Abstract
To date, more than 20 different vertebrate master sex-determining genes have been identified on different sex chromosomes of mammals, birds, frogs and fish. Interestingly, six of these genes are transcription factors (Dmrt1- or Sox3- related) and 13 others belong to the TGF-β signalling pathway (Amh, Amhr2, Bmpr1b, Gsdf and Gdf6). This pattern suggests that only a limited group of factors/signalling pathways are prone to become top regulators again and again. Although being clearly a subordinate member of the sex-regulatory network in mammals, the TGF-β signalling pathway made it to the top recurrently and independently. Facing this rolling wave of TGF-β signalling pathways, this review will decipher how the TGF-β signalling pathways cope with the canonical sex gene regulatory network and challenge the current evolutionary concepts accounting for the diversity of sex-determining mechanisms.
This article is part of the theme issue ‘Challenging the paradigm in sex chromosome evolution: empirical and theoretical insights with a focus on vertebrates (Part I)’.
Keywords: sex determination, fish, evolution, sex-determining genes
1. Introduction
The existence of two complementary sexes, male and female, is probably one of the most ancient and pervasive features in biology. Intriguingly, this apparently conserved ontogenetic process, triggering the differentiation of two highly specialized male and female reproductive organs, relies on astonishingly plastic, rarely considerably conserved and stochastic regulatory networks [1,2]. Furthermore, the initial triggers ahead of the molecular machinery that rules sex determination (SD) from a fundamentally bipotential genome, are extremely variable, and have evolved recurrently and independently [3].
Our understanding of the so-called proximate causes, the underlying mechanism, regulation and drivers for this observed variability, has been advanced through the characterization of a substantial number of master sex-determining (MSD) genes over a broad spectrum of species. This knowledge in turn improves our understanding of what organismic biologists designated as the ultimate causes, scenarios leading to transitions of SD mechanisms within and between diverse lineages. The diversity and extreme lability of these initial triggers can either come from the genome itself (genetic sex determination, GSD), or from ‘external’ signals (environmental sex determination, ESD). Importantly, the phylogenetic distribution of these triggers does not exhibit any evolutionary pattern. Therefore, numerous (and sometimes conflicting) scenarios and hypotheses have been proposed to explain the evolutionary forces underlying the diversity of SD triggers [4].
Adaptive hypotheses prevail for explaining the evolution of the vast diversity of sex-determining triggers (GSD or ESD), whose ultimate effect is to favour an equal sex ratio in a given population. Under this natural selection scenario, mutations which establish new SD mechanisms and confer fitness advantages to their carriers will increase in frequency until fixation in a given population, while the previous SD mechanism will be lost [5]. Fitness advantage and sexually antagonistic models predict that GSD is stabilized through the evolution of sex chromosomes, with sexual selection linking the genetic sex-determiner to a locus which is beneficial to the same sex or antagonistic in the opposite sex [6–10]. Recently, Perrin proposed sex as a bistable equilibrium (male versus female phenotypes) which can be triggered by any random process capable of tipping the balance in either direction from the undetermined state (random sex determination, RSD [11]).
The progressive elucidation of the molecular components constituting the SD pathways and their biological functions has resulted in the neutral process of genetic drift also being considered instrumental in generating the observed diversity of SD mechanisms. If only the final gene product of a regulatory network produces a phenotype, then changes in the upstream system can occur without necessarily impacting the final phenotype, and thus can evolve neutrally [12,13]. Furthermore, since downstream genes of sex-determining pathways seem to be more conserved than upstream ones, it was hypothesized that the SD cascade is built from a ‘bottom-up’ scenario, with novel top regulators being issued from downstream components of the cascade itself ([14] and this issue [15] for a revisit of that theory).
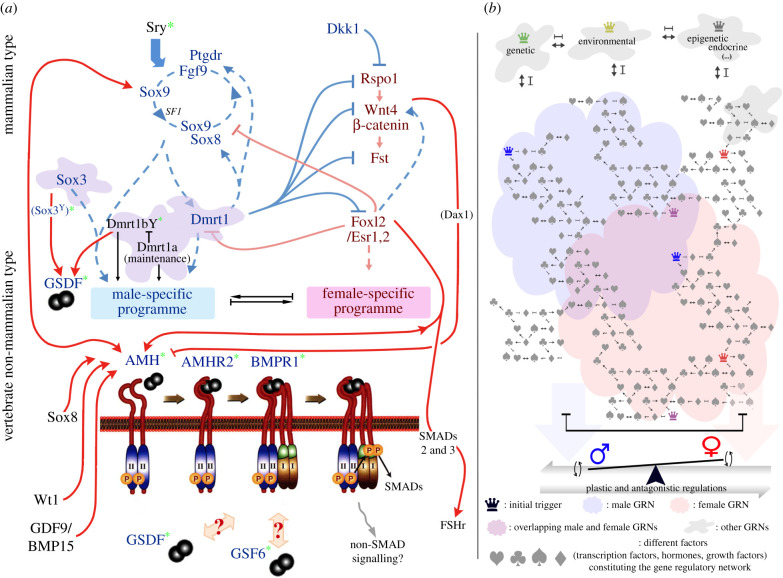
To date, more than 20 different vertebrate MSD genes have been identified on different sex chromosomes of mammals, birds, frogs and fish (table 1). Interestingly, six of these genes are transcription factors (Dmrt1- or Sox3- related) and 13 others belong to the TGF-β signalling pathway (Amh, Amhr2, Bmpr1b, Gsdf and Gdf6). This pattern suggests that only a limited group of factors/signalling pathways are prone to become top regulators, while other well-characterized and indispensable components of sex-determining pathways—e.g. Sox9 or Foxl2—have apparently not been recruited as master regulators in any species studied so far. Moreover, while TGF-β members are clearly subordinate in the mammalian sex-regulatory network, they have independently and recurrently made it to the top in fish (table 1). The biological importance of TGF-β members is unfortunately contrasted by the lack of information on how such signalling(s) is/are elicited and physiologically integrated during gonadal induction and development. Additionally, such a profusion of master regulators from TGF-β pathway-related members (figure 1a) draws attention to the evolutionary meaning of this convergent evolution.
Table 1.
Master sex-determining genes and systems in vertebrates. Table of the currently known and documented master sex-determining (MSD) genes in vertebrates. Of special interest, MSD genes belonging to the TGF-β are highlighted in red. MSD genes that arose after allelic diversification or gene duplication are highlighted in green and blue, respectively. Specific references are given; PC, personal communication. (Online version in colour.)
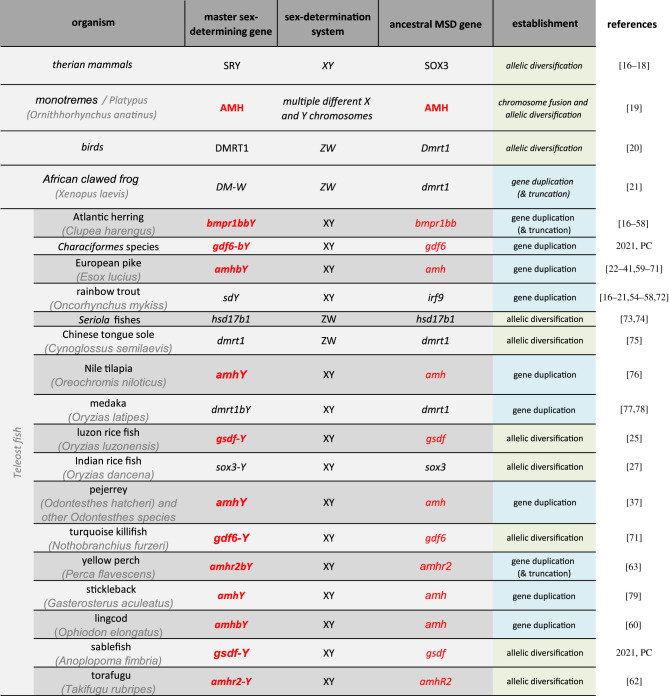 |
Figure 1.
Signalling pathways and gene regulatory networks of gonadal sex induction and maintenance in vertebrates. (a) Several genetic triggers regulate and temper modular sex-determining gene regulatory networks. Central to the male and female regulatory networks, respectively, are the evolutionarily conserved Dmrt1 and Foxl2 transcription factors, which cross-inhibit each other. Unstable equilibria between these conflicting (male (blue) and female (pink)) genetic pathways and the gene regulatory networks which underlie the phenotypic differentiation of the somatic gonad towards ovary or testis. The recurrent recruitment of secreted and diffusible cytokines of the TGF-β family to top positions in sex determination (green asterisk) forces us to reconsider our mechanistic view of sex determination. The pleiotropic nature of these factors, which act as hubs for integrating diverse signals, should make us consider SD from a developmental perspective, and sex as a threshold phenotype trait. (b) Sex differentiation is a threshold phenotypic trait resulting from fine regulation within and between intricate gene regulatory networks. The extreme genetic and phenotypic plasticity underlying sex determination, development and maintenance suggest that sex might also be considered as nothing more than a multilayer reaction norm. This multilayer reaction norm emerges from the integration of environmental stimuli (temperature, pH, etc.) and the genome (genomic and epigenomic variation) through (ambivalent) master triggers (crown symbols). (Online version in colour.)
Here, we will take advantage of this ‘evolution in motion’ scenario to decipher ‘how the TGF-β signalling pathways cope with the canonical sex gene regulatory network (GRN) and challenge the current evolutionary concepts accounting for the diversity of sex-determining mechanisms’.
2. Conserved critical roles for gonadal TGF-β signalling molecules
From sponges to mammals, the TGF-β superfamily of active polypeptides has attracted much attention for its pleiotropy. Members of this superfamily control a plethora of cellular processes from embryonic development to tissue homeostasis [80]. Based on sequence homology, TGF-β molecules are divided into: (i) TGF-β sensu stricto; (ii) bone morphogenetic proteins (BMPs); (iii) activins, inhibins and growth and differentiation factors (GDFs); and (iv) distant members. Astonishingly, despite the tremendous diversity of physiological responses which these family members elicit, the core of this signalling pathway is highly stereotyped. Canonical TGF-β members signal through heteromeric complexes composed of type I and II serine/threonine kinase receptors. Ligand binding induces the formation of a ternary complex, which then initiates intracellular signal transduction and transcriptional regulation of target genes via Smads proteins [80,81] (see lower part of figure 1a).
From an evolutionary perspective, the appearance of the TGF-β pathway is intrinsically linked to the emergence of metazoans [82], and the expansion of this pathway accompanied the generation of key vertebrate evolutionary novelties (see [83] for review). Nevertheless, due to an additional round of whole genome duplication (WGD) in teleosts on top of the ancient WGDs at the vertebrate root, the TGF-β pathway gene radiation is most extensive in this clade. Indeed, the repertoires of ligands and Type I and II receptors increased from 7, 3 and 2 in Drosophila, to 30, 7 and 5 in mammals and up to 43–50, 9–11 and 7–10 in teleosts, respectively [83]. This evolutionary expansion increases the number of players, relaxes the physiological constraints on them, and thereby provides an ideal playground for neutral processes of genetic drift to act on ‘dispensable’ copies. Obviously, teleosts seized this opportunity, as is apparent from their mechanisms of primary sex determination involving TGF-β signalling molecules (table 1 and figure 1a).
(a) . The anti-Müllerian hormone dual (Amh and AmhRII)
The anti-Müllerian hormone (Amh) is a distant member of the TGF-β family and belongs to the fourth subgroup; a subgroup which shows limited homology to members of the activin/inhibin and sensu stricto TGF-βs subgroups (figure 1a).
In mammals, Amh plays a major role in the regression of the Müllerian duct-forming part of the female reproductive tract during male embryo development ([84,85] for review). Amh has additionally been shown to regulate germ cell development and primordial follicle recruitment [86]. Furthermore, while Amh is not required for primary sex determination and testis development in mouse [87,88], mutations in the Amh pathway lead to disorders in male sexual development (the persistent Müllerian duct syndrome, see [88] for review). Contrastingly, Amh-related molecules play a central role in testis formation in non-mammalian vertebrates. For instance, in chicken embryonic gonads, Amh and AmhRII are more highly expressed in males than females, and their signalling is hypothesized to be responsible for organizing early testis structures in chickens and other birds [89]. The knock down of Amh in chicken embryos revealed that this molecule is required for embryonic urogenital system growth but not for sexual differentiation [90], and overexpression experiments suggest that, although it does not operate as a deterministic early testis activator, it affects downstream events, such as steroid production [91]. In amphibians, Amh is predominantly expressed in differentiating testes, but is not sexually dimorphic during the sex-determining stages [92,93]. Although Amh has so far never been located to amphibian sex chromosomes [93], Amh and AmhRII nevertheless show variable degrees of association with sex in different populations of common frog (Rana temporaria) [94]. Interestingly, in the platypus (Ornithorhynchus anatinus), the Amh-Y (located within an ancient bloc of genes on Y-chromosome #5) is the prime candidate for being the principal SD gene. While its candidacy awaits further functional characterization [89], this suggests that an ‘ancestral’ AMH may have functioned as a primary inducer of sex differentiation, and that this function may have been lost in therian mammals.
Fish do not have Müllerian ducts, but have an Amh homolog, which was first identified in the Japanese medaka (Oryzias latipes) [95]. Although being clearly a subordinate member of the sex regulatory network in therian mammals, the Amh/Amh-receptor system has made its way to the top in several teleost species (table 1 and figure 1a). In this regard, AMH ligand is the most successful, having risen to master sex determiner in several fish species, including the Patagonian pejerrey (Odontesthes hatcheri) [96], the Nile tilapia (Oreochromis niloticus) [76], the northern pike (Esox lucius) [59], and likely the lingcod (Ophiodon elongatus) [60] and the threespine stickleback (Gasterosteus aculeatus) [61]. Besides the ligand, a hypo-active version of the AMHRII on the X-chromosome of the tiger pufferfish (Takifugu rubripes) [62] also made it to the top (setting up a recessive allele as a sex determiner), while truncated AMHRII receptors determine sex in the yellow perch (Perca flavescens) [63] and a catfish (Y Guiguen and A Herpin 2021, personal communication). It is also worth noting that all of the above mentioned AMH pathway molecules (in pike, Nile tilapia, pejerrey, stickleback and lingcod, with the exception of AMHR2 in the pufferfish and likely AMH in monotremes) arose via the same mechanism of ‘sporadic’ gene duplication (see table 1). So far, no other components of this pathway (e.g. Smads, figure 1a) have been identified as MSD genes in any species.
(b) . Bone morphogenetic proteins/growth and differentiation factors, the new comer
In mouse embryos, several independent BMP signals are required for proper primordial germ cell (PGC) specification: primary induction of PGCs at the posterior proximal epiblast is driven by BMP4 [64], whereas PGC number is controlled synergistically by BMP2, BMP4 and BMP8b, as well as BMP7 signals [65,66]. In mice, GDF9 and BMP15 influence and coordinate the development of granulosa cells together with that of the oocytes they surround. As a result, GDF9 or BMP15 mutant mice are either sterile or sub-fertile, respectively [67], and decreased levels of the oocyte-secreted GDF9 and BMP15 factors result in polycystic ovarian syndrome [68]. Hence, while the BMP signalling pathway seems not to be involved in early SD events, it is implicated in mammalian germ cell specification and gametogenesis [69]. In zebrafish, whose MSD gene remains unknown, functional female-to-male sex reversal is observed in BMP15-deficient females [70], suggesting that BMP15 is required to maintain female sex fate in juveniles, although not required for primary female sex determination [70]. In contrast to mice, GDF9 does not seem to have any evident role in the maintenance of female sex differentiation or oogenesis in zebrafish [70]. Interestingly, zebrafish BMP15 mutant phenotype resembles that of mouse GDF9 mutants: early stage arrest in oocyte development and then degradation. Nevertheless, GDF9 mutant mice do not sex-reverse [68].
Intriguingly, although Gdf6 has no known involvement in any gonadal development processes, this gene has been recruited independently as a male determiner in the killifish (Nothobranchius furzeri) [71] and a Characiformes species (Y Guiguen 2021, unpublished data) following allelic diversification and gene duplication, respectively (table 1). Interestingly, in both cases, non-conservative amino acid changes and deletions at key residues likely altered protein interactions, particularly dimerization and receptor interaction [70]. Nevertheless, the downstream targets (activins, BMP or other GDFs and Smads) of the Gdf6 signalling route during fish SD remain unknown (figure 1a).
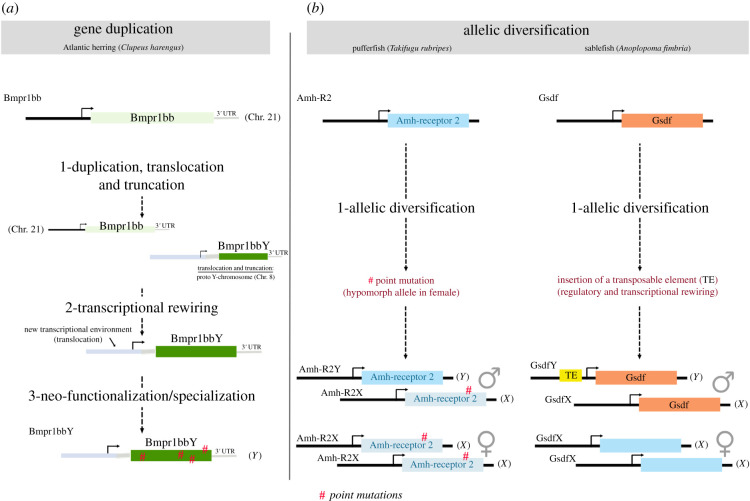
More recently, a truncated form of a BMP type I receptor, BMPR1BB, that originated by gene duplication has been identified as the MSD gene in Atlantic herring [22]. It was shown that BMPR1B could induce testis development by transducing AMH signalling in the absence ligands. This case brought another member in the BMP signalling pathways to the spotlight and also highlighted the crosstalk between AMH and BMP pathways.
(c) . Gonadal soma-derived factor, the teleost speciality
The gonadal soma-derived factor (Gsdf) is another example illustrating the importance of the TGF-β family in SD. Its biochemical function is not well understood; however, it is assumed to play a role in male gonad development because it is expressed exclusively in the early differentiating testes (of all fish analysed so far) [23,24]. Interestingly, the signalling modalities of Gsdf are challenging to decipher (figure 1a); while Gsdf is most homologous to inhibins, it nevertheless harbours a C-terminal LEFTY/DAN-like extension, and lacks any α-helix type I receptors epitopes (A Herpin 2021, unpublished data). Besides its proposed role in the downstream gonadal regulatory network [24], Gsdf has made it to the top of the SD pathway in Oryzias luzonensis [25] and in the sablefish ([26] and A Herpin 2021, unpublished data). Interestingly in O. latipes and O. dancena, two sister species of O. luzonensis, Gsdf is under direct transcriptional regulation of the MSD genes (Dmrt1bY and Sox3Y, respectively), indicating a conserved role for Gsdf as an important initiator of male sexual development ([24,27]; figure 1a).
3. Signalling specificity and crosstalk between different gonadal TGF-β signal transducing factors (Amh/AmhR2/BmpR1b/Gsdf/Gdf6)
The Amh/AmhR2/BmpR1b backbone, which has so far given rise to 5/2/1 MSD genes, respectively, (table 1), seems to be the main axis for eliciting gonadal TGF-β signalling (figure 1a). Therefore, it is of prime interest to understand how such a diversity of redundant and cross-talking pathways physiologically interact with the canonical gonadal GRN (see §5a and figure 1a).
Because Amh can directly interact with Sox9 [28], Foxl2 [29], Wt1 [30], Sox8 [31], Wnt4/Dax1 [32] and GDF9/BMP15 [33] (figure 1a), and because zebrafish amh/gsdf double mutant gonads are phenotypically no more compromised than either single mutant [34], it is likely that the gonadal Amh core signalling (Amh, AmhR2, BmpR1 and Smads 1 and 5) serves as the main regulatory hub for physiologically integrating multiple signals from a plethora of cross-talking pathways (figure 1a). However, the loss of AmhR2 in two fish clades, the Chimaeriformes and Cyprinidae [83], the function of a truncated AmhR2 as MSD in perch [63] and catfish, and the profound structural reorganization (implying impacted ligand/receptor interactions) of the Gdf6 ligands in killifish and a Characiformes species leave room for alternative routes, possibly relying on BmpR2 [34]. Indeed, in the Atlantic herring, the functional but truncated BmpR1bbY can phosphorylate downstream Smads 1 and 5 independently of the Amh-induced regulation [22]. These findings, together with the absence of any identified Smad effectors at the top of the SD pathway, suggest that gonadal TGF-β can also use non-Smad signalling (figure 1a).
Molecular specificity in the TGF-β family is not singularly achieved by ligand-receptor interactions, but by a network of interactions between multiple partners [35] (figure 1a,b). In this regard, the pleiotropic nature of the TGF-βs makes them the best candidates to influence the regulation of these pathways (figure 1b), and may thereby explain their prominence in fish SD.
4. How sex determination involving TGF-β molecules challenges the current evolutionary concepts accounting for the diversity of sex-determining mechanisms
(a) . Random sex determination: herring, pejerrey and the resonance from the noise
RSD conceptualises male versus female phenotypes as a bistable equilibrium which can be triggered by any random processes favouring a balanced sex ratio. Such a stochastic mechanism is predicted to evolve in species with large and genetically unstructured populations [11].
Surprisingly, in the Atlantic herring (a species with enormous population sizes (swarms of up to 4 billion individuals) and spawns (up to 200 000 eggs spawned per female), and without factors which indicate sexual selection and/or provide triggers for ESD such as morphological dimorphism, courtship behaviour, intraspecific social interactions and a structured environment) SD is nevertheless ruled by a strong genetic determiner: a truncated BMPR1BBY receptor carried by a well differentiated sex chromosome [22]. Intriguingly also, in the Argentinian silverside or pejerrey, the Amhy MSD gene is structurally well conserved and has strong genetic association with maleness within most of the clades of the Odontesthes genus [36], but can also function under an ESD (temperature) regime (e.g. in O. bonariensis [37]), with RSD at certain temperatures. These examples do not only reflect the obvious interplay of genetic and environmental factors (the so-called ‘developmental noise’) to produce a sexual phenotype, but also emphasize the importance of other factors including density, pH and temperature on the sexual fates of many fish—and reptile— species [38]. It also reveals that RSD processes might be sporadically resilient, but not perfectly random. Hence, even minimal deviations from optimal sex ratios might initiate evolutionary drift towards GSD, particularly when population sizes are large and/or natural selection is effective. Under almost all scenarios there will be a predisposition towards one sex or the other depending on the resonance emerging from these interactions—or developmental noise—with one system trumping the other according to the context. In these conditions, the pleiotropic nature of TGF-βs, being able to integrate multiple signals from ‘developmental noise’ might explain their recurrent recruitment to be MSD genes.
(b) . Fitness advantage and sexually antagonistic models
Classical models of sex chromosome evolution postulate that there first occurs an expansion of regions with reduced recombination near the MSD gene, and that the loss of recombination subsequently results in the gradual decay of the whole sex chromosome [39,40]. These models are based on the highly degenerated sex chromosomes of model species, and the universality of these models has recently been called into question by new findings in non-model species. For example, the MSD gene in northern pike (Amhby) arose greater than 65 Ma [41,59] and the surrounding Y-specific region remains restricted in size and without other protein coding genes. Furthermore, no male beneficial/female antagonistic genes have been found in this tiny SD region [59]. Interestingly, this very restricted sex-differentiated region encompassing amhby is a conserved feature of all Y-chromosomes in several species of this clade, illustrating that SD systems can be stable for over 50 Myr. These examples temper the ‘need’ for highly degenerated sex chromosomes as ‘evolutionary traps’ preventing the turnover of SD systems [3].
While increasing numbers of undifferentiated sex chromosomes have been recently identified, the most extreme case remains a conserved single nucleotide polymorphism (SNP) on Amhr2 which differentiates X and Y chromosomes in torafugu [62]. Another intriguing aspect of this case is that the recessive allele located on the X chromosome—with hypoactive function—is a novel mutation which became fixed as a sex determiner [62]. The novelty of the X copy is evidenced by the Y copy being phylogenetically closer to the canonical Amhr2 [62]. Several evolutionary models have been formulated to capture the dynamics of sex chromosome turnover (for a recent review, see [42]). The replacement of an ancestral sex-determining locus could happen via positive selection when the new MSD gene confers a fitness advantage [43], or when the new MSD gene is associated with strongly sexually antagonistic alleles [44]. The fugu recessive Amhr2 allele fulfils neither criteria, and likely increased in frequency to fixation via the neutral process of genetic drift. Nevertheless, it remains unclear how the system has stabilized and why the Y-chromosome allele persists. This case emphasizes the importance of considering neutral—non-adaptive—processes in the turnover of MSD genes. Moreover, the role of amhr2 in signal transduction implies that its position is downstream in the sex differentiation cascade. This, in turn, implies that two sexes can be produced from a shift in the balance of male/female promoting factors, and that these factors do not necessarily need to be upstream of key regulatory nodes (figure 1a,b). Moreover, the difference in signal transduction efficiency between the X and Y alleles of amhr2, which tips the balance towards either male or female development, illustrates that sex could be considered not as binary states but as a threshold phenotypic trait, and the process not a simple binary switch to a binary output.
(c) . Punctuated equilibrium versus gradual transformation
Current concepts of evolutionary biology propose that the evolution of phenotypic divergence either occurs after punctual but major changes, termed ‘punctuated equilibrium’, or through a continuum of gradual transformations, referred to as ‘phyletic gradualism’ [45–47]. By analogy, it is meaningful to ask whether the genetic changes underlying this phenotypic evolution occurred punctually after a single, major-effect mutation or if these phenotypic changes were underpinned by an accumulation of small-effect nucleotide alterations over time. In this perspective, the high turnover rate of the MSD genes offers an opportunity to compare the importance of these two scenarios. As far as we know, MSD genes arose following mainly two different types of evolutionary routes: (i) gene duplication (GD) followed by sub-/neo-functionalization and sometimes accompanied by translocation, or (ii) allelic diversification (AD) of a single locus (figure 2). Interestingly, with only a handful of exceptions (4 out of 12, table 1), the great majority of MSD genes belonging to the TGF-β family pathway arose via GD (table 1). Under this scenario, following a sudden duplication event, gradual neutral processes are thought to act on the dispensable gene copy (see the documented example of Dmrt1bY in medaka for instance, [15,48,49]).
Figure 2.
Documented evolutionary scenarios possibly explaining the different mechanisms accounting for the emergence of master sex-determining genes. Although not always predestined to exert a direct function during SD, common recurrent mechanisms of evolution underlay the emergence of MSD genes. (a) Gene duplication. In the Atlantic herring, the evolutionary process must have started with the emergence of a truncated copy of BMPR1BB that was partly duplicated and then translocated from chromosome 21 to chromosome 8. The truncation assigned a new protein function to the gene. At the same time, because of the loss of its 5′ end including the promoter, BMPR1BBY must have lost the expression regulation of the autosomal gene and most likely acquired a new spatial and temporal expression pattern required to trigger male gonad development. After translocation, Bmpr1bbY underwent rapid protein evolution. Functionally, the BMPR1BBY protein can replace the whole AMH/BMPR1b/AMHR2 pathway for SMAD phosphorylation [22]. (b) Allelic diversification. In the pufferfish, allelic diversification engendered two versions of the Amh receptor II (amhr2) that differ only by one amino acid located in the kinase domain (H384D). This hypomorphic mutation that confers lower receptor activity is encoded on the X chromosome. Quantitative variations in Amh signalling in females (homozygous for the hypomorphic amhr2 allele) versus males (heterozygous for the wild type and hypomorphic alleles), account for male gonadal development. In the sablefish, a unique insertion of a transposable element (TE) within the promoter of one of the two alleles of the Gsdf gene provoked regulatory reassignments and transcriptional rewirings between both GsdfX and GsdfY alleles. Otherwise both GsdfX and GsdfY alleles are functionally similar. Acquisition of a new spatial and temporal expression pattern together with quantitative variation in Gsdf signalling in males versus females likely account for male gonadal development (A Herpin 2021, personal communication). (Online version in colour.)
One of the exceptional cases which arose via AD is the MSD gene of the sablefish, GsdfY (table 1). While both GsdfY and GsdfX copies are nearly identical and function similarly, GsdfY gained its sex-determining function after the sole transcriptional rewiring initiated by the exaptation of a single transposable element (A Herpin 2021, personal communication). Hence, this case provides a paradigmatic functional example that different phenotypes—as extreme as sexual development—can evolve by altering the expression of functionally conserved proteins after mutations in the cis-regulatory regions of pleiotropic developmental regulatory genes and of targets genes within the vast regulatory network they control. While this case reveals that transitions between SD systems can happen rather abruptly (evolutionary speaking), it also shows that the Gsdf locus is an excellent candidate to evolve into an MSD gene, and constitutes a textbook example of the bottom-up hypothesis [14] (figure 1a).
(d) . The bottom-up theory put to test by the ‘outsiders’
A few years ago, we designated the nickname ‘usual suspects’ to genes recurrently identified as MSD genes across species [50]. These genes (e.g. Dmrt1, Sox- and TGF-β-related factors) have arisen following allelic variation or gene duplication (table 1) and are crucial subordinate players of the gonadal regulatory network ([51,52] for review; and figure 1a). Because these genes independently persist in a given population, and could emancipate any upstream regulatory element and take the leadership, it was theorized that SD pathways might preferentially evolve from the bottom to the top [14], tightly constrained by the need to cope with the pre-existing GRN [53]. While this theory fits perfectly well with empirical data from numerous species (table 1), it is unable to account for some ‘newcomer’ and ‘outsider’ genes that were previously not considered part of the SD pathway being recently identified as MSD genes.
Indeed, initially with the unusual SdY MSD gene in rainbow trout ([54]; see [97] this same issue), and now within the TGF-β family members themselves, ‘newcomer’ factors, like Gdf6, have also made it to the top (figure 1a). Even more intriguing, Gdf6 rose to the top independently in the killifish [71] and a Characiformes species (Y Guiguen 2021, personal communication), although it has no known role in gonadal developmental processes in other species. The unexpected entry and importance of Gdf6 in the SD network is probably due to it being a member of the vast and tightly connected TGF-β family, where multiple members hold crucial positions in the SD gene network. Thus, any mutation that would initiate novel interactions or change the direction or magnitude of existing interactions between Gdf6 and new partners within TGF-βs could have provided shortcuts for Gdf6 to join the SD network (figure 1a). Indeed, substantial amino acid changes have been observed in Gdf6 in both species [71]. This versatility of the TGF-β molecules (ligands and receptors), and flexibility of connectivity among them widens the options of which genes could become involved in SD since any factor that could bend the TGF-β GRNs could, in principle, be co-opted to be the MSD gene.
The existence of such unexpected events, where a factor that was not known a priori to be involved in any sex-related regulatory network was recruited independently as the MSD gene, also reflects our fragmented knowledge of TGF-β regulatory networks. It would therefore be unsurprising if other TGF-β factors/regulators of the TGF-β regulatory network were revealed as key regulators of SD.
(e) . A composite sex chromosome gathers components of the TGF-β signalling pathway in Rana temporaria
In a geographically restricted population of European common frogs (Rana temporaria), a neo-sex chromosome was formed by reciprocal translocation between LG2 (the original Y chromosome in a nearby population) and LG7 (an autosome) [94]. Interestingly, LG2 and LG7 show similar levels of male-female differentiation (by Fst), indicating allelic diversification is underway. This neo-sex chromosome likely spread through the population under positive selection because of the advantage of linking male beneficial genes (Amh from LG2 and AmhR2 from LG7) [55]. The linkage between Amh and AmhR2 would ease the fixation of coevolved alleles of the ligand and its receptor and thus create a strong masculinizing effect, reducing the chance of sex reversal. In R. temporaria, as with many amphibians and some teleost species, meiotic recombination depends on phenotypic sex rather than genotypic sex. Recombination is rare in males, except at extremities of chromosomes [56]. The occasional sex reversals, for which there exists limited empirical data, could, depending on their natural frequency, provide the opportunity for the X and Y chromosomes to coexist in phenotypic females, allowing the exchange of alleles between them. This ‘fountain of youth’ mechanism renews the Y-chromosome sequence and prevents the chromosome's degeneration. Over time, this mechanism helps maintain sex chromosome homomorphy [56]. In this population, the neo-sex chromosome therefore greatly reduces the chance of recombination between X and Y by hindering sex reversal, and thus indirectly promotes the degeneration of the Y. The coevolution of amh with its receptor may thereby direct the evolutionary fate of the sex chromosomes in this population.
5. Conclusion
Much of the pioneering work deciphering the ‘ultimate causes’ of transitions between SD mechanisms was conducted at a time when our knowledge of the ‘proximate causes’ was fragmented and predominantly based on a few model species. Today, with deepened knowledge of molecular and cell biological processes and the discovery of an incredible diversity of sex-determining genes, chromosomes and systems from a multitude of organisms (table 1), new light has been shed on the ‘proximate causes’ of SD, and the generality of the classic theories is now being questioned.
Although many studies still focus primarily on discovering new MSD genes, the elucidation of the overall genetic architecture of a threshold phenotypic trait as complex as sex cannot be restricted to the study of MSD genes. For example, little is known about the proximate consequences of when ‘masters change’. A few years back, based on the classical view of sexual differentiation, we would have predicted little downstream change, since ‘slaves remain’ [57]. This is because a new master being derived from the conserved downstream SD pathway supposedly meant that only minor regulatory adjustments would be required (bottom-up theory [14]). An important adjustment to this view of vertebrate systems was the recognition that, contrary to what has been found in C. elegans and Drosophila, SD cascades are nonlinear, and rather constitute a complex network of interacting factors, particularly known transcription factors (figure 1a). Thus, new MSD genes could readily emanate from factors regulating key nodes of the canonical gonadal GRN (the ‘usual suspects’ [1,51]) following transcriptional rewiring and/or sub- or neo-functionalization (figure 1b).
Today, the recurrent recruitment of secreted and diffusible cytokines from the TGF-β family and their receptors to the top of the SD pathway prompts us to reconsider the transcription factor-based mechanistic view of SD. Additionally, these new findings demonstrate that SD is more than a simple top-down determination/differentiation process (genetically and physiologically), and should rather be considered from a developmental perspective, with sex as a threshold phenotype (figure 1b).
Emerging from random but resilient processes, and integrating a multitude of signals, Bmpr1bbY and AmhY made it to the top in herring and pejerrey, respectively. Flouting the basic expectation of sex chromosome evolution, Amhr2Y behaves like an ‘ordinary’ recessive allele in fugu, modulating the strength of the male versus female signals. Similarly, Amhby in northern pike has acted as an MSD gene for more than 65 Myr without coupling with any male beneficial/female antagonistic partners. In a special population of common frogs, the coevolution of Amh and its receptor, together with Dmrt1, directed the spread of a neo-sex chromosome. The ‘outsider’ Gdf6, in an unexpected case of convergent evolution, became the central regulator of SD in two teleost species. And finally, GsdfY (the MSD gene of the sablefish and a unique functional example of a bona fide punctuated equilibrium process [45]) showed that the Gsdf locus is per se inclined to spawn a MSD gene.
The pleiotropic nature of the TGF-β signalling pathway (combining signalling molecules, ligands, integrators, the receptor, and effectors, transcription factors) makes this pathway particularly capable of re-structuring and fine-tuning intricate networks of gene regulation (figure 1a,b). While TGF-β family members clearly play a crucial role in integrating a plethora of signals, and have been functionally and genomically proven to be MSD genes, environmental factors can still override them and cause sex reversal both in the lab and in the wild. This plasticity in species with clear sex chromosomes and a GSD emphasizes that sex should no longer be viewed as a rigid and pre-determined path from genotype to phenotype, but rather as a multilayer reaction norm resulting from developmental noise, and which can be contingently modulated or totally ruled by genetic factors. In this perspective, sex is the net product of a variety of environmental, genomic, epigenomic and stochastic determinants (figure 1b). The master genetic trigger therefore has to cope with influences from factors which may disturb its action directly, or disturb the downstream action. TGF-β, with its tight-knit regulatory network, and surplus copies in teleosts, is probably especially suitable for the production of a phenotype as plastic as sex.
(a) . Sidebar: TGF-β in need of answers
TGF-β signalling is crucial for fish sex determination. An emerging concept is that the response of given cells to extrinsic signals does not only rely on the effect of a single pathway, but on the integration of multiple signals from a plethora of interconnected cross-talking pathways. While this broad picture appears to be well supported, a number of specific issues remain unaddressed, for instance: (1) How are the sex-determining function(s) of TGFβ-signalling molecules (Amh, Gsdf and Gdf6) accomplished during fish gonadal induction? (2) Do these molecules converge to a general ‘TGF-β hub’ which connects and integrates them all, or do they remain independent of each other? (3) How do(es) that gonadal TGF-β regulatory network(s) interact with the canonical gonadal gene regulatory network? (4) What is the evolutionary meaning of the recurrent convergent evolution toward establishing TGF-β signalling pathways to control sex determination in fish?
Data accessibility
This article has no additional data.
Authors' contributions
All of them wrote the paper (review).
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the AquaCRISPR (ANR-16-COFA-0004-01), the TUNESAL (HAVBRUKR Research Council of Norway, project 301602), and 111 (China, grant no. D20007) projects to A.H. Q.P. and T.K. are supported by the University of Lausanne and Q.P. is supported by a grant from the Swiss NSF (310030B_176406) to Laurent Keller.
References
- 1.Herpin A, Schartl M. 2015. Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 16, 1260-1274. ( 10.15252/embr.201540667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herpin A, et al. 2013. Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex determination. Mol. Biol. Evol. 30, 2328-2346. ( 10.1093/molbev/mst130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachtrog D, et al. 2014. Sex determination: why so many ways of doing it? PLoS Biol. 12, e1001899. ( 10.1371/journal.pbio.1001899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beukeboom L, Perrin N. 2014. The evolution of sex determination. Oxford, NY: Oxford University Press. [Google Scholar]
- 5.Charnov EL, Bull JJ. 1989. The primary sex ratio under environmental sex determination. J. Theor. Biol. 139, 431-436. ( 10.1016/S0022-5193(89)80063-3) [DOI] [PubMed] [Google Scholar]
- 6.Fisher RA. 1931. The evolution of dominance. Biol. Rev. 6, 345-368. ( 10.1111/j.1469-185X.1931.tb01030.x) [DOI] [Google Scholar]
- 7.Rice WR. 1987. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution 41, 911-914. ( 10.1111/j.1558-5646.1987.tb05864.x) [DOI] [PubMed] [Google Scholar]
- 8.Bull JJ. 1983. Evolution of sex determining mechanisms. Menlo Park, CA: Benjamin/Cummings Publishing Company. [Google Scholar]
- 9.Charlesworth D, Charlesworth B. 2005. Sex chromosomes: evolution of the weird and wonderful. Curr. Biol. 15, R129-R131. ( 10.1016/j.cub.2005.02.011) [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick M. 2017. The evolution of genome structure by natural and sexual selection. J. Hered. 108, 3-11. ( 10.1093/jhered/esw041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrin N. 2016. Random sex determination: when developmental noise tips the sex balance. Bioessays 38, 1218-1226. ( 10.1002/bies.201600093) [DOI] [PubMed] [Google Scholar]
- 12.Lynch M. 2007. The evolution of genetic networks by non-adaptive processes. Nat. Rev. Genet. 8, 803-813. ( 10.1038/nrg2192) [DOI] [PubMed] [Google Scholar]
- 13.Gempe T, Beye M. 2011. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 33, 52-60. ( 10.1002/bies.201000043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkins AS. 1995. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. Bioessays 17, 71-77. ( 10.1002/bies.950170113) [DOI] [PubMed] [Google Scholar]
- 15.Adolfi MC, Herpin A, Schartl M. 2021. The replaceable master of sex determination: bottom-up hypothesis revisited. Phil. Trans. R. Soc. B 376, 20200090. ( 10.1098/rstb.2020.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato Y, Shinka T, Sakamoto K, Ewis AA, Nakahori Y. 2010. The male-determining gene Sry is a hybrid of DGCR8 and SOX3, and is regulated by the transcription factor CP2. Mol. Cell. Biochem. 337, 267-275. ( 10.1007/s11010-009-0308-x) [DOI] [PubMed] [Google Scholar]
- 17.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. 1991. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117-121. ( 10.1038/351117a0) [DOI] [PubMed] [Google Scholar]
- 18.Kashimada K, Koopman P. 2010. Sry: the master switch in mammalian sex determination. Development 137, 3921-3930. ( 10.1242/dev.048983) [DOI] [PubMed] [Google Scholar]
- 19.Grafodatskaya D, Rens W, Wallis MC, Trifonov V, O'Brien PCM, Clarke O, Graves JAM, Ferguson-Smith MA. 2007. Search for the sex-determining switch in monotremes: mapping WT1, SF1, LHX1, LHX2, FGF9, WNT4, RSPO1 and GATA4 in platypus. Chromosome Res. 15, 777-785. ( 10.1007/s10577-007-1161-y) [DOI] [PubMed] [Google Scholar]
- 20.Shan Z, Nanda I, Wang Y, Schmid M, Vortkamp A, Haaf T. 2000. Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenet. Cell Genet. 89, 252-257. ( 10.1159/000015626) [DOI] [PubMed] [Google Scholar]
- 21.Yoshimoto S, et al. 2008. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl Acad. Sci. USA 105, 2469-2474. ( 10.1073/pnas.0712244105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafati N, et al. 2020. Reconstruction of the birth of a male sex chromosome present in Atlantic herring. Proc. Natl Acad. Sci. USA 117, 24 359-24 368. ( 10.1073/pnas.2009925117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautier A, Le Gac F, Lareyre J-J.. 2011. The gsdf gene locus harbors evolutionary conserved and clustered genes preferentially expressed in fish previtellogenic oocytes. Gene 472, 7-17. ( 10.1016/j.gene.2010.10.014) [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, et al. 2016. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 6, 19738. ( 10.1038/srep19738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, Naruse K, Hamaguchi S, Sakaizumi M. 2012. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics 191, 163-170. ( 10.1534/genetics.111.137497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rondeau EB, et al. 2013. Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics 14, 452. ( 10.1186/1471-2164-14-452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takehana Y, et al. 2014. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nat. Commun. 5, 4157. ( 10.1038/ncomms5157) [DOI] [PubMed] [Google Scholar]
- 28.De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P.. 1998. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol. Cell. Biol. 18, 6653-6665. ( 10.1128/MCB.18.11.6653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sacchi S, Marinaro F, Xella S, Marsella T, Tagliasacchi D, La Marca A.. 2017. The anti-Müllerian hormone (Amh) induces forkhead box L2 (FOXL2) expression in primary culture of human granulosa cells in vitro. J. Assist. Reprod. Genet. 34, 1131-1136. ( 10.1007/s10815-017-0980-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. 1998. Wilms' Tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93, 445-454. ( 10.1016/S0092-8674(00)81172-1) [DOI] [PubMed] [Google Scholar]
- 31.Schepers G, Wilson M, Wilhelm D, Koopman P. 2003. SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J. Biol. Chem. 278, 28 101-28 108. ( 10.1074/jbc.M304067200) [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Mizusaki H, Kawabe K, Kasahara M, Yoshioka H, Morohashi K. 2002. Concerted regulation of gonad differentiation by transcription factors and growth factors. Novartis Found. Symp. 244, 68-77; discussion 77–85, 253–257. [PubMed] [Google Scholar]
- 33.Roy S, Gandra D, Seger C, Biswas A, Kushnir VA, Gleicher N, Kumar TR, Sen A. 2018. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology 159, 3433-3445. ( 10.1210/en.2018-00609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Y-L, Batzel P, Titus T, Sydes J, Desvignes T, BreMiller R, Draper B, Postlethwait JH. 2019. A hormone that lost its receptor: anti-Müllerian hormone (Amh) in zebrafish gonad development and sex determination. Genetics 213, 529-553. ( 10.1534/genetics.119.302365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinck AP, Mueller TD, Springer TA. 2016. Structural biology and evolution of the TGF-β family. Cold Spring Harb. Perspect. Biol. 8, a022103. ( 10.1101/cshperspect.a022103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hattori RS, Somoza GM, Fernandino JI, Colautti DC, Miyoshi K, Gong Z, Yamamoto Y, Strüssmann CA. 2019. The duplicated Y-specific amhy gene is conserved and linked to maleness in silversides of the genus Odontesthes. Genes (Basel) 10, 679. ( 10.3390/genes10090679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Zhang Y, Sarida M, Hattori RS, Strüssmann CA. 2014. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS ONE 9, e102574. ( 10.1371/journal.pone.0102574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge C, Ye J, Zhang H, Zhang Y, Sun W, Sang Y, Capel B, Qian G. 2017. Dmrt1 induces the male pathway in a turtle species with temperature-dependent sex determination. Development 144, 2222-2233. ( 10.1242/dev.152033) [DOI] [PubMed] [Google Scholar]
- 39.Vicoso B, Charlesworth B. 2006. Evolution on the X chromosome: unusual patterns and processes. Nat. Rev. Genet. 7, 645-653. ( 10.1038/nrg1914) [DOI] [PubMed] [Google Scholar]
- 40.Singh ND, Koerich LB, Carvalho AB, Clark AG. 2014. Positive and purifying selection on the Drosophila Y chromosome. Mol. Biol. Evol. 31, 2612-2623. ( 10.1093/molbev/msu203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Q, et al. 2020. The rise and fall of the ancient northern pike master sex determining gene. bioRxiv, 2020.05.31.125336. ( 10.1101/2020.05.31.125336) [DOI] [Google Scholar]
- 42.Vicoso B. 2019. Molecular and evolutionary dynamics of animal sex-chromosome turnover. Nat. Ecol. Evol. 3, 1632-1641. ( 10.1038/s41559-019-1050-8) [DOI] [PubMed] [Google Scholar]
- 43.Bull JJ, Charnov EL. 1977. Changes in the heterogametic mechanism of sex determination. Heredity 39, 1-14. ( 10.1038/hdy.1977.38) [DOI] [PubMed] [Google Scholar]
- 44.van Doorn GS, Kirkpatrick M.. 2007. Turnover of sex chromosomes induced by sexual conflict. Nature 449, 909-912. ( 10.1038/nature06178) [DOI] [PubMed] [Google Scholar]
- 45.Eldredge N, Gould JS. 1972. Punctuated equilibria: an alternative to phyletic gradualism. San Francisco, CA: Freeman, Cooper and Company. [Google Scholar]
- 46.Mayr E. 1997. Evolution and the diversity of life: selected essays. Cambridge, MA: Harvard University Press. [Google Scholar]
- 47.Rhodes FHT. 1983. Gradualism, punctuated equilibrium and the Origin of Species. Nature 305, 269-272. ( 10.1038/305269a0) [DOI] [PubMed] [Google Scholar]
- 48.Schartl M, et al. 2018. Sox5 is involved in germ-cell regulation and sex determination in medaka following co-option of nested transposable elements. BMC Biol. 16, 16. ( 10.1186/s12915-018-0485-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herpin A, Braasch I, Kraeussling M, Schmidt C, Thoma EC, Nakamura S, Tanaka M, Schartl M. 2010. Transcriptional rewiring of the sex determining dmrt1 gene duplicate by transposable elements. PLoS Genet. 6, e1000844. ( 10.1371/journal.pgen.1000844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herpin A, Schartl M. 2011. Dmrt1 genes at the crossroads: a widespread and central class of sexual development factors in fish. FEBS J. 278, 1010-1019. ( 10.1111/j.1742-4658.2011.08030.x) [DOI] [PubMed] [Google Scholar]
- 51.Herpin A, Schartl M. 2008. Regulatory putsches create new ways of determining sexual development. EMBO Rep. 9, 966-968. ( 10.1038/embor.2008.182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herpin A, Schartl M. 2011. Sex determination: switch and suppress. Curr. Biol. 21, R656-R659. ( 10.1016/j.cub.2011.07.026) [DOI] [PubMed] [Google Scholar]
- 53.Marshall Graves JA, Peichel CL. 2010. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 11, 205. ( 10.1186/gb-2010-11-4-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertho S, et al. 2018. The unusual rainbow trout sex determination gene hijacked the canonical vertebrate gonadal differentiation pathway. Proc. Natl Acad. Sci. USA 115, 12 781-12 786. ( 10.1073/pnas.1803826115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charlesworth D, Charlesworth B. 1980. Sex differences in fitness and selection for centric fusions between sex-chromosomes and autosomes. Genet. Res. 35, 205-214. ( 10.1017/s0016672300014051) [DOI] [PubMed] [Google Scholar]
- 56.Perrin N. 2009. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043-3049. ( 10.1111/j.1558-5646.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- 57.Graham P, Penn JKM, Schedl P. 2003. Masters change, slaves remain. Bioessays 25, 1-4. ( 10.1002/bies.10207) [DOI] [PubMed] [Google Scholar]
- 58.Pettersson ME, et al. 2019. A chromosome-level assembly of the Atlantic herring genome—detection of a supergene and other signals of selection. Genome Res. 29, 1919-1928. ( 10.1101/gr.253435.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan Q, et al. 2019. Identification of the master sex determining gene in Northern pike (Esox lucius) reveals restricted sex chromosome differentiation. PLoS Genet. 15, e1008013. ( 10.1371/journal.pgen.1008013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rondeau EB, Laurie CV, Johnson SC, Koop BF. 2016. A PCR assay detects a male-specific duplicated copy of anti-Müllerian hormone (Amh) in the lingcod (Ophiodon elongatus). BMC Res. Notes 9, 230. ( 10.1186/s13104-016-2030-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peichel CL, et al. 2004. The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Curr. Biol. 14, 1416-1424. ( 10.1016/j.cub.2004.08.030) [DOI] [PubMed] [Google Scholar]
- 62.Kamiya T, et al. 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8, e1002798. ( 10.1371/journal.pgen.1002798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feron R, et al. 2020. Characterization of a Y-specific duplication/insertion of the anti-Mullerian hormone type II receptor gene based on a chromosome-scale genome assembly of yellow perch, Perca flavescens. Mol. Ecol. Resour. 20, 531-543. ( 10.1111/1755-0998.13133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. 1999. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 13, 424-436. ( 10.1101/gad.13.4.424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ying Y, Qi X, Zhao GQ. 2001. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc. Natl Acad. Sci. USA 98, 7858-7862. ( 10.1073/pnas.151242798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ross A, Munger S, Capel B. 2007. Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex. Dev. 1, 127-137. ( 10.1159/000100034) [DOI] [PubMed] [Google Scholar]
- 67.Su Y-Q, Sugiura K, Eppig JJ. 2009. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 27, 32-42. ( 10.1055/s-0028-1108008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAllister JM, Legro RS, Modi BP, Strauss JF. 2015. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol. Metabolism 26, 118-124. ( 10.1016/j.tem.2014.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pangas SA. 2012. Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells. Mol. Cell. Endocrinol. 356, 40-47. ( 10.1016/j.mce.2011.06.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dranow DB, Hu K, Bird AM, Lawry ST, Adams MT, Sanchez A, Amatruda JF, Draper BW. 2016. Bmp15 is an oocyte-produced signal required for maintenance of the adult female sexual phenotype in zebrafish. PLoS Genet. 12, e1006323. ( 10.1371/journal.pgen.1006323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichwald K, et al. 2015. Insights into sex chromosome evolution and aging from the genome of a short-lived fish. Cell 163, 1527-1538. ( 10.1016/j.cell.2015.10.071) [DOI] [PubMed] [Google Scholar]
- 72.Yano A, et al. 2012. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Curr. Biol. 22, 1423-1428. ( 10.1016/j.cub.2012.05.045) [DOI] [PubMed] [Google Scholar]
- 73.Koyama T, et al. 2019. A SNP in a steroidogenic enzyme is associated with phenotypic sex in Seriola fishes. Curr. Biol. 29, 1901-1909; e8. ( 10.1016/j.cub.2019.04.069) [DOI] [PubMed] [Google Scholar]
- 74.Purcell CM, Seetharam AS, Snodgrass O, Ortega-García S, Hyde JR, Severin AJ. 2018. Insights into teleost sex determination from the Seriola dorsalis genome assembly. BMC Genomics 19, 31. ( 10.1186/s12864-017-4403-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen S, et al. 2014. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 46, 253-260. ( 10.1038/ng.2890) [DOI] [PubMed] [Google Scholar]
- 76.Li M, et al. 2015. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 11, e1005678. ( 10.1371/journal.pgen.1005678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuda M, et al. 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559-563. ( 10.1038/nature751) [DOI] [PubMed] [Google Scholar]
- 78.Nanda I, et al. 2002. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl Acad. Sci. USA 99, 11 778-11 783. ( 10.1073/pnas.182314699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peichel CL, et al. 2020. Assembly of the threespine stickleback Y chromosome reveals convergent signatures of sex chromosome evolution. Genome Biol. 21, 177. ( 10.1186/s13059-020-02097-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herpin A, Lelong C, Favrel P. 2004. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev. Comp. Immunol. 28, 461-485. ( 10.1016/j.dci.2003.09.007) [DOI] [PubMed] [Google Scholar]
- 81.Saremba S, Nickel J, Seher A, Kotzsch A, Sebald W, Mueller TD. 2008. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 275, 172-183. ( 10.1111/j.1742-4658.2007.06187.x) [DOI] [PubMed] [Google Scholar]
- 82.Pang K, Ryan JF, Baxevanis AD, Martindale MQ. 2011. Evolution of the TGF-β signaling pathway and its potential role in the ctenophore, Mnemiopsis leidyi. PLoS ONE 6, e24152. ( 10.1371/journal.pone.0024152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng S, Long J, Liu Z, Tao W, Wang D. 2018. Identification and evolution of TGF-β signaling pathway members in twenty-four animal species and expression in tilapia. Int. J. Mol. Sci. 19, 1154. ( 10.3390/ijms19041154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.di Clemente N, Josso N, Gouédard L, Belville C.. 2003. Components of the anti-Müllerian hormone signaling pathway in gonads. Mol. Cell. Endocrinol. 211, 9-14. ( 10.1016/j.mce.2003.09.005) [DOI] [PubMed] [Google Scholar]
- 85.Adolfi MC, Nakajima RT, Nóbrega RH, Schartl M. 2019. Intersex, hermaphroditism, and gonadal plasticity in vertebrates: evolution of the Müllerian duct and Amh/Amhr2 signaling. Annu. Rev. Anim. Biosci. 7, 149-172. ( 10.1146/annurev-animal-020518-114955) [DOI] [PubMed] [Google Scholar]
- 86.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP.. 1999. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 140, 5789-5796. ( 10.1210/endo.140.12.7204) [DOI] [PubMed] [Google Scholar]
- 87.Xu H-Y, Zhang H-X, Xiao Z, Qiao J, Li R. 2019. Regulation of anti-Müllerian hormone (Amh) in males and the associations of serum AMH with the disorders of male fertility. Asian J. Androl. 21, 109-114. ( 10.4103/aja.aja_83_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mullen RD, Ontiveros AE, Moses MM, Behringer RR. 2019. AMH and AMHR2 mutations: a spectrum of reproductive phenotypes across vertebrate species. Dev. Biol. 455, 1-9. ( 10.1016/j.ydbio.2019.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cortez D, Marin R, Toledo-Flores D, Froidevaux L, Liechti A, Waters PD, Grützner F, Kaessmann H. 2014. Origins and functional evolution of Y chromosomes across mammals. Nature 508, 488-493. ( 10.1038/nature13151) [DOI] [PubMed] [Google Scholar]
- 90.Lambeth LS, Ayers K, Cutting AD, Doran TJ, Sinclair AH, Smith CA. 2015. Anti-Müllerian hormone is required for chicken embryonic urogenital system growth but not sexual differentiation. Biol. Reprod. 93, 138. ( 10.1095/biolreprod.115.131664) [DOI] [PubMed] [Google Scholar]
- 91.Lambeth LS, et al. 2016. Overexpression of anti-Müllerian hormone disrupts gonadal sex differentiation, blocks sex hormone synthesis, and supports cell autonomous sex development in the chicken. Endocrinology 157, 1258-1275. ( 10.1210/en.2015-1571) [DOI] [PubMed] [Google Scholar]
- 92.Piprek RP, Pecio A, Laskowska-Kaszub K, Kubiak JZ, Szymura JM. 2013. Sexual dimorphism of AMH, DMRT1 and RSPO1 localization in the developing gonads of six anuran species. Int. J. Dev. Biol. 57, 891-895. ( 10.1387/ijdb.130192rp) [DOI] [PubMed] [Google Scholar]
- 93.Kodama M, et al. 2015. Molecular cloning and characterization of anti-Müllerian hormone (AMH) from the Japanese wrinkled frog, Rana rugosa. Endocrinology 156, 1914-1923. ( 10.1210/en.2013-2053) [DOI] [PubMed] [Google Scholar]
- 94.Rodrigues N, Vuille Y, Brelsford A, Merilä J, Perrin N. 2016. The genetic contribution to sex determination and number of sex chromosomes vary among populations of common frogs (Rana temporaria). Heredity 117, 25-32. ( 10.1038/hdy.2016.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klüver N, Pfennig F, Pala I, Storch K, Schlieder M, Froschauer A, Gutzeit HO, Schartl M. 2007. Differential expression of anti-Müllerian hormone (amh) and anti-Müllerian hormone receptor type II (amhrII) in the teleost medaka. Dev. Dyn. 236, 271-281. ( 10.1002/dvdy.20997) [DOI] [PubMed] [Google Scholar]
- 96.Hattori RS, Gould RJ, Fujioka T, Saito T, Kurita J, Strüssmann CA, Yokota M, Watanabe S. 2007. Temperature-dependent sex determination in Hd-rR medaka Oryzias latipes: gender sensitivity, thermal threshold, critical period, and DMRT1 expression profile. Sex Dev 1, 138-146. ( 10.1159/000100035) [DOI] [PubMed] [Google Scholar]
- 97.Bertho S, Herpin A, Schartl M, Guiguen Y. 2021. Lessons from an unusual vertebrate sex-determining gene. Phil. Trans. R. Soc. B 376, 20200092. ( 10.1098/rstb.2020.0092) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.