fos mRNA levels at 60 min post-stress exposure as male rat c-Fos levels returned to baseline levels at the same time point (Bland et al., 2005). In the current study, c-Fos protein levels in the mPFC when measured at 2 h post-ketamine infusion were greater in female rats as compared to male rats. These results may indicate that females may have a heightened sensitivity to the effects of ketamine on behaviors regulated by the mPFC of the brain.
Possible explanations for female rats’ enhanced c-Fos response can be drawn from some similar patterns in other studies. Behavioral studies have demonstrated that female rodents are more sensitive to ketamine compared to male rats at the same dose (Carrier and Kabbaj, 2013, Dossat et al., 2018, Saland et al., 2016). For instance, studies have shown female rodents spent less time immobile in the forced swim test following ketamine administration (2.5 mg/kg and 3 mg/kg, IP) (Carrier and Kabbaj, 2013, Dossat et al., 2018). More specifically, females showed greater antidepressant effects at a lower dose (2.5 mg/kg, IP) than male rats in the forced swim test (Carrier and Kabbaj, 2013). In the same study, antidepressant effects of low-dose ketamine were dependent upon gonadal hormones, as ovariectomy abolished ketamine effects in female rats. In addition, the low dose of ketamine (2.5 mg/kg, IP) in this study produced a decreased latency in the novelty suppressed feeding task in females compared to no effect in males (Carrier and Kabbaj, 2013). This heightened sensitivity trend is also seen in the sucrose preference test as ketamine induced an increase in sucrose preference in females, but not males, at 2.5 mg/kg IP (Saland et al., 2016). Taken together, previous and current findings agree on the enhanced sensitivity to ketamine in females, similar to what we observed in c-Fos levels between male and female rats.
The extracellular signal-regulated kinase (ERK) has also been implicated in multiple roles in learning and memory, as ERK is suggested to be important for synaptic plasticity and the formation of memory (Sun and Nan, 2017). Indeed, studies have suggested ERK1/2 activation is necessary for synaptic plasticity in both the hippocampus and amygdala (English and Sweatt, 1997, Schafe et al., 2000). Accordingly, previous studies have shown that a single administration of ketamine (10 mg/kg, IP) can increase the phosphorylation of ERK in the mPFC of male rodents (Girgenti et al., 2017, Li et al., 2010, Park et al., 2014). In the current investigation, we measured two phosphorylated isoforms of ERK, pERK1 and pERK2, in the brain. A 10 mg/kg ketamine infusion reduced pERK1 and 2 levels while a 40 mg/kg ketamine infusion only increased pERK1 levels in the mPFC of male rats. Interestingly, neither dose of ketamine produced significant effects on pERK1 and 2 levels in the mPFC of female rats. Thus, 10 mg/kg ketamine infusion reduced locomotor activity and pERK levels in the mPFC of male rats. This finding is interesting because 40 mg/kg did not alter locomotor activity and pERK levels in male rats. However, it is difficult to discuss potential mechanisms by which pERK may modulate locomotor activity due to a lack of published work on this topic. It is possible that reduced pERK levels in the mPFC following a clinically relevant dose of ketamine infusion (10 mg/kg), which produces antinociception without severe dissociation (Radford et al., 2017, Radford et al., 2020), may serve as a neuronal marker for dose-dependent effects of ketamine. Because female rats are more sensitive to c-Fos elevation but less sensitive to pERK changes in the mPFC following IV ketamine infusion, different molecular pathways mediated by these two important proteins may play a role in ketamine’s behavioral disparities between male and female rats. Contrary to our findings, a previous investigation reported that low-dose ketamine injection (3 mg/kg, IP) had no effects on pERK levels in the mPFC but increased pERK levels in the hippocampus of both male and female mice (Dossat et al., 2018). Moreover, an antidepressant dose of ketamine (10 mg/kg, IP) injected to stress-naïve mice produced sex-specific effects (Thelen et al., 2019). Based on ketamine effects on spine density and synaptic plasticity-related proteins, the mPFC and hippocampus showed greater reactivity to ketamine in male and female mice, respectively, in that study. Taken together, we can conclude that ketamine may produce heterogeneous effects on pERK levels in different brain regions depending on the route and doses of ketamine (3 mg/kg, IP vs. 10 and 40 mg/kg IV infusion over 2 h) and the timing of protein assays (1 h vs. 2 h) after ketamine administration. Further study is necessary to investigate sex-related differences in the effects of IV ketamine infusion on pERK levels in neuroplasticity-related brain regions at different time points.
BDNF is a necessary molecule in neuroplasticity and has been widely implicated in learning and memory. There is evidence that BDNF plays a major role in ketamine’s effects and is linked directly to its antidepressant attributes. Indeed, behavioral effects of ketamine are blocked in BDNF knockout mice while an increase of BDNF release was found in control mice (Autry et al., 2011). Moreover, several investigations have also demonstrated increased BDNF levels in brain tissue upon ketamine administration in both the mPFC and hippocampus in rodents (Akinfiresoye and Tizabi, 2013, Garcia et al., 2008, Yang et al., 2013, Zhou et al., 2014). Most importantly, an investigation looking at potential sex differences after a low-dose ketamine injection detected an increase in BDNF in the PFC of female rats (Dossat et al., 2018). Furthermore, the same study observed a sex hormone effect as proestrus females exhibited higher levels of BDNF in the mPFC when compared to diestrus females. Contrary to the previous studies, we observed no change in BDNF levels in any of the regions except hippocampus of male rats. The lack of changes in BDNF levels following ketamine infusion could be attributed to the differences in route and duration of ketamine administration and timing of sample collection. Most previous studies found positive changes in BDNF when measured within 30–60 min after bolus ketamine injection, while our samples were analyzed 2 h post-ketamine infusion. Accordingly, previous reports describe no changes in hippocampal BDNF when measured between 4 h up to 10 days post-ketamine injection, further suggesting a transient regulation of BDNF levels after ketamine administration (Li et al., 2020, Medeiros et al., 2021, Saur et al., 2017). Thus, a subsequent study is warranted to investigate effects of IV ketamine infusion on BDNF levels in different brain regions of animals at multiple time points.
The interaction between gonadal hormones, including estrogen and progesterone, and ketamine in females remains elusive. In the current study, we observed a greater elevation of c-Fos levels in the mPFC of female rats in proestrus/estrus phases (high estrogen levels) when compared to those in diestrus phase (low estrogen levels). However, a study utilizing uncontrollable stress found c-Fos expression in the mPFC of female rats was opposite to our findings as diestrus female rats showed greater c-Fos expression than proestrus and estrus female rats (Bland et al., 2005). This suggests that gonadal hormones in female rats may differentially regulate c-Fos expression in the mPFC depending on the type of stimuli such as stress or ketamine. A potential mechanism that could cause an increase of c-Fos levels during the proestrus/estrus phases may be due the fluctuation of dendritic spine density in the mPFC during the natural estrous cycle in female rats, as dendritic spines are known to be key structures for neuroplasticity (Gipson and Olive, 2017). Studies confirmed that female rats in the proestrus phase had greater dendritic spine density compared to those in the diestrus phase (Chen et al., 2009, Sarkar and Kabbaj, 2016). Thus, the increased dendritic spine density in the mPFC could result in increased c-Fos expression when interacting with ketamine. Taken together, it is possible that circulating gonadal hormones may play an important role in regulating biological effects of ketamine in the mPFC of females.
The current study is not without limitations. First, measuring protein levels at single time point after the ketamine infusion has a limitation as those neuroplasticity related proteins can show dynamic temporal changes following a 2-hour ketamine infusion. Additionally, pERK levels were normalized with β-actin rather than total ERK levels. We chose this approach based on the prior studies that also used β-actin to normalize pERK levels in the spinal cord and brain tissue of rodents (Chen et al., 2021, Esmaili-Shahzade-Ali-Akbari et al., 2021, Xu et al., 2019, Zhang et al., 2019). However, because of a longer duration of IV ketamine infusion (2 h) employed in the current study, calculating the ratio of pERK/ERK would have provided more accurate information on functional changes of ERK proteins in these brain regions. Thus, further studies that examine multiple time points of phosphorylated as well as total protein levels beyond 2 h post-ketamine infusion are warranted. Second, the Western blot assay provides limited information on anatomical specificity and cell types in the brain regions investigated. In order to enhance anatomical specificity of mPFC, amygdala, and hippocampus, different techniques such as immunohistochemistry would provide more information. Thus, a future study using immunohistochemistry along with cell type-specific labeling of proteins in those regions is warranted. Third, sex-related effects of ketamine are known to be different in stressed vs. non-stressed animals. For instance, sex-related differences in depression-like behaviors and synaptic plasticity, including dendritic spine density and synaptic protein levels, following ketamine administration have been described in animals exposed to chronic stress (Sarkar and Kabbaj, 2016). Therefore, further investigation is necessary to better understand the relationship between stress and ketamine on synaptic plasticity in males and females. Finally, increased c-Fos levels in the PFC following 40 mg/kg ketamine infusion may be due to dissociative symptoms which increased locomotor activity especially in female rats. Indeed, previous studies reported a dose-dependent relationship between motor activity and c-Fos levels in multiple brain regions of male rats (Imre et al., 2006) and female mice (Nishizawa et al., 2000). Imre et al. reported that 12 and 16 mg/kg ketamine injection increased locomotor activity and c-Fos levels in the PFC, amygdala, and hippocampus of rats when measured at 2 hr post-ketamine. Higher doses of ketamine (20 and 50 mg/kg) injection produced robust dissociative stereotypy such as ataxia and head weaving, which were positively correlated with c-Fos immunoreactivity in the cortex of female mice (Nishizawa et al., 2000). Therefore, it is possible that increased c-Fos activity in the PFC of female rats may have been influenced by increased motor activity in the animals.
In conclusion, we found sex-related differences in the effects of IV ketamine infusion on locomotor activity and neuroplasticity-related proteins in multiple brain regions in male and female rats. Compared to male rats, female rats were more sensitive to c-Fos expression in the mPFC and amygdala following an IV ketamine infusion. Moreover, ketamine-induced c-Fos elevation in the mPFC was greater in female rats with high estrogen levels compared to those with low estrogen levels. Better understanding of the molecular mechanisms by which an IV ketamine infusion impacts neuroplasticity-related proteins differently between male and female rodents can facilitate the translation of preclinical findings to clinical research and practice.
CRediT authorship contribution statement
Michael Zhang: Methodology, Data acquisition and analysis, Software, Manuscript writing. Haley Spencer: Sample and data acquisition, Methodology. Rina Berman: Methodology, Manuscript writing. Kennett Radford: Conceptualization, Supervision. Kwang Choi: Conceptualization, Supervision, Manuscript writing.
Declaration of Competing Interest
None. The views expressed in this article are those of the authors and do not reflect official policy or position of the Uniformed Services University of the Health Sciences, the Department of the Navy, the Department of Defense, or the United States Government.
Acknowledgements
This work was financially supported by the TriService Nursing Research Program (TSNRP) - R061947719, USU Intramural Grant - T0613801, and the Center for the Study of Traumatic Stress (CSTS).
References
- Akinfiresoye L., Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology. 2013;230(2):291–298. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry A.E., Adachi M., Nosyreva E., Na E.S., Los M.F., Cheng P.F., Kavalali E.T., Monteggia L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bland S.T., Schmid M.J., Der-Avakian A., Watkins L.R., Spencer R.L., Maier S.F. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051(1–2):90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Carrier N., Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Chen H., Li X., Ma H., Zheng W., Shen X. Reduction in Nesfatin-1 Levels in the cerebrospinal fluid and increased nigrostriatal degeneration following ventricular administration of anti-nesfatin-1 antibody in mice. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.621173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.R., Yan Y.T., Wang T.J., Chen L.J., Wang Y.J., Tseng G.F. Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex. 2009;19(11):2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- D’Souza D., Sadananda M. Estrous cycle phase-dependent changes in anxiety- and depression-like profiles in the late adolescent Wistar-Kyoto rat. Ann. Neurosci. 2017;24(3):136–145. doi: 10.1159/000477151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degasperi A., Birtwistle M.R., Volinsky N., Rauch J., Kolch W., Kholodenko B.N. Evaluating strategies to normalise biological replicates of Western blot data. PLoS One. 2014;9(1):87293. doi: 10.1371/journal.pone.0087293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat A.M., Wright K.N., Strong C.E., Kabbaj M. Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology. 2018;130:30–41. doi: 10.1016/j.neuropharm.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English J.D., Sweatt J.D. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J. Biol. Chem. 1997;272(31):19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Esmaili-Shahzade-Ali-Akbari P., Hosseinzadeh H., Mehri S. Effect of suvorexant on morphine tolerance and dependence in mice: role of NMDA, AMPA, ERK and CREB proteins. Neurotoxicology. 2021;84:64–72. doi: 10.1016/j.neuro.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Fletcher C.V., Acosta E.P., Strykowski J.M. Gender differences in human pharmacokinetics and pharmacodynamics. J. Adolesc. Health. 1994;15(8):619–629. doi: 10.1016/s1054-139x(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Franceschelli A., Sens J., Herchick S., Thelen C., Pitychoutis P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Gallo F.T., Katche C., Morici J.F., Medina J.H., Weisstaub N.V. Immediate early genes, memory and psychiatric disorders: focus on c-Fos, Egr1 and Arc. Frontiers in Behavioral Neuroscience. 2018;12(79):79. doi: 10.3389/fnbeh.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L.S., Comim C.M., Valvassori S.S., Reus G.Z., Barbosa L.M., Andreazza A.C., Stertz L., Fries G.R., Gavioli E.C., Kapczinski F., Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(1):140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Gipson C.D., Olive M.F. Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav. 2017;16(1):101–117. doi: 10.1111/gbb.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti M.J., Ghosal S., LoPresto D., Taylor J.R., Duman R.S. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol. Dis. 2017;100:1–8. doi: 10.1016/j.nbd.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.Z., Benet L.Z., Schwartz J.B. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50(2):222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- Herdegen T., Kovary K., Buhl A., Bravo R., Zimmermann M., Gass P. Basal expression of the inducible transcription factors c-Jun, JunB, JunD, c-Fos, FosB, and Krox-24 in the adult rat brain. J. Comp. Neurol. 1995;354(1):39–56. doi: 10.1002/cne.903540105. [DOI] [PubMed] [Google Scholar]
- Honack D., Loscher W. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 1993;620(1):167–170. doi: 10.1016/0006-8993(93)90287-w. [DOI] [PubMed] [Google Scholar]
- Imre G., Fokkema D.S., Den Boer J.A., Ter Horst G.J. Dose-response characteristics of ketamine effect on locomotion, cognitive function and central neuronal activity. Brain Res. Bull. 2006;69(3):338–345. doi: 10.1016/j.brainresbull.2006.01.010. [DOI] [PubMed] [Google Scholar]
- LeFevre J., McClintock M.K. Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol. Reprod. 1988;38(4):780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Li M., Xie A., Liu Y., Zeng Q., Huang S., Huang Q., Shao T., Chen X., Liao Z., Cai Y., Xiao Z., Zhang X., Shen H. Ketamine Administration leads to learning-memory dysfunction and decreases serum brain-derived neurotrophic factor in rats. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.576135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loikas D., Wettermark B., von Euler M., Bergman U., Schenck-Gustafsson K. Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. BMJ Open. 2013;3(5) doi: 10.1136/bmjopen-2012-002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loikas D., Wettermark B., Von Euler M., Bergman U., Weitoft G.R., Schenck-Gustafsson K. Big gender differences in drug utilization. The underlying disease is an insufficient explanation according to a systematic analysis. Lakartidningen. 2011;108(40):1957–1962. [PubMed] [Google Scholar]
- Mankowitz S.L., Regenberg P., Kaldan J., Cole J.B. Ketamine for rapid sedation of agitated patients in the prehospital and emergency department settings: a systematic review and proportional meta-analysis. J. Emerg. Med. 2018;55(5):670–681. doi: 10.1016/j.jemermed.2018.07.017. [DOI] [PubMed] [Google Scholar]
- McDougall S.A., Park G.I., Ramirez G.I., Gomez V., Adame B.C., Crawford C.A. Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats. Eur. Neuropsychopharmacol. 2019;29(6):740–755. doi: 10.1016/j.euroneuro.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros G.C., Greenstein D., Kadriu B., Yuan P., Park L.T., Gould T.D., Zarate C.A., Jr. Treatment of depression with ketamine does not change plasma levels of brain-derived neurotrophic factor or vascular endothelial growth factor. J. Affect. Disord. 2021;280:136–139. doi: 10.1016/j.jad.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion G., Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci. Ther. 2013;19(6):370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T., Yamaguchi K., Furukawa H., Kameyama T. Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats. Eur. J. Pharmacol. 1984;105(3–4):197–206. doi: 10.1016/0014-2999(84)90610-1. [DOI] [PubMed] [Google Scholar]
- Niesters M., Martini C., Dahan A. Ketamine for chronic pain: risks and benefits. Br. J. Clin. Pharmacol. 2014;77(2):357–367. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa N., Nakao S., Nagata A., Hirose T., Masuzawa M., Shingu K. The effect of ketamine isomers on both mice behavioral responses and c-Fos expression in the posterior cingulate and retrosplenial cortices. Brain Res. 2000;857(1–2):188–192. doi: 10.1016/s0006-8993(99)02426-9. 〈http://www.ncbi.nlm.nih.gov/pubmed/10700567〉 [DOI] [PubMed] [Google Scholar]
- Palenicek T., Fujakova M., Brunovsky M., Balikova M., Horacek J., Gorman I., Tyls F., Tislerova B., Sos P., Bubenikova-Valesova V., Hoschl C., Krajca V. Electroencephalographic spectral and coherence analysis of ketamine in rats: correlation with behavioral effects and pharmacokinetics [Research Support, Non-U.S. Gov’t] Neuropsychobiology. 2011;63(4):202–218. doi: 10.1159/000321803. [DOI] [PubMed] [Google Scholar]
- Pare W.P., Redei E. Sex differences and stress response of WKY rats. Physiol. Behav. 1993;54(6):1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Park S.W., Lee J.G., Seo M.K., Lee C.H., Cho H.Y., Lee B.J., Seol W., Kim Y.H. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int. J. Neuropsychopharmacol. 2014;17(11):1831–1846. doi: 10.1017/S1461145714000534. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford K.D., Berman R.Y., Zhang M., Wu T.J., Choi K.H. Sex-related differences in intravenous ketamine effects on dissociative stereotypy and antinociception in male and female rats. Pharmacol. Biochem. Behav. 2020;199 doi: 10.1016/j.pbb.2020.173042. [DOI] [PubMed] [Google Scholar]
- Radford K.D., Park T.Y., Lee B.H., Moran S., Osborne L.A., Choi K.H. Dose-response characteristics of intravenous ketamine on dissociative stereotypy, locomotion, sensorimotor gating, and nociception in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2017;153:130–140. doi: 10.1016/j.pbb.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Radford K.D., Spencer H.F., Zhang M., Berman R.Y., Girasek Q.L., Choi K.H. Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats. Behav. Brain Res. 2020;378 doi: 10.1016/j.bbr.2019.112259. [DOI] [PubMed] [Google Scholar]
- Saland S.K., Kabbaj M. Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats. J. Pharmacol. Exp. Ther. 2018;367(3):393–404. doi: 10.1124/jpet.118.251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland S.K., Schoepfer K.J., Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner [Research Support, N.I.H., Extramural] Sci. Rep. 2016;6:21322. doi: 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol. Psychiatry. 2016;80(6):448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur L., Neves L.T., Greggio S., Venturin G.T., Jeckel C.M.M., Costa Da Costa J., Bertoldi K., Schallenberger B., Siqueira I.R., Mestriner R.G., Xavier L.L. Ketamine promotes increased freezing behavior in rats with experimental PTSD without changing brain glucose metabolism or BDNF. Neurosci. Lett. 2017;658:6–11. doi: 10.1016/j.neulet.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000;20(21):8177–8187. doi: 10.1523/jneurosci.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk E.S., Viscusi E.R., Buvanendran A., Hurley R.W., Wasan A.D., Narouze S., Bhatia A., Davis F.N., Hooten W.M., Cohen S.P. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 2018;43(5):456–466. doi: 10.1097/AAP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock S.A., Stollenwerk B., Redaelli M., Civello D., Lauterbach K.W. Sex differences in treatment patterns of six chronic diseases: an analysis from the German statutory health insurance. J. Womens Health. 2008;17(3):343–354. doi: 10.1089/jwh.2007.0422. [DOI] [PubMed] [Google Scholar]
- Sun J., Nan G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: a potential therapeutic target. Int. J. Mol. Med. 2017;39(6):1338–1346. doi: 10.3892/ijmm.2017.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen C., Flaherty E., Saurine J., Sens J., Mohamed S., Pitychoutis P.M. Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience. 2019;398:182–192. doi: 10.1016/j.neuroscience.2018.11.053. [DOI] [PubMed] [Google Scholar]
- Van der Heyden J.H., Gisle L., Hesse E., Demarest S., Drieskens S., Tafforeau J. Gender differences in the use of anxiolytics and antidepressants: a population based study. Pharmacoepidemiol. Drug Saf. 2009;18(11):1101–1110. doi: 10.1002/pds.1827. [DOI] [PubMed] [Google Scholar]
- Wilson C., Kercher M., Quinn B., Murphy A., Fiegel C., McLaurin A. Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol. Behav. 2007;91(2–3):202–207. doi: 10.1016/j.physbeh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Wright K.N., Hagarty D.P., Strong C.E., Schoepfer K.J., Kabbaj M. Sex-dependent ketamine addiction-like behavior profile following exposure to chronic mild stress. Chronic Stress. 2019;3 doi: 10.1177/2470547019832613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Fu S., Shi X., Liu R. Microglial BDNF, PI3K, and p-ERK in the spinal cord are suppressed by pulsed radiofrequency on dorsal root ganglion to ease sni-induced neuropathic pain in rats. Pain Res. Manag. 2019;2019 doi: 10.1155/2019/5948686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Hu Y.M., Zhou Z.Q., Zhang G.F., Yang J.J. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J. Med. Sci. 2013;118(1):3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr., Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Radford K.D., Driscoll M., Purnomo S., Kim J., Choi K.H. Effects of subanesthetic intravenous ketamine infusion on neuroplasticity-related proteins in the prefrontal cortex, amygdala, and hippocampus of Sprague-Dawley rats. IBRO Rep. 2019;6:87–94. doi: 10.1016/j.ibror.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang N., Yang C., Li X.M., Zhou Z.Q., Yang J.J. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry. 2014;29(7):419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]