Abstract
Various lymph node functions are regulated by the sympathetic nervous system as shown in rodent studies. If human lymph nodes show a comparable neural regulation, their afferent nerves could represent a potential therapeutic target to treat, for example, infectious or autoimmune disease. Little information is available on human lymph node innervation and the aim of this study is to establish a comprehensive and accurate representation of the presence and location of sympathetic nerves in human lymph nodes. Since previous studies mention sympathetic paravascular nerves to occasionally extent into T cell‐rich regions, the relation of these nerves with T cells was studied as well. A total number of 15 inguinal lymph nodes were resected from six donated human cadavers. Lymph node sections were stained with HE and a double T/B cell staining for evaluation of their morphology and to screen for general pathologies. A triple stain was used to identify blood vessels, sympathetic nerves and T cells, and, to study the presence and location of sympathetic nerves and their relation to T cells. To evaluate whether the observed nerves were en route to other structures or were involved in local processes, adjacent slides were stained with a marker for varicosities (synaptophysin), which presence is suggestive for synaptic activity. All lymph nodes contained sympathetic nerves, both as paravascular and discrete structures. In 15/15 lymph nodes, nerves were observed in their capsule, medulla and hilum, whereas only 13/15 lymph nodes contained nerves in their cortex. The amount of sympathetic nerves varied between compartments and between and within individuals. In general, if a lymph node contained more paravascular nerves in a specific compartment, more discrete nerves were observed as well. Occasionally, discrete nerves were observed in relation to T cells in lymphoid tissues of the cortex and medulla. Furthermore, discrete nerves were frequently present in the capsule and hilum. The presence of varicosities in a portion of these nerves, independently to their compartment, suggested a local regulatory function for these nerves. Human lymph nodes contain sympathetic nerves in their capsule, trabeculae, cortex, medulla and hilum, both as paravascular or as discrete structures. Discrete nerves were observed in relation to T cells and non‐T cell‐rich areas such as the hilar and capsular connective tissue. The presence of discrete structures suggests neural regulation of structures other than blood vessels, which was further supported by the presence of varicosities in a portion of these nerves. These observations are of relevance in further understanding neural regulation of lymph node immune responses and in the development of neuromodulatory immune therapies.
Keywords: adrenergic innervation, lymph node, neuroimmune regulation, sympathetic innervation, sympathetic nerves
Human lymph nodes contain a significant number of sympathetic nerves (black encircled, pink stained TH positive structures). These nerves can be observed surrounding vascular structures, but also as discrete entities in the capsule, cortex, medulla and hilum. In the medulla, these nerves are frequently observed in proximity with immune cells such as T lymphocytes (CD3 positive cells as shown in the right images). Sympathetic nerves in lymph nodes might contribute to neuro immune regulation of lymph node immune function.

1. INTRODUCTION
Lymph nodes are strategically located secondary lymphoid organs which filter lymph and initiate local adaptive immune responses. Although long considered to function independently, nowadays it is known that this response can be regulated by the autonomic nervous system. This neural regulation predominantly occurs via the sympathetic neurotransmitter noradrenalin (NA) which influences lymph node lymphocyte proliferation, antibody secretion, blood perfusion, inflammatory cytokine production, and lymphocyte migration and egression, as shown in various rodent studies (Guyot et al., 2019; Madden et al., 1989; Madden et al., 1994; Nakai et al., 2014; Rogausch et al., 2004; Suzuki et al., 2016). In rodents, sympathetic varicosities have been observed in relation to lymphocytes which could attribute to this neural regulation (Felten et al., 1984; Giron et al., 1980). If human lymph nodes show a comparable neural regulation, lymph node nerves could represent a potential therapeutic target to treat, for example, infectious or autoimmune disease.
So far only two studies report on sympathetic innervation of human lymph nodes. Both studies primarily observed sympathetic nerves surrounding vascular structures and both mentioned that infrequently nerves branched off to extent into T cell‐specific parenchymal regions (Fink & Weihe, 1988; Panucio et al., 1998). Since the description of innervation patterns in these studies was very concise and no specific T cell staining was performed, the distribution patterns of sympathetic fibers in human lymph nodes remains unclear as well as their relation with T cells. To further consider therapeutic neural modulation of human lymph node immune response, understanding of the sympathetic innervation patterns of human lymph node is essential. Therefore, the aim of the current study is to establish a comprehensive and accurate representation of the presence and location of sympathetic nerves in human lymph nodes and to determine their relation to T cells.
2. MATERIALS AND METHODS
2.1. Staining procedures
Inguinal lymph nodes of 6 formaldehyde perfused cadavers were studied. These included three male and three female cadavers with a median age of 75.5, ranging from 69 to 91 years. No scars or skin infections were observed in the inguinal‐ or pelvic region or on the lower extremities. Bodies were donated through a body donation program to the Anatomy department of the University Medical Center Utrecht, the Netherlands. Informed consent was obtained during life, allowing the use of these bodies for educational and research purposes. Whole body preservation was accomplished by perfusion with 3% formaldehyde via the femoral artery. For each cadaver, 2‐4 inguinal lymph nodes, resulting in a total number of 15 lymph nodes, were macroscopically located, resected and further processed for paraffin embedding by placing them in increasing percentages of ethanol, xylene, and paraffin. Paraffin blocks were cut on a microtome (Leica 2050 Super Cut, Nussloch, Germany) and a series of 5 μm thick sections (originating from one level of the middle portion of the lymph nodes) were placed on glass slides, air dried, and subsequently heat fixed for 2 hours on a slide drying table of 60°C (Medax, 14801, Kiel, Germany). Hematoxylin/Eosin (HE) and a double T and Bcell immunostaining (using antibodies against specific membrane proteins, being CD3 and CD20, respectively) were used to evaluate technical tissue quality and to screen for general histoarchitectural changes (technical details on staining methods are listed in the following paragraphs). To evaluate the presence of sympathetic nerves in relation to T cells and blood vessels, a triple stain was performed using the markers Tyrosine Hydroxylase (TH), CD3 and von Willebrand Factor (vWF), respectively. Adjacent slides were single stained with a general nerve marker (Protein Gene Product 9.5 (PGP9.5)) to confirm neural identity of TH‐immune reactive (IR) structures and with a marker for presynaptic vesicles (synaptophysin) to determine the presence of varicosities and thereby confirm that these nerves were not en route, but most likely innervated local tissue. Prior to histochemical and immunohistochemical staining, all tissue sections were dewaxed in xylene and rehydrated through graded alcohols and distilled water.
2.1.1. HE staining
Tissue sections were stained with hematoxylin (Klinipath, Olden, Belgium) for 10 min. After rinsing in running tap water sections were dipped in ethanol 50%, stained with eosin (Klinipath) for 1 min and dehydrated in a graded series of alcohol and xylene. The slides were cover slipped with Entellan (Merck, Darmstadt, Germany). All steps were performed at room temperature (RT).
2.1.2. Immune histochemical staining procedures
Tissue sections were pretreated with Heat Induced Epitope Retrieval (HIER) in citrate buffer (pH6.0) for 20 min at 95°C. Tris‐buffered saline with 0.05% Tween20 (TBS‐T) was used for all washing steps and were performed at room temperature (RT). Table 1 contains technical details on the used antibodies. For the sequential triple stain, the sections were incubated with 3% Normal Goat Serum (NGS) in TBS prior to incubation with Rabbit anti‐human CD3 antibody (1:50 in TBS‐T + 1% BSA, 90 min, RT, Dako, Glostrup, Denmark). CD3 was visualized with undiluted Brightvision Poly‐AP Goat‐anti‐Rabbit (ImmunoLogic, Amsterdam, the Netherlands) and PermaBlue Plus/AP (Diagnostics Biosystems, Pleasanton, CA). Next, a HIER step was performed in citrate buffer (pH 6.0, 15 min, 95°C) to remove all unbound antibodies but leave the chromogen in place (van der Loos, 2007). Staining was continued by incubating with 5% NHS in TBS and rabbit anti‐human TH (1:1500 in TBS‐T + 1% BSA, overnight at RT), Pel Freez, Rogers AR). TH was detected with Brightvision Poly‐AP Goat‐anti‐Rabbit (ImmunoLogic) and liquid permanent red (LPR) (Dako). Next a HIER step was performed in Tris‐EDTA buffer (pH9.0, 15 min, RT) to remove all unbound antibodies. Finally, the sections were incubated with 5% NHS in TBS and Rabbit anti‐human vWF (1:2000 in TBS‐T + 1% BSA, 60 min RT, Dako), Brightvision Poly‐AP Goat‐anti‐Rabbit (ImmunoLogic) and PermaGreen Plus/AP (Diagnostics Biosystems). Sections were then dried on a hot plate for 15 min at 60°C and cover slipped with Entellan (Merck).
TABLE 1.
Primary antibodies used for immune histochemical staining
| Host + Clone | Vendor | Dilution, incubation time and temperature | |
|---|---|---|---|
| Tyrosine hydroxylase | Rabbit Polyclonal | PelFreez (Rogers, USA) | 1:1500, o.n. RT |
| PGP9.5 | Rabbit Polyclonal | Dako (Glostrup, Denmark) | 1:2000, 48 hours, 4ºC |
| Synaptophysin | Mouse Monoclonal (SP11) | Thermo Fisher (Fremont, USA) | 1:300, o.n., 4ºC |
| CD3 | Rabbit Polyclonal | Dako (Glostrup, Denmark) | 1:50, 90 min, RT |
| CD20 | Mouse Monoclonal (L26) | Dako (Glostrup, Denmark) | 1:50, 90 min, RT |
| Von Willebrand Factor | Rabbit Polyclonal | Dako (Glostrup, Denmark) | 1:2000, 60 min, RT |
For the sequential double stain for T‐ and B cells, sections were incubated with 5% Normal Human Serum (NHS) in TBS prior to incubation with Mouse anti‐CD20 (1:50 in TBS‐T + 1%BSA, 90 min, RT, Dako). CD20 was visualized with undiluted Brightvision Poly‐AP Goat‐anti‐Mouse and PermaBlue plus/AP (Diagnostics biosystems). To remove all antibodies but leave the chromogen in place, a HIER step in citrate buffer was performed (pH 6.0, 15 min, 95°C). Next all sections were incubated with 3% NGS in TBS prior to incubation with Rabbit anti‐ human CD3 antibody (1:50 in TBS‐T + 1% BSA, 90 min, RT, Dako). Staining was continued with Brightvision Poly‐AP Goat‐anti‐Rabbit (ImmunoLogic) and LPR (Dako). Sections were dried on a hotplate for 15 min at 60°C and cover slipped with Entellan (Merck).
For PGP9.5 and synaptophysin single staining, sections were incubated with 5% Normal Human Serum (NHS) in TBS prior to incubation with rabbit anti human PGP9.5 antibody (1:2000 in TBS‐T + 3% BSA, 48 hr, 4°C, Dako) or Synaptophysin (1:300 in TBS‐T + 1% BSA, overnight 4°C, Thermo Fisher, Fremont, CA), followed by visualization with undiluted Brightvision Poly‐Alkaline Phosphatase (AP) Goat‐anti‐Rabbit (ImmunoLogic) and LPR (Dako). Sections were counterstained with hematoxylin (Klinipath), dried on a hotplate for 15 min at 60°C and cover slipped with Entellan (Merck).
Lymph node vessel wall innervation was used as an intrinsic positive control for both neural markers and intrinsic lymph node vessels and T cells for vWF and CD3, respectively. Human spleen sections that were previously confirmed to show proper staining for B cells, T cells and sympathetic nerves were included as a positive control for CD20, CD3, TH and PGP9.5, respectively. Beta cells in human pancreas sections were used as positive controls for synaptophysin. Negative controls were obtained by incubation with TBS‐3%BSA without primary antibodies.
2.2. Microscopic evaluation
All slides were evaluated by bright field microscopy and in case of small TH, PGP and synaptophysin‐IR structures, which were visualized with the chromogen LPR, by additional fluorescent microscopy (LPR has stable fluorescent features, allowing to alternately switching between bright field and fluorescent microscopy). All samples were studied using a DM6 microscope (Leica, Nussloch, Germany) and in case of fluorescent microscopy, an I3 fluorescent filter.
2.2.1. Location and quantification of sympathetic nerves
Brightfield tile scans (stitched overlapping images) were captured of all lymph nodes using a 10x objective. Tile scans were opened in 3D paint (Microsoft office) and all sympathetic nerves were encircled manually. This resulted in overview images of lymph nodes with their sympathetic nerve distribution clearly visible. The general amount of sympathetic nerves (irrespective to whether these were paravascular or discrete nerves) for each compartment, being the capsule, cortex, medulla and hilum, was then quantified by means of scoring according to the following grading scale: ‐: complete absence, +: minor amount, ++: moderate amount and +++: substantial amount. Prior to scoring, all lymph nodes were evaluated by the observers in order to obtain a general idea of minimal and maximal amounts for each compartment. Each sample was examined independently by two observers (CC and CM). When there was disagreement between the observers the samples were re‐examined and scored by consensus.
2.2.2. Image acquisition
Brightfield single images were captured at various magnifications using a DM6 microscope with a motorized scanning stage, a DFC7000T camera and LASX software (all from Leica, Nussloch, Germany).
3. RESULTS
3.1. General observations
In total, 15 lymph nodes were evaluated. All showed typical morphological lymph node characteristics such as a capsule with trabecula, a subcapsular sinus, a cortex, medulla and a hilum (Figure 1). Depending on the level/angle of the sections, the amount of cortical, medullar and hilar tissue slightly varied. To a certain extent all lymph nodes showed common age‐related histoarchitectural changes such as lymphocyte paucity (resulting in less demarcated B‐ and T cell regions), fibrosis and lipomatosis (Hadamitzky et al., 2010). No significant pathologies were observed.
FIGURE 1.
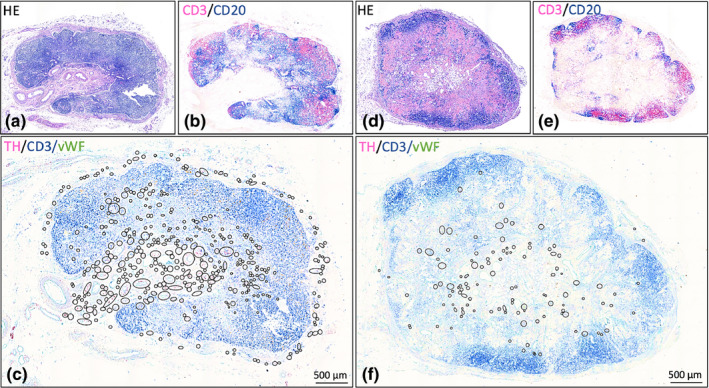
Lymph nodes with different quantities of sympathetic nerves. Hematoxylin / eosin and CD3/CD20 stained slides show the general lymph node morphology and distribution of lymphocytes, respectively. In overview images of TH/CD3/vWF stained slides, the presence of sympathetic nerves in the various compartments is indicated by black circles (in these overview images, nerves cannot be distinguished). More detailed images of sympathetic nerve distribution can be found in Figure 2. (a‐c): Lymph node (# 13) with high nerve quantity. (d‐f): Lymph node (# 15) with low nerve quantity. Black circled structures: sympathetic nerves ( Small circles: discrete nerve fibers or small nerves Larger circles: clusters of nerves / blood vessels with paravascular nerves)
3.2. Sympathetic nerve distribution
All lymph nodes contained sympathetic nerves in their capsule, cortex, medulla and hilum (Figure 1). The amount of sympathetic nerves per lymph node compartment varied between and within individuals. Table 2 contains an overview of the studied lymph nodes, including a semiquantitative evaluation of the amount of sympathetic nerves per compartment. Adjacent PGP‐stained slides confirmed the neural identity of the observed TH‐IR structures. In general, it was noticed that if a lymph node contained more paravascular nerves, the more discrete nerves were present as well.
TABLE 2.
Patient profiles and semi‐quantified data of lymph node sympathetic nerve distribution
| Cadaver | Sex | Age | Lymph node | Distribution of sympathetic nerves | |||
|---|---|---|---|---|---|---|---|
| Capsule | Cortex | Medulla | Hilum | ||||
| I | F | 81 | 1 (L) | ++ | ‐ | + | + |
| II | F | 91 | 2 (L) | + | + | ++ | ++ |
| 3 (R) | + | _ | ++ | ++ | |||
| III | M | 71 | 4 (L) | ++ | + | ++ | +++ |
| 5 (L) | ++ | ++ | ++ | +++ | |||
| 6 (L) | ++ | + | ++ | +++ | |||
| 7(R) | ++ | ++ | ++ | ++ | |||
| IV | M | 70 | 8 (L) | + | ++ | ++ | + |
| 9 (R) | + | ++ | ++ | ++ | |||
| V | M | 69 | 10 (L) | ++ | ++ | +++ | +++ |
| 11 (L) | +++ | +++ | +++ | +++ | |||
| 12 (R) | +++ | ++ | +++ | +++ | |||
| 13 (R) | +++ | ++ | +++ | +++ | |||
| VI | F | 86 | 14 (L) |
++ |
+ | + | ++ |
| 15 (R) | + | + | ++ | ++ | |||
Each compartment is evaluated as a distinct entity and the amount of nerves were scored as none (‐), the least amount of all lymph nodes (+), the most amount of all lymph nodes (+++) or valued as in between (++). This scoring method allows to compare various lymph nodes for the amount of sympathetic nerves in a specific compartment. Compartments within a lymph node cannot be compared. No distinction was made between paravascular and discrete nerves.
(L): from the left inguinal region (R): from the right inguinal region.
3.2.1. Capsule
All 15 lymph nodes showed a well‐developed capsule from which occasionally trabeculae emerged that penetrated the parenchyma. All capsules, and occasionally their trabeculae, contained paravascular and discrete sympathetic nerves (Figure 2d). Nerves were most frequently observed in the most outer layers of the capsule. Occasionally nerves were observed in deeper layers, but never in proximity to the subcapsular sinus.
FIGURE 2.
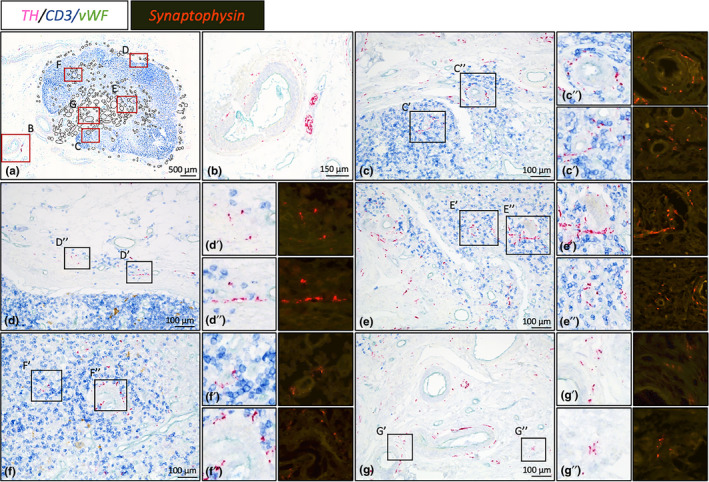
Magnified images of sympathetic innervation of lymph node # 13. Bright field images represent TH/CD3/vWF triple stained slides showing sympathetic nerves (in relation with), T cells and blood vessels, respectively. Synaptophysin stained slides show the presence of varicosities. (a): Overview image of lymph node #13. Black circled structures represent locations with sympathetic nerves. Red boxed areas are shown in more detail in figures B‐G. (b): Large sympathetic nerve bundle running with a lymph node artery towards the hilum. (c‐g): Nerves can be observed as paravascular or as discrete entities of which some are in close proximity with T cells. (c): Cortex, (d): Capsule, (e): Medulla, (f): Cortex, (g): Hilum
3.2.2. Cortex
In 13/15 lymph nodes, sympathetic nerves were present in the cortex. In most lymph nodes, cortical nerves were infrequently observed and showed a heterogeneously distribution, whereas in a few cases (the ones scored as ++ or +++), cortical nerves represented a more prominent feature with a homogeneous distribution. In the cortex nerves were observed either as paravascular nerves or as discrete nerves within T cell dense lymphoid tissue (Figure 2c,f). Occasionally paravascular nerves were observed to extent into T cell dense lymphoid tissue.
3.2.3. Medulla
Sympathetic nerves were observed in the medulla of all lymph nodes. In 2/15 LNs, medullary nerves were a rare observation whereas in other lymph nodes they appeared to represent a more residential feature. Nerves were observed surrounding vascular structures and in between the lymphoid tissue of the medullary cords (Figure 2e).
3.2.4. Hilum
All lymph nodes, contained sympathetic nerves in their hilum. In general, this compartment contained many blood vessels of various sizes with a paravascular sympathetic nerves (Figure 2g) and many fine discrete nerves in between its connective tissue (Figure 2g).
3.2.5. Synaptic activity
Synaptophysin‐IR was, irrespective to the compartment, observed in a portion of paravascular nerves and in discrete nerves (Figure 2c‐g).
4. DISCUSSION
The current study shows that human lymph nodes contain sympathetic nerves in their capsule, cortex, medulla and hilum, not only paravascular but also as discrete structures. Although these observations have been reported previously (Fink & Weihe, 1988; Panucio et al., 1998), this is the first study that visually showed sympathetic nerve distribution, semi‐quantified the amount for each compartment and showed that a portion of these nerves was in proximity with T cells.
Although previous studies suggest sympathetic innervation of human lymph node parenchyma to be a rare finding (Fink & Weihe, 1988; Panucio et al., 1998), the current study shows this represented a more common feature, especially in the medulla. Discrete sympathetic nerves were observed to be in close proximity with T cells and the presence of synaptophysin, and thus varicosities, suggests local neural activity. T cells are known to express beta (β) 2‐ adrenergic receptors (AR), which upon activation can regulate T cell differentiation, activation and effector function (recently reviewed by Qiao et al., 2018). Although the current study focused on the relation of sympathetic nerves with T cells, other immune cells, such as macrophages, dendritic cells and B cells express adrenergic receptors (Qiao, 2018) and might be under direct sympathetic control as well.
In addition to immune cells, reticular cells should also be taken into consideration as a neuro immune target. These cells represent the main cellular component of the reticular fiber network of secondary lymphoid organs and play a vital role in, for example, guided migration of immune cells by the production and secretion of specific chemokines (Murray et al., 2017). For rodents it has been shown that nerve endings are in contact with these reticular cells (Villaro et al., 1987), that these cells express adrenergic receptors and that their chemokine production is regulated by sympathetic nerves (Murray et al., 2017). The presence and function of similar adrenergic signaling components in the human reticular fiber network remains to be resolved.
Thus, lymph node immune function can be regulated directly via adrenergic receptors on various immune cells and indirectly via reticular cells. However, other sympathetic innervated structures might be involved in indirect regulation as well. These structures might include SMCs in capsule, trabeculae, hilum and vascular structures. All studied lymph nodes showed discrete sympathetic nerves in their capsules and occasionally in their trabeculae. These structures are known to contain a substantial amount of smooth muscle cells (SMCs) in addition to their fibrous connective tissue (Folse et al., 1975; Furuta, 1948) which most likely represent the neural target tissue of the observed fine discrete sympathetic nerves. In vitro electrostimulation of bovine capsular nerves resulted in contraction of these SMCs (Lobov & Pan’Kova, 2013) and hence contraction of the lymph node as a whole. Although the contribution of capsular and trabecular nerves to lymph node immune function remains to be resolved, one could assume that lymph node contraction affects, for example, lymph node lymph and blood flow, immune cell migration, and immune cell egression as described above.
A substantial amount of discrete sympathetic nerves was observed to reside in the connective tissue of the hilum. Interestingly, as shown by the presence of varicosities, these nerves are not en route to other structures, but most likely innervate the hilar connective tissue. Although the hilar connective tissue of normal lymph nodes does not contain nerves or cells that require neural regulation, it is known that superficial lymph nodes, and especially inguinal lymph nodes, can display SMCs as a result of local inflammation (Channer & Davies, 1985). If the observed discrete sympathetic nerves innervate hilar SMCs, their activation might result in hilar contraction, which in turn could affect hilar lymph and blood flow and hence lymph node function.
Sympathetic paravascular nerves were observed in all subjects of the current study. Although, these plexuses do not directly influence lymph node immune function, they regulate blood and lymph flow, and hence play an important role in regulating the entrance of antigen presenting cells via the lymph, the influx of immune cells from the blood and the migration of immune cells through the lymph node, all processes that should be optimal in order to conjoin the right cells for a properly aligned immune response (reviewed by Zhao et al., 2015).
Data from the current and previous studies (Fink & Weihe, 1988; Panucio et al., 1998) show that human lymph nodes do not contain a sympathetic subcapsular nerve plexus, a structure previously reported in mice (Felten et al., 1984). In mice the subcapsular plexus represents a prominent structure and varicosities have been observed in the cortical region just beneath this plexus. Although the function of this subcapsular plexus is unknown, this neural structure might be involved in species‐specific lymph node immune regulatory processes and should be taken into consideration when extrapolating animal data to humans.
Although in the current study sympathetic lymph node innervation was semi quantitatively analyzed at one specific level of the lymph nodes only (middle portion), the observations were considered to be representative for the whole lymph node since sections from another level of the same lymph nodes which were used in a preceding pilot study showed comparable sympathetic innervation patterns. This assumption was further supported by the fact that multiple lymph nodes of the same individual showed comparable numbers of nerves.
The variations of lymph node sympathetic nerve distribution between individuals as observed in the current study might be explained by the use of cadavers of different ages and with different clinical and social backgrounds, all known to alter sympathetic innervation patterns in secondary lymphoid organs (Bellinger et al., 1992; Sloan et al., 2007; Hoover et al., 2017). However, this study indisputably shows human lymph nodes to be sympathetically innervated and that based on its close relation with T cells and various other lymph node structures, it could play an essential role in regulation of the lymph node immune response.
5. CONCLUSION
Human lymph nodes contain sympathetic nerves in their capsule, trabeculae, cortex, medulla and hilum, both as paravascular or as discrete structures. Discrete nerves were observed in relation to T cells and non‐T cell‐rich areas such as the hilar and capsular connective tissue. The presence of discrete structures suggests neural regulation of structures other than blood vessels, and was confirmed by the presence of varicosities in a portion of these nerves. These observations are of relevance in further understanding neural regulation of lymph node immune responses and in the development of neuromodulatory immune therapies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTION
CC designed the study, performed data acquisition and analysis, designed the figures and wrote the manuscript. CM performed technical procedures and data acquisition, and helped writing the manuscript. DLB designed the study, performed technical procedures and helped writing the manuscript. BS evaluated the samples and helped writing the manuscript. RB supervised the project and helped writing the manuscript.
ACKNOWLEDGEMENT
The authors thank Suzanne Verlinde Schellekens of the Department of Anatomy at the University Medical Center Utrecht for her assistance in processing the tissue samples and staining procedures.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bellinger, D.L. , Ackerman, K.D. , Felten, S.Y. & Felten, D.L. (1992) A longitudinal study of age‐related loss of noradrenergic nerves and lymphoid cells in the rat spleen. Experimental Neurology, 116, 295–311. [DOI] [PubMed] [Google Scholar]
- Channer, J.L. & Davies, J.D. (1985) Smooth muscle proliferation in the hilum of superficial lymph nodes. Virchows Archiv A Pathological Anatomy and Histopathology, 406(3), 261–270. [DOI] [PubMed] [Google Scholar]
- Felten, D.L. , Livnat, S. , Felten, S.Y. , Carlson, S.L. , Bellinger, D.L. & Yeh, P. (1984) Sympathetic innervation of lymph nodes in mice. Brain Research Bulletin, 13(6), 693–699. [DOI] [PubMed] [Google Scholar]
- Fink, T. & Weihe, E. (1988) Multiple neuropeptides in nerves supplying mammalian lymph nodes : Messenger candidates for sensory and autonomic neuroimmunomodulation? Neuroscience Letters, 90(1–2), 39–44. [DOI] [PubMed] [Google Scholar]
- Folse, D.S. , Beathard, G.A. & Granholm, N.A. (1975) Smooth muscle in lymph node capsule and trabeculae. The Anatomical Record, 183(4), 517–521. [DOI] [PubMed] [Google Scholar]
- Furuta, W.J. (1948) The histologic structure of the lymph node capsule at the hilum. The Anatomical Record, 102(2), 213–223. [DOI] [PubMed] [Google Scholar]
- Giron, L.T. , Crutcher, K.A. & Davis, J.N. (1980) Lymph nodes—A possible site for sympathetic neuronal regulation of immune responses. Annals of Neurology, 8(5), 520–525. [DOI] [PubMed] [Google Scholar]
- Guyot, M. , Simon, T. , Ceppo, F. , Panzolini, C. , Guyon, A. , Lavergne, J. et al. (2019) Pancreatic nerve electrostimulation inhibits recent‐onset autoimmune diabetes. Nature Biotechnology, 37(12), 1446–1451. [DOI] [PubMed] [Google Scholar]
- Hadamitzky, C. , Spohr, H. , Debertin, A.S. , Guddat, S. , Tsokos, M. & Pabst, R. (2010) Age‐dependent histoarchitectural changes in human lymph nodes: An underestimated process with clinical relevance? Journal of Anatomy, 216(5), 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover, D.B. , Brown, T.C. , Miller, M.K. , Schweitzer, J.B. & Williams, D.L. (2017) Loss of sympathetic nerves in spleens from patients with end stage sepsis. Frontiers in Immunology, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov, G.i. & Pan’kova, M.n. (2013) Involvement of α‐adrenoceptors to the implementation of the contractile effects in the capsule of mesenteric lymph nodes in response to electrostimulation. Bulletin of Experimental Biology and Medicine, 154(5), 588–590. [DOI] [PubMed] [Google Scholar]
- Madden, K.S. , Felten, S.Y. , Felten, D.L. , Sundaresan, P.R. & Livnat, S. (1989) Sympathetic neural modulation of the immune system. I. Depression of T cell immunity in vivo and in vitro following chemical sympathectomy. Brain Behavior and Immunity, 3(1), 72–89. [DOI] [PubMed] [Google Scholar]
- Madden, K.S. , Moynihan, J.A. , Brenner, G.J. , Felten, S.Y. , Felten, D.L. & Livnat, S. (1994) Sympathetic nervous system modulation of the immune system. III. Alterations in T and B cell proliferation and differentiation in vitro following chemical sympathectomy. Journal of Neuroimmunology, 49(1–2), 77–87. [DOI] [PubMed] [Google Scholar]
- Murray, K. , Godinez, D.R. , Brust‐Mascher, I. , Miller, E.N. , Gareau, M.G. & Reardon, C. (2017) Neuroanatomy of the spleen: Mapping the relationship between sympathetic neurons and lymphocytes. PLoS One, 12(7), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai, A. , Hayano, Y. , Furuta, F. , Noda, M. & Suzuki, K. (2014) Control of lymphocyte egress from lymph nodes through Β2‐adrenergic receptors. Journal of Experimental Medicine, 211(13), 2583–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuncio, A.l. , De la pena, S. , Gualco, G. & Reissenweber, N. (1999) Adrenergic innervation in reactive human lymph nodes. Journal of Anatomy, 194, 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, G. , Chen, M. , Bucsek, M.J. , Repasky, E.A. & Hylander, B.L. (2018) Adrenergic signaling: A targetable checkpoint limiting development of the antitumor immune response. Frontiers in Immunology, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogausch, H. , Böck, T. , Voigt, K.‐H. & Besedovsky, H. (2003) The sympathetic control of blood supply is different in the spleen and lymph nodes. NeuroImmunoModulation, 11(1), 58–64. [DOI] [PubMed] [Google Scholar]
- Sloan, E.K. , Capitanio, J.P. , Tarara, R.P. , Mendoza, S.P. , Mason, W.A. & Cole, S.W. (2007) Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. Journal of Neuroscience, 27(33), 8857–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K. , Hayano, Y. , Nakai, A. , Furuta, F. & Noda, M. (2016) Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. Journal of Experimental Medicine, 213(12), 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loos, C.M. (2007) Multiple immunoenzyme staining: Methods and visualizations for the observation with spectral imaging. Journal of Histochemistry and Cytochemistry, 56(4), 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaro, A.C. , Sesma, M.P. & Vazquez, J.J. (1987) Innervation of mouse lymph nodes: Nerve endings on muscular vessels and reticular cells. American Journal of Anatomy, 179(2), 175–185. [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Liu, L. , Guo, B.o. & Zhu, B.o. (2015) Regulation of adaptive immune responses by guiding cell movements in the spleen. Frontiers in Microbiology, 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


