Abstract
The current pandemic forced us to introspect and revisit our armamentarium of medicinal agents which could be life‐saving in emergency situations. Oxygen diffusion‐enhancing compounds represent one such class of potential therapeutic agents, particularly in ischemic conditions. As rewarding as the name suggests, these agents, represented by the most advanced and first‐in‐class molecule, trans‐sodium crocetinate (TSC), are the subject of intense clinical investigation, including Phase 1b/2b clinical trials for COVID‐19. Being a successor of a natural product, crocetin, TSC is being investigated for various cancers as a radiosensitizer owing to its oxygen diffusion enhancement capability. The unique properties of TSC make it a promising therapeutic agent for various ailments such as hemorrhagic shock, stroke, heart attack, among others. The present review outlines various (bio)synthetic strategies, pharmacological aspects, clinical overview and potential therapeutic benefits of crocetin and related compounds including TSC. The recent literature focusing on the delivery aspects of these compounds is covered as well to paint the complete picture to the curious reader. Given the potential TSC holds as a first‐in‐class agent, small‐ and/or macromolecular therapeutics based on the core concept of improved oxygen diffusion from blood to the surrounding tissues where it is needed the most, will be developed in future and satisfy the unmet medical need for many diseases and disorders.
Keywords: Crocetin, crocin, glioblastoma multiforme, oxygen diffusion‐enhancing compounds, trans‐sodium crocetinate, TSC
1. INTRODUCTION
The COVID‐19 pandemic caused by the novel coronavirus, SARS‐CoV‐2, is far from getting over. The conflicting reports on disease severity, susceptibility, symptoms, potential treatment, and wary statistics are truly worrisome. One of the most challenging aspects of the COVID‐19 is the 'silent or happy hypoxia'. The ability of the virus to compromise patient's oxygen‐sensing capability until the situation becomes worse, at times beyond control, puzzled the medical fraternity. A recent report diligently questioned the validity of the target oxygen saturation in COVID‐19 patients in view of the indirect evidences from recently concluded studies (Shenoy et al., 2020). The worsening clinical scenario due to the presence of comorbidities such as hypertension, diabetes, cardiovascular disease and others, along with multiorgan involvement leading to failure possibly due to local hypoxemia, hypercoagulability, and/or poorly understood etiological origins are definitive concerns (Zaim et al., 2020). Dhont et al. (2020) religiously reviewed the pathophysiology of the 'silent or happy' hypoxemia in COVID‐19, and presented the disconnect between the severity of the hypoxemia and comparatively milder respiratory discomfort experienced by the patients. Of the several potential therapeutic strategies, supplemental oxygenation is the key to healing of the lungs by improving lung oxygenation mechanics and associated events. Summarily, gas exchange abnormalities coupled with the intravascular thrombi and compromised cortical feedback due to anxiety complicate the clinical presentation and the ensuing treatment such as mechanical ventilation.
Intrigued by the facts and observations related to COVID‐19 hypoxemia, the authors reasoned if somehow the gas‐exchange abnormalities could be reversed and possibly restored, the multiple organ damage would be prevented or minimized. The literature search on 'oxygen diffusion‐enhancing compounds' pointed to an interesting report which described the evaluation of compounds for improved oxygen diffusion in plasma (Kuryel & Akgerman, 1978). The discussion on the theoretical aspects of one‐dimensional mass transport of oxygen from red blood cells (RBCs) to plasma, then through vascular wall followed by interstitial spaces and ultimately to the cells in tissues and organs, highlighted the importance of oxygen diffusion coefficient in plasma. A total of four compounds – procaine (local anesthetic drug, 1, Figure 1), clofibrate (antihyperlipidemic drug, 2), vitamin A (3) and crocetin (natural apocarotinoid acid, 4) were evaluated in vitro using Clark‐type polarographic electrode for measuring the oxygen diffusion coefficient in human plasma under unsteady state. The best compound, clofibrate (2), increased the oxygen diffusion coefficient to an extent of 58.5% (0.125 mg/ml of plasma); other tested compounds were effective as well, at the concentrations used.
FIGURE 1.
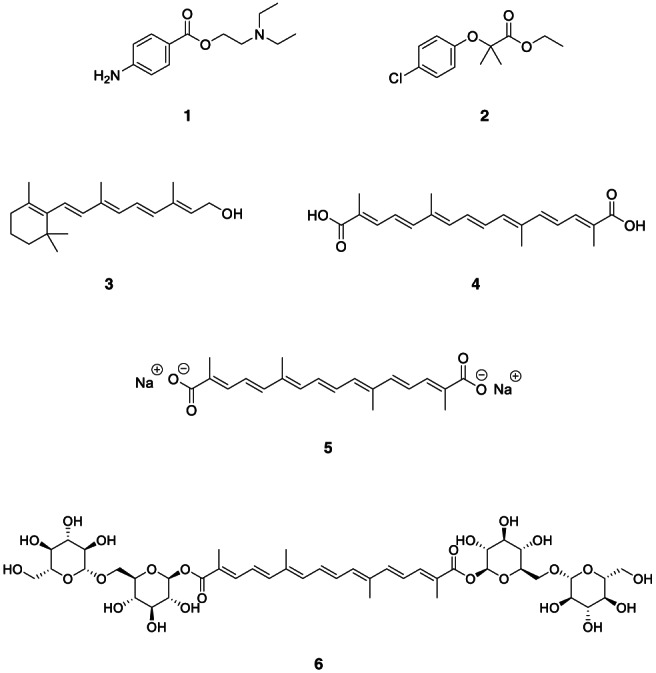
Molecular structures of oxygen diffusion‐enhancing compounds
Interestingly, 1, a carboxylic acid ester, is highly unstable in human plasma (t1/2 = <5 min), which is hydrolyzed to p‐aminobenzoic acid by plasma esterases (Hartman, 2003) Due to its extremely short half‐life, it is reasonable to assume that the observed oxygen diffusion‐ enhancing effect of 1 could arise from its polar acid metabolite. On the similar lines, 2, an ester prodrug, is relatively unstable in plasma (Du et al., 2003), generating its alkanoic acid metabolite. The structural features common to all four molecules, thus, include – (1) a polar group (either —COOH or —CH2OH) capable of forming stronger H‐bond(s) with suitable H‐bond acceptor(s) and (2) structural rigidity imparted by the polyene system in 3 and 4. The precise mechanism(s) of oxygen diffusion improvement by the identified leads was missing from the original report. Few follow‐up articles by the same group delineated the detailed mechanism of increased O2 diffusivity from plasma which involved the physical alteration in the plasma water structure upon addition of the oxygen diffusion‐enhancing compounds (Gainer, 2008). The H‐bonding groups (—COOH/—CH2OH) and the structural rigidity in these molecules have a significant impact on the plasma water structure with direct impact on solute (e.g., O2) diffusivity. Surprisingly, solute diffusivity alterations by physical means has been largely overlooked as seen from dearth of literature reports investigating it.
Further literature search on 4 and its analogs and derivatives as promising oxygen diffusion‐enhancing agents led us to a very recent update featuring trans‐sodium crocetinate (TSC), a disodium salt of 4 (5, Figure 1) as an Investigational New Drug for international phase 1b/2b COVID‐19 clinical program based on (Diffusion Pharmaceuticals, 2020). Additionally, 5 is a clinical candidate for oncology indication (Overview/Trans Sodium Crocetinate (TSC), 2020) (Glioblastoma multiforme – Phase 3; pancreatic cancer and brain metastasis – Phase 1), stroke (Stroke Program, 2020) and related disorders, owing to its ability to enhance oxygen diffusion in various tissues and organs. It has been shown to improve the outcome of radiotherapy due to increased oxygenation of the otherwise hypoxic cancerous tissue being treated. Gainer (2008) have been investigating naturally‐occurring, first‐in‐class agent 4 since 1970s for its unique ability to enhance oxygen diffusion in plasma across various therapeutic categories and indications such as ischemic stroke and haemorrhagic shock. Several reviews and meta‐analyses discussed the pharmacology and therapeutic utility of 4 and related compounds such as 5 and 6 (crocin, crocetin glycoside, Figure 1). The recently proposed indication of 5, that is, COVID‐19, can certainly be the game‐changer. The effective treatment of hypoxia, particularly in acute respiratory distress syndrome (ARDS) could save millions of lives. It is no surprise that this class of agents will open up many more therapeutic opportunities due to their unique ability. Substantial resources will be invested by the global pharmaceutical industry in the discovery of agents capable of enhancing oxygen diffusion in plasma for varied therapeutic end‐points, particularly the ones with unmet medical need.
The present review is aimed at providing the latest updates on the utility of first‐in‐class clinical candidate 5 and related compounds for treating the most intractable and difficult‐to‐treat conditions resulting from cellular oxygen deficiency, or hypoxia. An in‐depth overview on the pharmacological attributes, clinical development, therapeutic indications and (bio)synthetic modalities of these agents is presented. It is going to generate substantial interest in the drug discovery and development community and medical fraternity for the obvious reasons. Successful completion and the desired outcome of the several ongoing clinical trials of 5, including the international phase 1b/2b study for COVID‐19 will certainly endorse the promise offered by this unique class of small‐molecule agents.
1.1. Crocetin: Natural sources
Crocetin (4) is one of the major active ingredients found in saffron (dried stigmas of Crocus sativus L., Family: Iriaceae), along with crocin (6) which is a diester of crocetin with gentobiose, along with various other natural products. Other source of 4 includes fruits of Gardenia jasminoides (Family: Rubiaceae). Both 4 and 6 are responsible for imparting coloration to the saffron and its preparations. The medicinal properties of saffron have been reported in an ancient Chinese compendium in 1956 as a blood vitalizer and calming fright. It has also been used in Tibet as a tonic for the cardiovascular and nervous system. Modern studies showed that 4, along with its derivatives could be useful in the treatment of various diseases such as cancer, coronary heart diseases and hypertension (Xi & Qian, 2006). As mentioned earlier, various investigations demonstrated the effectiveness of 4 in increasing oxygen diffusivity and oxygen consumption by the body tissues (Xi & Qian, 2006). However, saffron is the most expensive spice in the world. The copious amount of water needed, coupled with a difficult irrigation process for saffron cultivation are the key reasons for its abysmally higher cost (Xi & Qian, 2006). Today, 1 kg of saffron costs ~US $200 (estimate). However, 4 has a carotenoid‐like structure, a C20 carbon chain with seven double bonds and a carboxylic acid group at both the termini. This has a direct impact on its aqueous solubility (Gainer, 2000; Lautenschläger et al., 2014; Mir et al., 2020). Thus, solving the solubility issue holds the key to fully optimizing the medicinal utility of this product (Wang et al., 2020). One feasible solution that has been widely reported is using trans‐sodium crocetinate (5). It is a sodium salt of 4. The trans form is supposedly more effective. It is well‐known that the cis form counterbalances the increase in oxygen diffusivity shown by the trans form (Gainer, 2000). Conventionally, 5 is extracted from saffron repeatedly using warm, distilled water. Interested readers are invited to read up on the extraction methods through the scientific literature (Gainer, 2000; Lautenschläger et al., 2014; Mir et al., 2020). The main problem with the conventional extraction method is the use of natural saffron which is quite expensive. Thus, the bio/synthetic methods have been pursued over the years to yield the product of interest due to synthetically‐challenging chemical strategies.
1.2. Approaches to synthetic Crocetin and its intermediates
The literature reports indicated few de novo multi‐step approaches to synthetic 4, 5 and/or related compounds. The all‐trans C20 chain was systematically built using repetitive addition of building blocks mimicking the addition of isoprenoid units in biosynthetic pathways.
1.2.1. Route 1
The retrosynthetic strategy leading to 4 and 5 is shown in Figure 2. Compound 5 was derived from its corresponding di‐ester 18 by alkaline hydrolysis (yield 58–65%), which in turn, was obtained as the double condensation product of C10 di‐aldehyde (15) and C5 phosphorane salt (16) (Frederico et al., 2003). The conversion of C10 di‐ester (13) to 15 involved intermediate reduced product, C10 di‐alkanol (14). The mono condensation of 11 with phosphorane salt (12) yielded 13. Intermediate 16 was derived from tiglic acid (17) in four steps in reasonable yield (32%) (Buchta & Andree, 1960; Duffield & Pettit, 2001; Gainer & Grabiak, 2007; Ingold & Roberts, 1971; Inhoffen et al., 1953; Isler et al., 1957). The di‐ester 18 was purified using silica gel column chromatography to obtain pure all‐trans isomer as a brick‐red solid. The contaminating isomers were removed in the process (Gainer & Grabiak, 2007). The final step, that is, saponification of 18 to generate 5, proved to be cumbersome. Of the several methods tried, MeOH/40% NaOH under reflux conditions gave good results; commercial process may use EtOH/i‐PrOH instead of MeOH. The insolubility of 5 in most NMR solvents then presented difficulty in the structural characterization; HPLC, UV, IR and elemental analysis were used for this purpose (Gainer & Grabiak, 2007; Gainer & Lanz, 2013).
FIGURE 2.
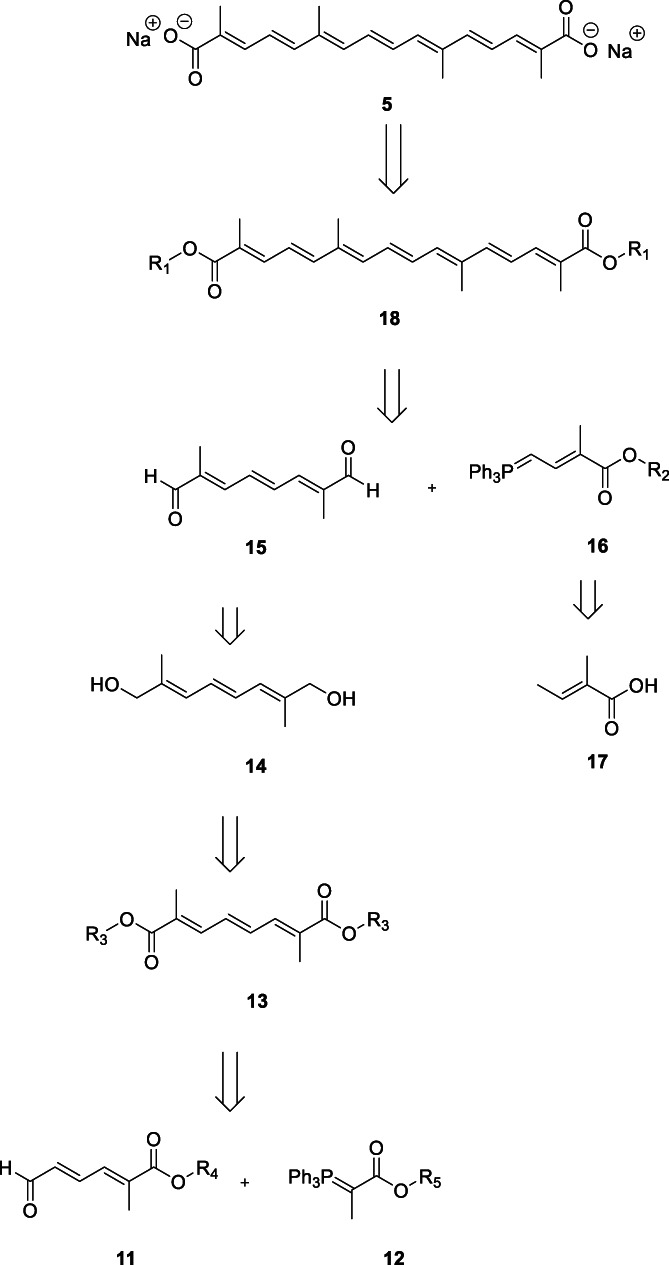
Retrosynthetic strategy leading to compounds 4 and 5
The synthetic scheme leading to major intermediate 11 (Scheme 1) began with the oxidative ring‐opening reaction of furan in presence of Br2/MeOH leading to (E)‐1,1,4,4‐tetramethoxybut‐2‐ene (8), which was further subjected to acidic hydrolysis catalyzed by cation‐exchange sulfonic acid resin, Amberlyst‐15, yielding monoprotected aldehyde (E)‐4,4‐dimethoxybut‐2‐enal (9) (Frederico et al., 2003; Gainer & Grabiak, 2007). Intermediate 9 was combined with the corresponding ylide (Scheme 1) to give 10 in moderate (45%) yield, which was then deprotected to obtain 11. The ylide was generated by treating ethyl bromoacetate with PPh3 and MeI in a four‐step process with 87% yield (Gainer & Grabiak, 2007; Jansen & Lugtenbura, 1994). Overall, the discussed retrosynthetic strategy formed the basis of the first synthetic route leading to 5 (reported in 2003) starting from furan (7, Scheme 1) with overall yield of 1.5% (Frederico et al., 2003). Appreciably large number of synthetic steps, lower yields, higher cost with associated scale‐up issues prompted the development of alternate, more efficient, high‐yielding and environment‐friendly synthetic routes for 5 and its intermediates as well as derivatives.
SCHEME 1.
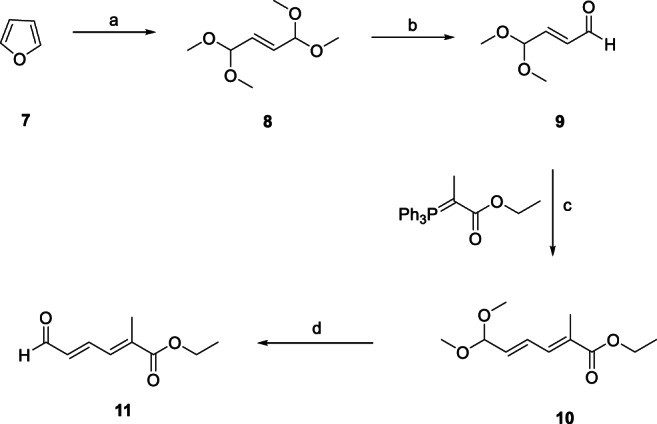
Synthesis of major intermediate 11. aReagents and Conditions. a. Br2/MeOH, Na2CO3 (Yield: 77%); b. Amberlyst‐15, H2O, Acetone (Yield: 72%); c. CH2Cl2 (Yield: 45%); d. Amberlyst‐15, H2O, Acetone (Yield: 42–65%)
The di‐aldehyde 15 (Figure 2), being one of strategically‐important intermediates and supposedly the bottleneck in the synthetic route to 5, due to 16% yield starting from 7 (Scheme 1) was revisited for optimizing the synthesis. Scheme 2 depicts the alternate pathway to obtain 15 starting from 1,4‐dichlorobut‐2‐ene (19), which in turn was generated following chlorination of 1,3‐butadiene (Gainer & Grabiak, 2007), followed by isomerization using FeCl3 or a quaternary salt such as trioctylethylammonium bromide (Gordon, 1974). Condensation of 19 with triethyl phosphite gave tetraethyl but‐2‐ene‐1,4‐diyl(E)‐bis(phosphonate) (20) in high yield (94%). Further reaction of 20 with methyl glyoxal generated intermediate (2E,4E,6E)‐1,1,8,8‐tetramethoxy‐2,7‐dimethylocta‐2,4,6‐triene (21) and its isomers in reasonably higher yield (Babler, 1992; Gainer & Grabiak, 2007). The acidic hydrolysis led to a mixture of desired and undesired isomers of 15, which was reacted with pTSA to convert part of the undesired isomers to the desired ones, thereby modestly improving the yield (59% to 67%) (Gainer & Grabiak, 2007).
SCHEME 2.
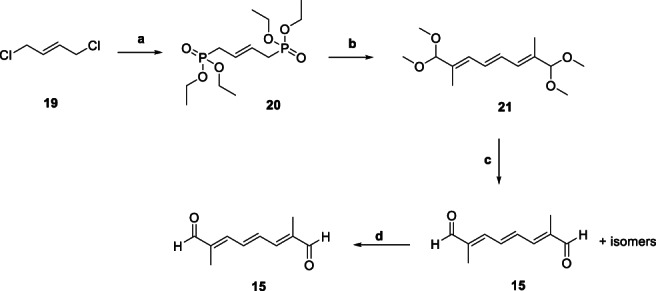
Synthesis of key intermediate 15 (route 1). aReagents and Conditions. a. Triethyl phosphite, 140 °C (Yield: 94%); b. NaOH, K2CO3, toluene/cyclohexane (Yield: 66%); c. AcOH, THF/H2O (Yield: 59%); d. pTSA, 1,4‐dioxane (Yield: 67%)
Alternatively, 15 could be assembled starting from acetaldehyde diethyl acetal (22, Scheme 3), wherein 22 was condensed with (E)‐1‐ethoxyprop‐1‐ene (23) to yield 1,1,3‐triethoxy‐3‐methylbutane (24) in great yield (81–88%); the reaction was catalyzed by Lewis acid such as FeCl3 or AlCl3 under inert atmosphere. In the next step, 24, under pyrolytic conditions aided by catalysts isoquinoline and pTSA, generated (E)‐1‐ethoxy‐2‐methylbuta‐1,3‐diene (25). Further reaction of 25 with EtOH and trichloroisocyanuric acid under phase‐transfer catalytic conditions (PTC: cetyltrimethylammonium bromide) gave (E)‐4‐chloro‐1,1‐diethoxy‐2‐methylbut‐2‐ene (26). Reaction of 26 with PPh3 generated the corresponding phosphonium salt (27), which was reacted with H2O2 to give the condensed intermediate 28. The acid‐catalyzed hydrolysis of 28 yielded 15 in reasonable yield (Chen et al., 2016).
SCHEME 3.
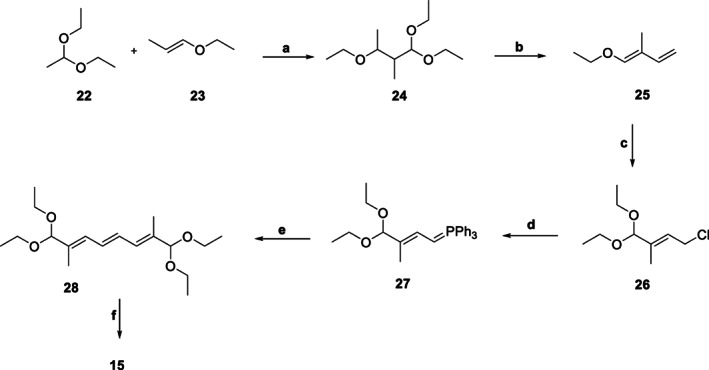
Synthesis of key intermediate 15 (route 2). aReagents and Conditions. a. FeCl3/AlCl3, 0 to −10 °C (Yield: 81%); b. isoquinoline/pTSA 180–220 °C; c. i) EtOH, PTC, KOAc, toluene; ii) trichloroisocyanuric acid, 0–5 °C; iii) NaHCO3; (Yield: 75%); d. PPh3, MeOH (Yield: 99%); e. 0–5 °C, 35% H2O2; f. H+ (Yield: 80%)
The comparative analysis of the two routes leading to 15 highlighted route 2 (Scheme 3) as a clear winner with respect to the individual and overall yields (Chen et al., 2016). The first step in Route 1 (Scheme 2) used environmentally hazardous and costly liq. Br2, which could present challenges on the industrial production side in addition to the cost issue. Overall, the use of environmentally benign starting materials, reagents and intermediates coupled with the relatively higher yields makes Route 2 as the preferred choice. Nonetheless, attempts were made to devise alternative synthetic strategies which could potentially avoid the use of liq. Br2. One such pathway used 2,5‐dimethoxy‐2,5‐dihydrofuran (29, Scheme 3) (obtained by oxidation of furan in MeOH) instead of 7 (Scheme 1) to generate 8 (Scheme 4) (Wegner et al., 2004). The best part of the process is much lesser consumption of MeOH (1:2.4 versus 1:4 29:MeOH) in a continuous process as compared to its batch version (1.5:10 29:MeOH) without compromising the yield (Wegner et al., 2004). Interestingly, 8 could be synthesized in electrochemical oxidation process based on 1,4‐dialkoxy‐1,3‐butadiene (30, Scheme 4) as the KSM along with excess MeOH in the electrolyte solution containing Methyltributylammonium methylsulphate as one of the electrolyte salts (Richter et al., 2007). The environment‐friendly process avoids use of furan as well as liq. Br2 and can be carried out at atmospheric pressure in a continuous fashion using an undivided flow‐through cell, resulting in better yield (62%) of 8. Moreover, a suitable ionic liquid could be used as an electrolyte salt in the process.
SCHEME 4.
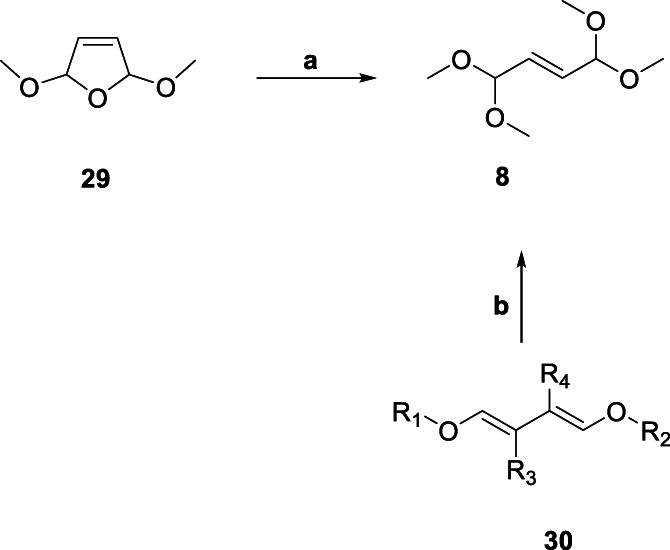
Synthesis of key starting material 8 (route 2). aReagents and Conditions. a. Amberlyst 15/Lewatit S100, 15–30 °C; b. Electrochemical oxidation, MTBS, 1 atm
1.3. Biological production of Crocetin Dialdehyde (31)
As an alternative to synthetic chemistry methods, biological production of crocetin dialdehyde (31, Figure 3) using genetic engineering and recombinant DNA technology approaches offers a great avenue. The key intermediate 31 could be produced in genetically engineered strains of Escherichia coli and plant tissues. The saffron (Crocus sativus) gene CCD2, which encodes for an enzyme that cleaves carotenoids (carotenoid cleavage deoxygenase 2, CCD2, EC 1.14.99.36) could be cloned and then overexpressed in a microorganism such as E. coli or plant tissue such as corn endosperm, both of which accumulated zeaxanthin (32), which was then cleaved to 31 in E. coli and to 4 (Figure 1) in the corn endosperm (Giuliano et al., 2018). The E. coli too must be genetically modified to accumulate zeaxanthin (Li et al., 2015). The gene CCD2 was identified and isolated from the stigmas of saffron and then cloned onto the vector p‐ThioDAN1, which allowed its expression in E. coli when induced from arabinose.
FIGURE 3.
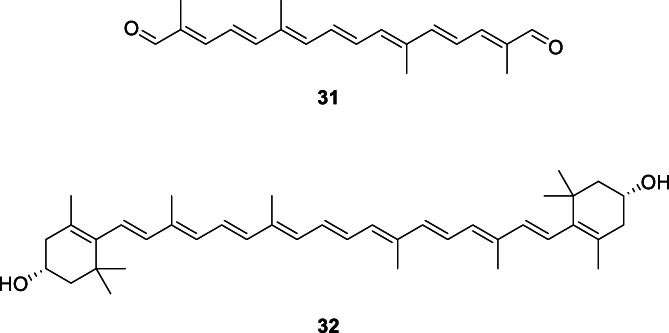
Molecular structures of crocetin dialdehyde and its precursor, zeaxanthin
Very recently, Song et al. (2020) revisited the bioproduction aspects of 4 and reported its overproduction in engineered Saccharomyces cerevisiae starting from glucose. The authors systematically optimized the overproduction and achieved a titer of 12.43 ± 0.62 mg/L for 4 in a 5‐L bioreactor, the highest crocetin titer so far achieved in microorganisms. In a similar study, Liu, Xue, et al. (2020) constructed a stable and temperature‐responsive S. cerevisiae strain for biosynthesis of 4. Multiplex genome integration technology based on CRISPR‐Cas9 was put to use for increasing CCD2 and ALDH gene copy numbers, yielding the final engineered strain (TL009) capable of producing 139.67 ± 2.24 μg/g (dry‐cell‐weight basis) of 4.
Overall, the biological production of 31 or 4 offers several obvious advantages compared to the chemical pathways such as absence of hazardous chemical waste, environmentally benign process, among others. Such processes, once developed and optimized, are preferred to the lengthy, yet low‐yielding chemical synthesis.
1.4. Pharmacological aspects of Crocetin and TSC
1.4.1. Mechanism of action
Crocetin exhibits enhanced oxygen diffusion from plasma owing to its unique ability to alter the water structure physically. Figure 4 shows the schematic representation of crocetin's mechanism of action, which is solely dependent on its ability to make the plasma water more structured via improved H‐bonding (Diffusion Pharmaceuticals TSC MOA, 2020). Understanding the overall oxygen transport to and from the erythrocytes is crucial for delineating the crocetin's mechanism of oxygen diffusion‐enhancing action. At the outset, O2 dissociates from the hemoglobin, crosses the erythrocyte membrane, diffuses through the so‐called transition layer in plasma, vascular wall and the cell membrane of the target cell and finally reaches to the mitochondria (Gainer, 2008). Several studies have demonstrated that the diffusion through the transition layer in the plasma is the biggest hurdle (upto an extent of 70–90%) for O2 transport (Holland et al., 1985; Huxley & Kutchai, 1981). This difficulty encountered by O2 is mainly due to the H‐bonding among the plasma water molecules. The physics behind the diffusivity phenomenon highlights the crucial role of plasma thickness and the solute concentration gradient. Manipulation of the solute (e.g., O2) diffusivity by altering the water structure in plasma using small molecules seemed to be a distant dream for the researchers.
FIGURE 4.
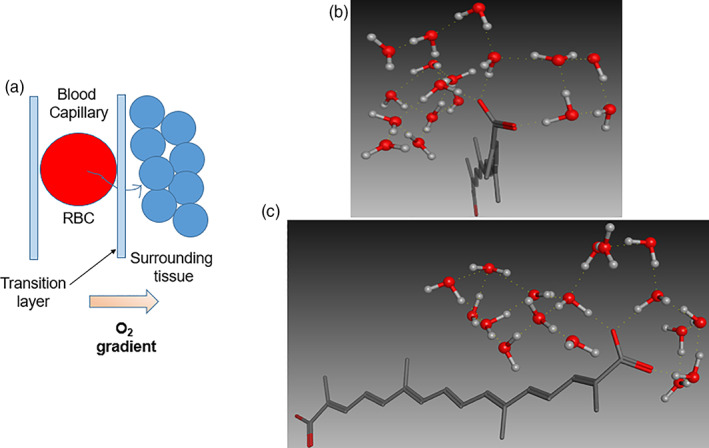
Schematic representation of crocetin's molecular mechanism of oxygen diffusion‐ enhancing action. (a). Diffusion of O2 from erythrocytes through transition layer (plasma), out of vascular wall into the surrounding tissue; (b). Front‐view of ordered water structure surrounding the crocetin molecule (polar heads and hydrophobic interior); (c). Side‐view od ordered water structure surrounding crocetin molecule. Water molecules are shown in element color (ball‐and‐stick model); crocetin is shown in element color (capped stick) with non‐polar H atoms hidden for clarity
Crocetin's unique ability of facilitating the definitive ordering of water molecules in plasma led to substantial reduction in the plasma fluid density, further decreasing the flow resistance. The structurally rigidity and hydrophobicity of crocetin's interior part repel water molecules and the polar heads in turn, create an ordered structure of water molecules via H‐bonding (Figure 4). This process had a direct positive impact on the diffusivity of solutes such as glucose, O2, and others, present in the plasma (Holland et al., 1985). Overall, 4 causes enhanced O2 diffusion by ordering the plasma water structure due to its unique set of structural features.
1.4.2. Crocetin in neurodegenerative disorders
Crocetin and related compounds are known to possess significant free‐radical scavenging activity. This is overtly beneficial in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease as long as the molecule is capable of crossing the blood–brain barrier (BBB). Very recently, Fernández‐Albarral et al. (2020) reviewed the therapeutic potential of saffron and its constituents, particularly 4 and 6 in neurodegenerative retinal diseases. The authors succinctly narrated the pharmacological and therapeutic properties, along with the historical importance of the saffron extract and its constituents, mainly 4 and 6. Interested readers may refer to the cited references therein for thorough overview and in‐depth understanding. Briefly, 6 is converted to 4 in vivo (humans and animals) during intestinal absorption. Surprisingly, owing to its poor binding to human serum albumin despite the acidic character, 4 is distributed widely to bodily tissues including the BBB. In sharp contrast to a popular belief that acids are prevented from entering the CNS, 4 traverses the BBB via passive transcellular diffusion and accumulates in the brain, possibly due to its polyene character and rigid structural framework (crossref. 30). Interestingly, both 4 and 6 demonstrated modulations of amyloidogenic pathway and tau misprocessing in Alzheimer's disease (AD) neuronal cell culture models SH‐SY5Y overexpressing amyloid precursor protein (APP) and PC12 expressing hyperphosphorylated tau (Chalatsa et al., 2019). Iranshahy and Javadi (2019), from a Traditional Persian Medicine (TPM) perspective, extensively reviewed diet therapy for the treatment of AD, which included 4, 6 and saffron, among others.
The antioxidant potential of saffron constituents is of particular interest, for example, 6 exerts multivalent effects at several targets and pathways in context with the cellular redox signaling (Bukhari et al., 2018; Korani et al., 2019). There is a significant overlap in its redox properties and anti‐inflammatory effects. This is not surprising, given the deep involvement of the free‐radicals in several inflammatory pathways. Overall, the neuroprotection offered by the saffron constituents due to their antioxidant capacity, is of immense benefit in the neurodegenerative disorders.
1.5. Crocetin in cancer and other inflammatory disorders
Owing to a long history dating back to 1970s and its unique mode of O2‐diffusion enhancement action, 5 is at the centre stage of clinical development for various cancers. In addition, it is being investigated for improving the outcome of radiotherapy for inoperable cancers such as glioblastoma multiforme (GBM) (Gainer et al., 2017) and other difficult‐to‐treat cancers. Khorasanchi et al. (2018) conducted a non‐systematic literature review of the antitumor potential of saffron and concluded that it exhibited antitumor activity against solid as well as haematologic malignancies. In yet another interesting review, extremely beneficial pharmacological activities of 4 such as increased O2 transport in shock, decreased pro‐inflammatory cytokines, protection against oxidative stress as well as apoptosis induction in cancer cells were revisited (Hashemi & Hosseinzadeh, 2019). On the similar lines, Colapietro et al. (2019) compiled an up‐to‐date overview of the preclinical studies on saffron and its constituents in tumor models and the underlying mechanisms, which included, but not limited to, cell proliferation inhibition, increased cell differentiation, cell cycle progression and cell growth modulation, alterations in tumor metabolism, immune modulation as well as stimulation of cell‐to‐cell communications. Further molecular and biochemical details related to cancer chemoprevention associated with carotenoid intake could be found somewhere else (Milani et al., 2017; Moradzadeh et al., 2018; Moradzadeh et al., 2019; Naeimi et al., 2019; Patel et al., 2017). The anti‐inflammatory and immunoregulatory properties of saffron and its constituents were summarized by Zeinali et al. (2019). The immune pathways, both humoral and cellular, involving interleukins, NF‐κB and associated inflammatory cascades including inducible nitric oxide synthase (iNOS), cyclooxygenase‐2 (COX‐2), myeloperoxidase (MPO), phospholipase A2, and so forth, were thoroughly discussed in context with the beneficial effects of 4, 6 and others.
Colapietro et al. (2020) demonstrated the utility of 4 against GBM, both in vitro and in vivo. The in vitro studies in four GBM cell lines ‐ U251, U87, U138, and U373, demonstrated its antiproliferative and pro‐differentiative actions with definite effects on cell surface and epigenetic markers. In xenograft studies, the effects of treatment with 4 were comparable to the standard‐of‐care therapy, that is, temozolomide, but better than radiotherapy alone. The phase 1/2 study comprising of an open, single arm design (59 patients) strongly supported the beneficial effects of TSC addition during radiation therapy for the treatment of GBM (Gainer et al., 2017).
Zhang et al. (2020) discovered the protective effects of 4 against radiation‐induced injury (RII) in intestinal epithelial cells in vitro (Zhang et al., 2020). This is a desirable activity, particularly in patients suffering for abdominal and/or pelvic cancers; no appropriate treatment exists for RII in such patients. The beneficial effects of 4 were due to its antioxidant, antiapoptotic as well as anti‐inflammatory effects as shown by the authors in rat intestinal epithelial IEC‐6 cells at therapeutically‐achievable dose of 4, that is, 10 μM. This could have a great impact on strategies for mitigating the post‐radiation therapy damage in these patients. In fact, the inherent radiosensitizing ability of 4 could be an added advantage.
In an interesting investigation, 4 was shown to improve Dengue virus (DENV)‐induced liver injury in immunocompetent mouse model of DENV infection with observed liver injury without any significant effect on viral load (Sreekanth et al., 2020). Major inhibitory effects were observed on DENV‐induced apoptosis, pro‐inflammatory cytokine expressions and NF‐κB nuclear translocation. The observation could extrapolate to other pathies of inflammatory origin involving major organs. In fact, Liu, Dong, et al. (2020) clearly showed the attenuation of oxidative stress, inflammation and apoptosis in As2O3‐induced nephrotoxicity in rats. Michael et al. (2020) proved the mitigating effects of 4 in vivo on ischemia reperfusion injury in the renal tissue via potential restoring effects on expression of microRNAs MiR‐127, ‐132, and 21. Crocetin derivatives 33 and 34, previously reported as more soluble versions of 4, with better antiproliferative and anti‐inflammatory activities than 4 are shown in Chart 1 (Wang et al., 2020). Recently, Nalini et al. (2020) reviewed the role of herbal nutraceuticals including 5 as potent and safe therapeutics to battle tumor hypoxia via their effect on the oncogenic hypoxia‐inducible factor 1 (HIF‐1) pathway. The end‐result is the improved outcome of the cancer chemotherapy when used with these nutraceuticals as adjuncts.
CHART 1.
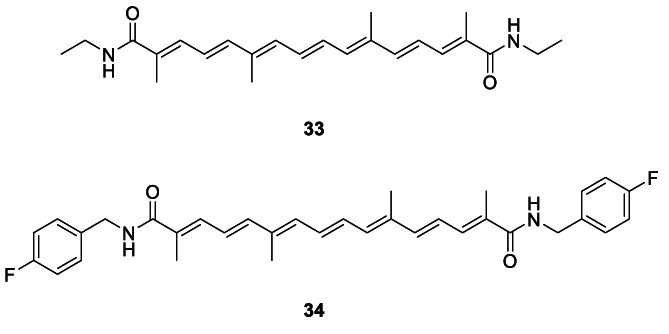
Crocetin derivatives
1.6. Crocetin in metabolic disorders and associated complications
The antioxidant nature of saffron constituents can be exploited for metabolic disorders and underlying consequences. On these lines, Ghaffari and Roshanravan (2019) recently updated the biological properties of saffron including anti‐atherogenic, antihypertensive as well as antihyperlipidaemic. Further intensive efforts are needed to make these agents as a clinical reality for cardiovascular complications. A report by Razavi and Hosseinzadeh (2017) and other related article (Shafiee et al., 2017) attempted to view the therapeutic utility of saffron from the metabolic syndrome perspective and concluded that the antidiabetic and anti‐obesity properties were crucial along with anti‐inflammatory and anti‐oxidant benefits to curb the cluster of disorders, that is, metabolic syndrome, all at once as a preventive or therapeutic modality.
Recently, Heitmar et al. (2019) narrated the utility of saffron in ocular diseases, for example, age‐ related macular degeneration, glaucoma, and diabetic maculopathy, among others, as demonstrated by the systematic clinical investigations. Special attention was devoted to the ensuing molecular and/or pharmacological mechanisms. Analogous clinical studies have confirmed the efficacy and safety of saffron supplementation in human pathologies such as cardiac ischemia besides AD and related macular degeneration (Broadhead et al., 2016; Hatziagapiou et al., 2019). Relating to the present‐day COVID‐19 cardiovascular complications, especially hypoxia‐induced multi‐organ damage as a result of microcoagulopathies, Akbari et al. (2018) provided a glimpse of the regulatory and protective effects of 6 on ischemia/reperfusion (I/R) injury in multiple O2‐dependent organs such as intestine, liver, kidneys, heart and brain. The more water‐soluble salt of 4, that is, TSC (5), was reviewed long ago for its potential to treat hypoxia/ischemia and hemorrhagic shock (Gainer, 2008). Rameshrad et al. (2018) highlighted the importance of 4, 6 and one of the monoterpenoids from saffron, that is, safranal, from various therapeutic standpoints, including hypoxia‐induced organ damage such as the one seen in COVID‐19. Wu et al. (2020) highlighted the importance of 5 in offering protection against cerebral I/R injury (CIRI) by putting brakes on several underlying pathological events. In addition, 5 alleviated CIRI‐induced myocardial oxidative stress and ensuing apoptosis via SIRT3/FOXO3a/SOD2 signaling pathway (Chang et al., 2019).
1.7. Crocetin: Additional indications
Moratalla‐López et al. (2019) reviewed the bioactivity and bioavailability of saffron flower metabolites used as food and cosmetic ingredients and phytopharmaceuticals, including esters of 4, safranal, picrocrocin, kaemferol, delphinidin, among others. The updates on the analytical methods for their identification and quantitation with reference to bioactivity and bioavailability were provided. In a related review, Mykhailenko et al. (2019) provided a comprehensive outlook on bioactive compounds and their pharmacological activities of the Crocus genus. Of particular interest was the coverage of antiparasitic, immunomodulatory, antimicrobial, and antidepressant activities as well as the interspecies differences in the chemical composition and the ensuing alterations in the pharmacological activities.
In an interesting study involving murine models of lens‐induced myopia, Mori et al. (2019) demonstrated the suppression of refractive shift and axial elongation following oral administration of 4. The authors screened a large number of natural products for discovering agent that might control myopia; 4 turned out to be the best via in vitro dose‐dependent activation of early growth response protein 1 (Egr‐1), a myopia suppressive factor. The findings were confirmed by in vivo studies in C57BL/6 J mice, confirming the beneficial effect of 4 against myopia progression.
1.8. Crocetin: Physicochemical, pharmacokinetic, and toxicity aspects
The natural source of 4, that is, saffron, is a coloring agent in food preparations. Daily saffron consumption saffron up to 1.5 g was considered relatively safe. Daily doses beyond 5 g were harmful, while awfully higher doses of 20 g or so proved lethal (Hashemi & Hosseinzadeh, 2019). Oral administration of Crocetin glycoside, 6, was advantageous over its metabolite 4 since it led to the higher serum concentrations of 4 in rats compared to 4 itself (Zhang et al., 2017). Additionally, lower aqueous solubility of 4 (1.238 μg/mL, ~3.7 nM) is a major concern for its utility as a promising therapeutic agent. To overcome the solubility issue of 4, Chu et al. (2018) synthesized its amide prodrugs, which significantly enhanced its solubility (10–70‐fold). The lipophilic amide derivatives were appreciably more active than 4 (3.5–4‐fold) as anticancer and anti‐inflammatory agents without being toxic to the normal cells.
The physicochemical and the pharmacokinetic properties have a significant impact on the formulation aspects of small‐molecule candidates and vice versa. Wong et al. (2020) attempted the trans‐BBB delivery of 4 utilizing γ‐cyclodextrin (γ‐CD) for treating AD owing to its neuroprotective effects (antioxidant and amyloid‐β fibril formation inhibition). The i.v. formulation was based on improved solubility of the nontoxic 4:γ‐CD complex. The pharmacokinetics and biodistribution studies unequivocally demonstrated the BBB permeation of the complex as well as superior activity in cell‐based assays. Overall, the study opened up new avenues for exploitation of the neuroprotective activity of 4 for the treatment of neurodegenerative diseases and disorders. Formulation studies involving nanotherapeutic approaches may offer promising alternatives to the conventional drug delivery strategies.
Recently granted/published patents/patent applications involving use and clinical trials of 5 emphasize its therapeutic potential and clinical use (Table 1) for varied indications. Several of these clinical trials established the safety and efficacy of TSC for varied indications. One of the Phase 2 trials, NCT03763929, investigating TSC efficacy and safety for suspected stroke treatment was halted due to lack of expected enrollment owing to the COVID‐19 pandemic (as per the update on 2020, December 17). The results of previously completed phase 1/2 clinical trial, NCT00725881, centered on safety and efficacy of TSC following intravenous administration, were not posted by the sponsor. On similar lines, Kermani et al. (2017) reported the effects of daily administration of crocin (6, Figure 1) (100 mg/day for 6 weeks) on components of metabolic syndrome in a randomized controlled clinical trial involving 48 patients. The study demonstrated that 6 was well tolerated without any complication during and after 6 weeks of oral administration.
TABLE 1.
Summary of recently granted patents/patent applications involving TSC applications and ongoing/recently concluded clinical trials
| Sr. no. | Title | Patent/NCT no. (patent Grant year) | Details | Reference |
|---|---|---|---|---|
| Patents | ||||
| 1 | Trans carotenoids, their synthesis, formulation and uses | US9950067B2 (2018) | Improvement of O2 diffusivity between RBCs and body tissues | Gainer & Lanz, 2018 |
| 2 | Oral formulations of bipolar trans carotenoids | US10016384B2 (2018) | Drug delivery system for bipolar carotenoids containing carotenoid, cyclodextrin and coating | Gainer & Murray, 2018 |
| 3 | Bipolar trans carotenoid salts and their uses | US9604899B2 (2017) | Methods of making and solubilizing trans carotenoid salt compounds | Gainer & Grabiak, 2017 |
| 4 | Combination of oxidizing agents, photosensitizers and wound healing agents for oral disinfection and treatment of oral diseases |
JP6095710B2 (2017) |
Use of crocetin as radiosensitizer for photodynamic therapy by generating reactive oxygen species upon exposure to light or radiation, e.g. UV or visible light; photocleavage of nucleic acids with an agent | Piergallini & Loupis, 2015 |
| 5 | Compositions and methods for treating cancer with atypical BRaf mutations | AU2018269982 | Use of TSC as a radiosensitizer | Gary et al., 2018 |
| 6 | Use of bipolar trans carotenoids as a pretreatment and in the treatment of peripheral vascular disease | US8901174B2 (2014) | TSC for treatment of a) angina; b) reduction of athero‐sclerotic plaque; c)chronic ocular disease; d) macular degeneration; e) diabetic retinopathy; f) ischemic osteonecrosis or g) peritoneal ischemia | Gainer, 2014 |
| 7 | Diffusion enhancing compounds and their use alone or with thrombolytics | US10130689B2 (2018) | Application of diffusion‐enhancing compounds alone or with thrombolytic agents for the treatment of ischemic disorders (myocardial infarction or stroke) | Gainer, 2018 |
| 8 | Use of bipolar trans carotenoids with chemotherapy and radiotherapy for treatment of cancer | US20190083439A1 | Use of TSC for the treatment of several cancers such as pancreatic and brain cancers | Gainer, 2019 |
| Clinical trials | ||||
| 4 | Efficacy and Safety of Trans Sodium Crocetinate (TSC) for Treatment of Suspected Stroke (PHAST‐TSC) | NCT03763929 |
Recruiting; Assessment of potential efficacy and safety of 5 as early treatment for both ischemic and hemorrhagic stroke when administered while subject is in ambulance being transported to hospital. Outcome: Trial halted due to lack of enrollment owing to pandemic |
NCT03763929, 2020 |
| 5 | Safety and Efficacy Study of Trans Sodium Crocetinate (TSC) in Newly Diagnosed Glioblastoma (GBM) Biopsy‐Only Subjects (INTACT) | NCT03393000 | Active, not recruiting; Open‐label, randomized, controlled, phase 3 safety and efficacy registration trial | NCT03393000, 2020 |
| 6 | Trans Sodium Crocetinate (TSC) Study of Intra‐tumoral Oxygen Concentration, Safety, and Pharmacokinetics in Patients With High Grade Glioma | NCT00826930 | Terminated due to business decision; Evaluation of the impact of 5 on O2 levels in brain tumor tissue in patients with high grade glioma. (Indication: Radiation sensitizer) | NCT00826930, 2020 |
| 7 | Safety and Efficacy of Trans Sodium Crocetinate (TSC) With Radiation and Temozolomide in Newly Diagnosed Glioblastoma | NCT01465347 |
Completed; Evaluation of effect of 5 on survival and tumor response in patients with GBM while establishing an acceptable patient risk profile. Outcome: Beneficial effects on TSC addition to radiotherapy demonstrated |
NCT01465347, 2020 |
| 8 | Safety, Efficacy, and Pharmacokinetics (PK) Study of Trans Sodium Crocetinate (TSC) in Patients With Intermittent Claudication | NCT00725881 |
Completed; Evaluation of safety and PK of multiple, once‐daily, intravenous doses of trans sodium crocetinate (TSC). The effectiveness of TSC in alleviating the symptoms of intermittent claudication (IC) will also be assessed. Outcome: Trial results not posted |
NCT00725881, 2020 |
2. CONCLUSION AND FUTURE PERSPECTIVES
Both 4 and its salt 5 are the subject of intense biological, biochemical, pharmacological, toxicological, biomanufacturing as well as therapeutic investigations lately. The renewed interest in these widely explored and exploited therapeutic entities clearly demonstrate the potential they hold; more so in the current pandemic times. The ultimate use of 5 as a radiosensitizer is particularly more rewarding for inaccessible and inoperable tumors, such as GBM. In addition, the antioxidant potential, ability to cross BBB and accumulate in the brain is so very motivating from the neurodegenerative diseases and disorders perspective, given the lack of promising treatment modalities for such conditions. In future, such an approach based on use of TSC along with radiation therapy, for inoperable and inaccessible solid tumors could bring a radical transformation in oncology. The medical fraternity has waited so long for therapeutic options which could restore the organ functioning post‐ischemia by regularizing or reversing the damage caused by lack of oxygen in the damaged tissue. Extension of therapeutic approaches based on enhanced oxygen diffusion by small molecules will certainly contribute to treat such conditions as ischemic stroke, myocardial infarction and several other cardiovascular complications.
The dangerous precipitation of the so‐called cytokine storm with major contribution from the immune system and the ensuing inflammatory component in conditions, such as ARDS is therapeutically challenging, wherein the body has to deal with multiple attacks from within and outside; even the multiple administered drugs fail to tame it down. The pharmacological utility of 4 and 5 in such conditions requiring antioxidant, anti‐inflammatory as well as antiapoptotic actions, is particularly valuable. These compounds offer a promise to treat COVID‐19 and related diseases including emerging viral infections. The nutraceutical status of 4 is the best part, which in a way, certifies its relatively safer character. The tip of the iceberg is just unraveled. There is much more beneath our current state of knowledge. Further systematic medicinal chemistry and biochemical explorations involving the molecular targets and the associated structural requirements will dictate tailor‐made derivatives or analogs with imparted specialized abilities. The current surge in the biosynthetic and chemical synthetic routes will make it possible, delivering better new chemical entities with defined therapeutic profiles. Crocetin and derivatives are here to stay for a very long time on the horizon of medicinal agents.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors are thankful to Prof. Aniruddha B. Pandit, Vice Chancellor, Institute of Chemical Technology (ICT), for the literature search facilities and encouragement throughout the preparation of the manuscript.
Shah HM, Jain AS, Joshi SV, Kharkar PS. Crocetin and related oxygen diffusion‐enhancing compounds: Review of chemical synthesis, pharmacology, clinical development, and novel therapeutic applications. Drug Dev Res. 2021;82:883–895. 10.1002/ddr.21814
Hriday M. Shah and Ashvi S. Jain contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Akbari, G. , Ali Mard, S. , & Veisi, A. (2018). Comprehensive review on regulatory effects of crocin on ischemia/reperfusion injury in multiple organs. Biomedicine & Pharmacotherapie, 99, 664–670. [DOI] [PubMed] [Google Scholar]
- Babler, J. H. (1992). Method of making 2,7‐dimethyl‐2,4,6‐octatrienedial and derivatives thereof. U.S. Patent 5107030A.
- Broadhead, G. K. , Chang, A. , Grigg, J. , & McCluskey, P. (2016). Efficacy and safety of saffron supplementation: Current clinical findings. Critical Reviews in Food Science and Nutrition, 56(16), 2767–2776. [DOI] [PubMed] [Google Scholar]
- Buchta, E. , & Andree, F. (1960). Eine Totalsynthese des “all”‐trans‐Crocetin‐dimethylesters. Chemische Berichte, 93, 1349–1353. [Google Scholar]
- Bukhari, S. I. , Manzoor, M. , & Dhar, M. K. (2018). A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomedicine & Pharmacotherapy, 98, 733–745. [DOI] [PubMed] [Google Scholar]
- Chalatsa, I. , Arvanitis, D. A. , Koulakiotis, N. S. , Giagini, A. , Skaltsounis, A. L. , Papadopoulou‐Daifoti, Z. , Tsarbopoulos, A. , & Sanoudou, D. (2019). The Crocus sativus compounds trans‐crocin 4 and trans‐crocetin modulate the amyloidogenic pathway and tau misprocessing in Alzheimer disease neuronal cell culture models. Frontiers in Neuroscience, 13, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, G. , Chen, Y. , Zhang, H. , & Zhou, W. (2019). Trans sodium crocetinate alleviates ischemia/reperfusion‐induced myocardial oxidative stress and apoptosis via the SIRT3/FOXO3a/SOD2 signaling pathway. International Immunopharmacology, 71, 361–371. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Lv, C. , Pi. S. , LU, D. , & Ouyang, P. (2016). Method for synthesizing 2,7‐dimethyl‐2,4,6‐octatriene‐1,8‐dialdehyde. European Patent 2799420B1.
- Chu, Y. , Gao, J. , Niu, J. , Huang, Y. F. , Chen, M. , Wang, M. Z. , Shang, Q. , Lu, W. Q. , Peng, L. H. , & Jiang, Z. H. (2018). Synthesis, characterization and inhibitory effects of crocetin derivative compounds in cancer and inflammation. Biomedicine & Pharmacotherapy, 98, 157–164. [DOI] [PubMed] [Google Scholar]
- Colapietro, A. , Mancini, A. , D'Alessandro, A. M. , & Festuccia, C. (2019). Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anticancer Agents in Medicinal Chemistry, 19(1), 38–47. [DOI] [PubMed] [Google Scholar]
- Colapietro, A. , Mancini, A. , Vitale, F. , Martellucci, S. , Angelucci, A. , Llorens, S. , Mattei, V. , Gravina, G. L. , Alonso, G. L. , & Festuccia, C. (2020). Crocetin extracted from saffron shows antitumor effects in models of human glioblastoma. International Journal of Molecular Sciences, 21(2), 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhont, S. , Derom, E. , Van Braeckel, E. , Depuydt, P. , & Lambrecht, B. N. (2020). The pathophysiology of 'happy' hypoxemia in COVID‐19. Respiratory Research, 21(1), 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffusion Pharmaceuticals . Diffusion pharmaceuticals files IND for international phase 1b/2b COVID‐19 clinical program with TSC. (2020). https://investors.-diffusionpharma.com/News/-news-details/2020/Diffusion-Pharma-ceuticals-Files-IND-for-International-Phase-1b2b-COVID-19-Clinical-Program-With-TSC/default.aspx
- Diffusion Pharmaceuticals TSC MOA . (2020) https://youtu.be/lIuHSA2I6sY
- Du, L. , Xu, Y. , & Musson, D. G. (2003). Simultaneous determination of clofibrate and its active metabolite clofibric acid in human plasma by reversed‐phase high‐performance liquid chromatography with ultraviolet absorbance detection. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 794(2), 343–351. [DOI] [PubMed] [Google Scholar]
- Duffield, J. J. , & Pettit, G. R. (2001). Synthesis of (7S,15S)‐ and (7R,15S)‐dolatrienoic acid. Journal of Natural Products, 64(4), 472–479. [DOI] [PubMed] [Google Scholar]
- Fernández‐Albarral, J. A. , de Hoz, R. , Ramírez, A. I. , López‐Cuenca, I. , Salobrar‐García, E. , Pinazo‐Durán, M. D. , Ramírez, J. M. , & Salazar, J. J. (2020). Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regeneration Research, 15(8), 1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederico, D. , Donate, P. M. , Constantino, M. G. , Bronze, E. S. , & Sairre, M. I. (2003). A short and efficient synthesis of crocetin‐dimethyl ester and crocetindial. Journal of Organic Chemistry, 68(23), 9126–9128. [DOI] [PubMed] [Google Scholar]
- Gainer, J. , & Grabiak, R. (2007). Bipolar trans carotenoid salts and their uses. U.S. Patent 20070161610A1.
- Gainer, J. L. (2000). Trans‐sodium crocetinate, methods of making and methods of use thereof. U.S. Patent 6060511A
- Gainer, J. L. (2008). Trans‐sodium crocetinate for treating hypoxia/ischemia. Expert Opinion on Investigational Drugs, 17, 917–924. [DOI] [PubMed] [Google Scholar]
- Gainer, J. L. (2014). Use of bipolar trans carotenoids as a pretreatment and in the treatment of peripheral vascular disease. U.S. Patent 8901174B2.
- Gainer, J. L. (2018). Diffusion enhancing compounds and their use alone or with thrombolytics. U.S. Patent 10130689B2.
- Gainer, J. L. (2019). Use of bipolar trans carotenoids with chemotherapy and radiotherapy for treatment of cancer. U.S. Patent 20190083439A1.
- Gainer, J. L. , & Grabiak, R. C. (2017). Bipolar trans carotenoid salts and their uses. U.S. Patent 9604899B2.
- Gainer, J. L. , & Lanz, M. (2013). Trans carotenoids, their synthesis, formulation and uses. Eur. Patent 2540696A1.
- Gainer, J. L. , & Lanz, M. (2018). Trans carotenoids, their synthesis, formulation and uses. U.S. Patent 9950067B2.
- Gainer, J. L. , & Murray, R. (2018). Oral formulations of bipolar trans carotenoids. U.S. Patent 10016384B2.
- Gainer, J. L. , Sheehan, J. P. , Larner, J. M. , & Jones, D. R. (2017). Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. Journal of Neurosurgery, 126(2), 460–466. [DOI] [PubMed] [Google Scholar]
- Gary, D. , & Dean, W. , Saurabh, S. (2018). Compositions and methods for treating cancer with atypical Braf mutations. Australian Patent 2018269982.
- Ghaffari, S. , & Roshanravan, N. (2019). Saffron; an updated review on biological properties with special focus on cardiovascular effects. Biomedicine & Pharmacotherapy, 109, 21–27. [DOI] [PubMed] [Google Scholar]
- Giuliano, G. , Ferrante, P. , Frusciante, S. , Diretto, G. , Pietrella, M. , & Al‐Babili, S. (2018). Carotenoid dioxygenase and methods for the biotechnological production in microorganisms and plants of compounds derived from saffron. U.S. Patent 9969989B2.
- Gordon, R. (1974). Isomerization of 1,2‐dichloro‐3‐butene to 1,4‐dichloro‐2‐butene. U.S. Patent 3836592A.
- Hartman, D. A. (2003). Determination of the stability of drugs in plasma. Current Protocols in Pharmacology, 7, 6. [DOI] [PubMed] [Google Scholar]
- Hashemi, M. , & Hosseinzadeh, H. (2019). A comprehensive review on biological activities and toxicology of crocetin. Food and Chemical Toxicology, 130, 44–60. [DOI] [PubMed] [Google Scholar]
- Hatziagapiou, K. , Kakouri, E. , Lambrou, G. I. , Bethanis, K. , & Tarantilis, P. A. (2019). Antioxidant properties of Crocus sativus L. and its constituents and relevance to neurodegenerative diseases; focus on Alzheimer's and Parkinson's disease. Current Neuropharmacology, 17(4), 377–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmar, R. , Brown, J. , & Kyrou, I. (2019). Saffron (Crocus sativus L.) in ocular diseases: A narrative review of the existing evidence from clinical studies. Nutrients, 11(3), 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, R. A. , Shibata, H. , Scheid, P. , & Piiper, J. (1985). Kinetics of O2 uptake and release by red cells in stopped‐flow apparatus: Effects of unstirred layers. Respiration Physiology, 59(1), 71–91. [DOI] [PubMed] [Google Scholar]
- Huxley, V. H. , & Kutchai, H. (1981). The effect of red cell membrane and a diffusion boundary layer on the rate of oxygen uptake by human erythrocytes. The Journal of Physiology, 316, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold, K. V. , & Roberts, B. P. (1971). Free radical substitution reactions (p. p22). New York: John Wiley & Sons. [Google Scholar]
- Inhoffen, H. H. , Isler, O. , Bey, G. V. D. , Raspe', G. , Zeller, P. , & Ahrens, R. (1953). Synthesen in der Carotinoid‐Reihe XXVI. Totalsynthese des Crocetin‐dimethylesters. Justus Liebigs Annalen der Chemie, 580, 7–19. [Google Scholar]
- Iranshahy, M. , & Javadi, B. (2019). Diet therapy for the treatment of Alzheimer's disease in view of traditional Persian medicine: A review. Iranian Journal of Basic Medical Sciences, 22(10), 1102–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler, O. , Gutmann, H. , Montavon, M. , Rüegg, R. , Ryser, G. , & Zeller, P. (1957). Synthesen in der carotinoid‐reihe. 10. Mitteilung. Anwendung der Wittig‐reaktion zur synthese von estern des bixins und crocetins. Helvetica, 40, 1242–1249. [Google Scholar]
- Jansen, F. J. H. M. , & Lugtenbura, J. (1994). Synthesis of isotopically labelled carotenoids: Investigations on structure and function of carotenoproteins at the atomic level. Pure & Applied Chemistry, 66, 963–972. [Google Scholar]
- Kermani, T. , Kazemi, T. , Molki, S. , Ilkhani, K. , Sharifzadeh, G. , & Rajabi, O. (2017). The efficacy of crocin of saffron (Crocus sativus L.) on the components of metabolic syndrome: A randomized controlled clinical trial. Journal of Research in Pharmacy Practice, 6(4), 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasanchi, Z. , Shafiee, M. , Kermanshahi, F. , Khazaei, M. , Ryzhikov, M. , Parizadeh, M. R. , Kermanshahi, B. , Ferns, G. , Avan, A. , & Hassanian, S. M. (2018). Crocus sativus a natural food coloring and flavoring has potent anti‐tumor properties. Phytomedicine, 43, 21–27. [DOI] [PubMed] [Google Scholar]
- Korani, S. , Korani, M. , Sathyapalan, T. , & Sahebkar, A. (2019). Therapeutic effects of crocin in autoimmune diseases: A review. BioFactors (Oxford, England), 45(6), 835–843. [DOI] [PubMed] [Google Scholar]
- Kuryel, R. , & Akgerman, A. (1978). Compounds that increase oxygen diffusion in plasma. Atherosclerosis, 29(2), 131–139. [DOI] [PubMed] [Google Scholar]
- Lautenschläger, M. , Lechtenberg, M. , Sendker, J. , & Hensel, A. (2014). Effective isolation protocol for secondary metabolites from saffron: Semi‐preparative scale preparation of crocin‐1 and trans‐crocetin. Fitoterapia, 92, 290–295. [DOI] [PubMed] [Google Scholar]
- Li, X. R. , Tian, G. Q. , Shen, H. J. , & Liu, J. Z. (2015). Metabolic engineering of Escherichia coli to produce zeaxanthin. Journal of Industrial Microbiology & Biotechnology, 42(4), 627–636. [DOI] [PubMed] [Google Scholar]
- Liu, P. , Xue, Y. , Zheng, B. , Liang, Y. , Zhang, J. , Shi, J. , Chu, X. , Han, X. , & Chu, L. (2020). Crocetin attenuates the oxidative stress, inflammation and apoptosis in arsenic trioxide‐induced nephrotoxic rats: Implication of PI3K/AKT pathway. International Immunopharmacology, 88, 106959. [DOI] [PubMed] [Google Scholar]
- Liu, T. , Dong, C. , Qi, M. , Zhang, B. , Huang, L. , Xu, Z. , & Lian, J. (2020). Construction of a stable and temperature‐responsive yeast cell factory for crocetin biosynthesis using CRISPR‐Cas9. Frontiers in Bioengineering and Biotechnology, 8, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael, C. P. , Derpapas, M. , Aravidou, E. , Sofopoulos, M. , Michael, P. , Polydorou, A. , Vezakis, A. , & Fragulidis, G. P. (2020). The carotenoid compound of saffron crocetin alleviates effects of ischemia reperfusion injury via a mechanism possibly involving MiR‐127. Cureus, 12(2), e6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani, A. , Basirnejad, M. , Shahbazi, S. , & Bolhassani, A. (2017). Carotenoids: Biochemistry, pharmacology and treatment. British Journal of Pharmacology, 174(11), 1290–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir, M. A. , Ganai, S. A. , Mansoor, S. , Jan, S. , Mani, P. , Masoodi, K. Z. , Amin, H. , Rehman, M. U. , & Ahmad, P. (2020). Isolation, purification and characterization of naturally derived Crocetin beta‐d‐glucosyl ester from Crocus sativus L. against breast cancer and its binding chemistry with ER‐alpha/HDAC2. Saudi Journal of Biolological Sciences, 27(3), 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradzadeh, M. , Kalani, M. R. , & Avan, A. (2019). The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: A mini review. Journal of Cellular Biochemistry, 120(4), 4732–4738. [DOI] [PubMed] [Google Scholar]
- Moradzadeh, M. , Sadeghnia, H. R. , Tabarraei, A. , & Sahebkar, A. (2018). Anti‐tumor effects of crocetin and related molecular targets. Journal of Cellular Physiology, 233(3), 2170–2182. [DOI] [PubMed] [Google Scholar]
- Moratalla‐López, N. , Bagur, M. J. , Lorenzo, C. , Salinas, M. , & Alonso, G. L. (2019). Bioactivity and bioavailability of the major metabolites of Crocus sativus L. flower. Molecules, 24(15), 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, K. , Kurihara, T. , Miyauchi, M. , Ishida, A. , Jiang, X. , Ikeda, S. I. , Tori, H. , & Tsubota, K. (2019). Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens‐induced myopia. Scientific Reports, 9(1), 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykhailenko, O. , Kovalyov, V. , Goryacha, O. , Ivanauskas, L. , & Georgiyants, V. (2019). Biologically active compounds and pharmacological activities of species of the genus crocus: A review. Phytochemistry, 162, 56–89. [DOI] [PubMed] [Google Scholar]
- Naeimi, M. , Shafiee, M. , Kermanshahi, F. , Khorasanchi, Z. , Khazaei, M. , Ryzhikov, M. , Amir, A. , Gorji, N. , & Hassanian, S. M. (2019). Saffron (Crocus sativus) in the treatment of gastrointestinal cancers: Current findings and potential mechanisms of action. Journal of Cellular Biochemistry, 120(10), 16330–16339. [DOI] [PubMed] [Google Scholar]
- Nalini, D. , Selvaraj, J. , & Kumar, G. S. (2020). Herbal nutraceuticals: Safe and potent therapeutics to battle tumor hypoxia. Journal of Cancer Research and Clinical Oncology, 146(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00725881 . 2020. Safety, efficacy, and pharmacokinetics (PK) study of trans sodium crocetinate (TSC) in patients with intermittent claudication (Diffusion Pharmaceuticals, Inc.).
- NCT00826930 . 2020. Trans sodium crocetinate (TSC) study of intra‐tumoral oxygen concentration, safety, and pharmacokinetics in patients with high grade glioma (Diffusion Pharmaceuticals, Inc.).
- NCT01465347 . 2020. Safety and efficacy of trans sodium crocetinate (TSC) with radiation and temozolomide in newly diagnosed glioblastoma. (Diffusion Pharmaceuticals, Inc).
- NCT03393000 . 2020. Safety and efficacy study of trans sodium crocetinate (TSC) in newly diagnosed glioblastoma (GBM) biopsy‐only subjects (INTACT) (Diffusion Pharmaceuticals, Inc).
- NCT03763929 . 2020. Efficacy and safety of trans sodium crocetinate (TSC) for treatment of suspected stroke (PHAST‐TSC) (Diffusion Pharmaceuticals, Inc).
- Overview/Trans Sodium Crocetinate (TSC) (2020). https://www.diffusion-pharma.com/prod-uct-pipeline/overview-trans-sodium-crocetinate-tsc/
- Patel, S. , Sarwat, M. , & Khan, T. H. (2017). Mechanism behind the anti‐tumour potential of saffron (Crocus sativus L.): The molecular perspective. Critical Reviews in Oncology/Hematology, 115, 27–35. [DOI] [PubMed] [Google Scholar]
- Piergallini, R. , & Loupis, N. (2015). Combination of oxidizing agents, photosensitizers and wound healing agents for oral disinfection and treatment of oral diseases. U.S. Patent 8986746B2.
- Rameshrad, M. , Razavi, B. M. , & Hosseinzadeh, H. (2018). Saffron and its derivatives, crocin, crocetin and safranal: A patent review. Expert Opinion on Therapeutic Patents, 28(2), 147–165. [DOI] [PubMed] [Google Scholar]
- Razavi, B. M. , & Hosseinzadeh, H. (2017). Saffron: A promising natural medicine in the treatment of metabolic syndrome. Journal of the Science of Food and Agriculture, 97(6), 1679–1685. [DOI] [PubMed] [Google Scholar]
- Richter, I. , Puetter, H. , Griesbach, U. , & Gerlach, T. (2007). Process for preparing 1,1,4,4‐tetraalkoxybut‐2‐ene derivatives. Can. Patent 2617556A1.
- Shafiee, M. , Aghili Moghaddam, N. S. , Nosrati, M. , Tousi, M. , Avan, A. , Ryzhikov, M. , Parizadeh, M. R. , Fujji, H. , Rajabian, M. , Bahreyni, A. , Khazaei, M. , & Hassanian, S. M. (2017). Saffron against components of metabolic syndrome: Current status and prospective. Journal of Agricultural and Food Chemistry, 65(50), 10837–10843. [DOI] [PubMed] [Google Scholar]
- Shenoy, N. , Luchtel, R. , & Gulani, P. (2020). Considerations for target oxygen saturation in COVID‐19 patients: Are we under‐shooting? BMC Medicine, 18(1), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, T. , Wu, N. , Wang, C. , Wang, Y. , Chai, F. , Ding, M. , Li, X. , Yao, M. , Xiao, W. , & Yuan, Y. (2020). Crocetin overproduction in engineered Saccharomyces cerevisiae via tuning key enzymes coupled with precursor engineering. Frontiers in Bioengineering and Biotechnology, 8, 578005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekanth, G. P. , Chuncharunee, A. , Yenchitsomanus, P. T. , & Limjindaporn, T. (2020). Crocetin improves dengue virus‐induced liver injury. Viruses, 12(8), 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroke Program . (2020). https://www.diffusionpharma.com/stroke-program/
- Wang, M. Z. , Gao, J. , Chu, Y. , Niu, J. , Chen, M. , Shang, Q. , Pen, L. H. , & Jiang, Z. H. (2020). Synthesis of crocetin derivatives and their potent inhibition in multiple tumor cells proliferation and inflammatory property of macrophage. BMC Complementary Medicine and Therapies, 20(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, C. , Paust, J. , & Ernst, H. (2004). Preparation of 1,1,4,4‐tetramethoxy‐2‐butene. U.S. Patent 6673971B2.
- Wong, K. H. , Xie, Y. , Huang, X. , Kadota, K. , Yao, X. S. , Yu, Y. , Chen, X. , Lu, A. , & Yang, Z. (2020). Delivering crocetin across the blood–brain barrier by using γ‐cyclodextrin to treat Alzheimer's disease. Scientific Reports, 10(1), 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Xiong, X. , Wu, X. , Ye, Y. , Jian, Z. , Zhi, Z. , & Gu, L. (2020). Targeting oxidative stress and inflammation to prevent ischemia–reperfusion injury. Frontiers in Molecular Neuroscience, 13, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, L. , & Qian, Z. (2006). Pharmacological properties of crocetin and crocin (digentobiosyl ester of crocetin) from saffron. Natural Product Communications, 1(1). 10.1177/1934578X0600100112 [DOI] [Google Scholar]
- Zaim, S. , Chong, J. H. , Sankaranarayanan, V. , & Harky, A. (2020). COVID‐19 and multiorgan response. Current Problems in Cardiology, 45(8), 100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinali, M. , Zirak, M. R. , Rezaee, S. A. , Karimi, G. , & Hosseinzadeh, H. (2019). Immunoregulatory and anti‐inflammatory properties of Crocus sativus (saffron) and its main active constituents: A review. Iranian Journal of Basic Medical Sciences, 22(4), 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Chen, K. , Wang, J. , Zheng, Z. , Luo, Y. , Zhou, W. , Liang, J. , Sha, W. , & Chen, H. (2020). Protective effects of crocetin against radiation‐induced injury in intestinal epithelial cells. BioMed Research International, 2020, 2906053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Fei, F. , Zhen, L. , Zhu, X. , Wang, J. , Li, S. , Geng, J. , Sun, R. , Yu, X. , Chen, T. , Feng, S. , Wang, P. , Yang, N. , Zhu, Y. , Huang, J. , Zhao, Y. , Aa, J. , & Wang, G. (2017). Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1044–1045, 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


