Abstract
Introduction
Mechanical bowel preparation (MBP) prior to rectal surgery is widely used. Based on retrospective data many guidelines recommend mechanical and oral antibiotic bowel preparation (MOABP) to reduce postoperative complications and specifically surgical site infections (SSIs). The primary aim of this study is to examine whether MOABP reduces complications of rectal surgery.
Methods and analysis
The MOBILE2 (Mechanical Bowel Preparation and Oral Antibiotics vs Mechanical Bowel Preparation Only Prior Rectal Surgery) trial is a multicentre, double-blinded, parallel group, superiority, randomised controlled trial comparing MOABP to MBP among patients scheduled for rectal surgery with colorectal or coloanal anastomosis. The patients randomised to the MOABP group receive 1 g neomycin and 1 g metronidazole two times on a day prior to surgery and patients randomised to the MBP group receive identical placebo. Based on power calculations, 604 patients will be enrolled in the study. The primary outcome is Comprehensive Complication Index within 30 days after surgery. Secondary outcomes are SSIs within 30 days after surgery, the number and classification of anastomosis dehiscences, the length of hospital stay, mortality within 90 days after surgery and the number of patients who received adjuvant treatment if needed. Tertiary outcomes are overall survival, disease-specific survival, recurrence-free survival and difference in quality-of-life before and 1 year after surgery. In addition, the microbiota differences in colon mucosa are analysed.
Ethics and dissemination
The Ethics Committee of Helsinki University Hospital approved the study. The findings will be disseminated in peer-reviewed academic journals.
Trial registration number
Keywords: colorectal surgery, surgery, gastrointestinal tumours
Strengths and limitations of this study.
To our knowledge, there are no large prospective trials among patients with rectal resection and colorectal/coloanal anastomosis comparing mechanical and oral antibiotic bowel preparation with mechanical bowel preparation only.
Appropriate subgroup analyses have been planned according to neoadjuvant treatment and height of the anastomoses.
Routine postoperative abdominal imaging with per rectal contrast medium or sigmoidoscopy will be performed for all patients to ensure the healing of anastomosis.
Analyses of microbiota will provide more data of effects of antibiotics on different bacteria in the colonic mucosa and lumen. Microbiota characteristics will be correlated with the clinical data to link specific bacteria with the outcomes.
The multicentre prospective study setting with double-blinding and randomisation will minimise the potential bias associated with previous retrospective studies.
Introduction
There is a long history of research into the benefits of preparing the bowel before colorectal surgery.1 Decades ago, mechanical bowel preparation (MBP) was thought to reduce surgical site infections (SSIs) and the risk of anastomotic leakage. However, later evidence showed that cleansing of the bowel does not, in itself, reduce the quantity of bacteria in the intestinal mucosa.2 Thus it has been hypothesised that providing patient oral antibiotics could be beneficial in combination or after MBP, which would reduce the number of bacteria in the intestinal mucosa. The theory proposes that the cleansing of the bowel simply allows the antibiotics to reach the mucosa.2 As early as 1973, Nichols et al published their small prospective, randomised controlled trial (RCT) comprising only 20 patients comparing MBP to MBP with oral antibiotics (mechanical and oral antibiotic bowel preparation, MOABP).3 They focused mainly on microbial changes inside the bowel and there was no significant reduction of SSIs in the MOABP group.3 Furthermore, another small prospective randomised trial from the 1970s studied MOABP.4 It found MOABP to be beneficial in terms of SSI, but intravenous antibiotic prophylaxis was not used, and the rate of SSIs in the control group was 43% which is not in line with contemporary numbers.4 A RCT containing mainly rectal resections (70%) reported that there were significantly less SSIs in patients undergoing MOABP (oral neomycin and metronidazole) than MBP alone.5 Ever since, studies questioning the benefits of cleansing the bowel have been published.6 Until quite recently, several extensive retrospective series focusing on colorectal surgery have been published. In those studies, patients who underwent MOABP have been compared with patients who received no bowel preparation (NBP) before surgery. These studies have shown a significant difference in favour of the patients who received MOABP.7–10 According to an earlier meta-analysis, MOABP compared with NBP reduced SSIs, but not overall complications or anastomotic leakages.11 Another meta-analysis included only RCTs, but since the RCTs comparing MOABP to NBP did not exist at the time, they performed an indirect comparison of these two and showed by network meta-analysis that MOABP reduced the rates of SSI compared with NBP.12
In the modern era with enhanced recovery after surgery (ERAS) protocols, there has not been RCTs until recently, as the MOBILE trial was published.13 In contrast to retrospective series, no difference was found between MOABP and NBP among patients undergoing colon resection in terms of SSIs or overall morbidity.13 Another recent study, the ORALEV trial among patients undergoing colon surgery, found a significant reduction in SSI rates in those receiving oral antibiotics comparing to patients not receiving oral antibiotics. However, no MBP was used in either group in the ORALEV trial.14
For rectal surgery, the situation is different; the risk of leakage is higher for low colorectal and coloanal anastomoses, and on the other hand, a protective ostomy is often used. Usually, if protective ostomy is anticipated, MBP has been widely used to clean the section of bowel between the stoma and the anastomosis. At one point, a Cochrane review concluded that MBP offers no benefit in rectal surgery either.15 However, the GRECCAR study group showed that MBP reduces infectious complications after rectal surgery (34% vs 16%).16 The American Society of Colon and Rectal Surgeons updated their recommendation in 2019; MOABP is typically recommended also for elective rectal resections (Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B).17 The ERAS society concluded that the quality of evidence is low, yet recommending oral antibiotics in addition to MBP, and furthermore concluding that oral antibiotics should not be given if MBP is not performed.18 There are no high-quality RCTs comparing MOABP to MBP including only patients undergoing rectal surgery.
This multicentre prospective randomised MOBILE2 trial compares MOABP to MBP in patients undergoing rectal surgery. The aim of the study is to examine if oral antibiotics reduce the overall complications, SSIs or anastomotic leakages after rectal surgery, and also if there are any adverse effects related to the oral antibiotics.
Methods and analysis
Study design
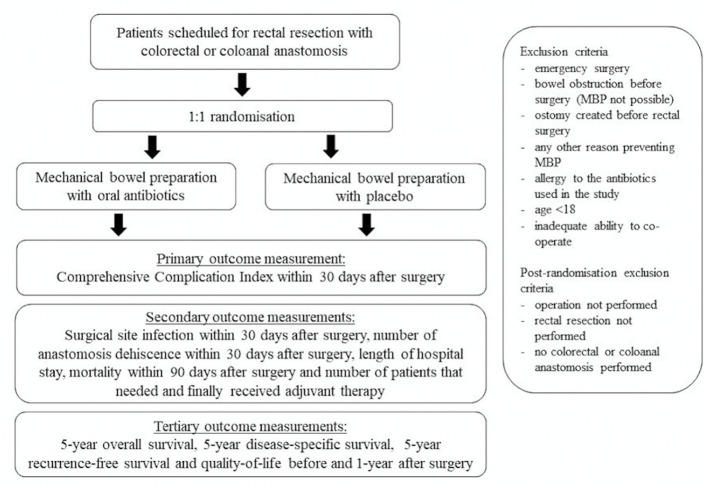
The MOBILE2 trial is a multicentre, double-blinded, parallel group, randomised superiority trial comparing MOABP with MBP in patients with rectal cancer scheduled for anterior rectal resection with planned colorectal or coloanal anastomosis. The study is carried out in three Finnish university hospitals (Helsinki, Turku and Tampere University Hospitals) with an option to include more centres along the trial. A flowchart of the trial is shown in figure 1.
Figure 1.
Flowchart of patient recruitment and randomisation. MBP, mechanical bowel preparation.
Inclusion criteria
Patients scheduled for an anterior resection (resection of the rectum and performing colorectal or coloanal anastomosis) due to a rectal tumour are eligible for the study. Tumours 15 cm or less from anal verge at MRI are considered as rectal tumours.
Exclusion criteria
The exclusion criteria are: (1) emergency surgery, (2) bowel obstruction before the surgery (MBP not possible), (3) ostomy created before the rectal surgery, (4) any other reason preventing MBP, (5) allergy to the antibiotics used in the study, (6) age <18 or (7) inadequate ability to cooperate. Postrandomisation exclusion criteria (patients will be excluded from the analysis if even one of these conditions is met): (1) the surgery was not performed, (2) the rectal resection was not performed or (3) colorectal/coloanal anastomosis was not created (eg, an end colostomy was performed instead).
Trial intervention
MOABP group: bowel preparation using MBP and oral antibiotics.
MBP group: Bowel preparation using MBP and placebo.
The MBP is performed using polyethylene glycol (PEG). The patients drink 2 L of PEG and 1 L of clear fluids of the patient’s choice. The MBP can be started 2 days before the surgery at 15:00 and must be completed by 15:00 on a day prior to the surgery. Thereafter, the patients take 1 g of neomycin or placebo orally at 15:00 and 23:00 and 1 g of metronidazole or placebo orally at 15:00 and 23:00. Patients receive perioperative prophylactic intravenous antibiotics (cefuroxime 1.5 g, metronidazole 500 mg) approximately 1 hour before surgery. The intravenous antibiotics are repeated if surgery is still ongoing 3 hours after the first intravenous dose. Diverting ostomy is used in cases of low colorectal or coloanal anastomosis (<6 cm from anal verge) or if there is leakage in the intraoperative air leak test or some other reason why surgeon considers diverting ostomy necessary.
Microbiota analysis
Faecal samples as well as biopsies of the colonic mucosa are collected from the patients randomised at Helsinki University Hospital. Samples are taken at the planning visit, at the surgery and 6 and 12 months after the surgery. Biopsies are collected directly in RNALater solution and stored at −20°C and faecal samples are stored at –80°C until analysis. The intestinal microbiota analyses are performed with established protocols in use in the Human Microbiome Research Program, University of Helsinki, as described previously.19 The primary analysis method is high-throughput sequencing of the 16S rRNA gene amplicons to assess the taxonomic composition and diversity of bacterial population. Based on this information, a more detailed analysis by using metagenomic sequencing can be considered.
Randomisation procedure, stratification and masking
The patients are individually randomised 1:1 to MOABP group and MBP group. The randomisation sequence is generated with a computer using variable block size. Based on the randomisation sequence, antibiotics or placebo are packed at Helsinki University Hospital’s pharmacy’s clinical trials unit in numerically numbered vials, which are grouped based on stratification. No person outside the pharmacy’s clinical trial unit has access to randomisation sequence or knows whether the vials contain placebo or antibiotics. Pharmacy’s clinical trial unit is not involved in the trial, except for producing and packing of the study medicine and placebo. Placebos are identical to oral antibiotics. The surgeon responsible for operation (or if absent, another surgeon at the unit) will obtain the informed consent. Allocation will take place at the preoperative appointment, after the patient has given their written consent to participate in the study (model consent form in Finnish: online supplemental appendix 1). The patient is allocated by giving a vial from correct stratification group to the patient in numerical order.
bmjopen-2021-051269supp001.pdf (75.5KB, pdf)
The patient population is stratified for centre as well as according to the distance of the lower edge of the tumour from the anal verge (measured from rectal MRI) and the preoperative treatment they receive. Four different stratification groups are created for each participating centre: group A—<10 cm from anal verge, no preoperative treatment or short course radiotherapy (SCRT) with immediate surgery; group B—<10 cm from anal verge, long course chemoradiotherapy (LCCRT) or SCRT with long waiting time; group C—≥10 cm from anal verge, no preoperative treatment or SCRT with immediate surgery and group D—≥10 cm from anal verge, LCCRT or SCRT with long waiting time.
The study is double-blinded. Patients, treating physicians, ward nurses, data collectors and outcome analysers are blinded to the allocated intervention. Once all the data have been collected and is in the analysis phase, patients will be sorted to groups A and B, but these will not yet be connected to whether the patient received a placebo or antibiotics. Only after the results have been analysed for the main and secondary outcomes will the blinding be completely eliminated. The patients undergo MBP and take their medication either at the ward or at home, according to the nurses’ instructions. Emergency envelopes will be stored at the ward, at a specific marked location, in case information about which medicine the patient has received is suddenly needed during treatment.
Outcomes
The primary outcome is Comprehensive Complication Index (CCI) within 30 days after surgery.20 The secondary outcomes are (1) SSI within 30 days after surgery (according to the Centers for Disease and Control and Prevention criteria),21 including superficial incisional infection, deep incisional infection and organ/space infection, (2) the number and classification of anastomosis dehiscence within 30 days of procedure, (3) the length of hospital stay, (4) mortality within 90 days after surgery (any cause) and (5) the number of patients who received adjuvant treatment divided by the number of patients that needed it within 6 months of the procedure. Adjuvant therapy indications follow the national recommendation of colorectal cancer treatment.22 Tertiary outcomes (long-term follow-up) are (1) 5-year overall survival, (2) 5-year disease-specific survival, (3) 5-year recurrence-free survival and (4) difference in quality-of-life questionnaire (36-Item Short Form Survey (SF-36), Quality of Life Questionnaire Core 30 (QLQ-C30), Quality of Life Questionnaire Colorectal 29 (QLQ-CR29), Low Anterior Resection Syndrome (LARS) scores before the surgery and 1 year after the surgery). Potential adverse effects of antibiotics (diarrhoea, Clostridium difficile infections or allergic reactions) will be collected.
Sample size calculation
In Helsinki University Hospital, the number of leakages/abscesses related to low anterior resections performed in 2005–2011 was 12.8%, but minor wound complications were not reported.23 In our previous randomised MOBILE study comparing MOABP to NBP, patients undergoing colon resection had leakage/abscess rate of approximately 6%–8% and a CCI of 9–10 with SD 13–16.13 Based on these figures, we estimate that the CCI is higher in rectal surgery than in colon surgery and the SD may also be higher. Sample size was calculated with the aim of showing a difference of 5 CCI points between the groups (hypothesis: 12.5 points in the MOABP group, 17.5 points in the MBP group). The SD is estimated to be 18 in both groups. With a power of 90% and a margin of error of 5%, 574 patients need to be recruited (Willcoxon-Mann-Whitney test). About 5% of patients are estimated to become lost to follow-up, resulting in a final sample size of 604 patients.
Follow-Up
Normal postrectal surgery follow-up will be scheduled. At 6–8 weeks after surgery, the patients will have an abdominal imaging with per rectal contrast medium or, alternatively, a sigmoidoscopy. At this time, it is checked whether the patient has experienced any SSIs, other complications, reoperations or died. With regard to long-term outcomes (tertiary outcomes), recurrences are checked from patient records and, if necessary, by ordering patient records from other hospitals, and furthermore the patient can be contacted by letter or telephone if needed. Patients will also be asked to complete quality-of-life questionnaires (SF-36, QLQ-C30, QLQ-CR29) before surgery and at 1-year after surgery.
Data collection and statistical analysis plan
Data will be collected by using paper case report forms by the colorectal surgeons responsible for patient care. The data for these forms are manually transferred to electronical form. Only the study personnel will have access to final trial dataset. The study personnel have signed the confidentiality agreement of study documents. Statistical analyses are made with SPSS software. The primary outcome will be analysed using either the Mann-Whitney U test or the t-test, bootstrapped or log-transformed if necessary. Secondary outcomes will be analysed using either the t-test or the Mann-Whitney U test for continuous variables, depending on the distribution, and using χ2 or Fisher’s exact test for categorical variables. If necessary, log-transformation can be performed on non-normally distributed continuous variables, or a bootstrapped t-test can be used. A more detailed classification of SSIs will be reported (superficial, deep, organ/space). Tertiary outcomes will be analysed separately at a later date, when at least 1-year (for QOL) or 5-year follow-up (survival analyses) is available for all patients. Kaplan-Meier and the log-rank test will be used for survival analyses.
The following subgroup analyses will be performed for primary outcome and first secondary outcome (SSIs within 30 days): (1) tumour location (lower edge < or ≥10 cm from anal verge, based on pre-op MRI), (2) long-term chemoradiotherapy before the surgery (yes/no), (3) protective ostomy (yes/no) and (4) surgical approach (minimally-invasive/open surgery).
Schedule
The treatment of rectal cancers in Southern Finland has been centralised to Helsinki University Hospital. About 300 rectal cancer surgeries take place at the centre annually. Other research locations include Turku University Hospital and Tampere University Hospital. At least 2/3 of the rectal cancer operations are performed with primary anastomosis. Thus approximately 300 patients will be recruited annually and the estimated time for recruitment is 2 years. The recruitment started on March 2020. After completing the recruitment of patients, the data will be analysed and reported in international peer-reviewed journal by the study team.
Patient and public involvement
Patients were not involved in the design of the study or assessment of the burden of the interventions. On recruitment, patients are informed of the current knowledge on bowel preparation prior rectal resection. The risks and benefits of the trial intervention are explained to the patients.
Ethics and dissemination
The study will be conducted in accordance with the principles of Declaration of Helsinki and ‘good clinical practice’ certification is demanded for all the study personnel. The research is defined as clinical drug trial. The research plan has been evaluated by the Finnish National Committee on Medical Research Ethics (TUKIJA) and Finnish Medicines Agency (FIMEA) has been notified. The EUDRA CT number for the clinical drug trials has been applied (No 2018-004355-20). The research plan was further approved by the local ethics committee of Helsinki University Hospital and in each participating centres’ institutional review board (Helsinki University Hospital, Tampere University Hospital and Turku University Hospital). The trial has been registered at ClinicalTrials.gov (NCT04281667) before its initiation. The study is monitored by Clinical Research Institute HUCH (HYKS Instituutti). Monitoring is performed in accordance with currently valid rules and regulations, Good Clinical Practice.
Discussion
While American Society of Colon and Rectum Surgeons recommends using MOABP for all colorectal operations, there is no randomised controlled evidence supporting the recommendation.17 In fact, in 2017 the European Society of Coloproctology collaborating group found out that only 17% of a large cohort of patients with colorectal cancer mainly in Europe received MOABP before colorectal resection.24 Another survey from Europe found out that MOABP was used in 6%–19% of the cases by European surgeon responders,25 while nearly 80% of surgeons in the USA reported routine use of MOABP.26
In the retrospective analysis of the 2015 American College of Surgeons National Surgical Quality Improvement Program database comparing the four strategies in (MOABP, oral antibiotics alone, MBP alone, no preparation), MOABP was associated with the greatest risk reduction in SSIs and anastomotic leak when used prior to elective left-sided restorative colorectal surgery with pelvic anastomosis with and without faecal diversion in both laparoscopic and open setting.12
In a Canadian retrospective study of consecutive series of patients undergoing rectal surgery, MOABP with neomycin and metronidazole was associated with a significant reduction in superficial SSIs but not in organ/ space infections.27
After commencing the MOBILE2 trial, a trial from Russia was published, where patients undergoing rectal surgery were randomised to MOABP or MBP. The study reported a statistical difference in in SSIs favouring MOABP, but the study included only 116 patients, metronidazole was not included in the intravenous antibiotic prophylaxis, the study was not blinded and patients with no bowel anastomosis were included.28
The SELECT trial compared prospectively selective decontamination of digestive tract (SDD) with an oral suspension containing amphotericin B, colistin sulphate and tobramycin to no decontamination.29 The patients with left colectomy received MBP, but patients undergoing right colectomy did not. Overall rate of infectious complications was smaller in SDD group, but no difference was noted in the anastomotic dehiscence rates. The results were not reported according to the side of colectomy, and it remains unclear whether the SDD affects right-sided versus left-sided colectomies uniformly. By contrast the subgroup analysis of MOBILE trial reported no difference in SSIs either right or left colectomy.30
Although being a large multicentre trial, the study is limited by its sample size, which may not be sufficient for detecting subtle differences in the outcomes or in subgroup analyses. The study also includes both open and laparoscopic approaches, and results might not be generalisable to other centres where this ratio differs from the trial significantly. On the other hand, this is a double-blinded placebo-controlled multicenter randomised trial providing the highest level of evidence on the subject.
There is a similar, although smaller, RCT to the MOBILE2 recruiting at the moment in France (PREPACOL2, NCT03491540) and we are also looking forward to their results in the future.
Finally, there seems to be many retrospective analyses, meta-analyses of the retrospective series, expert opinions and recommendations, but little high-quality evidence on the role of oral antibiotic preparation before rectal surgery.
Supplementary Material
Footnotes
Twitter: @LauraKoskenvuo, @CHaapamaki
Contributors: LK, PL, PV, MH, RS, CH, AL and VS have contributed to the design of the trial protocol. The protocol was written by LK and VS and it was critically revised and accepted by all authors.
Funding: This work was supported by Cancer Society of Finland. Open access funded by Helsinki University Library.
Competing interests: LK reports grants from Mary and Georg Ehrnrooth’s foundation and Cancer Foundation Finland. VS reports grants from Helsinki University Hospital Research grants, Academy of Finland, Cancer Society of Finland, Mary and Georg Ehrnroot’s foundation, Vatsatautien tutkimussäätiö and Finska Läkaresällskapet. RS reports grants from Academy of Finland, Sigrid Juselius Foundation and Novo Nordisk Foundation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Poth EJ. Historical development of intestinal antisepsis. World J Surg 1982;6:153–9. 10.1007/BF01654682 [DOI] [PubMed] [Google Scholar]
- 2.Fry DE. Colon preparation and surgical site infection. Am J Surg 2011;202:225–32. 10.1016/j.amjsurg.2010.08.038 [DOI] [PubMed] [Google Scholar]
- 3.Nichols RL, Broido P, Condon RE, et al. Effect of preoperative neomycin-erythromycin intestinal preparation on the incidence of infectious complications following colon surgery. Ann Surg 1973;178:453–62. 10.1097/00000658-197310000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke JS, Condon RE, Bartlett JG, et al. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg 1977;186:251–9. 10.1097/00000658-197709000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg 2002;45:173–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Ram E, Sherman Y, Weil R, et al. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg 2005;140:285–8. 10.1001/archsurg.140.3.285 [DOI] [PubMed] [Google Scholar]
- 7.Morris MS, Graham LA, Chu DI, et al. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg 2015;261:1034–40. 10.1097/SLA.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 8.Kim EK, Sheetz KH, Bonn J, et al. A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg 2014;259:310–4. 10.1097/SLA.0b013e3182a62643 [DOI] [PubMed] [Google Scholar]
- 9.Toneva GD, Deierhoi RJ, Morris M, et al. Oral antibiotic bowel preparation reduces length of stay and readmissions after colorectal surgery. J Am Coll Surg 2013;216:756–62. 10.1016/j.jamcollsurg.2012.12.039 [DOI] [PubMed] [Google Scholar]
- 10.Scarborough JE, Mantyh CR, Sun Z, et al. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of Colectomy-Targeted ACS NSQIP. Ann Surg 2015;262:331–7. 10.1097/SLA.0000000000001041 [DOI] [PubMed] [Google Scholar]
- 11.Rollins KE, Javanmard-Emamghissi H, Acheson AG, et al. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg 2019;270:43–58. 10.1097/SLA.0000000000003145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh JWT, Phan K, Hitos K, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Netw Open 2018;1:e183226. 10.1001/jamanetworkopen.2018.3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (mobile): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840–8. 10.1016/S0140-6736(19)31269-3 [DOI] [PubMed] [Google Scholar]
- 14.Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729–38. 10.1016/S2468-1253(20)30075-3 [DOI] [PubMed] [Google Scholar]
- 15.Guenaga KKFG, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2009:CD001544. 10.1002/14651858.CD001544.pub3 [DOI] [PubMed] [Google Scholar]
- 16.Bretagnol F, Panis Y, Rullier E, et al. Rectal cancer surgery with or without bowel preparation: the French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863–8. 10.1097/SLA.0b013e3181fd8ea9 [DOI] [PubMed] [Google Scholar]
- 17.Migaly J, Bafford AC, Francone TD, et al. The American Society of colon and rectal surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 2019;62:3–8. 10.1097/DCR.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019;43:659–95. 10.1007/s00268-018-4844-y [DOI] [PubMed] [Google Scholar]
- 19.Lahtinen P, Jalanka J, Hartikainen A, et al. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther 2020;51:1321–31. 10.1111/apt.15740 [DOI] [PubMed] [Google Scholar]
- 20.Slankamenac K, Nederlof N, Pessaux P, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–62. 10.1097/SLA.0000000000000948 [DOI] [PubMed] [Google Scholar]
- 21.National Healthcare Safety Network . Centers for disease control and prevention. surgical site infection event (SSI), 2021. Available: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf [Accessed 11 Feb 2021].
- 22.HUS FICAN Southin nimeämä hoitosuositustyöryhmä. Kolorektaalisyövän kansalliset hoitosuositukset. Helsinki: Terveysportti 2019 [2.4.2019]. Available: https://www.terveysportti.fi/dtk/ltk/avaa?p_artikkeli=hsu00007 [Accessed 11 Jan 2021].
- 23.Räsänen M, Carpelan-Holmström M, Mustonen H, et al. Pattern of rectal cancer recurrence after curative surgery. Int J Colorectal Dis 2015;30:775–85. 10.1007/s00384-015-2182-1 [DOI] [PubMed] [Google Scholar]
- 24.2017 European Society of Coloproctology (ESCP) collaborating group . Association of mechanical bowel preparation with oral antibiotics and anastomotic leak following left sided colorectal resection: an international, multi-centre, prospective audit. Colorectal Dis 2018;20 Suppl 6:15–32. 10.1111/codi.14362 [DOI] [PubMed] [Google Scholar]
- 25.Battersby CLF, Battersby NJ, Slade DAJ, et al. Preoperative mechanical and oral antibiotic bowel preparation to reduce infectious complications of colorectal surgery - the need for updated guidelines. J Hosp Infect 2019;101:295–9. 10.1016/j.jhin.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 26.McChesney SL, Zelhart MD, Green RL, et al. Current U.S. pre-operative bowel preparation trends: a 2018 survey of the American Society of colon and rectal surgeons members. Surg Infect 2020;21:1–8. 10.1089/sur.2019.125 [DOI] [PubMed] [Google Scholar]
- 27.Ghuman A, Kasteel N, Brown CJ, et al. Surgical site infection in elective colonic and rectal resections: effect of oral antibiotics and mechanical bowel preparation compared with mechanical bowel preparation only. Colorectal Dis 2020;22:1686–93. 10.1111/codi.15153 [DOI] [PubMed] [Google Scholar]
- 28.Rybakov E, Nagudov M, Sukhina M, et al. Impact of oral antibiotic prophylaxis on surgical site infection after rectal surgery: results of randomized trial. Int J Colorectal Dis 2021;36:323–30. 10.1007/s00384-020-03746-0 [DOI] [PubMed] [Google Scholar]
- 29.Abis GSA, Stockmann HBAC, Bonjer HJ, et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (select trial). Br J Surg 2019;106:355–63. 10.1002/bjs.11117 [DOI] [PubMed] [Google Scholar]
- 30.Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation in right and left colectomy: subgroup analysis of mobile trial. BJS Open 2021;5. 10.1093/bjsopen/zrab011. [Epub ahead of print: 05 03 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051269supp001.pdf (75.5KB, pdf)