Abstract
Objective: To evaluate the efficacy and safety of a 16-hr multilayer-release methylphenidate (PRC-063) in a community-based adult ADHD population. Method: In a double-blind study, 375 participants were randomized to one of four fixed doses of PRC-063 or placebo. The primary outcome was the ADHD-Rating Scale-5 (RS). The first 50% of double-blind completers were invited to participate in a 6-month dose-optimized open-label study to assess response and safety. Results: In total, 333 participants completed the double-blind trial; 184 entered the open-label study. PRC-063 produced greater symptom reduction in ADHD-RS-5 total score from baseline compared with placebo in the double-blind study (least-square [LS] mean = −4.7 [−7.7, −1.6], p = .003). The most frequent adverse events were headache, insomnia, and decreased appetite. No significant sleep quality impact was observed (p = .123). Significant improvements in ADHD-RS-5 scores from baseline continued through the open-label study (p < .0001), coincident with dose optimization. Conclusion: PRC-063 was well tolerated and significantly improved ADHD symptomatology in adults.
Keywords: attention deficit hyperactivity disorder, methylphenidate, stimulant, adult ADHD
Introduction
ADHD in adults has been estimated to have a prevalence of 4.4% and to be associated with a considerable burden of illness (Karlsdotter et al., 2016; Matza et al., 2005) and functional impairment (Able et al., 2007; Adler et al., 2008), in particular during periods of transition such as early morning or late in the day (Brown et al., 2017). In a recent survey, 44% of adults reported that duration of effect was among the top three reasons for choosing their current treatment regimen (Brown et al., 2017). Duration of effect was key in the decision to take more than one medication in 67% of respondents. Respondents also reported that lack of early morning and late night symptom control impaired their relationships, ability to carry out responsibilities, emotional responses, and mood. Management of symptoms in adults with ADHD requiring multiple daily dosing or a combination of short-acting and long-acting pharmacotherapy is likely to be associated with poor adherence (Gajria et al., 2014; Kooij et al., 2013).
Most short-acting products have a duration of action ranging from 4 to 6 hr, and long-acting stimulants are typically effective for 10 to 12 hr, whereas most adults have at least a 16-hr day. Some of the most stressful and attention demanding activities of an adult’s day occur in the early morning or evening, such as driving (Cox et al., 2012), parenting (Chronis-Tuscano & Stein, 2012), and activities of daily living (e.g., getting out of the house on time or paying bills). If adults with ADHD do not have adequate symptom control during these critical hours, they are going to be most symptomatic during those transitions that they find most problematic. Impairment is driven as much by how poorly someone functions at their worst, as it is by how well they are doing at their best. Failure to obtain an adequate response during key periods of the day is a serious barrier to normalization. In an adult population, adequate management of ADHD symptoms would ideally require a once-daily therapy with a rapid onset of action (≤1 hr) and a long duration of effect, extending to the end of the patient’s day (Spencer et al., 2008; T. Wigal et al., 2018b).
Up until recently, available long-acting stimulant preparations had a duration of action of 14 hr at most. In an adult workplace environment study of lisdexamfetamine, effects were noted from 2 hr after taking medication, up to 14 hr postdose (T. Wigal et al., 2010). A recently marketed extended-release mixed amphetamine salts preparation was shown to have an onset of action of 2 to 4 hr and a duration of action of 16 hr (T. Wigal et al., 2018a, 2018b). This mixed amphetamine salt compound is currently the only 16-hr amphetamine-based preparation available.
PRC-063 (marketed as FOQUEST® in Canada and as ADHANSIA XR™ in the USA) is a once-daily extended-release formulation of methylphenidate hydrochloride (MPH) designed to provide a rapid onset of action, along with continued symptom control throughout the day and evening. Following administration, PRC-063 demonstrates a bimodal plasma concentration-time profile of MPH with an initial peak at 1.6 hr, and a second, higher peak at 12.5 hr. MPH plasma levels then slowly decline with residual levels of approximately 18% at 24 hr post-administration. Steady state is achieved by 3 days of once-daily dosing (Quinn et al., 2016).
A previous double-blind (DB), controlled, crossover study evaluated the onset and duration of action of PRC-063 in adult ADHD patients in a controlled, simulated adult workplace environment (S. B. Wigal et al., 2020). PRC-063 demonstrated statistically significant improvements relative to placebo in the mean Permanent Product Measure of Performance (PERMP) scores in a simulated adult workplace environment day within 1 hr and up to 16-hr post-dose, exceeding currently available MPH or amphetamine formulations approved for ADHD in having both a quick onset and a 16 hr duration of action (S. B. Wigal et al., 2020). While the primary objective of that study was to determine the pharmacodynamic profile of this stimulant and response on laboratory workplace measures, the objectives of the studies reported here were to look at outcome as measured by a variety of standardized scales in a large community sample at different doses, and then followed-up naturalistically over time in an open-label (OL), dose-optimized study.
Two studies are reported here: a large, community-based randomized, DB, parallel, fixed-dose study to assess the efficacy and safety of PRC-063 in adults with ADHD compared with placebo, and a 6-month dose-optimized OL follow-up to characterize longer term safety and effectiveness. We report the first large, multi-site pivotal trial of a methylphenidate product with both a rapid onset of action and 16-hr duration.
Method
Study Conduct
These studies were conducted at 34 sites in the United States and Canada from April 2014 to January 2015. The study protocols were approved by an Institutional Review Board (IRB Services, Aurora, ON, or local IRB as required). All study participants signed an Authorization of Release of Personal Health Information for Research Purposes form and provided written informed consent prior to enrollment in the DB study and again prior to enrollment in the OL study. All documentation and procedures related to the studies were executed in accordance with Good Clinical Practice (GCP) guidelines as required by the Declaration of Helsinki 1964 and all of its amendments to this date, and the International Conference on Harmonization (ICH) Guideline for GCP (CPMP/ICH/135/95) of the European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products, ICH of Pharmaceuticals for Human Use.
DB Study (NCT02139124)
Participants
In total, 465 adults ≥18 years of age meeting the Diagnostic and Statistical Manual of Mental Disorders (5th ed., American Psychiatric Association, DSM-5) diagnostic criteria for ADHD were screened for study entry. Diagnoses were confirmed using the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID; Conners et al., 2004; Epstein & Kollins, 2006). Eligible participants included those with a post-washout baseline ADHD DSM-5 rating scale score ≥24 (Dupaul et al., 2016), who were either treatment naïve or dissatisfied with their current ADHD pharmacotherapy (310 participants [82.7%] were treatment naïve and 65 participants [17.3%] had previously received an ADHD medication that was discontinued at or prior to Visit 1). Participants had to have an IQ ≥ 80 on the Kaufman Brief Intelligence Test–2 (KBIT-2; Bain & Jaspers, 2010), a negative pregnancy test, and demonstrate that they could successfully swallow an empty 100 mg study capsule.
Participants were excluded from the study if they had a psychiatric comorbidity that required treatment (as judged by the investigator), history of allergy to stimulants or serious adverse reactions to MPH or were known to have a poor response to MPH treatment. Other exclusion criteria included, but were not limited to, a history of, or risk for, cardiovascular disease (including known family history of sudden cardiac death or ventricular arrhythmia), use of other psychotropic drugs, medications known to interact with MPH or herbal remedies, clinically significant laboratory abnormalities, or current suicide risk. Patients were allowed to take melatonin if they had been on a stable dose for at least 4 weeks.
Participant baseline clinical characteristics and demographics are summarized in Table 1.
Table 1.
Participant demographics and baseline clinical characteristics of the randomized population in the Double-Blind study.
| Characteristics | PRC-063 |
|||||
|---|---|---|---|---|---|---|
| Placebo | 25 mg/day | 45 mg/day | 70 mg/day | 100 mg/day | All PRC doses | |
| Randomized (n) | 78 | 77 | 73 | 73 | 74 | 297 |
| Completed (n, %) | 69 (88.5%) | 73 (94.8%) | 69 (94.5%) | 61 (83.6%) | 61 (82.4%) | 264 (88.9%) |
| Discontinued (n, %) | 9 (11.5%) | 4 (5.2%) | 4 (5.5%) | 12 (16.4%) | 13 (17.6%) | 33 (11.1%) |
| Age (M ± SD) | 37.4 ± 12.4 | 36.3 ± 12.5 | 36.0 ± 11.8 | 35.1 ± 11.2 | 35.3 ± 11.7 | 35.7 ± 11.8 |
| Sex (n, %) | ||||||
| Male (n, %) | 35 (44.9%) | 37 (48.1%) | 37 (50.7%) | 38 (52.1%) | 30 (40.5%) | 142 (47.8%) |
| Female (n, %) | 43 (55.1%) | 40 (51.9%) | 36 (49.3%) | 35 (47.9%) | 44 (59.5%) | 155 (52.2%) |
| Race (n, %) | ||||||
| White | 64 (82.1%) | 69 (89.6%) | 57 (78.1%) | 68 (93.2%) | 59 (79.7%) | 253 (85.1%) |
| Black/African American | 9 (11.5%) | 8 (10.4%) | 13 (17.8%) | 1 (1.4%) | 10 (13.5%) | 32 (10.8%) |
| Asian | 3 (3.8%) | 0 | 2 (2.7%) | 3 (4.1%) | 2 (2.7%) | 7 (2.4%) |
| Native American | 1 (1.3%) | 0 | 0 | 1 (1.4%) | 0 | 1 (0.3%) |
| Other | 1 (1.3%) | 0 | 1 (1.4%) | 0 | 3 (4.1%) | 4 (1.3%) |
| BMI (kg/m2; M ± SD) | 29.6 ± 8.7 | 29.1 ± 6.4 | 27.9 ± 6.1 | 27.7 ± 5.9 | 29.4 ± 7.3 | 28.6 ± 6.5 |
| ADHD subtype (n, %) | ||||||
| Inattentive | 22 (28.2%) | 22 (28.6%) | 17 (23.3%) | 14 (19.2%) | 19 (25.7%) | 72 (24.2%) |
| Hyperactive-impulsive | 2 (2.6) | 0 | 0 | 1 (1.4%) | 2 (2.7%) | 3 (1.0%) |
| Combined type | 54 (69.2%) | 55 (71.4%) | 56 (76.7%) | 58 (79.5%) | 53 (71.6%) | 222 (74.7%) |
| Duration of ADHD diagnosis, years (M ± SD) | 14.9 ± 15.6 | 14.2 ± 13.1 | 11.8 ± 13.0 | 13.1 ± 13.4 | 14.0 ± 15.4 | 13.3 ± 13.7 |
Note. BMI = body mass index.
Study design
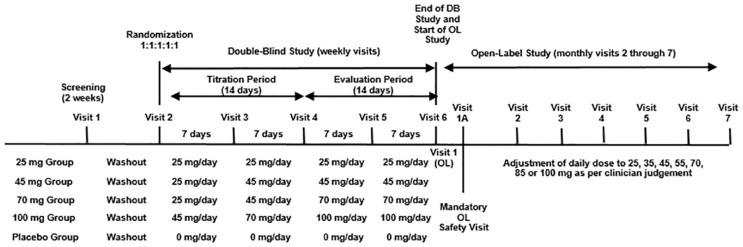
The study phases are illustrated in Figure 1. Following screening (up to 14 days) participants were washed out of any previous psychotropic medication for at least 7 days and then titrated over 14 days to their randomized dose of drug or placebo. Eligible participants were randomized by an integrated web response system in a 1:1:1:1:1 ratio to receive 25, 45, 70, or 100 mg/day of PRC-063 (MLR methylphenidate, Purdue Pharma [Canada], Pickering, Ontario) or matching placebo. Participants randomized to higher dose groups had their PRC-063 dose increased weekly until their randomized dose was achieved. Participants who did not enroll in the OL study completed a 14-day safety follow-up.
Figure 1.
Study timetable for both Double-Blind and Open-Label studies. Note. DB = double-blind; OL = open label.
Outcome measures
The primary endpoint was the between-treatment comparison of the change from baseline ADHD-Rating Scale-5 (RS) total score at the end of the study (Dupaul et al., 2016). The ADHD-RS-5 is a clinician-administered scale evaluating 18 symptoms of ADHD on a 4-point scale of severity (0–3), with lower scores indicating less severe ADHD symptoms. The ADHD-RS-5 was completed by the clinician at every visit.
Secondary endpoints included the within-treatment change from baseline on the ADHD-RS-5 and between-treatment differences on the Conners’ Adult ADHD Rating Scale–Self: Short Form (CAARS-S) and -Observer: Short Form (CAARS-O; Conners et al., 1999). The CAARS-S, completed by the participant, and CAARS-O, completed by a consistent observer, are 26-item tools that measure outcome in the following domains: inattention and memory problems, hyperactivity and restlessness, impulsivity and emotional liability, and problems with self-concept. Severity of each item is scored from 0 (“not at all”) to 3 (“very true”); the total raw score is corrected for gender and age to derive a t score value, and t scores >65 indicate a clinically significant issue. Participants and observers completed the CAARS at home twice during each week between clinic visits throughout the DB study. Treatment response was also measured using the Clinical Global Impression Scale of Improvement (CGI-I; assessed at Visits 3 through 6; Guy, 1976). The CGI-I is a 7-point scale in which participants are rated from “very much improved” to “very much worse” compared with baseline behavior. Participants rated as “much improved” or “very much improved” were considered to be responders. Clinicians measured improvement from the patient’s off-medication baseline.
The Pittsburgh Sleep Quality Index (PSQI) was administered to assess sleep quality at screening (over the past 6 months), Visit 2 (randomization; to establish a washout baseline), and Visit 6 (end of study; on study medication). The PSQI provides results in seven categories rated from 0 to 3 and a total Global PSQI score of up to 21, with lower scores indicating better sleep quality. A participant is considered to have “good sleep” with a score ≤5 and “poor sleep” with a score >5 (Buysse et al., 1989).
Additional secondary outcomes assessing functional impairment, executive function, and quality of life were assessed at baseline and end of study, results of which will be reported in a separate manuscript.
Safety and tolerability endpoints
Participants were assessed by the Principal Investigator (PI) at each site for treatment-emergent adverse events (TEAEs) throughout the DB study. At each contact with the participant, the PI sought information on TEAEs by examination or questioning in a general, nondirected manner. Vital signs (blood pressure, pulse, respiration rate, and weight) and the Columbia Suicidality Severity Rating Scale (C-SSRS; Madan et al., 2016) scores were assessed at all visits. Physical examinations, clinical laboratory tests (including serum pregnancy tests), and 12-lead electrocardiograms (ECGs) were evaluated at screening and end of study.
Statistical analyses
Sample size calculations were based on detecting the mean difference between all active doses combined and placebo for the primary efficacy endpoint of the ADHD-RS-5 total score. Assuming an effect size (ES) of 0.62, with a common standard deviation (SD) of 12.8, 300 completed participants were needed (60 participants in each of five treatment groups) to maintain a two-sided family-wise Type 1 error rate of 0.05, with 80% power. Based on a potential dropout rate of 17%, this study planned to randomize 360 participants.
The primary efficacy analysis was the ADHD-RS-5 total score at end of study (after 2 weeks of titrated treatment and 2 weeks of stable dose) based on an analysis of covariance (ANCOVA) model that included terms for treatment and baseline clinician ADHD-RS-5 total score as a covariate. Differences in least-square (LS) means were calculated for the separate dose levels compared with placebo along with two-sided 95% confidence intervals (CIs) for the treatment differences. The primary analysis involved multiple testing, wherein each active dose was tested against placebo separately. Therefore, Dunnett’s adjustments for multiple, pairwise, mean comparisons were used to compare the ADHD-RS-5 total scores for each of the four active treatments with placebo. For the Dunnett’s test, the family-wise Type 1 error rate was set at 0.05 (two-sided). The full analysis (FA) population, defined as all randomized subjects who received any amount of study medication and had an ADHD-RS-5 assessment during the 2-week, stable dose period, was used for the primary analysis, based on observed data. Sensitivity analyses were performed on the per protocol population (observed data) and the all randomized population using the Markov Chain Monte Carlo (MCMC) method to impute missing data. The ADHD-RS-5 subscale scores were analyzed similarly to the total scores as secondary analyses, using the FA population and observed data. The total score and the subscale scores from each visit earlier than Visit 6 were compared using this same model. In addition, a linear dose response was tested at Visit 6 to evaluate for a linear relationship between ADHD-RS-5 scores and dose level. Other secondary analyses included LS means of change from baseline for the separate dose groups, as well as all active doses compared with placebo. CGI-I scores were summarized categorically by visit and compared between dose groups on an ordinal scale using Wilcoxon rank sum tests. CAARS-S and CAARS-O subscale t scores between each dose group and placebo were compared using two-sample t tests, changes from baseline were compared using the ANCOVA model specified above and two-sided, 95% CIs were provided on the treatment difference. Safety endpoints are presented for the safety population (those taking at least one dose of the study medication) using descriptive statistics.
OL Study (NCT02168127)
Participants
Approximately the first 50% of participants to complete the DB study (whether they had been randomized to PRC-063 or placebo) were eligible to be enrolled in the OL extension study, provided the participants continued to satisfy the eligibility criteria of the DB study.
Study design
Participants provided informed consent and were initiated on a daily dose of PRC-063, as determined by the investigator, on the day following the end of the DB study, regardless of whether the participant previously received active or placebo treatment. Blinding for the DB study was not broken until after completion of the OL study. There were seven available PRC-063 doses in the OL study: 25, 35, 45, 55, 70, 85, and 100 mg/day. Participants returned 7 days following study initiation for a dose evaluation visit. Investigators used their clinical judgment to determine the optimal dose for individual participants. Participants were subsequently followed monthly up to 6 months postenrollment, with dose adjustments occurring as required. A 14-day safety follow-up telephone contact occurred following the 6-month, end of study visit.
During the 6-month OL study, ADHD-RS-5, PSQI, vital signs, C-SSRS scores, concomitant medications, and TEAEs were assessed at screening and all subsequent visits. Clinical laboratory tests (including serum pregnancy tests) were conducted at V1 and the final visit. Additional outcomes of functional impairment, executive function, and quality of life were assessed at the end of OL study and will be reported in a separate manuscript.
ADHD-RS-5 scores were summarized by visit, as well as baseline scores from the DB study for the subset of participants who entered the OL study. Changes from baseline were calculated and paired t tests were used to compare the baseline and follow-up scores within each dose group. Adverse events were summarized in the dose group corresponding to the last dose the participant took prior to the AE start date. The incidence of TEAEs was tabulated within each treatment group and for all participants combined.
Results
DB Study
Participant disposition
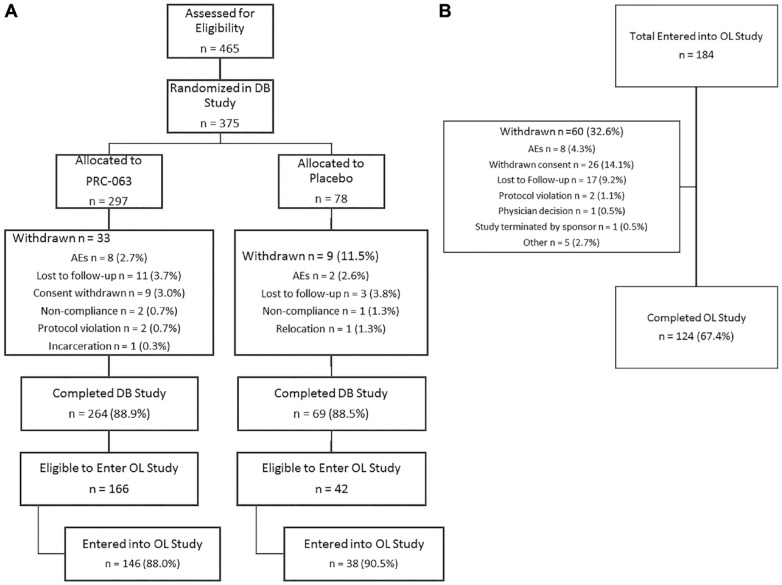
Participant disposition is shown in Figure 2A. Of the 375 participants randomized, 42 withdrew after randomization. Lost to follow-up was the primary reason for premature termination. The percentage of participants who discontinued was similar among those who received PRC-063 (11.1%) and placebo treatment (11.5%). There were more participants who discontinued at higher doses, and more of these discontinuations were due to TEAEs or being lost to follow-up.
Figure 2.
CONSORT diagram of participant disposition during: A. Double-Blind study; B. Open Label study.
Efficacy
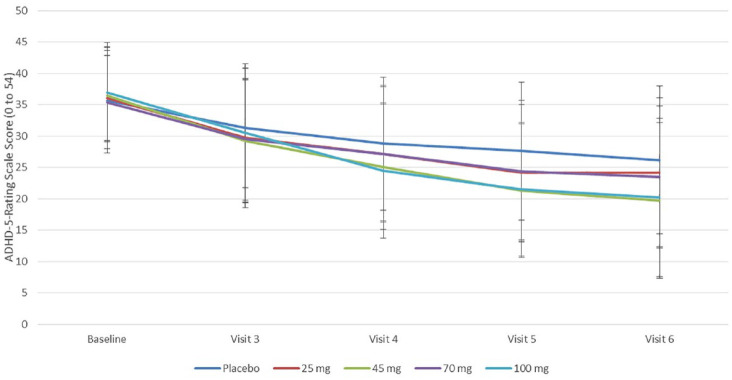
Participants receiving PRC-063 had a statistically significant improvement in ADHD symptomatology compared with participants receiving placebo at Visit 6, as measured by the change from baseline ADHD-RS-5 total score (LS mean [95% CI] between-treatment difference of −4.7 [−7.7, −1.6], p = .003, ES = –.40; Figure 3). Of the four active treatment groups, only the 45 mg (LS mean [95% CI] treatment difference of −6.9 [−11.5, −2.2]) and 100 mg groups (−8.1 [−12.9, −3.2]) had statistically significant between-treatment differences from placebo. At end of the DB study, all treatment groups were significantly improved from baseline (p < .0001; Table 2), with a 39.9% decrease from baseline in ADHD-RS-5 total scores in the PRC-063 group compared with a 26.9% decrease for the placebo group. All active treatment groups had greater LS mean improvements from baseline than the placebo group. Of participants receiving active PRC-063, 38.7% achieved ADHD-RS-5 total scores of ≤18 compared with 23.1% in the placebo group. The linear dose effect on response was statistically significant, as shown by the linear contrast between dose groups (p = .0002).
Figure 3.
Clinician-rated total ADHD-RS-5 score by treatment group Visit 2 (Baseline) through Visit 6 during the Double-Blind study.
Table 2.
Summary of efficacy endpoints by treatment group at the end of the Double-Blind study.
| Efficacy Endpoints | Double-blind study |
|||||
|---|---|---|---|---|---|---|
| Placebo (n = 78) | 25 mg/day (n = 77) | 45 mg/day (n = 73) | 70 mg/day (n = 73) | 100 mg/day (n = 74) | All PRC-063 subjects (n = 297) | |
| ADHD-RS-5 total score | ||||||
| Observed score at baseline | 35.7 | 36.1 | 36.5 | 35.4 | 37.0 | 36.3 |
| SD | 8.42 | 8.14 | 7.19 | 7.44 | 7.94 | 7.68 |
| Observed score at EOS | 26.1 | 24.2 | 19.9 | 24.0 | 18.7 | 21.8 |
| SD | 12.0 | 11.9 | 12.5 | 11.3 | 11.5 | 12.0 |
| p value vs. baseline | <.001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| CAARS-Self, t score | ||||||
| Observed score at EOS | 60.7 | 58.8 | 55.8 | 60.0 | 57.4 | 58.0 |
| SD | 13.1 | 12.1 | 13.8 | 11.6 | 12.2 | 12.5 |
| p value vs. baseline | — | NS | 0.0380 | NS | NS | NS |
| CAARS-Observer, t score | ||||||
| Observed score at EOS | 60.1 | 58.1 | 56.8 | 58.5 | 59.4 | 58.2 |
| SD | 12.3 | 12.7 | 13.2 | 13.3 | 10.5 | 12.5 |
| p value vs. baseline | — | NS | NS | NS | NS | NS |
| CGI-I at EOS n (%) patients | ||||||
| Very much improved | 10 (12.8) | 10 (13.0) | 23 (31.5) | 11 (15.1) | 16 (21.6) | 60 (20.2) |
| Much improved | 13 (16.7) | 25 (32.5) | 16 (21.9) | 15 (20.5) | 25 (33.8) | 81 (27.3) |
| Minimally improved | 18 (23.1) | 17 (22.1) | 18 (24.7) | 19 (26.0) | 9 (12.2) | 63 (21.2) |
| No change | 25 (32.1) | 19 (24.7) | 11 (15.1) | 15 (20.5) | 10 (13.5) | 55 (18.5) |
| Minimally worse | 2 (2.6) | 1 (1.3) | 1 (1.4) | 2 (2.7) | 1 (1.4) | 5 (1.7) |
| Much worse | 1 (1.3) | 1 (1.3) | 0 | 0 | 0 | 1 (0.3) |
| Very much worse | 0 | 0 | 0 | 0 | 0 | 0 |
Note. RS = Rating Scale; CAARS-Self = Conners’ Adult ADHD Rating Scale–Self: Short Form; CAARS-Observer = Conners’ Adult ADHD Rating Scale-Observer: Short Form; CGI-I = Clinical Global Impression Scale of Improvement; EOS = end of study; NS = not significant.
PRC-063 produced improvements compared with placebo on the ADHD Index t scores of the CAARS-S, but not the CAARS-O (Table 2). Mean t scores declined in all treatment groups through the course of the study. The LS mean [95% CI] difference from placebo in change from baseline was only significantly different than placebo on the CAARS-S ADHD Index in the 45 mg group (−4.6 [−8.5, −0.7], p = .022) and the 100 mg group (−4.2 [−8.2, −0.2], p = .042). At the end of study, both the mean ADHD Index t scores were below 65 for both placebo and active PRC-063 treatment groups on the CAARS-S and CAARS-O (Table 2).
At the end of the DB study, based on categorical analysis of the CGI-I score, 47.5% of participants on active PRC-063 were responders, rated as “much” or “very much improved,” versus 29.5% on placebo (p = .002, comparing ranked scores to placebo using Wilcoxon rank sum tests).
The mean ± SD global PSQI scores were 8.8 ± 3.7 and 8.4 ± 3.7 at baseline and 8.1 ± 3.6 and 7.3 ± 4.1 at end of study, for active PRC-063 and placebo treatment groups, respectively. The mean global PSQI score for the PRC-063 treatment group was not significantly different from placebo at end of study (p = .123).
Safety and tolerability
During the DB study, the frequency of TEAEs were lowest in the placebo group (44.9%) and increased in frequency with increasing daily dose of PRC-063 (51.9%, 65.8%, 69.9%, and 74.3% in the 25, 45, 70, and 100 mg group, respectively). The percent of participants experiencing severe TEAEs was 2.6% for the placebo treatment group and 4.0% for the PRC-063 treatment group. One serious adverse event (SAE) of “uterine cancer” occurred during this study, which was deemed to have “no reasonable possibility” of relatedness to the study medication by the investigator.
The most common adverse events (occurring in ≥5% of participants receiving PRC-063) from each group are listed in Table 3. The most frequent adverse events for PRC-063 and placebo were headache, insomnia, and decreased appetite. For participants in the PRC-063 treatment groups, the higher the dose of the treatment group, the greater the frequency in reports of decreased appetite, whereas reports of insomnia occurred at a similar frequency, irrespective of medication dose.
Table 3.
Summary of treatment-emergent adverse events (TEAEs) for placebo and all PRC-063-treated participants (safety population) and list of TEAEs occurring in ≥ 5% of the study population receiving PRC-063 for both Double-Blind and Open-Label studies.
| TEAEs | Placebo (n = 78) |
All PRC-063 treatments (n = 297) double-blind study |
All PRC-063 treatments (n = 184) open-label study |
||||||
|---|---|---|---|---|---|---|---|---|---|
| # | n | % | # | n | % | # | n | % | |
| Any TEAE | 77 | 35 | 44.9 | 516 | 194 | 65.3 | 455 | 145 | 78.4 |
| Treatment-related TEAEs | 48 | 25 | 32.1 | 392 | 158 | 53.2 | 282 | 118 | 63.8 |
| Severe TEAEs | 2 | 2 | 2.6 | 17 | 12 | 4.0 | 9 | 7 | 3.8 |
| TEAEs leading to withdrawal | 2 | 2 | 2.6 | 8 | 8 | 2.7 | 9 | 9 | 4.9 |
| Serious TEAEs | 0 | 0 | 0 | 1 | 1 | 0.3 | 4 | 4 | 2.2 |
| TEAEs occurring in ≥5% participants receiving study treatment | |||||||||
| Headache | 10 | 9 | 11.5 | 58 | 52 | 17.5 | 24 | 20 | 10.8 |
| Insomnia | 3 | 3 | 3.8 | 52 | 47 | 15.8 | 33 | 28 | 15.1 |
| Decreased appetite | 2 | 2 | 2.6 | 33 | 33 | 11.1 | 15 | 15 | 8.1 |
| Dry mouth | 3 | 3 | 3.8 | 27 | 27 | 9.1 | 13 | 12 | 6.5 |
| Nausea | 2 | 2 | 2.6 | 20 | 18 | 6.1 | 15 | 13 | 7.0 |
| Initial insomnia | 1 | 1 | 1.3 | 19 | 18 | 6.1 | 25 | 22 | 11.9 |
| Irritability | 4 | 4 | 5.1 | 17 | 16 | 5.4 | 14 | 12 | 6.5 |
Note. TEAEs = treatment-emergent adverse events.
Suicidal ideation was reported at a single visit for one participant (PRC-063 dose 100 mg), and suicidal behavior was reported at a single visit for another participant (placebo). Both participants completed the study without further reports of suicidal ideation or behavior.
Weight decreased in a near-dose-dependent fashion in participants receiving PRC-063 (change from baseline [SD] of −0.47 [1.55], −0.71 [1.54], −1.73 [5.78], −1.54 [2.15] kg for the 25, 45, 70, and 100 mg groups, respectively) and increased (change from baseline 0.62 [1.72] kg) in those receiving placebo. Minor increases in systolic and diastolic blood pressure were observed (maximum change from baseline 1.60 [11.2] mm Hg and 1.75 [7.36] mm Hg, respectively). There were no clinically significant changes from baseline in laboratory findings or vital signs observed in any of the treatment arms or during either study.
Open-Label Study
Participants and exposure
Of the first 208 participants who completed the DB study and were eligible for entry, 184 (88%) entered into the 6-month OL study, 38 of whom were previously on placebo, and 146 of whom were previously receiving active PRC-063 (Figure 2A). Of the 184 who entered, 60 were withdrawn and 124 completed the study (Figure 2B). The mean starting dose of PRC-063 in the OL study, based on the investigators’ clinical judgment, was 37.8 ± 12.0 mg/day (range = 25–85 mg/day). One week following OL study initiation, participants’ mean dose was adjusted to 50.8 ± 14.1 mg/day and, by the end of the first month of the OL study, the mean ± SD dose of PRC-063 for all participants had been increased to 64.0 ± 19.8 mg/day (range = 25–100 mg/day). By Month 2, the mean daily dose (71.1 ± 21.3 mg/day) had reached 97% of the optimized daily dose (73.3 ± 21.8 mg/day). More than 80% of participants were optimized on doses of 55 mg/day or greater. Of those participants who received active PRC-063 in the DB study, 89% (n = 131) were optimized at a different dose during OL [56% of participants (n = 83) received a lower than optimal dose, and 33% (n = 48) received a higher than optimal dose during the DB study].
Outcomes
Significant improvements on the ADHD-RS-5 total score from baseline and end of the DB study were observed within 1 month of entering the OL study (p < .0001), were sustained at each monthly visit throughout the OL study, and showed improvements from baseline of 24.4 (ES = 3.32) and from the end of the DB of 11.6 (ES = 1.23), respectively, for participants at Month 6 (p < .0001; Table 4). By the end of the OL study, 75.5% of participants had an ADHD-RS-5 score of 18 or less. Results in the OL study were similar regardless of whether participants were exposed to placebo or active medication in the DB study. Following 2 months of OL treatment, improvements on ADHD-RS-5 total score were ~86% of that achieved after 6 months OL treatment. Compared with prestudy baseline values, at the end of the 6-month OL study, there was a 68.3% improvement in ADHD symptoms (Table 4).
Table 4.
Summary of ADHD-RS-5 scores over the course of the Open Label study by month, overall and by Double-Blind study treatment allocation. Results are presented from Double-Blind baseline as well as end of Double-Blind study.
| ADHD-RS-5 mean score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DB treatment allocation | Parameter | Baseline | End of DB | Week 1 OL | Month 1 OL | Month 2 OL | Month 3 OL | Month 4 OL | Month 5 OL | Month 6 OL |
| Placebo | n (count) | 38 | 38 | 27 | 36 | 33 | 32 | 32 | 31 | 29 |
| M | 34.3 | 26.4 | 25.9 | 18.3 | 13.1 | 13.0 | 12.6 | 14.0 | 12.7 | |
| SD | 8.6 | 9.8 | 9.8 | 10.4 | 7.8 | 6.3 | 6.3 | 6.4 | 6.6 | |
| Active | n (count) | 146 | 146 | 105 | 144 | 131 | 115 | 106 | 102 | 95 |
| M | 36.1 | 22.0 | 21.8 | 15.8 | 12.9 | 12.0 | 11.7 | 10.8 | 10.9 | |
| SD | 7.7 | 12.3 | 11.0 | 10.1 | 8.1 | 8.2 | 8.0 | 6.3 | 7.8 | |
| All subjects | n (count) | 184 | 184 | 132 | 180 | 164 | 147 | 138 | 133 | 124 |
| M | 35.8 | 22.9 | 22.6 | 16.3 | 13.0 | 12.2 | 11.9 | 11.5 | 11.3 | |
| SD | 7.9 | 11.9 | 10.9 | 10.2 | 8.0 | 7.9 | 7.6 | 6.4 | 7.6 | |
Note. RS = Rating Scale; DB = double-blind; OL = open-label.
During the 6-month OL study, 78.4% of participants experienced TEAEs, of which 4.9% led to study withdrawal. The most common TEAEs for participants in the OL study were insomnia, initial insomnia, headache, and decreased appetite (Table 3). There were four SAEs reported (Bell’s palsy, dizziness, left breast cancer, and ruptured patellar tendon), and all were deemed to have “no reasonable possibility” of relatedness to the study medication. During the OL study, the mean global PSQI score for all participants was 5.4 ± 3.2, an improvement from the end of study values during the DB study for all PRC-063-treated participants. Suicidal ideation was reported by one participant at Month 5 in the OL study while receiving 45 mg/day. The participant completed the OL study without further reports of suicidal ideation or behavior.
Discussion
Following 4 weeks of treatment in this randomized, placebo-controlled, fixed-dose, community-based study, PRC-063 significantly improved the severity of ADHD symptoms in adults, as rated by clinicians using the ADHD-RS-5. This improvement was statistically superior to that observed with placebo treatment in the same study. These results were supported by clinicians’ global ratings of ADHD disease state, wherein nearly 50% of participants who received study drug were identified as responders on the CGI-I. This study failed to show statistical separation on the self and observer report of the CAARS, though it was not powered to do so. Overall, participants receiving PRC-063 showed a mean decrease in ADHD symptom severity from baseline of 39.9%, greater than the minimum 25% to 30% decrease from baseline ADHD-RS score previously defined as a clinically detectable response to treatment (Goodman et al., 2010). Although the randomized, fixed-dose, parallel design is not optimal for reporting overall improvement across doses, this is nonetheless a robust improvement as compared with similar trials. Similar improvements in ADHD-RS scores were achieved with SHP465, triple-bead mixed amphetamine salts, another long-acting stimulant with demonstrated efficacy 16 hr postdose (T. Wigal et al., 2018b).
Although the study did show response and separation from placebo on two key outcomes of efficacy, not all individual dose groups separated from placebo. Even though both the 25 and 70 mg dose groups improved 32.7% and 33.6%, respectively, from baseline, they were numerically, but not statistically, superior to placebo.
There was a high placebo response rate of 27.5% improvement from baseline. Although placebo response rates of 17% to 40% have been previously observed in multisite clinical studies in adults with ADHD, depending on the definitions used (Medori et al., 2008; Waxmonsky et al., 2011), a review of 10 ADHD clinical development programs consisting of 17 clinical trials found increasing placebo and ADHD medication response rates, whereas the ES for each treatment arm comparison remained the same over the decade (Khan et al., 2017). In a retrospective cohort analysis of the placebo arm of a metadoxine study for adult ADHD (Ben-Sheetrit et al., 2020), it was found that the placebo response was higher for clinician-rated than self-rated measures. The authors suggest a possible additive effect, whereby participant expectations of response may also be amplified by clinician expectations, leading to higher improvements in the clinician ratings. Given that the ADHD-RS-5 and the CGI-I are both clinician-rated measures, it is possible that such mechanisms were in effect here. They also suggested an unintended effect of therapeutic interactions with study staff as a mechanism for enhancing the placebo response, which certainly may have occurred with the intensity of the DB study weekly visits.
A secondary contributor to the lack of separation of some strengths from placebo may have been the randomized, fixed-dose design itself. Although often required by regulators, the procedure of assigning ADHD participants to a random dose, instead of titrating to an optimized dose for that individual patient, likely contributes to lower levels of efficacy. Based on doses subsequently selected in the OL study through optimization, 89% of the patients in the DB study were randomized to a dose that was too low—mitigating optimal effect—or too high—increasing the rates of drop out and adverse events. Thus, although the study was conducted in a large community sample, the treatment protocol during the DB study did not follow current treatment guidelines for ADHD which stress the importance of dose optimization (Canadian ADHD Resource Alliance [CADDRA], 2018; National Institute for Health and Care Excellence (NICE), 2018).
Our power analysis likely underestimated the sample size required for a five-group study with a high placebo response rate and considerable inter-individual variability in dose response to stimulants, decreasing the likelihood of measuring a clinically meaningful response within a relatively small number of participants randomized to a dose inappropriate to their needs.
When the data from the OL study in a subset of the same participants is examined, it showed that when the dose of PRC-063 was individually titrated and optimized, ADHD symptom severity continued to decrease until more than 75% of participants had ADHD-RS-5 total scores of 18 or less, indicating symptom remission. By 2 months, the mean OL dose reached approximately 97% of the final optimized OL dose, suggesting that the majority of participants were dose optimized by that time. This appeared to correlate with the decrease in symptom severity, which appeared to plateau at Month 2, with a 64% improvement from baseline, which was maintained for the remainder of the OL study. This supports the importance of individualized dose optimization, allowing participants to derive maximal effect from the medication. The fact that the time-course of continued improvement in the OL study matches the time-course of the dose optimization protocol suggests that the continued improvement observed was based on participants receiving an appropriate dose, and not a delay in onset of efficacy. Slow improvement over months of treatment is not characteristic of stimulants—where onset of action is generally almost immediate from the point of dose optimization—nor is it characteristic of the placebo response—typically most robust early on in treatment and attenuating over time. This also suggests that the low ES of the DB study of 0.40 can be partially explained by a majority of patients being under or over medicated. Other factors contributing to the robust response in OL versus DB would include selection bias of responders entering OL, the expectation of active treatment, and improved response especially in those who were randomized to very low doses or placebo in the DB.
The study medication was well tolerated, with no significant differences compared with placebo in sleep quality, satisfaction with appetite, or overall TEAEs. Only three TEAEs occurred in >10% of participants on PRC-063: headache, insomnia, and decreased appetite. The primary safety and tolerability profile of PRC-063 was similar to other long-acting psychostimulants in terms of type and frequency of TEAEs (T. Wigal et al., 2018b). The rates of TEAEs such as headache, decreased appetite, and dry mouth were reduced in the OL study once dose optimization occurred. There were no significant differences in global sleep quality (PSQI) score in the DB study between those receiving PRC-063 and those receiving placebo, and sleep outcome did not worsen in the OL study. Although melatonin was allowed for subjects who had taken a stable dose for at least 4 weeks prior to DB study entry, only a small number of subjects (2.7%) took melatonin during the trial.
Limitations
The conclusions are limited by some aspects of the study design. First, due to the magnitude of the placebo response, the DB study was not adequately powered to assess the effect of the individual PRC-063 doses. The limitations of the forced dose randomization design are more evident when considered in light of the outcomes observed in the dose-optimized OL study. Given the high inter-individual variability in dose response, the DB study does not reflect clinical outcomes that may be achieved for patients randomized to optimized doses of this 16-hr MPH versus placebo if each patient were maintained for a sufficient period of time. Although the OL study demonstrates the potential impact of optimally dosed PRC-063, it is limited by the lack of a blinded placebo control. Although it is acknowledged that more sophisticated methods of analyzing the data are possible, the results reported here are based on the statistical analysis plan proposed in the original protocol for regulatory purposes, and are therefore simple, generalizable, and repeatable by design.
Conclusion
These studies demonstrated the efficacy, safety, and tolerability of a novel multilayer-release 16-hr MPH treatment on core symptoms of ADHD, and the potential for maximal improvement with dose optimization. The medication was well tolerated, with an AE profile comparable with what has been seen in other stimulant studies in adults. Treatment with PRC-063 did not seem to occur at the expense of sleep, with a majority of patients considered to have normal sleep at the end of treatment. Adults may benefit from 16-hr formulations which cover the duration of a typical adult day, without the challenges of having to augment with additional doses of stimulants. To obtain a better understanding of the actual efficacy of PRC-063, a double-blind, randomized dose-optimized study would be needed.
Acknowledgments
Under the direction of the authors, medical writing was provided by Marsha Haynes, PhD (of Keen Scientific Services, Inc., Toronto, ON). Jodan Ratz, PhD, of Purdue Pharma (Canada) reviewed and edited the manuscript for scientific accuracy. The authors also acknowledge Joseph L. Reiz, BSc, for his contributions to the clinical development program for PRC-063.
Author Biographies
Margaret D. Weiss, MD, PhD, FRCP(C), is currently the director of clinical research in child psychiatry at Cambridge Health Alliance, Cambridge, MA. She is the author of the Weiss Functional Impairment Rating Scale, a widely used measure translated into 20 languages. She is on the advisory council of the Canadian Attention Deficit Disorder Resource Alliance (CADDRA) and the American Professional Society of ADHD and Related Disorders (APSARD).
Ann C. Childress, MD, is a psychiatrist and president at the Center for Psychiatry and Behavioral Medicine in Las Vegas, Nevada. She is an adjunct associate professor of psychiatry at Touro University Nevada. She has published numerous scientific papers and has also been an investigator for more than 150 studies of major depressive disorder, schizophrenia, and ADHD, among others.
Graeme A.E. Donnelly, MSc, is a clinical research scientist with 19 years of experience developing the ADHD therapeutic portfolio for Purdue Pharma Canada.
Footnotes
Authors’ Note: These studies have been presented in part as posters at the American Psychiatric Association Annual Meeting, May 16–18, 2016, Atlanta, GA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, analyses, authorship and/or publication of this article: M.D.W. has received consultant fees or received honoraria from Janssen, Purdue Pharma (Canada), Purdue Pharma L.P., Shire, Rhodes, Mundipharma, NLS Pharma, and Akili. A.C.C. has received research support from Akili, Alcobra, Arbor, Forest, Ironshore, KemPharm, Lilly, Lundbeck, Medgenics, Neos, Neurovance, NextWave, NLS, Noven, Otsuka, Pearson, Pfizer, Purdue Pharma (Canada), Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus, Theravance, and Tris; been a consultant for and received honoraria from Kempharm, Ironshore, Neos, Pfizer, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus and Tris; received travel support from Ironshore, NextWave, Pfizer, and Shire; has received writing assistance on projects from Arbor, Ironshore, Neos, NextWave, Pfizer, Rhodes Pharmaceuticals L.P., Shire and Tris; received payment for lectures from Arbor, Neos, Pfizer, Shire and Tris; and has been an advisory board member for Arbor, Ironshore, Neos, Neurovance, NextWave, Noven, Pfizer, Purdue Pharma L.P. and Rhodes Pharmaceuticals L.P. G.A.E.D. is an employee of Purdue Pharma (Canada).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Purdue Pharma (Canada).
ORCID iD: Margaret D. Weiss  https://orcid.org/0000-0001-8153-8213
https://orcid.org/0000-0001-8153-8213
References
- Able S. L., Johnston J. A., Adler L. A., Swindle R. W. (2007). Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychological Medicine, 37(1), 97–107. 10.1017/S0033291706008713 [DOI] [PubMed] [Google Scholar]
- Adler L. A., Spencer T. J., Levine L. R., Ramsey J. L., Tamura R., Kelsey D., . . . Biederman J. (2008). Functional outcomes in the treatment of adults with ADHD. Journal of Attention Disorders, 11(6), 720–727. 10.1177/1087054707308490 [DOI] [PubMed] [Google Scholar]
- Bain S. K., Jaspers K. E. (2010). Test Review: Review of Kaufman Brief Intelligence Test, Second Edition: Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman Brief Intelligence Test, Second Edition. Bloomington, MN: Pearson, Inc. Journal of Psychoeducational Assessment, 28(2), 167–174. 10.1177/0734282909348217 [DOI] [Google Scholar]
- Ben-Sheetrit J., Peskin M., Newcorn J. H., Daniely Y., Shbiro L., Rotem A., Weizman A., Manor I. (2020). Characterizing the placebo response in adults with ADHD. Journal of Attention Disorders, 24(3), 425–433. 10.1177/1087054718780328 [DOI] [PubMed] [Google Scholar]
- Brown T. E., Flood E., Sarocco P., Atkins N., Khachatryan A. (2017). Unmet medication coverage needs among adults with attention deficit/hyperactivity disorder (ADHD). Psychopharmacology Bulletin, 47(4), 18–28. https://www.ncbi.nlm.nih.gov/pubmed/28936005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D. J., Reynolds C. F., 3rd, Monk T. H., Berman S. R., Kupfer D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Canadian ADHD Resource Alliance. (2018). Canadian ADHD practice guidelines (4th ed.). [Google Scholar]
- Chronis-Tuscano A., Stein M. A. (2012). Pharmacotherapy for parents with attention-deficit hyperactivity disorder (ADHD): Impact on maternal ADHD and parenting. CNS Drugs, 26(9), 725–732. 10.2165/11633910-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Conners C. K., Epstein J., Johnson D. E. (2004). Conners’ adult ADHD diagnostic interview DSM-IV. Multi-Health Systems. [Google Scholar]
- Conners C. K., Erhardt D., Sparrow E. P. (1999). Conners’ adult ADHD rating scales, technical manual. Multi-Health Systems. [Google Scholar]
- Cox D. J., Davis M., Mikami A. Y., Singh H., Merkel R. L., Burket R. (2012). Long-acting methylphenidate reduces collision rates of young adult drivers with attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology, 32(2), 225–230. 10.1097/JCP.0b013e3182496dc5 [DOI] [PubMed] [Google Scholar]
- Dupaul G. J., Power T. J., Anastopoulos A. D., Reid R. (2016). ADHD rating Scale-5 for children and adolescents: Checklists, norms, and clinical interpretation. Guilford. [Google Scholar]
- Epstein J. N., Kollins S. H. (2006). Psychometric properties of an adult ADHD diagnostic interview. Journal of Attention Disorders, 9(3), 504–514. 10.1177/1087054705283575 [DOI] [PubMed] [Google Scholar]
- Gajria K., Lu M., Sikirica V., Greven P., Zhong Y., Qin P., Xie J. (2014). Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder—A systematic literature review. Neuropsychiatric Disease and Treatment, 10, 1543–1569. 10.2147/NDT.S65721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D., Faraone S. V., Adler L. A., Dirks B., Hamdani M., Weisler R. (2010). Interpreting ADHD rating scale scores: Linking ADHD rating scale scores and CGI levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Primary Psychiatry, 17(3), 44–52. [Google Scholar]
- Guy W. (1976). ECDEU assessment manual for psychopharmacology—Revised (DHEW Publ No ADM 76-338). U.S. Department of Health, Education, and Welfare, Public Health Services, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs. [Google Scholar]
- Karlsdotter K., Bushe C., Hakkaart L., Sobanski E., Kan C. C., Lebrec J., . . . Deberdt W. (2016). Burden of illness and health care resource utilization in adult psychiatric outpatients with attention-deficit/hyperactivity disorder in Europe. Current Medical Research and Opinion, 32(9), 1547–1556. 10.1080/03007995.2016.1189892 [DOI] [PubMed] [Google Scholar]
- Khan A., Mar K. F., Brown W. A. (2017). Does the increasing placebo response impact outcomes of adult and pediatric ADHD clinical trials? Data from the US Food and Drug Administration 2000–2009. Journal of Psychiatric Research, 94, 202–207. [DOI] [PubMed] [Google Scholar]
- Kooij J. J., Rosler M., Philipsen A., Wachter S., Dejonckheere J., van der Kolk A., . . . Schauble B. (2013). Predictors and impact of non-adherence in adults with attention-deficit/hyperactivity disorder receiving OROS methylphenidate: Results from a randomized, placebo-controlled trial. BMC Psychiatry, 13, Article 36. 10.1186/1471-244X-13-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A., Frueh B. C., Allen J. G., Ellis T. E., Rufino K. A., Oldham J. M., Fowler J. C. (2016). Psychometric reevaluation of the Columbia-Suicide Severity Rating Scale: Findings from a prospective, inpatient cohort of severely mentally ill adults. The Journal of Clinical Psychiatry, 77(7), e867–e873. 10.4088/JCP.15m10069 [DOI] [PubMed] [Google Scholar]
- Matza L. S., Paramore C., Prasad M. (2005). A review of the economic burden of ADHD. Cost Effectiveness and Resource Allocation, 3, Article 5. 10.1186/1478-7547-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medori R., Ramos-Quiroga J. A., Casas M., Kooij J. J., Niemela A., Trott G. E., . . . Buitelaar J. K. (2008). A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry, 63(10), 981–989. 10.1016/j.biopsych.2007.11.008 [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2018). Attention deficit hyperactivity disorder: Diagnosis and management (NG87). nice.org.uk/guidance/ng87 [PubMed]
- Quinn D. M., Bode T. D., Donnelly G., Reiz J. L. (2016). 6.49 Pharmacokinetics of a novel, extended-release formulation of methylphenidate (PRC-063) in adolescents with attention-deficit/hyperactivity disorder and in healthy adults at steady state. Journal of the American Academy of Child & Adolescent Psychiatry, 55, S219–S220. 10.1016/j.jaac.2016.09.368 [DOI] [Google Scholar]
- Spencer T. J., Adler L. A., Weisler R. H., Youcha S. H. (2008). Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: A randomized, double-blind, multicenter, placebo-controlled study. The Journal of Clinical Psychiatry, 69, 1437–1448. [DOI] [PubMed] [Google Scholar]
- Waxmonsky J. G., Waschbusch D. A., Glatt S. J., Faraone S. V. (2011). Prediction of placebo response in 2 clinical trials of lisdexamfetamine dimesylate for the treatment of ADHD. The Journal of Clinical Psychiatry, 72(10), 1366–1375. 10.4088/JCP.10m05979pur [DOI] [PubMed] [Google Scholar]
- Wigal S. B., Wigal T., Childress A., Donnelly G. A. E., Reiz J. L. (2020). The time course of effect of multilayer-release methylphenidate hydrochloride capsules: A randomized, double-blind study of adults with ADHD in a simulated adult workplace environment. Journal of Attention Disorders, 24(3), 373–383. 10.1177/1087054716672335 [DOI] [PubMed] [Google Scholar]
- Wigal T., Brams M., Frick G., Yan B., Madhoo M. (2018. a). A randomized, double-blind study of SHP465 mixed amphetamine salts extended-release in adults with ADHD using a simulated adult workplace design. Postgraduate Medicine, 130(5), 418–493. [DOI] [PubMed] [Google Scholar]
- Wigal T., Brams M., Gasior M., Gao J., Squires L., Giblin J., &. 316 Study Group. (2010). Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: Novel findings using a simulated adult workplace environment design. Behavioral and Brain Functions, 6, Article 34. 10.1186/1744-9081-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal T., Childress A., Frick G., Yan B., Wigal S., Madhoo M. (2018. b). Effects of SHP465 mixed amphetamine salts in adults with ADHD in a simulated adult workplace environment. Postgraduate Medicine, 130(1), 111–121. [DOI] [PubMed] [Google Scholar]