Abstract
Background:
Acute kidney injury (AKI) is a recognized complication in critically ill patients. The epidemiology of AKI varies worldwide, depending on the diagnostic criteria used and the setting. The International Society of Nephrology has called for a reduction in preventable deaths from AKI to zero by the year 2025. It is suspected that the majority of AKI cases are in limited-resource countries, but the true burden of AKI in these settings remains unknown.
Objective:
We aimed to determine, using standardized KDIGO (Kidney Disease Improving Global Outcomes) criteria, the prevalence of AKI, associated factors, and clinical characteristics of adult (≥18 years) patients admitted to intensive care units (ICUs) at a tertiary hospital in Tanzania.
Design:
Prospective observational study from November 2017 to May 2018.
Methods:
In all, 320 patients admitted to medical and surgical ICUs were consecutively enrolled. Baseline, clinical, and laboratory data were collected on admission and during their ICU stay. Serum creatinine and urine output were measured, and KDIGO criteria were used to determine AKI status.
Results:
More than half (55.3%) of ICU patients were diagnosed with AKI. Of these, 80% were diagnosed within 24 hours of admission. Acute kidney injury stage 3 accounted for 35% of patients with AKI. Patients with AKI were older, more likely to have cardiovascular comorbidities, and with higher baseline serum levels of creatinine, potassium, universal vital assessment admission scores, and total white cell count ≥12. Sepsis (odds ratio [OR] = 3.81; confidence interval [CI] = 1.21-11.99), diabetes (OR = 2.54; CI = 1.24-5.17), and use of vasopressors (OR = 3.78; CI = 1.36-10.54) were independently associated with AKI in multivariable logistic regression. Less than one-third of those who needed dialysis received it. There was 100% mortality in those who needed dialysis but did not receive (n = 19).
Limitations:
Being based at a referral center, the findings do not represent the true burden of AKI in the community.
Conclusion:
The prevalence of AKI was very high in ICUs in Northern Tanzania. The majority of patients presented with AKI and were severely ill, suggesting late presentation, underscoring the importance of prioritizing prevention and early intervention. Further studies should explore locally suitable AKI risk scores that could be used to identify high-risk patients in the community health centers from where patients are referred.
Keywords: acute kidney injury, risk factors, intensive care unit, low-income countries
Abrégé
Contexte:
L’insuffisance rénale aiguë (IRA) est une complication reconnue chez les patients gravement malades et son épidémiologie varie dans le monde en fonction du contexte et des critères diagnostiques utilisés. L’International Society of Nephrology (ISN) en appelle à une réduction à zéro des décès évitables dus à l’IRA d’ici 2025. On soupçonne que la majorité des cas d’IRA se trouvent dans des pays disposant de ressources limitées, mais le fardeau réel de la maladie dans ces contextes demeure inconnu.
Objectifs:
Notre objectif était de déterminer, avec les critères normalisés KDIGO, la prévalence de l’AKI, les facteurs associés et les caractéristiques cliniques des patients adultes admis aux unités de soins intensifs (USI) d’un hôpital de soins tertiaires en Tanzanie.
Type d’étude :
Étude observationnelle prospective couvrant la période de novembre 2017 à mai 2018.
Méthodologie:
Les 320 patients admis aux USI médicale et chirurgicale ont été inscrits de façon consécutive. Les données initiales, cliniques et de laboratoire ont été recueillies à l’admission et au cours du séjour à l’USI. Le taux de créatinine sérique et le débit urinaire ont été mesurés et le stade de l’IRA a été déterminé avec les critères KDIGO.
Résultats:
Plus de la moitié (55,3 %) des patients admis aux USI avaient reçu un diagnostic d’IRA, et 80 % de ceux-ci avaient été diagnostiqués dans les 24 heures suivant leur admission. L’IRA de stade 3 représentait 35 % des patients diagnostiqués. Les patients atteints d’IRA étaient plus âgés, présentaient des taux initiaux de créatinine et de potassium sériques plus élevés, et étaient plus susceptibles d’avoir une maladie cardiovasculaire concomitante, de même qu’un score d’évaluation universelle des signes vitaux à l’admission et un nombre total de leucocytes d’au moins 12. Une analyse de régression logistique multivariée a permis d’associer de façon indépendante l’IRA à la septicémie (RC: 3,81; IC: 1,21–11,99), au diabète (RC: 2,54; IC: 1,24–5,17) et à l’utilisation de vasopresseurs (RC: 3,78; IC: 1,36–10,54). Moins d’un tiers des patients ayant besoin de dialyse en avaient reçu. Tous les patients ayant besoin de dialyse, mais n’en ayant pas reçu (n = 19) sont décédés.
Limites:
L’étude s’est tenue dans un seul centre, les conclusions ne sont donc pas représentatives du véritable fardeau que représente l’IRA dans la communauté.
Conclusion:
La prévalence de l’IRA était très élevée dans les unités de soins intensifs du nord de la Tanzanie. La majorité des patients à l’étude souffraient d’IRA et étaient gravement malades, ce qui suggère une présentation tardive et souligne l’importance de prioriser la prévention et les interventions précoces. D’autres études devraient explorer les scores de risque localement accessibles qui pourraient être utilisés pour cerner les patients à haut risque dans les centres de santé communautaire d’où ils sont aiguillés.
Introduction
Acute kidney injury (AKI) is an important contributor toward poor patient outcomes worldwide and a recognized complication in critically ill patients, with a high incidence and poor short-term and long-term outcomes. 1 The incidence of AKI worldwide is estimated at 13.3 million people per year, leading to about 1.7 million deaths in a year. 2 The incidence of AKI in intensive care units (ICUs) varies widely across studies, reported to range between 5% and 67%.1,3-6A trend toward complicated AKI in the critically ill has been documented, with a significant proportion of these patients in hemodynamic instability and requiring renal replacement therapy (RRT). 7 A cross-sectional study in 97 centers worldwide found a high incidence of 57.3% of AKI in critically ill patients. 1 Data on incidence in low-income countries continue to be scarce, yet developing countries are reported to contribute to 85% of AKI cases worldwide. 2 Importantly, in the above-mentioned report, only 2 African countries were included. 1
In a neigboring country, Congo, the cummulative incidence of AKI in critically ill patients was as high as 52.7%. 8 This was associated with a significantly decreased mean survival time of 3 days versus 15 days in stage 3 and no-AKI groups, respectively. 8
In North Africa, 40% of the patients admitted to ICUs had AKI on admission, which was associated with worse outcomes. 9 Another study in Uganda found the incidence and in-hospital mortality of AKI in septic patients to be 16% and 21%, respectively. 10 Following the general trend in developing countries, there is a scarcity of data on AKI epidemiology in Tanzania and none found in the subset of critically ill adult patients in which AKI burden is known to be even higher.
Factors associated with an increased risk of AKI in critically ill patients include increasing age; infections, particularly sepsis; comorbidities such as diabetes and heart failure; higher severity of disease scores and baseline creatinine at admission; use of nephrotoxic drugs and vasopressors; and high-risk surgeries.11,12 In low-resource settings (LRS), patients are usually younger and risk factors involve common infections, sepsis, diarrheal illnesses, herbal intoxication, and toxins, whereas in high-resource settings (HRS), cases are usually older and involve chronic illnesses and organ failures. 13 Sepsis has been documented extensively across both settings.3,10,14,15
Given the myriad number of risk factors for AKI in Northern Tanzania, we thus expect a significant burden. However, historically, there has been limited documented patients with AKI diagnosis, both regionally and locally, including the previous dialysis initiative. 16 There is a great need to define the magnitude and relevant associated factors to understand and address poor outcomes in critically ill patients, an important step for improving outcomes and achieving the proposed goal of zero preventable deaths from AKI by the year 2025l. 2 Using standardized KDIGO (Kidney Disease Improving Global Outcomes) criteria, we aimed to prospectively determine the burden and important associated factors of AKI in patients admitted to ICUs at a referral hospital in Northern Tanzania.
Methods
This prospective cohort study was conducted in 4 medical and surgical ICUs at Kilimanjaro Christian Medical center (KCMC), a referral hospital in Northern Tanzania between November 2017 and May 2018. The KCMC is a tertiary center that services a population of over 15 million and is the main public hospital providing care for this population. On admission to ICUs, patients were seen and screened by a study research assistant (RA) within 24 hours, usually within 12 hours. Adult (≥18 years) patients admitted to the ICUs from whom consent was obtained were eligible for inclusion. Participants were excluded if they were discharged within 24 hours, were missing critical creatinine values for AKI diagnosis, had chronic kidney disease (CKD), or were on maintenance dialysis.
A piloted case report form was used to collect demographic information, baseline characteristics, medical history, admission vitals, exposures and risk factors for AKI, comorbidities, and lifestyle habits. Data were collected on admission and updated during the ICU period from clinical records and laboratory investigations. A detailed explanation of the clinical variables can be found in the Supplementary Material provided. Blood samples were taken for serum creatinine (SCr), which was measured at the KCMC laboratory, accredited through the Southern African Development Community Accreditation Services (SADCAS). The initial SCr taken on enrollment served to determine those with AKI on admission. Creatinine measurement was subsequently repeated for study purposes at 48 hours after admission. Urine output (UO) was monitored and recorded daily in the ICU, and any reduction in UO prompted additional SCr measurements. The KDIGO criteria were used to determine the proportion of patients with AKI. The criteria are normally based on a comparison with a known baseline SCr, to determine mild forms of AKI. This baseline SCr was obtained from previous patient records, where available. However, in this setting, it is not uncommon for patients not to have a previous documented prehospitalization creatinine, necessitating an estimation of a reference baseline SCr.17,18 In such a case, a reference SCr was used, which was either the admission SCr for those with no AKI on admission or otherwise estimated. Guidance for this is provided by KDIGO where an estimated baseline is obtained through a back-calculation using the Modification of Diet in Renal Disease (MDRD) equation and a glomerular filtration rate (GFR) of 75 mL/min/1.73 m2. 17 This estimation was needed for about a half (48%) of the cohort.
All patients with a prior diagnosis of CKD were excluded. Where it was previously undocumented, but CKD suspected, the diagnosis was based on clinical grounds using a combination of factors, including duration of illness, renal recovery, and radiological investigations assessing kidney size and architecture, urine, and hematological indices. A final decision was made in discussion with a nephrologist and renal team.
The requirement of RRT was defined as per protocol in use at KCMC renal department and includes the standard indications provided in the Supplemental Material. All decisions to start RRT were in discussion with the nephrologist. Assessment of the severity of illness on admission to ICU was done using the universal vital assessment (UVA) score at admission. This assessment method was previously validated in pooled studies in sub-Saharan Africa. 19 This score is cost-friendly and applicable in LRS and had an area under the receiver operating characteristic curve (AUC) of 0.77, which demonstrated superiority over Quick Sepsis-Related Organ Failure Assessment (AUC 0.69) and Modified Early Warning Score (AUC 0.70) in risk-stratifying patients and predicting mortality. 19 A Supplemental Material has been provided for further details on methodology.
The data were analyzed using STATA version 12IC. Summarization of numeric variables was done using measures of central tendency with their respective measures of dispersions. Categorical variables were summarized using frequencies and percentages and compared using a χ2 test or Fisher exact test (where applicable). The t test was used to compare difference in means for normally distributed variables and Kruskal-Wallis test for non-normal distribution. Logistic regression analysis was used to determine independent predictors of AKI. Variables of clinical relevance that were significant in univariate analysis were included in further multivariate logistic regression analysis, guided by previous similar studies. 3 A value of P ≤ .05 was considered to be statistically significant.
Ethical approval number 2077 has been obtained from the College Research Ethical Review Committee at Kilimanjaro Christian Medical University College (KCMUCo). Permission to conduct the study was concurrently obtained from the hospital administration. All participants or their designated next of kin provided written informed consent. The related study materials can be obtained on request from the corresponding author, as well as from KCMUCo.
Results
A total of 519 participants admitted were screened during the enrollment months between November 2017 and May 2018. Age was the most frequent reason for exclusion. Of the remaining 320 participants, more than half (55.3%) were diagnosed with AKI. The majority of these were diagnosed with AKI on admission (84.2%, n=320). (For more information, see Figure S1).
The cohort consisted mostly of middle-aged (median, 51 years; interquartile range [IQR], 35-70 years) male (57.2%) individuals, largely from the Kilimanjaro region (72.8%), with low levels of education (49.1%). Many participants had comorbidities, mainly cardiovascular-related (34.4%), mostly including hypertension, heart failure, and cerebrovascular diseases. Diabetes was reported in 15.6% of participants. Sepsis, cardiovascular diseases, diabetes, vasopressor use, and nephrotoxins were strongly associated with AKI in this univariate analysis. Patients with AKI had higher average values of potassium and were significantly more likely to have leukocytosis (white cell count ≥12). Nearly 80% of patients had either intermediate or high UVA admission scores. This was significantly associated with AKI. Hemoglobin values did not differ significantly by AKI status. Table 1 summarizes the clinical characteristics of the study cohort.
Table 1.
Clinical Characteristics of the Study Participants by AKI Status.
| Clinical characteristics | Total n (%) |
AKI-free patients n (%) |
AKI patients n (%) |
P value |
|---|---|---|---|---|
| Agea,b | 51 (35.70) | 46 (34.63) | 57 (37.73) | <.01 |
| Male | 183 (57) | 84 (46) | 99 (56) | .61 |
| Comorbidities | ||||
| Cancer | 20 (6) | 10 (7) | 10 (6) | .62 |
| CVD b | 110 (34) | 34 (24) | 76 (43) | .001 |
| Diabetes | 50 (16) | 14 (10) | 36 (20) | .01 |
| Liver disease b | 28 (9) | 11 (8) | 17 (10) | .55 |
| Nephrotoxic medications | 43 (13) | 12 (8) | 31 (18) | .03 |
| Surgical intervention | 96 (30) | 50 (35) | 46 (26) | .08 |
| Vasopressor use c | 34 (11) | 5 (3) | 29 (17) | <.001 |
| Anemia (Yes) d | 189 (59) | 82 (57) | 107 (60) | .39 |
| Glasgow Coma Scale (<9) e | 122 (38) | 50 (35) | 72 (41) | .24 |
| Infection present | 76 (24) | 24 (17) | 52 (29) | .008 |
| Sepsis | 26 (8) | 4 (3) | 22 (12) | <.01 |
| Universal vital assessment Admission score | <.01 | |||
| Low risk | 66 (21) | 38 (27) | 28 (16) | |
| Intermediate risk | 84 (26) | 43 (30) | 41 (23) | |
| High risk | 168 (53) | 62 (43) | 106 (60) | |
| Unknown | 2 (0.6) | 0 | 2 (1) | |
| Serum creatinine at ICU admission (µmol/L) a | 90 (65,141.5) | 66 (52,76) | 129 (97,206) | <.001 |
| Highest potassium in ICUa,f | 4.54 (3.96,5.38) | 4.3 (3.87,4.87) | 4.81 (4.08,5.66) | <.001 |
| Hemoglobin at admissiona,d | 11.6 (9.1,13.8) | 11.3 (8.6,13.8) | 11.9 (9.3,13.7) | .39 |
| White cell count | .02 | |||
| <12 | 207 (65) | 103 (72) | 104 (59) | |
| ≥12 | 106 (33) | 35 (24) | 71 (40) | |
| Unknown | 7 (2) | 5 (4) | 2 (1) | |
Note. Data presented as n (%); threshold values for anemia according to World Health Organization guidelines, overall male <13g/dL, female <12 g/dL. A detailed definition of variables has been provided in the Supplementary Material submitted separately. AKI = acute kidney injury; CVD = cardiovascular disease, a composite of heart failure, stroke, and/or hypertension; ICU = intensive care unit.
Median (interquartile range). N = 320, unless specified (bn = 319; cn = 315; dn = 311; en = 318; fn = 262).
P value given to 2 decimal places, or <.01 and <.001 for very small P values.
KDIGO stages 2 and 3 made up 60% of patients with AKI (stage 3 alone comprised 35% of all patients with AKI). Age, sepsis, hyperkalemia, oliguria, hypotension, and use of vasopressor in ICU showed a strong association with increasing severity of AKI. Furthermore, having multiple cardiovascular comorbidities was significantly associated with increasing severity of AKI (Table 3, Supplementary Material).
In a multivariate logistic regression analysis, sepsis (odds ratio [OR] = 3.8; confidence interval [CI] = 1.21-11.99), diabetes (OR = 2.5; CI = 1.24-5.17), and use of vasopressors (OR = 3.8; CI = 1.36-10.54) were found to be significantly associated with AKI over the study period (Table 2).
Table 2.
Multivariate Logistic Regression of Associated Factors With Acute Kidney Injury (N = 320).
| Characteristic | Odds ratio | P value | 95% confidence interval |
|---|---|---|---|
| Age | 1.01 | .13 | 0.99-1.02 |
| Gender | 0.96 | .86 | 0.59-1.55 |
| Sepsis | 3.81 | .02 | 1.21-11.10 |
| Nephrotoxic medication | 1.09 | .74 | 0.65-1.82 |
| Vasopressor use in intensive care unit | 3.78 | .01 | 1.36-10.54 |
| Hypertension | 1.26 | .46 | 0.68-2.35 |
| Diabetes | 2.54 | .01 | 1.25-5.17 |
| Heart failure | 1.67 | .20 | 0.77-3.60 |
Only 9 (32.1%) of the 28 patients who had an indication for RRT actually received it. Guidelines used for RRT initiation are provided in the Supplemental Material. The most common indication for RRT was symptomatic patients with uremia (46.4%, n = 13) (Table 4, Supplemental Material).
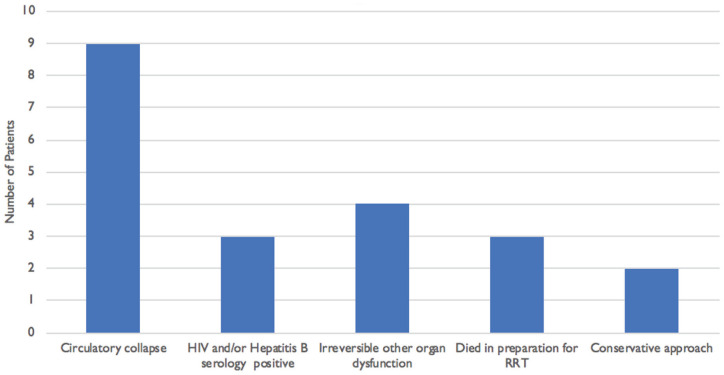
Of the 28 patients who needed dialysis, 23 patients died, comprising 4 who received dialysis and all (19) who did not receive dialysis. There are several reasons why those who needed dialysis did not receive. These included hemodynamic instability, death before the dialysis session, and other delays (Figure 1).
Figure 1.
Reasons for patients not receiving dialysis despite need, n = 19. Reasons are not mutually exclusive.
Note. RRT = renal replacement therapy.
Discussion
To our knowledge, this is the first study in Tanzania to look at the burden of AKI in critically ill adult patients. The ICU setting proved to be an ideal starting point to explore the burden of AKI. Our findings revealed a high burden of AKI, comprising over half of the study cohort. Although higher than what we expected, this finding is not surprising. We know that despite a scarcity of previous data in our region, the AKI burden has been noted to have a strong correlation with poor socioeconomic status. 20 There are several possibilities why the outcome of the dialysis initiative at KCMC years earlier did not yield many patients for dialysis. 16 It is possible that the diagnosis of AKI in this setting may have been suboptimal. This may be explained by the lack of adequate knowledge and education on AKI among health care workers. A survey at KCMC showed 89.4% of the workers had poor knowledge and could not detail the definition of AKI according to standard guidelines. In this survey, only 3.9% had received training on AKI. 21 This supports another survey where health care workers and nephrology representatives from different settings said the lack of awareness to AKI was up to 73%. 22 Adding to diagnostic challenges is the finding that death sometimes occurs in the first 24 hours, and hence some cases of AKI may have been previously missed. This emphasizes the need for early routine creatinine investigations in all ICUs.
The majority of AKI in our cohort was present at admission, supporting findings that AKI is predominantly community-acquired, mirroring similar settings.2,23,24 This is an important finding and has direct implications for prevention of AKI in this setting, which should be a community-based approach. There is a need to look closely into AKI recognition and management in community health centers and peripheral facilities in future research.
Risk factors identified for AKI closely mirror what has been found in other studies and include sepsis, hypotension, nephrotoxic medication, and comorbidities such as heart failure, hypertension, and diabetes.1,8,9,25 Our findings could reflect an unrealized large burden of AKI in ICU incorporating both patients with infections and comorbid cardiovascular diseases (CVDs) traditionally seen in the West. This pattern of AKI found in a tertiary center in Northern Tanzania differs from the typical description of AKI in LRS ascribed to infections and affecting younger individuals. 13 Our cohort included 34.4% of patients with CVD and an additional 15% with diabetes—having these comorbidities was significantly associated with AKI. Based on the current findings, it becomes important to recognize the contribution of CVDs to AKI. Where resources are limited, clinicians should be alert in prioritizing investigations and monitoring this group of patients. The fact that a prehospitalization creatinine is not always available in up to 50% of participants26,27 adds a challenge to the diagnosis of AKI particularly in limited-resource settings, where two-thirds of AKI is community-acquired. 17 The KDIGO and other guidelines have endorsed the use of the MDRD equation to estimate a reference baseline,17,28 which we adopted in this study. There are other ways of calculating an estimate, including the use of a GFR of 100 mL/min/1.73 m2 instead, which proved more accurate in a recent study. 18 It is worth mentioning that this study population was composed of young patients admitted with malaria in Malaysia, 18 where the more accurate outcome with the higher GFR could be expected. On the contrary, our cohort consisted of an older group, which was mostly middle-aged and elderly where the original GFR proposed by KDIGO may be still appropriate. 26 Nevertheless, although widely used in many studies,1,27,29,30 this formula has been shown to underestimate or overestimate the prevalence.27,31,32 Overall, research into better estimation methods and validation is needed. 26
In this study, 28 patients required RRT, of which only 9 received RRT. As discussed above, these patients were very ill, with circulatory collapse and irreversible organ dysfunction. The current mode of dialysis available is intermittent hemodialysis. Expanding services to include continuous hemodialysis may be beneficial for the subset of patients who did not have dialysis due to hemodynamic instability. Continuous renal replacement therapy has been shown to yield a lower mortality in severely ill patients. 33 In Tanzania, health care is partly subsidized; however, there is a substantial cost for patients to cover. This setting is one among few at the time of the study to have a dialysis center. However, resources were limited, and all dialysis was done in the main dialysis area, with no isolation unit, making it difficult to cater for patients with hepatitis. In this case, an alternative plan to another unit was inevitable, contributing to delays. The finding that a few succumbed in preparation for dialysis needs to be understood within the context, where late presentation is common, and both diagnosis and management decisions may take longer. The study did not look at the time to RRT initiation; however, it is an area for further evaluation. The fact that many patients do not have medical coverage and come from impoverished backgrounds makes RRT unaffordable to many. The average cost per dialysis was estimated at US $176 in the capital city, Dar-es-salaam. 34 In this center, fees for emergency RRT were waived to patients. This is not the case in many parts of the country. Given these economic implications among other challenges, investing in AKI prevention is paramount and a logical step that could move us closer to zero by 2025, an initiative by International Society of Nephrology to reach “0 preventable deaths from AKI by 2025.” 17 For this setting, this means 2 things: first, the need to develop a locally applicable risk score for AKI to identify patients at high risk to revert complications with AKI while channeling limited resources to those who are at high risk; second, the finding that most patients presented with AKI, some very advanced, suggests there is a need to look closely into AKI recognition and management in peripheral small facilities from where patients are referred in future research.
Limitations
We acknowledge that this was a hospital-based single-center study in a tertiary hospital and was therefore limited to sick patients who are referred. Hence, we could not estimate the true burden of AKI in the community. However, there are very few hospitals in the region, and these refer to our center, enabling us to capture the burden of AKI in critically ill patients in the region. Second, the UO monitoring was not ideal in some ICUs; however, all ICUs during the study period had UO recorded every 24 hours, which reflects the ideal scenario in resource-constraint areas. Third, the reference serum creatinine was not available in some patients, and a calculated baseline based on the MDRD equation assuming a GFR of 75 mL/min/1.73 m2 was used instead. Hence, the occurrence of AKI could have been underestimated or overestimated by a small proportion.27,31,32 The sample size was not very large; however, we used several methods to ensure a minimum loss to follow-up in the overall cohort. Finally, we did not follow up patients who recovered for long-term outcomes of AKI.
Conclusions
Acute kidney injury is a significant problem affecting over half of ICU patients. Associated factors are numerous, and rather a combination of infections and comorbidities, confirming the impact of the double burden affecting developing countries. Most patients were admitted with kidney injury; hence, studies are needed to estimate the burden of AKI in lower health facilities and communities.
Supplemental Material
Supplemental material, sj-pdf-1-cjk-10.1177_20543581211027971 for Acute Kidney Injury and Associated Factors in Intensive Care Units at a Tertiary Hospital in Northern Tanzania by Neema W. Minja, Huda Akrabi, Karen Yeates and Kajiru Gad Kilonzo in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: This study has been approved by the College Research and Ethics Review Committee (CRERC) at Kilimanjaro Christian Medical College (KCMUCo). (A Research Ethical Clearance certificate No. 2077 was issued). Permission to conduct research at the hospital was obtained from the hospital, Kilimanjaro Christian Medical Centre Ref. No: KCMC/P.1/Vol.IX. Consent to participate in the study was sought from participants or their next of kin.
Consent for Publication: Approval for publication was given by the National Institute for Medical Research (NIMR) Ref. No NIMR/HQ/P.12 VOL XXXII/43.
Availability of Data and Materials: Additional data and materials for the study are available through Supplementary Material submitted, as well as from the KCMUCo and corresponding author upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Neema W. Minja  https://orcid.org/0000-0003-2272-9153
https://orcid.org/0000-0003-2272-9153
Kajiru Gad Kilonzo  https://orcid.org/0000-0002-0367-5826
https://orcid.org/0000-0002-0367-5826
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hoste E, Bagshaw S, Bellomo R, Cely C. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411-1423. http://link.springer.com/article/10.1007/s00134-015-3934-7. Accessed June 15, 2021. [DOI] [PubMed] [Google Scholar]
- 2. Mehta RL, Cerdá J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616-2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 3. Andrikos E, Tseke P, Balafa O, et al. Epidemiology of acute renal failure in ICUs: a multi-center prospective study. Blood Purif. 2009;28:239-244. doi: 10.1159/000231986. [DOI] [PubMed] [Google Scholar]
- 4. Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11(3):R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis. Nat Rev Nephrol. 2011;7(4):209-217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2014;87. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613-1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 8. Masewu A, Makulo JR, Lepira F, et al. Acute kidney injury is a powerful independent predictor of mortality in critically ill patients: a multicenter prospective cohort study from Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2016;17(1):118. doi: 10.1186/s12882-016-0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abd Elhafeez S, Tripepi G, Quinn R, et al. Risk, predictors, and outcomes of acute kidney injury in patients admitted to intensive care Units in Egypt. Sci Rep. 2017;7:1-8. doi: 10.1038/s41598-017-17264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagasha P, Nakwagala F, Kwizera A, Ssekasanvu E, Kalyesubula R. Acute kidney injury among adult patients with sepsis in a low-income country: clinical patterns and short-term outcomes. BMC Nephrol. 2015;16:4. doi: 10.1186/1471-2369-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012. doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos PR, Monteiro DLS. Acute kidney injury in an intensive care unit of a general hospital with emergency room specializing in trauma: an observational prospective study. BMC Nephrol. 2015;16:30. doi: 10.1186/s12882-015-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cerdá J, Lameire N, Eggers P, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881-886. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 14. Uchino S, Kellum JA, Bellomo R, Doig GS, Tan I, Bouman C. Acute renal failure in critically ill patients: a multinational, multicentre study. JAMA. 2005;294:813-838. [DOI] [PubMed] [Google Scholar]
- 15. Oluseyi A, Ayodeji A, Ayodeji F. Aetiologies and short-term outcomes of acute kidney injury in a tertiary centre in Southwest Nigeria. Ethiop J Health Sci. 2016;26(1):37-44. doi: 10.4314/ejhs.v26i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kilonzo KG, Ghosh S, Temu SA, et al. Outcome of acute peritoneal dialysis in northern Tanzania. Perit Dial Int. 2012;32(3):261-266. doi: 10.3747/pdi.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellum J, a Lameire N, Aspelin P, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. doi: 10.1038/kisup.2012.7. [DOI] [Google Scholar]
- 18. Cooper DJ, Plewes K, Grigg MJ, et al. An evaluation of commonly used surrogate baseline creatinine values to classify AKI during acute infection. KI Reports. 2021;6(3):645-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore CC, Hazard R, Saulters KJ, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub-Saharan Africa. BMJ Glob Health. 2017;2(2):e000344. doi: 10.1136/bmjgh-2017-000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25(8):1834-1841. doi: 10.1681/ASN.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jonathan A, Kilonzo K. Level of knowledge on acute kidney injury among selected health care workers in Moshi. Moshi, Tanzania: Kilimanjaro Christian Medical University College; 2016. [Google Scholar]
- 22. Lunyera J, Kilonzo K, Lewington A, Yeates K, Finkelstein FO. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67:834-840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 23. Mehta RL, Burdmann EA, Cerdá J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387. doi: 10.1016/S0140-6736(16)30240-9. [DOI] [PubMed] [Google Scholar]
- 24. Fouda H, Ashuntantang G. The epidemiology of acute kidney injury in a tertiary hospital in cameroon: a 13 months review. J Nephrol Ther. 2016;6:3-7. doi: 10.4172/2161-0959.1000250. [DOI] [Google Scholar]
- 25. Bouchard J, Acharya A, Cerda J, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324-1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouchard J. Estimating baseline serum creatinine for assessning acute kidney injury: not a one size fits all approach. KI Reports. 2021;6:562-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bagshaw SM, Uchino S, Cruz D, et al. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24(9):2739-2744. doi: 10.1093/ndt/gfp159. [DOI] [PubMed] [Google Scholar]
- 28. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Závada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25(12):3911-3918. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 30. Maccariello E, Soares M, Valente C, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. 2007;33:597-605. doi: 10.1007/s00134-007-0535-0. [DOI] [PubMed] [Google Scholar]
- 31. Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5(7):1165-1173. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernier-Jean A, Beaubien-Souligny W, Goupil R, et al. Diagnosis and outcomes of acute kidney injury using surrogate and imputation methods for missing preadmission creatinine values. BMC Nephrol. 2017;18:1-9. doi: 10.1186/s12882-017-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kellum JA, Angus DC, Johnson JP, et al. Continuous versus intermittent renal replacement therapy: a meta-analysis. Intensive Care Med. 2002;28:29-37. doi: 10.1007/s00134-001-1159-4. [DOI] [PubMed] [Google Scholar]
- 34. Mushi L, Marschall P, Fleßa S. The cost of dialysis in low and middle-income countries: a systematic review. BMC Health Serv Res. 2015;15:1-10. doi: 10.1186/s12913-015-1166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cjk-10.1177_20543581211027971 for Acute Kidney Injury and Associated Factors in Intensive Care Units at a Tertiary Hospital in Northern Tanzania by Neema W. Minja, Huda Akrabi, Karen Yeates and Kajiru Gad Kilonzo in Canadian Journal of Kidney Health and Disease