Abstract
This proof‐of‐concept study sought to determine the effects of standard of care (SOC) and a topically applied concentrated surfactant gel (SG) on the total microbial load, community composition, and community diversity in non‐healing diabetic foot ulcers (DFUs) with chronic biofilm infections. SOC was provided in addition to a topical concentrated SG, applied every 2 days for 6 weeks. Wound swabs were obtained from the base of ulcers at baseline (week 0), week 1, mid‐point (week 3), and end of treatment (week 6). DNA sequencing and real‐time quantitative polymerase chain reaction (qPCR) were employed to determine the total microbial load, community composition, and diversity of patient samples. Tissue specimens were obtained at baseline and scanning electron microscopy and peptide nucleic acid fluorescent in situ hybridisation with confocal laser scanning microscopy were used to confirm the presence of biofilm in all 10 DFUs with suspected chronic biofilm infections. The application of SG resulted in 7 of 10 samples achieving a reduction in mean log10 total microbial load from baseline to end of treatment (0.8 Log10 16S copies, ±0.6), and 3 of 10 samples demonstrated an increase in mean Log10 total microbial load (0.6 log10 16S copies, ±0.8) from baseline to end of treatment. Composition changes in microbial communities were driven by changes to the most dominant bacteria. Corynebacterium sp. and Streptococcus sp. frequently reduced in relative abundance in patient samples from week 0 to week 6 but did not disappear. In contrast, Staphylococcus sp., Finegoldia sp., and Fusobacterium sp., relative abundances frequently increased in patient samples from week 0 to week 6. The application of a concentrated SG resulted in varying shifts to diversity (increase or decrease) between week 0 and week 6 samples at the individual patient level. Any shifts in community diversity were independent to changes in the total microbial loads. SOC and a topical concentrated SG directly affect the microbial loads and community composition of DFUs with chronic biofilm infections.
Keywords: diabetic foot ulcer, biofilm, Poloxomer‐188, concentrated surfactant gel
1. INTRODUCTION
Biofilms are ubiquitous in nature, but they also impact human health both positively and negatively. In healthcare, there is increasing recognition that biofilms are a growing concern where they contribute to chronic and persistent infections, 1 in addition to scant reports which have demonstrated that biofilms may be further implicated as a contributor to delayed wound healing. 2 , 3 , 4 , 5 , 6
A hallmark feature of biofilms is their increased tolerance to antimicrobial therapeutics and host defences. 7 Physical removal of infected wound tissue wherever possible in tandem with multi‐faceted strategies often represents the optimal therapeutic approach. 8 , 9 , 10 Wound debridement and or physical removal of tissue alone may not be successful in removing all biofilm aggregates because of the macrospatial variation within tissues, 11 the inability of the treating clinician to visually identify and target biofilm aggregates accurately, or where debridement is contraindicated (ie, in the presence of severe arterial disease/inadequate perfusion). To augment debridement/standard of care (SOC) in the management of chronic wound infections, clinicians have increasingly adopted the widespread use of topical antimicrobial and non‐antimicrobial agents to target biofilm, despite limited robust data on their effectiveness. 12
A limited number of research groups have performed human in vivo proof‐of‐concept studies, utilising molecular DNA sequencing and microscopy approaches to explore the effects of topical antimicrobial wound agents on microbial (planktonic and biofilm) communities. 13 , 14 , 15 These studies have illustrated that topical antimicrobial therapy can lead to alterations in microbial community composition, diversity and load, and biofilm architecture. Recently, a non‐antimicrobial, concentrated surfactant gel (SG) demonstrated an ability to eliminate bacterial biofilms in a porcine skin explant model with daily application and wiping over 3‐day treatment period. 16 In this proof‐of‐concept study, we sought to explore the effects of SOC in addition to topical application of a concentrated SG on non‐healing diabetic foot ulcers (DFUs) with suspected chronic biofilm infections. Specimens were subjected to analysis using DNA sequencing, real‐time quantitative polymerase chain reaction (quantitative PCR [qPCR]), scanning electron microscopy (SEM) and peptide nucleic acid fluorescent in situ hybridisation (PNA‐FISH) with confocal laser scanning microscopy (CLSM). Wound metrics and rates of healing were outside the scope of this proof‐of‐concept study and are not included.
2. METHODS
2.1. Study design
A proof‐of‐concept study was undertaken in an out‐patient High‐Risk Foot Service in Sydney, Australia (Liverpool Hospital). Individuals aged over 18 years presenting with a DFU were prospectively recruited over an 18‐month period. Participants were eligible if they had a non‐healing neuropathic or neuroischaemic DFU in the presence of standard care, with a high clinical suspicion for a localised chronic biofilm infection 17 not requiring systemic antibiotic therapy. Eligible DFUs included wound – grades 1 and 2 (excluding exposed deep structures or bone involvement), ischaemia – grades 0–2, infection grade 0, as per the risk stratification of Wound, Ischaemia, and foot Infection classification (WIfI). 18 Sensory neuropathy was defined as abnormal vibration sensation, abnormal sensation with 10‐g Semmes‐Weinstein monofilament, and/or absence of Achilles deep tendon reflexes. 19 Non‐healing of a DFU was defined as follows: as a DFU of greater than 6 weeks duration failing to respond to standard care, in addition to no observed changes in wound metrics over a lead in period of 4 consecutive weeks prior to enrolment. SOC was defined as being; weekly treatments by a podiatrist performing appropriate wound bed preparation through sharp conservative debridement or curettage, wound cleansing with NaCl 0.9%, and the use of a non‐adherent absorbent wound dressing. Offloading of plantar DFUs through a removable cast boot (DH Offloading Walker, Össur Australia) and for non‐plantar DFUs, a post‐op shoe (Darco all‐purpose boot, Össur Australia).
Exclusion criteria were clinical signs and current diagnosis of an acute infection as per the International Working Group on the Diabetic Foot – diabetic foot infection guidelines, 20 known diabetic foot osteomyelitis that was associated with an ulcer, or patients who had received any new topical or systemic antimicrobial therapy 2 weeks prior to enrolment. The reason for excluding patients with acute clinical infection was the assumption that these wounds would be driven predominantly by planktonic microorganisms.
In addition to SOC, patients received the study topical agent (concentrated SG, Plurogel®, Medline Industries Inc), which was applied to the surface of DFUs every 2 days up to a maximum of 6 weeks. A non‐adherent absorbent dressing pad (Zetuvit, Hartmann Australia) was applied over the topical concentrated SG for exudate management. Tissue curettage (4‐mm dermal ring curette, Kai Medical) and wound swabs (DNA/RNA Shield Collection Tube w/Swab, Zymo Research, Irvine, CA) of the ulcer base were collected at each specified timepoint following cleansing with NaCl 0.9% and conservative sharp debridement. Wound swabs were collected using the Levine technique. Sample and data collection timepoints were week 0 (baseline), week 1, week 3 (Midpoint), and week 6 (end of treatment). The presence or absence of biofilms in DFUs was confirmed through scanning electron microscopy (SEM) and/or peptide nucleic acid fluorescent in situ hybridisation (PNA‐FISH) with CLSM. Microbial community composition and any shifts in diversity and load were explored through DNA sequencing (16S rRNA) and qPCR. Clinical metadata was collected from electronic patient medical records.
2.2. Topical concentrated SG (Pluorgel, Medline Industries)
Plurogel is an amorphous, water‐soluble, concentrated non‐ionic poloxamer 188‐based gel that utilises Micelle Matrix Technology.
2.3. Outcomes
Outcomes of the study were: confirmation of biofilm within DFU samples: differences in total microbial load (Log10 16S copies) between study timepoints, changes to microbial community composition and diversity pre‐ and post‐treatment with a concentrated SG.
2.4. Specimen collection, processing, and analysis workflow for DNA sequencing, qPCR, SEM, and PNA‐FISH
Sample collection and storage, DNA extraction, next‐generation DNA sequencing, sequence analysis and quality control, qPCR, and the characterisation and visualisation of biofilms in DFUs using SEM and PNA‐FISH have been previously described 14 , 15 , 21 and are available in full in S1 to S8. All tissue samples were stored until the end of the study recruitment period and processed in bulk. Participant identifiers and group allocation were not disclosed to the scientist undertaking the analyses.
2.5. Statistics
Raw data (total bacterial 16S copy numbers) were log transformed to create normally distributed data. A one‐way analysis of variance (ANOVA) was used to analyse the differences between the mean Log10 values of three‐independent study timepoints. For all comparisons and modelling in this exploratory, proof‐of‐concept study, the level of alpha significance was set at <0.10. Data are given as mean, median, interquartile range (IQR), confidence intervals (CI), and standard deviation (±). Data were analysed through Statistical Package for Social Sciences Version 25 (IBM Corp., Armonk, NY). The R Statistical Package (R Core Development Team, 2017) was used to generate microbial relative abundance and alpha diversity plots and to compute genomic related data statistical analysis. Analysis of similarity (AnoSim) 22 using the Bray–Curtis dissimilarity matrix was performed to differences in microbial community composition pre and post treatment.
2.6. Human research ethics
Ethics approval for this study was granted by the South West Sydney Local Health District Research and Ethics Committee (2018/ETH00597). Informed written consent was obtained prior to enrolment in keeping with ethics compliance and the study was conducted.
3. RESULTS
A total of 11 patients with 11 non‐healing DFUs and suspected chronic biofilm infections were enrolled. One participant (P_2) was withdrawn from the study after being lost to follow‐up after developing an acute diabetic foot infection requiring admission and surgical debridement of the DFU 1 week after onset of study treatment. One wound swab and one dermal ring tissue curette were obtained from each ulcer at baseline (week 0), week 1, week 3 (midpoint) and week 6 (end of treatment). Patient demographics and wound metrics are shown in Table 1. The presence of biofilm was confirmed in all 10 participants with non‐healing DFUs with suspected chronic biofilm infections using SEM and PNA‐FISH (Figure 1).
TABLE 1.
Clinical metadata of eleven patients with non‐healing DFUs and suspected chronic biofilm infections
| Characteristics | n = 11 |
| Mean age (years) | 55.7 (±8.6) |
| Gender (male/female) | 9/2 |
| Type of diabetes (type 1/type 2) | 0/11 |
| Duration of diabetes (years) | 21.3 (±7.8) |
| Comorbidities | |
| Loss of protective sensation | 11 |
| Ankle brachial index |
R: 1.07 (±0.19) L: 1.10 (±0.14) |
| Toe brachial index |
R: 0.79 (±0.35) L: 0.75 (±0.53) |
| Chronic kidney disease (stage 5) | 1/11 |
| Ischemic heart disease | 1/11 |
| Smoking status (never/current/past) | 7/2/2 |
| Wound characteristics | |
| Duration of DFU prior to enrolment (weeks) | 53 (±49) |
| Initial WiFi score |
200 (n = 8) 100 (n = 3) |
| Risk of amputation at 1 year |
Low (n = 8) Very low (n = 3) |
| DFU location |
Plantar Forefoot (n = 6) Dorsal forefoot (n = 1) Plantar midfoot (n = 2) Plantar hindfoot (n = 2) |
| Baseline wound surface area (mm2) | 738.45 (±437.6) |
| End of treatment wound surface area (mm2) | 538.6 (±286) |
FIGURE 1.
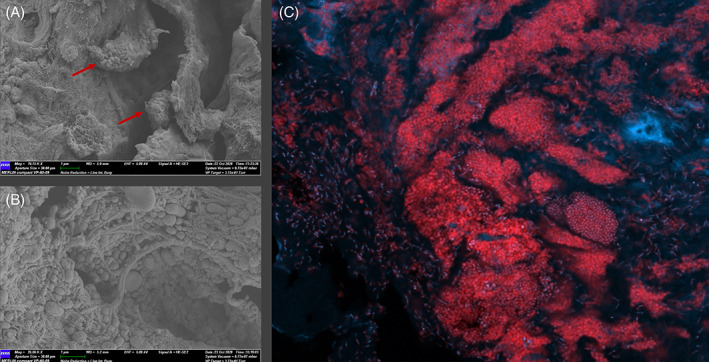
Microscopy of select patient samples to illustrate the confirmed presence of biofilm. All images obtained at baseline (week 0). A, Scanning electron microscopy image from the wound bed surface of a DFU tissue specimen (P_11) at 19000× magnification. Red arrows identify two small separate biofilm clusters with aggregates containing both coccoid and rod‐shaped microorganisms surrounded by a dense matrix. B, Scanning electron microscopy image from the wound bed surface of a DFU tissue specimen (P_8) showing a large aggregate of mixed microbial cells, both coccoid and rod‐shaped microorganisms. C, Demonstration of spatial organisation of bacteria using PNA‐FISH with confocal laser scanning microscopy in a non‐healing DFU (P_7) with suspected chronic biofilm infection. Tissue sections were stained with the universal bacterial probe Texas Red to illuminate bacterial cells within the tissue (red), followed by 40,6‐diamidino‐2‐phenylindole (DAPI), which was used as a counterstain to illuminate host cells such as neutrophils (blue)
3.1. Total microbial load of DFUs
The application of SG resulted in 7 of 10 samples achieving a reduction in mean log10 total microbial load from baseline to end of treatment (0.8 Log10 16S copies, ±0.6), and 3 of 10 samples demonstrated an increase in mean Log10 total microbial load (0.6 log10 16S copies, ±0.8) from baseline to end of treatment (Figure 2). The mean total microbial loads of all 10 patients grouped at baseline was 6.4 Log10 (±1), at midpoint 6.1 Log10 (±0.7) and end of treatment 6.0 Log10 (±0.9) (Figure 1). There was no statistical difference in the mean Log10 values between study timepoints (P = .63). Individual‐level data on Log10 values are further provided as supplementary information (S9).
FIGURE 2.
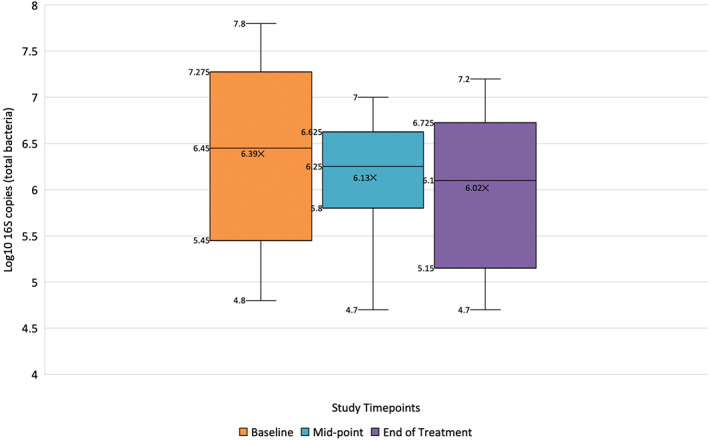
Mean, median, and interquartile range Log10 16S copies at baseline (week 0), midpoint (week 3), and end of treatment (week 6) in 10 patients receiving standard of care in addition to topical concentrated SG
3.2. Microbial community composition and diversity of DFUs treated with a topical concentrated SG
Wound swabs were collected from each patient at the intervals of week 0 (baseline), week 1, week 3, and week 6 (end of treatment). One patient (P_9) had missing data for week 1 (Figure 3). Data on the bacteria identified are presented as sub‐operational taxonomic units (sOTUs). OTUs are used to categorise bacteria based on their DNA sequence similarity. The top five most commonly sequenced and abundant (average abundance in %) sOTUs from patients at week 0 (pre‐treatment) were Corynebacterium sp., (25.9%, ±31.1), Streptococcus sp., (24.2%, ±27.7), Fusobacterium sp., (10.1%, ±15.3), Staphylococcus sp., (6.8%, ±14.7), and Anaerococcus sp., (4.7%, ±9.3) (Supplementary data).
FIGURE 3.
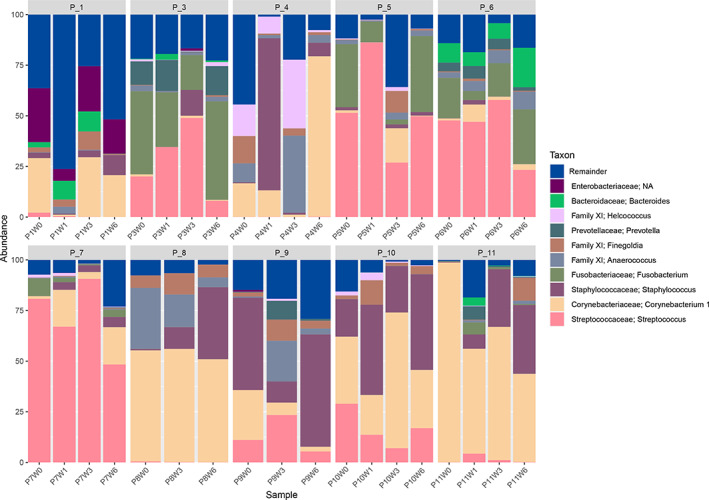
Bar charts of microbial composition (and their relative abundance in %) at the genus level at timepoints week 0 (baseline), week 1, week 3 (mid‐point), and week 6 (end of treatment). A cut‐off was applied to the top 10 most abundant taxa across patients, with all other taxa represented by the “remainder” group. Data are shown for each individual patient
To determine if the beta diversity changed over time, variations of microbial communities between samples were determined following the onset of SOC and a concentrated SG. Community composition between baseline and end‐of‐treatment samples for the entire dataset (week 0 vs week 6) were analysed using AnoSim with a Bray‐Curtis dissimilarity matrix. This identified no difference in the overall composition between samples for the study group (R = −0.069, P = .883). Generally, temporal changes to community composition were highly heterogeneous and only evident at the individual patient level.
As reported in previous molecular study designs, 4 , 15 sOTUs in this study either increased or decreased in their relative abundance; however, the most predominant sOTUs were generally not transient between timepoints. Any individual patient‐level changes in community composition were underpinned by alterations in the relative abundance of the most predominant sOTUs in the data set. Both Corynebacterium sp. and Streptococcus sp. frequently reduced in patient samples from week 0 to week 6 but did not disappear (ie, were not eradicated by treatment). In contrast, Staphylococcus sp., Finegoldia sp., and Fusobacterium sp. relative abundances frequently increased in patient samples from week 0 to week 6 (Supplementary data). Additionally, there were other sOTUs outside of the top five most abundant sOTUs who similarly increased or decreased in relative abundance. sOTUs of low relative abundance or uncommon sOTUs were most likely to be transient, either appearing or disappearing at each timepoint (Supplementary data).
To determine if the alpha diversity (within sample richness and evenness) changed over time, the number of observed sOTUs (Figure 4A) and Shannon index (Figure 4B) (total number of sOTUs and their evenness within the samples) was plotted for each time interval for each individual patient sample. The application of a concentrated SG resulted in varying shifts to diversity (increase or decrease) between week 0 and week 6 samples at the individual patient level. Any shifts in community diversity were independent to changes in the total microbial loads. For example, if community diversity increased, total microbial load did not increase, and vice versa. Despite any alterations in community diversity (Figure 4B), these changes were typically transient, occurring at time intervals of week 1 and week 3. However, by week 6, in most cases (excluding P_4, P_7 and, P_11), community diversity re‐established itself to similar levels noted at baseline.
FIGURE 4.
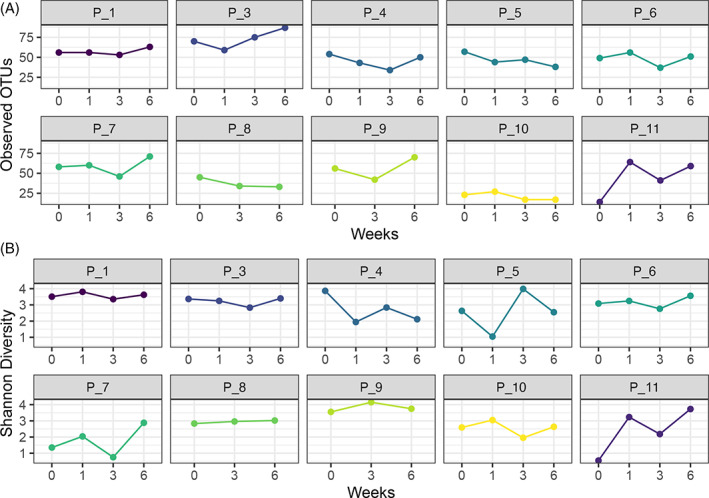
Comparison of alpha diversity in patient samples. A, Sample richness determined by the number of observed sOTUs and B, Shannon diversity, number of observed sOTUs, and their evenness
4. DISCUSSION
Microbial biofilms that form on human tissue have been implicated as causes of chronic infection. Previous studies in the areas of DFUs 3 , 6 , 13 , 14 , 15 and diabetic foot osteomyelitis 21 , 23 have identified that biofilms are often (but not always) polymicrobial in nature. Several alterations to growth, behaviour, and function render them highly tolerant to many antimicrobial treatments and host defences. 7 , 24 Physical removal and/or disruption of biofilms plays a pivotal role in wound care and this is typically undertaken by means of surgical or sharp conservative debridement. One limitation to physical debridement performed by a clinician is the inability to visualise or appreciate where biofilm aggregates are located within a wound bed. This may lead to an inability to remove all locally infected chronic tissue. 25 In this study, we sought to determine the effects of a non‐antimicrobial, concentrated SG on microbial communities (both planktonic and biofilm) in non‐healing DFUs in addition to SOC.
We demonstrate that SOC and a concentrated SG is able to reduce the total microbial load of DFUs with suspected chronic biofilm infection (mean 0.8 Log10 16S copies, ±0.6). This is similar to the in vivo effects achieved by an antimicrobial based wound agent in two previously published studies on DFUs complicated by biofilms. 13 , 14 We hypothesise that the concentrated SG facilitates autolytic debridement, sequestering microorganisms that are removed when the gel is wiped away at each visit. Further, non‐ionic surfactants aid in solubilising disaggregation of proteins, and can prevent protein adhesion to surfaces. 26 Microbial biofilms are typically defined by their surface association, aggregation and production of extracellular matrix. The concept that a non‐ionic surfactant can interfere with these aggregations, or solubilise their matrix, appears feasible. 16 However, we are not able to hypothesise if this in turn directly effects host wound repair.
Supporting clinical evidence does seem to suggest that topical agents may play an important role in improving wound healing rates in wounds complicated by chronic biofilm infections. One such clinical study by Kim and colleagues (2018) 27 of 43 patients (22 experimental, 21 control) with chronic wounds were randomised to a 12‐week treatment with a high osmolarity topical surfactant wound gel (experimental) or a broad‐spectrum antimicrobial ointment (control). Outcomes were to assess the effects of treatment on wound healing rates (wound size reduction and wound closure rates). Wound size in patients treated with a high osmolarity topical surfactant wound gel decreased significantly with a 71% reduction in wound area compared with 24% for the control (P < .001). Furthermore, 53% of patients treated with the high osmolarity topical surfactant wound gel achieved closure by 12 weeks as opposed to 17% who were treated with a broad‐spectrum antimicrobial ointment (P < .01).
However, no single DFU in this study exhibited eradication of microorganisms, nor was any Log10 reduction greater than 1.6 Log10. This trend has been observed by our group in three previous in vivo testing on DFUs with chronic biofilm infections. 13 , 14 , 28 The summation of our groups' in vivo human DFU studies have illustrated that no one topical antimicrobial has achieved reductions in the total microbial loads of >2.5 Log10. This may suggest one of two (or both) scenarios occur. Firstly, any effects to either planktonic or biofilm communities occur at the superficial wound surface interface, and thus any microorganisms spatially distributed in deeper tissue remain unaffected. Secondly, the concentrated surfactant has a minor affect to biofilm and removes mostly planktonic microorganisms.
Following treatment with a concentrated SG, we demonstrate reductions in the relative abundance of the most predominant sOTUs identified in patient samples; Corynebacterium sp. and Streptococcus sp. One exception to this finding was the increased relative abundance of Staphylococcus sp. post‐treatment, and it seems this microorganism was unaffected by SOC and a concentrated SG. Similar findings have been reported elsewhere. 15 The increased relative abundance of Staphylococcus sp. was typically observed in wounds that were the predominant sOTUs that decreased in abundance following treatment. This can be seen in patient samples P_8 to P_11 where reductions in the abundance of Corynebacterium sp. and Streptococcus sp. were met with the increased abundance of Staphylococcus sp. The latter patient samples (P_9 to P_11) had concomitant increases in microbial loads (S9). This diversity shift may have occurred as nutrient availability increased or where mutual benefit arose. 29
This study identified that SOC and a concentrated SG were able to reduce the total microbial loads and alter community composition and diversity over a 6‐week treatment period. The shifts in microbial communities and reductions in the most abundant bacteria identified in this study are similar to findings reported by Loesche and colleagues. 4 The study by Loesche and colleagues identified shifts in microbial communities were associated with faster healing and improved outcomes, and community stability was associated with non‐healing. Interestingly, the onset of SOC and treatment with a concentrated SG seem to cause a disruption to community composition and diversity in most patients, but this effect seems to stabilise as community diversity re‐establishes itself to similar levels noted at baseline. Future studies of larger sample sizes with longer treatment/data collection durations and data on wound metrics are needed. This may help determine if disruption and then stabilisation of microbial community composition and diversity in individual patients correlates to non‐healing‐healing‐non‐healing, or if continual disruption to microbial community composition and diversity leads to greater healing outcomes.
4.1. Study limitations
The primary aim of this proof‐of‐concept study was to ascertain the effects (if any) of a topical concentrated SG on microbial biofilm communities and not the effect on wound healing. As a proof‐of‐concept design with 10 patients, the small sample size is not sufficiently powered to correlate against wound healing data and thus our results provide a snapshot to support larger study designs of which the results can be generalised to the population affected by DFUs.
Without controlled conditions afforded by in vitro biofilm models, planktonic microorganisms likely contaminated samples. The total microbial load of DFUs likely reflects the presence of both planktonic and biofilm cells. However, our visualisation techniques (SEM and PNA‐FISH) support the predominance of biofilm aggregates within tissue specimens, identifying significant aggregates of microbial cells. PNA‐FISH utilises composite sections at different depths of tissue to offset limitations of SEM. PNA‐FISH was more accurate in this study in determining the extent of microbial aggregation in DFU specimens. When viewing tissue specimens under SEM, we commonly encountered difficulties regarding the spatial distribution of biofilms within tissue. These difficulties were predominantly associated with a large percentage of aggregates not being readily visible on the most outer surface of the tissue sections. Instead, many were located at deeper orientations or in pockets/crevices of tissue. This may explain a potential limitation of using topically applied agents for use in wounds, which may not have an ability to penetrate tissue or access deeper orientated microbial cells.
There are limitations of qPCR (based on 16S rRNA gene) with its inability to distinguish from viable and non‐viable cells in samples predominantly composed of biofilm phenotype cells with low metabolism. The log reductions noted in this study therefore represents the minimal response and we acknowledge that some of the bacteria detected by qPCR could be dead, resulting in a lower calculable Log10 for the topical concentrated SG.
As a research group, we have previously utilised molecular and microscopy approaches to explore the effects of different topical agents on the wound microbiome. We commonly used tissue specimens for both qPCR and 16S rRNA sequencing under the assumption that a wound swab may not detect microorganisms that have invaded deeper into tissue. Our rationale for using wound swabs in this study is supported from recent evidence identifying that wound swabs from DFUs using the Levine method were found to effectively detect bacteria at a similar or higher frequency to those recovered from tissue biopsy samples when using 16S rRNA sequencing. 30
CONFLICT OF INTEREST
MM received an investigator‐initiated research grant from Medline Industries for this study. All other authors have nothing to declare.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGEMENT
This work was supported by an investigator‐initiated study grant from Medline Industries.
Malone M, Radzieta M, Schwarzer S, Jensen SO, Lavery LA. Efficacy of a topical concentrated surfactant gel on microbial communities in non‐healing diabetic foot ulcers with chronic biofilm infections: A proof‐of‐concept study. Int Wound J. 2021;18:457–466. 10.1111/iwj.13546
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Vickery K. Special issue: microbial biofilms in healthcare: formation, prevention and treatment. Materials (Basel). 2019;12(12):2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schierle C, De la Garza M, Mustoe T, Galiano R. Staphylococcal biofilms impair wound healing by delaying re‐epithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354‐359. [DOI] [PubMed] [Google Scholar]
- 3. Kalan L, Loesche M, Hodkinson BP, et al. Redefining the chronic‐wound microbiome: fungal communities are prevalent, dynamic and associated with delayed healing. mBio. 2016;7(5):e01058‐e01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marano JR, Wallace JH, Wijeratne D, Fear WM, Wong SH, O'Handley R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci Rep. 2015;5:13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johani K, Malone M, Jensen S, et al. Microscopy visualisation confirms multi‐species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J. 2017;14(6):1160‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan J, Bassler BL. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe. 2019;26(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Høiby N, Bjarnsholt T, Moser C, et al. ESCMID* guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infec. 2015;21(1):S1‐S25. [DOI] [PubMed] [Google Scholar]
- 9. Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25(5):744‐757. [DOI] [PubMed] [Google Scholar]
- 10. Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time‐dependent therapeutic window. J Wound Care. 2010;19(8):320‐328. [DOI] [PubMed] [Google Scholar]
- 11. Price LB, Liu CM, Frankel YM, et al. Macro‐scale spatial variation in chronic wound microbiota: a cross‐sectional study. Wound Repair Regen. 2011;19(1):80‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwarzer S, James GA, Goeres D, et al. The efficacy of topical agents used in wounds for managing chronic biofilm infections: a systematic review. J Infect. 2020;80(3):261‐270. [DOI] [PubMed] [Google Scholar]
- 13. Malone M, Jensen SO, Gosbell IB, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non‐healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother. 2017;72(4):2093‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malone M, Schwarzer S, Radzieta M, et al. Effect on total microbial load and community composition with two vs six‐week topical Cadexomer iodine for treating chronic biofilm infections in diabetic foot ulcers. Int Wound J. 2019;16(6):1477‐1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalan L, Zhou M, Labbie M, Willing B. Measuring the microbiome of chronic wounds with use of a topical antimicrobial dressing—a feasibility study. PLoS One. 2017;12(11):e0187728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant‐based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J. 2017;14(2):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulton AJM, Armstrong DG, Hardman MJ, et al. Diagnosis and Management of Diabetic Foot Infections. Arlington (VA): American Diabetes Association; 2020. [PubMed] [Google Scholar]
- 18. Mills JL Sr, Conte MS, Armstrong DG, et al. Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59(1):220‐34.e1‐2. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med. 1998;158:289‐292. [DOI] [PubMed] [Google Scholar]
- 20. Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(S1):e3280. [DOI] [PubMed] [Google Scholar]
- 21. Malone M, Fritz BG, Vickery K, et al. Analysis of proximal bone margins in diabetic foot osteomyelitis by conventional culture, DNA sequencing and microscopy. APMIS. 2019;127(10):660‐670. [DOI] [PubMed] [Google Scholar]
- 22. Clarke KR, Warwick RM. Similarity‐based testing for community pattern: the two‐way layout with no replication. Mar Biol. 1994;118:167‐176. [Google Scholar]
- 23. Johani K, Fritz BG, Bjarnsholt T, et al. Understanding the microbiome of diabetic foot osteomyelitis: insights from molecular and microscopic approaches. Clin Microbiol Infec. 2018;25(3):332‐339. [DOI] [PubMed] [Google Scholar]
- 24. Orazi G, O'Toole GA. “It takes a village”: mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J Bacteriol. 2019;202(1):e00530‐e00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC. Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care. 2014;23(9):S4‐S8. [DOI] [PubMed] [Google Scholar]
- 26. Treter J, Bonatto F, Krug C, Soares GV, Baumvol IJR, Macedo AJ. Washing‐resistant surfactant coated surface is able to inhibit pathogenic bacteria adhesion. App Surf Sci. 2014;1(303):147‐154. [Google Scholar]
- 27. Kim D, Namen Ii W, Moore J, Buchanan M, Hayes V, Myntti MF. Hakaim A. clinical assessment of a biofilm‐disrupting agent for the management of chronic wounds compared with standard of care: a therapeutic approach. Wounds. 2018;30(5):120‐130. [PubMed] [Google Scholar]
- 28. Johani J, Malone M, Jensen SO, et al. Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: from in vitro to in vivo. J Antimicrob Chemother. 2018;73(2):494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Travis J, Malone M, Hu H, et al. The microbiome of diabetic foot ulcers: a comparison of swab and tissue biopsy wound sampling techniques using 16S rRNA gene sequencing. BMC Microbiol. 2020;20:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


