Abstract
Pembrolizumab, an anti-programmed death-1 specific monoclonal antibody is a second-line treatment for metastatic urothelial carcinoma. Physicians should be aware of adverse immune-related events associated with the use of immune checkpoint inhibitors, particularly adrenocortical insufficiency, which poses a risk of death.
We report a case of secondary adrenocortical insufficiency due to isolated adrenocorticotropic hormone deficiency with empty sella syndrome after pembrolizumab treatment in a patient with metastatic renal pelvic cancer. Fortunately, a therapeutic effect was observed 4 months after discontinuation of pembrolizumab, and a durable antitumor response has persisted for 5 months.
Keywords: Pembrolizumab, Adrenocortical insufficiency, Isolated adrenocorticotropic hormone deficiency, Empty sella syndrome, Immune-related adverse events
Introduction
The prognosis of patients with metastatic urothelial carcinoma (mUC) improved after the optimization of pembrolizumab.1 There are several immune-related adverse events (irAEs), including adrenocortical insufficiency. The rate of adrenocortical insufficiency was reported to be 0.4%.1 Adrenocortical insufficiency is a rare irAE; however, its management requires quick decisions, discontinuation of pembrolizumab, and administration of steroids.2 We report the rare case of a Japanese man with metastatic renal pelvic cancer who exhibited pembrolizumab-related adrenocortical insufficiency due to isolated adrenocorticotropic hormone (ACTH) deficiency (IAD) with empty sella syndrome (ESS).
Case presentation
A 75-year-old Japanese man referred to our hospital complaining hematuria. The patient was diagnosed with non-metastatic left renal pelvis cancer and underwent laparoscopic radical nephroureterectomy. The histological diagnosis was urothelial carcinoma (pT2). Three months after surgery, CT and cystoscopy showed metastasis to the paraaortic lymph nodes, and multifocal bladder cancer. Gemcitabine and cisplatin (GC) were administered as first-line treatment. The paraaortic lymph nodes decreased after 3 courses of GC, but swelled again after 6 courses of GC. Pembrolizumab was administered as a second line treatment.
After 6 courses of pembrolizumab, the patient was referred to our hospital with anorexia. The patient was hospitalized for further examination, and administration of pembrolizumab was discontinued. Two days after administration, the patient experienced disturbance of consciousness with fever, low blood pressure (systolic blood pressure: 90 mmHg), and hypoxemia (saturation of percutaneous oxygen: 88% in room air). Computed tomography (CT) revealed bilateral pleural effusion. The patient was diagnosed with hypoaldosteronism, and hydrocortisone sodium succinate (200 mg/day) was administered intravenously for three days. The patient was then administered oral hydrocortisone, 10 mg in the morning and 5 mg at night. The patient's vital signs and complaints, including anorexia, gradually improved. The initial chemistry panel showed normal free T3 (3.52 pg/ml, normal range: 1.71–3.71 pg/ml), free T4 (0.94 ng/dl, normal range: 0.7–1.48 ng/dl), and thyroid-stimulating hormone (2.24 μIU/ml, normal range: 0.35–4.94 μIU/ml). ACTH was undetectable (<1.5 pg/dl, normal range: 7.2–63.3 pg/dl), as was cortisol (<1.0 μg/dl, normal range: 3.7–19.4 μg/dl), and these results were confirmed three days after starting treatment by outsourcing the examination. Thus, the patient was diagnosed with adrenocortical insufficiency secondary to pembrolizumab administration. Brain magnetic resonance imaging (MRI) revealed atrophy of the anterior lobe of the pituitary (Fig. 1), although this was not noted on brain MRI during a routine health examination when the patient was 64 years old. The patient's cortisol levels reached the normal range, at 15.1 μg/dl, 3 weeks after starting treatment. The patient continued to receive oral hydrocortisone (10 mg in the morning and 5 mg at night), and cortisol levels remained within the normal range.
Fig. 1.
Sagittal view of cranial magnetic resonance imaging; atrophy of the anterior lobe of the pituitary (arrow) is evident.
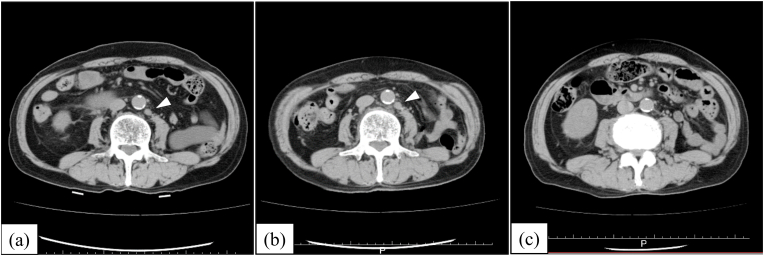
After the diagnosis of hypoaldosteronism and discontinuation of pembrolizumab, the paraaortic lymph nodes continued to swell gradually, but started to decrease 4 months later (Fig. 2). The patient is alive 24 months after diagnosis, and maintains a partial response without drug administration.
Fig. 2.
Horizontal view of a computed tomography scan (a) upon administration of pembrolizumab, (b) upon discontinuation of pembrolizumab, and (c) at 4 months after discontinuing pembrolizumab. The metastatic paraaortic lymph nodes (arrowhead) are visible in (a) and (b). The lesions were not detected in (c).
Discussion
In this case, secondary adrenocortical insufficiency due to ICI-related IAD was diagnosed, and steroid administration improved the patient's prognosis. ICI-related IAD is rare, with a rate of 0.87% in a retrospective cohort study.2 The main IAD treatment is long-term steroid administration.2 Moreover, ESS, which can be primary or secondary, was also observed in this case. Secondary ESS can occur in the pituitary by spontaneous necrosis, by infective, autoimmune, and traumatic causes, or by radiotherapy, drugs, and surgery.3 The patient had no history of brain radiation therapy or intracranial surgery. In addition, ESS was not noted on brain MRI during a prior routine health examination. Although hypophysitis generally exhibits enlargement of the pituitary, later-stage hypophysitis was reported to result in atrophy of the pituitary or in ESS.4 Therefore, we speculated that IAD and atrophy of the pituitary were induced by auto-immune hypophysitis, leading to secondary ESS.
An antitumor effect was observed and prolonged for several months after discontinuing the administration of pembrolizumab due to secondary adrenocortical insufficiency. Evidence regarding the prognosis of patients after discontinuation of immune checkpoint inhibitors is lacking. A study on lung cancer patients treated with nivolumab revealed that prolonged binding of nivolumab was reported more than 20 weeks after the last infusion.5 Similar prolonged effects are expected with pembrolizumab, but the duration of the prolonged antitumor effect was unclear in this case. If recurrence lesions are seen, re-administration of pembrolizumab or other chemotherapy for mUC may be necessary. If pembrolizumab is necessary, regular endocrinological hormone follow-up, especially for the pituitary, should be conducted.
Conclusions
We report a case of secondary adrenocortical insufficiency with empty sella syndrome after pembrolizumab treatment in a patient with metastatic renal pelvic cancer.
Physicians should consider initiating steroid administration when adrenocortical insufficiency is suspected during treatment with immune checkpoint inhibitors. Although this patient needed to continue steroid medication, therapeutic effects were observed, and a durable antitumor response was observed after discontinuation of pembrolizumab.
Consent
Verbal informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Bellmunt J., de Wit R., Vaughn D.J. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Percik R., Shlomai G., Tirosh A. Isolated autoimmune adrenocorticotropic hormone deficiency: from a rare disease to the dominant cause of adrenal insufficiency related to check point inhibitors. Autoimmun Rev. 2020;19(2):102454. doi: 10.1016/j.autrev.2019.102454. [DOI] [PubMed] [Google Scholar]
- 3.De Marinis L., Bonadonna S., Bianchi A., Maira G., Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005;90(9):5471–5477. doi: 10.1210/jc.2005-0288. [DOI] [PubMed] [Google Scholar]
- 4.Chang J., Tran J., Kamel D., Basu A. Nivolumab-induced hypophysitis leading to hypopituitarism and secondary empty sella syndrome in a patient with non-small cell lung cancer. BMJ Case Rep. 2019;12(3) doi: 10.1136/bcr-2018-228135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osa A., Uenami T., Koyama S. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3(19) doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]