Abstract
Papillon-Lefevre syndrome (PLS) (OMIM: 245000) is a rare autosomal recessive disorder characterized by palmoplantar hyperkeratosis and early onset periodontitis, resulting in the premature loss of the deciduous and permanent teeth. PLS is caused by mutations in the cathepsin C (CTSC) gene (OMIM: 602365), which has been mapped to chromosome 11q14–q21. Genetic analysis can help early and rapid diagnosis of PLS. Here we report on a Chinese PLS pedigree with two affected siblings. We have identified two novel compound heterozygous mutations c.763T>C (p.C255R) and c.1015C>A (p.R339S) in the CTSC gene. The two mutations expand the spectrum of CTSC mutations in PLS.
Keywords: Cathepsin C, Palmoplantar keratoderma, Papillon-Lefevre syndrome, Periodontitis
INTRODUCTION
Papillon-Lefevre syndrome (PLS) (OMIM: 245000) is a rare autosomal recessive disorder characterized by palmoplantar hyperkeratosis and progressive periodontitis, which consequently results in the premature loss of the deciduous and permanent dentitions1. Palmoplantar keratosis, varying from mild psoriasiform scaly skin to overt hyperkeratosis, typically develops within the first three years of life1. The prevalence of PLS is reported to be 1 to 4 cases per million in the general population with no gender preference2. The disease is caused by mutations in the cathepsin C (CTSC) gene which located on chromosome 11q14—q213. CTSC is a lysosomal protease and has an essential role in antibacterial effects and stimulation of cytokines through the activation of serine proteases within polymorphonuclear cells and macrophages4. Despite existing functional abnormalities in neutrophils and natural killer cells of patients with PLS, the systemic immunodeficiency is relatively mild in PLS5. Here we report on a Chinese PLS pedigree with two affected siblings and the identification of two novel mutations in the CTSC gene.
CASE REPORT
Patient 1
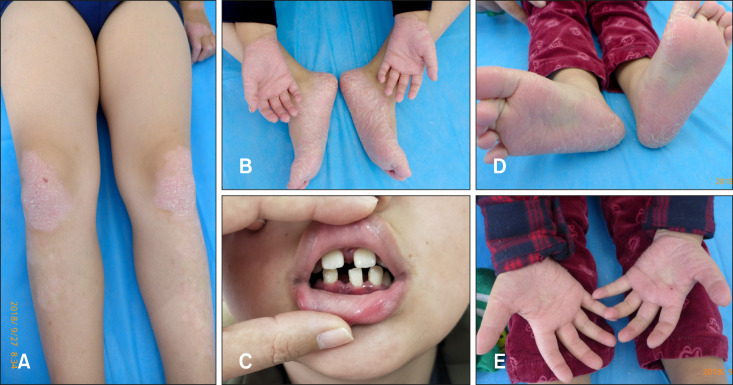
A 9-year-old boy presented with hyperkeratosis on palms and soles at four months after birth, the skin lesions had gradually spread to the knees and elbows. Physical examination revealed bilateral, symmetrical and demarcated hyperkeratotic plaques and fissures on the palms and soles. There were symmetrical psoriasiform lesions on his knees and elbows (Fig. 1A, B). The boy also complained of periodontal problems. Dental history indicated the boy had severe periodontal disease (Fig. 1C), deciduous teeth started to lose since the age of four, and the disease led to all deciduous teeth loss before 9-year-old. Intraoral examination revealed permanent teeth erupted prematurely and most of them were loose, and the gingiva was edematous with formation of periodontal pockets. Dental X-ray (orthopantomogram view) found alveolar bones resorption and affected teeth lacked of osseous support.
Fig. 1. (A) Psoriasiform lesions on knees of patient 1. (B) Hyperkeratosis on palms and soles of patient 1. (C) Dental finding of patient 1. (D) Hyperkeratosis on soles of patient 2. (E) Hyperkeratosis on palms of patient 2.
Patient 2
A 2-year-old boy, the younger brother of the first patient, was also referred to our institution for the similar symptoms as his brother (Fig. 1D, E). Palmoplantar hyperkeratosis started at the age of 3 months. At the present stage, the boy had no obvious periodontal problems or loss of deciduous teeth. His medical history showed he had no serious infectious disease; his unaffected parents were not intermarriage.
Genetic analysis
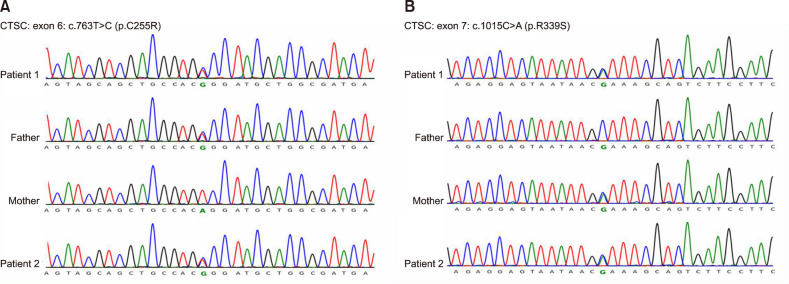
Genomic DNA was isolated from peripheral blood of the two patients and their unaffected parents, and whole-exome sequencing was performed, then all mutations had been verified by direct Sanger sequencing. Genetic analysis discovered novel compound heterozygous missense mutations in the siblings: c.763T>C (p.C255R) in exon 6 (Fig. 2A) and c.1015C>A (p.R339S) in exon 7 (Fig. 2B). Their father and mother were heterozygous for c.763T>C and c.1015C>A, respectively. Both of them were carriers of CTSC gene mutation. We also used the SIFT and Polyphen software to predict the phenotypic effects caused by p.C255R and p.R339S amino acid mutations, which were probably damaging, suggesting that the above heterozygous mutation might affect the normal function of CTSC protein.
Fig. 2. Genetic analysis revealed compound heterozygous mutations including two missense changes in the two patients. Their father and mother were heterozygous for c.763T>C and c.1015C>A, respectively. Both of them were carriers of CTSC gene mutation. (A) c.763T>C in exon 6 (p.C255R). (B) c.1015C>A in exon 7 (p.R339S).
Based on the clinical manifestations and genetic analysis, the diagnosis of PLS was made. The two patients were advised to maintain their oral hygiene. Oral acitretin was prescribed at a dose of 10 mg/day to the elder brother, his skin lesions showed a marked improvement after 1 month. The younger brother was only given topical emollients due to his young age.
We received the patient's consent form about publishing all photographic materials.
DISCUSSION
Nowadays, There were 77 different mutations in the CTSC gene have been reported worldwide (Human Gene Mutation Database), most of them are located in exons 5 to 7, which encode the heavy chain region of the cathepsin C protein and the heavy chain region is important for enzyme activity6,7. What we have reported the p.C255R and p.R339S mutations in the two patients are also located in this region, and we used bioinformatics analysis to predict phenotypic effects that were probably damaging. These mutations led to changes in peptidase C1A and papain C-terminal functional domain, which were supposed to be highly conserved, so we suggested these changes might damage the protein's function and the enzyme activity significantly.
In the past, most commonly, PLS patients were reported to be born to consanguineous parents, correlating with the described mutations are homozygous8. However, in our cases, the two patients are belonging to compound heterozygous missense mutations, their unaffected parents were non-consanguineous. It was reported that 17% of the PLS patients are prone to develop varying degrees of infections from skin pyoderma to fatal infections like multiple abdominal abscesses due to the impairment of immune system. Among them, pyogenic liver abscess is increasingly recognized as a complication of PLS9. In the present cases, the two novel mutations led to typical dental and skin lesions, while the siblings did not have recurrent infections. The mutations c.763T>C (p.C255R) and c.1015C>A (p.R339S) did not show more severe phenotypes than other mutations in the CTSC gene.
It is reported that mutations in the CTSC gene can lead to three closely related conditions, such as PLS, Haim-Munk syndrome and prepubertal periodontitis. Interestingly, Haim-Munk syndrome is characterized by the presence of arachnodactyly, acroosteolysis, and onychogryphosis in addition to palmoplantar keratoderma and periodontitis, while prepubertal periodontitis is usually without skin manifestations2. Phenotypic variants of the CTSC mutations may be affected by other genetic or environmental factors. Further gene mutation investigations are necessary to better clarify the relationship between the genotypes and phenotypes of patients with PLS.
In conclusion, we revealed two novel compound heterozygous mutations c.763T>C (p.C255R) and c.1015C>A (p.R339S). Although the latter mutation occurred in the same location as the identified pathogenic mutation, it had different amino acid missense mutation. The two mutations expand the spectrum of CTSC mutations in PLS.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by CAMS Innovation Fund for Medical Sciences (grant numbers CIFMS-2017-I2M-1-017).
References
- 1.Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23:421–424. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]
- 2.Selvaraju V, Markandaya M, Prasad PV, Sathyan P, Sethuraman G, Srivastava SC, et al. Mutation analysis of the cathepsin C gene in Indian families with Papillon-Lefèvre syndrome. BMC Med Genet. 2003;4:5. doi: 10.1186/1471-2350-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laass MW, Hennies HC, Preis S, Stevens HP, Jung M, Leigh IM, et al. Localisation of a gene for Papillon-Lefèvre syndrome to chromosome 11q14-q21 by homozygosity mapping. Hum Genet. 1997;101:376–382. doi: 10.1007/s004390050645. [DOI] [PubMed] [Google Scholar]
- 4.Wani AA, Devkar N, Patole MS, Shouche YS. Description of two new cathepsin C gene mutations in patients with Papillon-Lefèvre syndrome. J Periodontol. 2006;77:233–237. doi: 10.1902/jop.2006.050124. [DOI] [PubMed] [Google Scholar]
- 5.Roberts H, White P, Dias I, McKaig S, Veeramachaneni R, Thakker N, et al. Characterization of neutrophil function in Papillon-Lefèvre syndrome. J Leukoc Biol. 2016;100:433–444. doi: 10.1189/jlb.5A1015-489R. [DOI] [PubMed] [Google Scholar]
- 6.Nagy N, Vályi P, Csoma Z, Sulák A, Tripolszki K, Farkas K, et al. CTSC and Papillon-Lefèvre syndrome: detection of recurrent mutations in Hungarian patients, a review of published variants and database update. Mol Genet Genomic Med. 2014;2:217–228. doi: 10.1002/mgg3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart PS, Zhang Y, Firatli E, Uygur C, Lotfazar M, Michalec MD, et al. Identification of cathepsin C mutations in ethnically diverse papillon-Lefèvre syndrome patients. J Med Genet. 2000;37:927–932. doi: 10.1136/jmg.37.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ketterer S, Gomez-Auli A, Hillebrand LE, Petrera A, Ketscher A, Reinheckel T. Inherited diseases caused by mutations in cathepsin protease genes. FEBS J. 2017;284:1437–1454. doi: 10.1111/febs.13980. [DOI] [PubMed] [Google Scholar]
- 9.Almuneef M, Al Khenaizan S, Al Ajaji S, Al-Anazi A. Pyogenic liver abscess and Papillon-Lefèvre syndrome: not a rare association. Pediatrics. 2003;111:e85–e88. doi: 10.1542/peds.111.1.e85. [DOI] [PubMed] [Google Scholar]