Abstract
To evaluate the ocular safety of intravitreal carboplatin and digoxin injections as a new intravitreal chemotherapy option for retinoblastoma tumor vitreous seeds. Eighteen rabbits were divided randomly into three groups to receive intravitreal injection of Digoxin (6 rabbits), Carboplatin (7 rabbits), or Saline (5 rabbits). In every group, one eye randomly treated with 10 µg Digoxin in 0.1 cc or 1 µg Carboplatin or Saline, and the contralateral eye was considered as the control. All groups underwent three consecutive injections of the drugs with 1‐week intervals. Baseline electroretinography (ERG) was recorded from both eyes of all the animals prior to injection and was repeated 1st day, 1st week, and 1st month after the last injection. All rabbits were sacrificed 1 month after the last injection, and histological studies were done. Mean a and b wave amplitudes decreased significantly at 1st day, 1st week, and 1st month after the last intravitreal injection of 10 µg Digoxin in comparison with other groups (p‐value: .02). Contradictory, 1 µg Carboplatin injected eyes had minimal ERG changes. There were some nonspecific ERG changes with unclear clinical significance in non‐injected contralateral control eyes of Digoxin and Carboplatin groups in comparison with the control eyes of the Saline group. Histological studies revealed considerable neural retinal atrophy in injected eyes of the Digoxin group. Intravitreal 10 µg Digoxin might have more local ocular toxicity in comparison with intravitreal Carboplatin in albino rabbit eyes. Future studies should assess the induced toxicity of intravitreal injection of these drugs on the non‐injected contralateral eye.
Keywords: carboplatin, digoxin, intravitreal injection, rabbits, retinoblastoma
Mean a and b wave amplitudes decreased significantly at 1st day, 1st week, and 1st month after the last intravitreal injection of 10 µg Digoxin in comparison with other groups. Intravitreal 10 µg Digoxin might have more local ocular toxicity in comparison with intravitreal Carboplatin in albino rabbit eyes based on ERG and histopathological findings.

Abbreviations
- ERG
electroretinography
- IIRC
International Intraocular Retinoblastoma Classification
- RPE
retinal pigment epithelium
1. INTRODUCTION
Retinoblastoma is the most prevalent childhood primary intraocular malignant tumor with incidence of 1 per 15,000–20,000 live births. 1 Although systemic chemotherapy has become the cornerstone in the management of retinoblastoma 2 it is often tough to achieve complete tumor control in the presence of the vitreous seeds. 3 , 4
The rate of eye salvage varies based on the International Intraocular Retinoblastoma Classification (IIRC). 5 It can be as high as 100% for group A eyes, 96% for group B eyes, 90% for group C eyes, and 48% for group D eyes. 6 The lower eye salvage rate for group D eyes (defined by the presence of diffuse vitreous seeds) is usually due to active vitreous seeds. 3 , 4 Intra‐arterial chemotherapy increased group D's eye salvage levels to 70%. (64 percent for vitreous seeds and 83 percent for subretinal seeds). 7 , 8 , 9
Vitreous seeds are hard to manage with chemotherapy because the vitreous is avascular and chemotherapy cannot reach the optimum therapeutic levels. 10 Moreover, due to blood ocular barriers, systemic administrated chemotherapeutic drugs do not diffuse in to the vitreous cavity adequately. 11 on the other hand, systemic chemotherapy can also be associated with serious systemic risks such as myelosuppression, nephrotoxicity, ototoxicity, sepsis, second tumors, and death, preventing higher doses of medicines. 12
External beam radiotherapy has been reported as a reliable method to treat vitreous seeds with a salvage rate of 91% 13 ; however, it was associated with remarkable side effects such as secondary malignancies besides having other ocular side effects. 14 Various local approaches such as intraarterial, 15 intravitreal, 16 and periocular 17 chemotherapy have been developed to improve the delivery of greater concentrations of drugs into the eye. 3 , 4 As a result, direct intravitreal injection is the optimal method for obtaining adequate concentrations in the vitreous cavity. 3 , 4
The rationale for local retinoblastoma therapy is to deliver higher concentrations of chemotherapy adjacent to tumor cells, minimizing systemic adverse reactions. 18
Melphalan is currently the most common medication used for intravitreal chemotherapy in retinoblastoma. 19 The adverse eye effects of Melphalan, such as toxicity to the posterior and anterior segments, 20 , 21 , 22 , 23 prompted researchers to look for other possible chemotherapeutic agents for intravitreal injection. 24 , 25 Moreover, a second chemotherapeutic agent is usually required to reduce the number of injections, and serve as an alternative in cases with intravitreal melphalan resistance. 19 Thiotepa, 26 Methotrexate, 27 Topotecan, 10 , 19 Etoposide, 2 Carboplatin, 18 , 28 and Digoxin 29 were evaluated as other intravitreal chemotherapeutics in retinoblastoma. Among these alternatives the in vivo safety studies on repeated intravitreal injection of Digoxin and Carboplatin, as available agents especially in developing countries, is limited.
Carboplatin is an essential part of the most active chemotherapy regimens for retinoblastoma care (Vincristine, Etoposide, Carboplatin‐ VEC). 17 Lower toxicity and higher efficacy of Carboplatin, make it a good possible option for intravitreal chemotherapy. 2 There are several studies that show the effectiveness of intravitreal injection of Carboplatin, 12 , 18 , 30 however, there are limited data regarding the ocular safety and possible toxicity after repeated intravitreal injections. 28 , 31 , 32
Digoxin is a cardiac glycoside traditionally used in the treatment of heart failure and arrhythmia. 33 Anti‐proliferative and cytotoxic effects of Digoxin were shown in several experimental studies. 33 , 34 , 35 , 36 In addition, the anti‐tumor activity of Digoxin on retinoblastoma cells was demonstrated in vitro in previous investigations. 37 One case of retinoblastoma treated with oral and intraarterial Digoxin was reported in a clinical study and there was a modest intraarterial response and no therapeutic response with systemic Digoxin. 38 Only one study in the literature assessed the safety of a single intravitreal Digoxin injection in preclinical retinoblastoma models. 29
In this experimental study, we intended to compare the ocular safety of repeated intravitreal injections of 1 µg Carboplatin and 10 µg Digoxin in healthy albino rabbits based on the previous studies. 29 , 31 We aimed to assess the possible toxicity of these drugs on the retina based on the electroretinography (ERG) findings as a functional test, as well as structural evaluations on histopathological specimens from enucleated eyes.
2. MATERIALS AND METHODS
This investigation was conducted in the animal laboratory of Farabi eye hospital, Tehran, Iran, between August and October 2018. This study was approved by the ethical committee of the Farabi Eye Research Center and Tehran University of medical science. All the steps of this research were in line with the guidelines of the Vision and Ophthalmology Research Association Statement on the Use of Animals in Ophthalmic and Vision Research (ARVO).
Previous studies demonstrated overall similarities between rabbit and human eyes. At the anatomical and histological levels, different parts of the human and rabbit eyes especially the vitreous matrices, are sufficiently similar to make the rabbit a promising animal model for the study. 39 Therefore, eighteen New Zealand rabbits, weighing between 2 and 3 kg, were used to evaluate the safety of repeated intravitreal injections of Carboplatin and Digoxin. Baseline ocular examination with hand‐held slit lamp and indirect ophthalmoscopy and ERG using the electrophysiological test system (Metrovision, France) were done for all animals. Rabbits with documented baseline anterior or posterior segment abnormalities in the eye were excluded. They are repeated at 1st day, 1st week, and 1st month after the last injection. The following the interventions conjunctival injection, corneal status, lens condition, any pathologic findings in the retina, and anterior and posterior segments reaction were evaluated.
2.1. Treatment groups
The rabbits were randomly divided to receive an intravitreal injection of 1 µg Carboplatin (Thymo organ pharmazie GmbH) diluted with 0.1 cc Saline (N = 7), or 10 µg Digoxin (Sterop) diluted with 0.1 cc Saline (N = 6), or 0.1 cc Saline alone (N = 5). In each group, one eye randomly treated with intravitreal injection and the contralateral eye was considered as the non‐dosed control (without any intervention). All groups underwent three consecutive injections of the drugs with 1‐week intervals.
For the Carboplatin group, the dose chosen dose was close to the maximum tolerated dose to achieve the most effective concentration (1 µg), based on previous investigations. 28 , 30 , 31 , 32 For the Digoxin group, the dosage was chosen based on the amount of Digoxin that could result in pharmacologically active amounts in the vitreous of a rabbit (1.5 ml) for at least 4 h after injection, as well as a low systemic exposure as a proxy of cardiac toxicity for its direct impact in the translation to clinics, which was the end goal of these studies. 40
As a result, the dose was determined by the biological activity threshold, or IC50, and we chose the value obtained by Antczak et al. (10 µg). 37
2.2. Intravitreal injection technique
The rabbits were anesthetized with a mixture of 25 mg/kg Ketamine 10% (Alfasan) and 2 mg/kg Xylazine 2% (Alfasan). The injection was performed under a sterile condition after anterior chamber paracentesis (0.05 ml) using a 29‐gauge needle. All the injections were performed through 1.5 mm posterior to the limbus into the midvitreous. The needle was held in place for 15 s after injection to prevent reflux from the entrance site.
2.3. Electroretinography
The full‐field ERG measures the retina's mass electrical response to photic stimulation. It is a test that evaluates the function of the retina in human patients and laboratory animals. 41
The a‐wave is the first large negative component, followed by the b‐wave which is a positive wave and usually larger in amplitude. The a‐wave, also known as the “late receptor potential,” reflects the overall physiological health of the photoreceptors in the outer retina. In contrast, the b‐wave reflects the health of the inner layers of retina, including the Muller cells and bipolar cells. 42
Flash electroretinography (ERG‐Metrovision) was recorded at baseline, 1 day, 1 week, and 1 month after the last injection to evaluate the possible toxicity on the retina's function. Before the test, the rabbits were dark‐adapted for 45 min and prepared under dim red light. Animals were anesthetized before recording ERG, with an intramuscular injection of the ketamine and xylazine (25/2 mg/kg). Pupils were dilated using tropicamide (1%) and tetracaine hydrochloride (0.5%). The retinal electrical response was recorded by Goldring recording electrode (4 mm, Roland Consult). After placement of the reference electrode (stainless steel needle electrode) at the base of the ear subcutaneously and the ground electrode on the tail, the main recording electrode was inserted on the corneal surface. The scotopic ERG was recorded by applying eight‐light stimuli with 125 cdsm−2. The average of the responses from four separate light stimuli was documented.
2.4. Histological assessments
All animals were euthanized with overdosing of thiopental sodiumone month after the last injection and enucleated. Then the eyes were stored in Davidson's fixative solution. After the fixation, the specimens were divided into two parts by an anterior‐posterior incision. Then the histological processing was applied and 4‐µm‐thick cut specimens were stained with hematoxylin and eosin and observed under a light microscope (Olympus BX41). All specimens were studied by the same pathologist (FA), and all ocular layers were evaluated for the presence of any kind of inflammation, hemorrhage, congestion, necrosis, degeneration, and atrophic changes. The retina was assessed carefully for the thickness of different layers, presence or absence of retinal pigment epithelium (RPE), photoreceptors, and ganglion cells.
2.5. Statistical analysis
All data were analyzed using SPSS software (Version 22.0. Armonk: IBM Corp.). Wilcoxon test was used for evaluating the ERG value changes within groups. It was also used for the difference of the injected eye versus contralateral (control) eye in different follow‐ups in each group. We used the Mann–Whitney test to compare ERG values between the groups. A p‐value less than .05 was considered statistically significant.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), 43 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019). 44
3. RESULTS
3.1. Clinical evaluation
Twenty rabbits were randomly divided into three groups of Digoxin (7 rabbits), Carboplatin (7 rabbits), and Salin (6 rabbits); however, one rabbit from the Digoxin group and one from the Saline group died on the first day with unknown reasons. The other rabbits completed the experimental period. No obvious changes in food or water intake as a sign of the general toxicity were observed. In the ocular examinations of the rabbits at baseline, 1st day, 1st week, and 1st month after the last injection, we did not find any noticeable findings such as corneal opacity, cataract, hemorrhage, or inflammation in the anterior chamber and vitreous cavity. There was no phthisis bulbi in any of our groups.
3.2. ERG findings
In the Digoxin group, mean a‐wave amplitude in injected eyes at the 1st day (−19.8 ± 16.6 µv, p‐value: .02), 1st week (−23.7 ± 23.8 µv, p‐value: .02) and 1st month (−18.4 ± 17.8 µv, p‐value: .02) after the last injection were significantly lower than baseline (−33.8 ± 6.7 µv) (Figures 1 and 2, Table 1 and Table S1).
FIGURE 1.
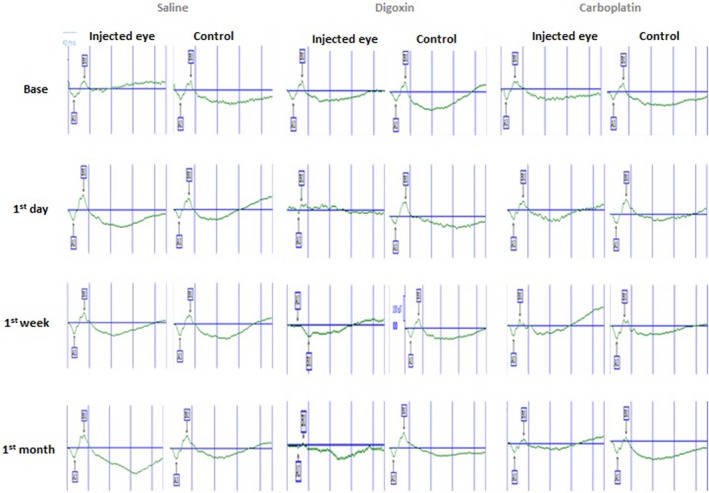
Scotopic electroretinographs (ERG) of injected and contralateral control eyes of three rabbits in Saline, Digoxin and Carboplatin groups at baseline, 1st day, 1st week, and 1st month after the last injection
FIGURE 2.

The linear graphs of ERG values demonstrating change in their means at baseline, 1st day, 1st week, and 1st month after the last injection in each group of injected and contralateral control eyes
TABLE 1.
Detailed scotopic electroretinographic (ERG) results including mean±standard deviation (SD) of a‐wave amplitudes (µv), b‐wave amplitudes (µv), a‐wave latency (ms) and b‐wave latency (ms) at baseline, 1st day, 1st week, and 1st month after the last injection in each group in comparison with their contralateral control eyes
| Injected eye | Control eye | p‐value between injected & control eye | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a† (µv) (mean ± SD) | b‡ (µv) (mean ± SD) | al§ (ms) (mean ± SD) | bl¶ (ms) (mean ± SD) | a (µv) (mean ± SD) | b (µv) (mean ± SD) | al (ms) (mean ± SD) | bl (ms) (mean ± SD) | a | b | al | bl | |
| Base | ||||||||||||
| Saline | −37.6 ± 6.1 | 73.7 ± 23.6 | 13.7 ± 1.5 | 34.7 ± 2.2 | −38.06 ± 6.7 | 77.7 ± 29.8 | 13.9 ± 1.8 | 35.6 ± 1.5 | 0.75 | 0.91 | 0.83 | 0.2 |
| Digoxin | −33.8 ± 6.7 | 77.7 ± 24.2 | 14.4 ± 0.7 | 34.9 ± 2.8 | −34.4 ± 9.9 | 82.1 ± 30.1 | 14.7 ± 0.48 | 36.04 ± 1.9 | 0.87 | 0.42 | 0.2 | 0.1 |
| Carboplatin | −34.4 ± 6.5 | 67.1 ± 12.1 | 13.6 ± 0.9 | 36.2 ± 1.4 | −35.9 ± 1.2 | 72.8 ± 14.5 | 14.08 ± 0.75 | 36.1 ± 1.5 | 0.52 | 0.74 | 0.36 | 0.56 |
| 1st day | ||||||||||||
| Saline | −33.5 ± 8.5 | 83.4 ± 26.5 | 14.3 ± 0.7 | 34.5 ± 1.8 | −35.1 ± 4.9 | 81.6 ± 19.03 | 13.9 ± 0.8 | 34.4 ± 1.3 | 0.91 | 0.6 | 0.2 | 0.7 |
| Digoxin | −19.8 ± 16.6 | 44.9 ± 38.5 | 16.4 ± 6.2 | 32.3 ± 10.5 | −34.8 ± 10.9 | 80 ± 19.4 | 15.08 ± 0.89 | 37.6 ± 0.4 | 0.004* | 0.004* | 0.26 | 0.004* |
| Carboplatin | −33.3 ± 5.1 | 76 ± 18.2 | 14.1 ± 0.6 | 32.9 ± 3.4 | −36.6 ± 2.8 | 88.2 ± 9.5 | 13.8 ± 0.6 | 34.3 ± 2.4 | 0.22 | 0.18 | 0.4 | 0.7 |
| 1st week | ||||||||||||
| Saline | −40.5 ± 11.9 | 81 ± 20.9 | 13.9 ± 0.4 | 35.5 ± 1.6 | −46.5 ± 13.2 | 91.2 ± 22.3 | 13.9 ± 0.21 | 35.2 ± 2.01 | 0.17 | 0.17 | 0.27 | 1.00 |
| Digoxin | −23.7 ± 23.8 | 42.6 ± 43.8 | 13 ± 7 | 27.8 ± 16 | −46.7 ± 7.03 | 84.3 ± 19.5 | 15.1 ± 1.2 | 35.9 ± 3.2 | 0.004* | 0.004* | 1.00 | 0.05 |
| Carboplatin | −39.2 ± 9 | 70.1 ± 16.5 | 14 ± 0.7 | 34.3 ± 3.6 | −33.5 ± 7.2 | 57.1 ± 10.3 | 14.2 ± 0.57 | 34.7 ± 3.5 | 0.4 | 0.01* | 0.21 | 0.4 |
| 1st month | ||||||||||||
| Saline | −34.7 ± 6.1 | 77.8 ± 20.7 | 13.9 ± 1.2 | 33.6 ± 2.9 | −36.1 ± 7.1 | 77.9 ± 21.7 | 13.8 ± 1.2 | 34.08 ± 2.66 | 0.25 | 0.91 | 0.83 | 0.82 |
| Digoxin | −18.4 ± 17.8 | 41.5 ± 41.4 | 14.4 ± 7.1 | 29.4 ± 13.2 | −34.2 ± 3.3 | 75.2 ± 20.7 | 13.8 ± 1.1 | 35.5 ± 1.8 | 0.004* | 0.004* | 0.47 | 0.1 |
| Carboplatin | −33.4 ± 8.3 | 74.2 ± 20.5 | 13.9 ± 1 | 35.2 ± 1.7 | −33.9 ± 6.9 | 80.2 ± 17.7 | 14.08 ± 1.4 | 36.2 ± 1.1 | 0.65 | 0.4 | 0.74 | 0.6 |
p‐value for comparison of ERG values between injected eye and contralateral control eye at different intervals after the last injection was demonstrated in each group.
a†: a‐wave amplitude (µv).
b‡: b‐wave amplitude (µv).
al§: a‐wave latency (ms).
bl¶: b‐wave latency (ms).
Statistical significant p‐value less than .05.
3.3. Histological findings
Based on histopathological findings, there were no distinguishable histological changes in both the Saline group and the contralateral control eyes of each group (Figure 3A and B).
FIGURE 3.

(A) Retinal histological specimen of a control eye, stained with hematoxylin and eosin. (B) Retinal histological specimen of the Saline‐injected eye. (C) Retinal histological specimen of the Digoxin‐injected eye (retinal atrophy is an apparent finding). (D) Retinal histological specimen of the Carboplatin‐injected eye. Abbreviations: GCL, Ganglion Cells Layer; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer, photoreceptors: photoreceptors layer
In the Digoxin group, substantial neural retinal atrophy was seen in five of six specimens (83.3%). The thickness of the ellipsoid zone, ganglion cells layer, inner, and outer nuclear layer was considerably reduced. However, the retinal pigment epithelium (RPE) layer remained unchanged in most of the specimens (Figure 3C). Additionally, in two specimens, chronic vitritis with lymphoplasmacytic infiltration was found in the posterior pole, especially around the optic disc (optic neuritis) (Figure 4A). Furthermore, severe choroidal congestion with or without ciliary body congestion was seen in all of the specimens of the Digoxin injected eyes (Figure 4B).
FIGURE 4.

(A) Optic disc histological specimen of the Digoxin‐injected eye. (B) Obvious choroidal congestion in the Digoxin‐injected eye
In the Carboplatin group, there was no sign of inflammation in the anterior and posterior segments of the eyes. All specimens had normal retinal thickness, ganglion cells, photoreceptor morphology, pigmented epithelial cells, and nuclear layers. No evidence of optic nerve edema, neuritis, or atrophy, as well as retinal hemorrhages, was identified (Figure 3D).
4. DISCUSSION
In this experimental study, we investigated the ocular safety of repeated intravitreal injections of 1 µg/0.1 cc Carboplatin and 10 µg/0.1 cc Digoxin as an available potential candidates for intravitreal chemotherapy in the treatment of retinoblastoma. Our results showed that repeated intravitreal injections of 10 µg Digoxin could have noticeable intraocular toxicity based on ERG and histological investigations in albino rabbit eyes. Contradictory, 1 µg Carboplatin injected eyes had minimal ERG and pathologic changes.
ERG values, including mean a‐wave amplitude and mean b‐wave amplitude decreased significantly after repeated intravitreal injections of 10 µg Digoxin at 1st day, 1st week, and 1st month after the last injection in comparison with the Carboplatin and the Saline groups. ERG results in the Digoxin group were consistent with the histological findings, which revealed neural retinal atrophy as well as severe choroidal congestion. Furthermore, chronic inflammation around the optic disc was found in two eyes. The reduction in both amplitudes may be the result of the injected volume or a toxic effect of the agent; however, the ERGs remained unchanged in the Saline group, despite receiving three consecutive injections with the same volume. Therefore, the elevation of IOP from increased intraocular volume after injection was not the cause of these findings.
To the best of our knowledge, there was only one study in the literature evaluating the ocular safety of intravitreal injection of Digoxin in rabbit eyes. In consistent with our findings, Winter et al. 29 revealed that retinal toxicity appeared after three consecutive injections of the 1 µg Digoxin based on ERG changes and histologic findings. They encountered severe retinal damage and complete loss of a‐ and b‐wave in ERG 1 week after an intravitreal injection of 10 µg Digoxin. In contrast to their experience, we did not see such profound damage with repeated intravitreal injection of 10 µg Digoxin. In the pharmacokinetic evaluation of their study on 17 rabbits with a single intravitreal injection of 10 µg Digoxin, Digoxin was not detected in vitreous and retina of contralateral non‐injected eyes. Interestingly, we found some ERG changes in the non‐injected control eyes in the Digoxin and Carboplatin groups in comparison with the contralateral non‐injected eyes of the Saline group. The repeated intravitreal injection of the agents used in our study might be an explanation for these ERG changes in non‐injected eyes. We assume that repeated intravitreal injection of these chemo‐agents may cause damage to the ocular‐blood barrier that leads to the systemic absorption of these drugs. 45 The clinical significance of these changes should be assessed in future studies.
In contradiction to Digoxin, repeated intravitreal injections of 1 µg Carboplatin showed promising results according to functional and morphologic findings. There were more studies on the ocular safety of intravitreal Carboplatin in literature with different doses. Susskind et al. have shown that Carboplatin is less toxic to RPE cells in comparison with Melphalan and Topotecan. 4 Francis et al. revealed that Carboplatin led to minimal changes in electroretinogram after intraarterial chemotherapy compared to Melphalan and Topotecan. 46
According to our results, Zlioba et al. 31 evaluated intravitreal Carboplatin toxicity in rabbit eyes and showed no ocular toxicity based on histological and electroretinographic observations for doses up to 3 μg. Pochop et al. 32 investigated the retinal toxicity of repeated intravitreal injection (4 times with 2 weeks intervals) of 8 µg Carboplatin. They demonstrated reduced dark‐adapted b‐wave amplitudes and light‐adapted b‐wave and a‐wave amplitudes in electroretinography studies. However, they didn't find remarkable histopathologic retinal change compatible with drug toxicity. In contrast to our study, they also found some significant ERG changes in the control eyes that were injected with Saline. Hence, they attributed these changes to transient retina ischemia caused by rapid elevation of intraocular pressure. They suggested the 8 µg of Carboplatin as the highest possible safe dose for intravitreal injection.
Retinal toxicity, along with widespread outer retina disruption, has been observed for intravitreal injection of Carboplatin at the dose of 10 µg or higher. 18 Carboplatin has shown impressive outcomes in terms of the efficacy/toxicity balance in a transgenic model by Harbour et al. They used different doses of Carboplatin, 1.4 and 4 μg, with different intervals for intravitreal injections and revealed that low doses every week seem to be equally efficient as high doses every 2 weeks with a lower chance for retinal toxicity. Serial doses of intravitreal Carboplatin can effectively inhibit tumor growth in a dose‐dependent manner.
Recently Lemaître et al. 28 evaluated the effectiveness and safety of intravitreal injection of Melphalan, Topotecan, and Carboplatin, alone or in combination in the animal model of LHBetaTag retinoblastoma mice. They found that weekly intravitreal Carboplatin, either 1.5 or 4 μg could reduce tumor burden significantly (80%). The sequential (injection every 2 weeks) administration of Carboplatin 4 μg plus Topotecan 0.1 μg also showed similar efficacy (about 80%). However, reduced retinal toxicity (approximatively 25% of eyes with a decrease in retinal thickness) was induced at weekly 1.5 μg Carboplatin as well as biweekly combination therapy, while an important percentage of toxicity (62.5% of the eyes with a decrease in retinal thickness) was observed with 4 μg of Carboplatin weekly injection based on optical coherence tomography findings. They proposed that the cumulative injected dose (addition of all the repeated doses), as well as the time interval between two injections (frequency), impact the efficacy/toxicity balance.
Besides, they found ocular complications related to the intravitreal injection technique, including media opacity and retinal detachment, which were not seen in our study.
The main drawback of animal studies is the translation to clinics for retinoblastoma treatment. The direct extrapolation of the doses used in the animal eye to the children's eye in order to treat retinoblastoma is debatable. Preclinical animal trials are necessary to examine the outcome of various chemotherapy agents on the eyes, but more things we need to learn about the application to clinics. All these rabbits had healthy eyes and we do not know whether the safety profile observed in this study will be the same in the eyes with retinoblastoma or not. We also did not assess the efficacy of the dose–response of drugs on tumor cells, and further investigation is needed to assess the efficacy as well as the safety of these drugs in transgenic retinoblastoma models.
Certain drawbacks of our study were the low sample size and no assessment of the intravitreal concentration of drugs. Moreover, we did not evaluate the dose–response for each drug in this survey. This research examined the functional and anatomical changes up to 1 month after the last injection, and did not rule out the possibility of long‐term toxicity or rehabilitation following such repeated injections. In addition, the effect of each injection on ERG parameters was not assessed separately.
As ERG is primarily a functional test of the status of the photoreceptors and bipolar cells, the normal ERG results do not exclude possible damage at the level of the retinal ganglion cells or their axons, although this was not seen in histological evaluations. Conversely, safety based on histological findings by light microscopy cannot rule out possible changes at the submicroscopic level. Therefore, it is better to design a study to perform immunocytochemical analysis on the histopathologic sections to evaluate the possible damage to retinal microstructures.
5. CONCLUSIONS
Intravitreal 10 µg Digoxin might have more local ocular toxicity in comparison with intravitreal Carboplatin in albino rabbit eyes based on ERG and histopathological findings. Future studies should evaluate the possible effects of intravitreal chemotherapy, specially on non‐injected fellow eyes of the retinoblastoma rabbit models.
DISCLOSURE
The authors declare that there are no conflicts of interest regarding the publication of this paper.
ETHICS APPROVAL STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Not applicable.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
We wish to record our deep sense of gratitude and profound thanks to Dr Naderi A. (Dr of Physiology) and Aghajanpour L. for their help in the laboratory setting.
Khodabande A, Ghassemi F, Asadi Amoli F, et al. Ocular safety of repeated intravitreal injections of Carboplatin and Digoxin: A preclinical study on the healthy rabbits. Pharmacol Res Perspect. 2021;9:e00814. 10.1002/prp2.814
Funding information
The authors received no financial support for the research, authorship, and publication of this article.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Gudiseva HV, Berry JL, Polski A, Tummina SJ, O'Brien JM. Next‐generation technologies and strategies for the management of retinoblastoma. Genes. 2019;10(12):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohney BG, Elner VM, Smith AB, et al. Preclinical acute ocular safety study of combined intravitreal carboplatin and etoposide phosphate for retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2017;48(2):151‐159. [DOI] [PubMed] [Google Scholar]
- 3. Karkhaneh R, Pourmostadam B, Ghassemi F, Movassat M, Roohipoor R, Ahmadabadi MN. Toxic effect of intravitreal carboplatin detected by flattenedelecteroretinogram in a patient with retinoblastoma treated by chemoreduction and local treatments. Iran J Ophthalmol. 2010;22(3):57‐60. [Google Scholar]
- 4. Susskind D, Hagemann U, Schrader M, Januschowski K, Schnichels S, Aisenbrey S. Toxic effects of melphalan, topotecan and carboplatin on retinal pigment epithelial cells. Acta Ophthalmol. 2016;94(5):471‐478. [DOI] [PubMed] [Google Scholar]
- 5. Murphree AL. Intraocular retinoblastoma: the case for a new group classification. Ophthalmolo Clin North Am. 2005;18(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 6. Shields CL, Mashayekhi A, Au AK, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276‐2280. [DOI] [PubMed] [Google Scholar]
- 7. Abramson DH, Marr BP, Dunkel IJ, et al. Intra‐arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2‐year results. Br J Ophthalmol. 2012;96(4):499‐502. [DOI] [PubMed] [Google Scholar]
- 8. Shields CL, Bianciotto CG, Jabbour P, et al. Intra‐arterial chemotherapy for retinoblastoma: report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129(11):1399‐1406. [DOI] [PubMed] [Google Scholar]
- 9. Munier FL, Beck‐Popovic M, Balmer A, Gaillard M‐C, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31(3):566‐573. [DOI] [PubMed] [Google Scholar]
- 10. Rao R, Honavar SG, Sharma V, Reddy VAP. Intravitreal topotecan in the management of refractory and recurrent vitreous seeds in retinoblastoma. Br J Ophthalmol. 2018;102(4):490‐495. [DOI] [PubMed] [Google Scholar]
- 11. Gombos DS, Cauchi PA, Hungerford JL, Addison P, Coen PG, Kingston JE. Vitreous relapse following primary chemotherapy for retinoblastoma: is adjuvant diode laser a risk factor? Br J Ophthalmol. 2006;90(9):1168‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray TG, Roth DB, O'Brien JM, et al. Local carboplatin and radiation therapy in the treatment of murine transgenic retinoblastoma. Arch Ophthalmol. 1996;114(11):1385‐1389. [DOI] [PubMed] [Google Scholar]
- 13. Shields CL, Ramasubramanian A, Thangappan A, et al. Chemoreduction for group E retinoblastoma: comparison of chemoreduction alone versus chemoreduction plus low‐dose external radiotherapy in 76 eyes. Ophthalmology. 2009;116(3):544‐551.e1. [DOI] [PubMed] [Google Scholar]
- 14. Kleinerman RA, Tucker MA, Tarone RE, et al. Risk of new cancers after radiotherapy in long‐term survivors of retinoblastoma: an extended follow‐up. J Clin Oncol. 2005;23(10):2272‐2279. [DOI] [PubMed] [Google Scholar]
- 15. Shields CL, Alset AE, Say EAT, Caywood E, Jabbour P, Shields JA. Retinoblastoma control with primary intra‐arterial chemotherapy: outcomes before and during the intravitreal chemotherapy era. J Pediatr Ophthalmol Strabismus. 2016;53(5):275‐284. [DOI] [PubMed] [Google Scholar]
- 16. Manjandavida FP, Shields CL. The role of intravitreal chemotherapy for retinoblastoma. Indian J Ophthalmol. 2015;63(2):141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shields CL, Fulco EM, Arias JD, et al. Retinoblastoma frontiers with intravenous, intra‐arterial, periocular, and intravitreal chemotherapy. Eye. 2013;27(2):253‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harbour JW, Murray TG, Hamasaki D, et al. Local carboplatin therapy in transgenic murine retinoblastoma. Invest Ophth Vis Sci. 1996;37(9):1892‐1898. [PubMed] [Google Scholar]
- 19. Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132(8):936‐941. [DOI] [PubMed] [Google Scholar]
- 20. Francis JH, Schaiquevich P, Buitrago E, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology. 2014;121(9):1810‐1817. [DOI] [PubMed] [Google Scholar]
- 21. Xue K, Ren H, Meng F, Zhang R, Qian J. Ocular toxicity of intravitreal melphalan for retinoblastoma in Chinese patients. BMC Ophthalmol. 2019;19(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chao A‐N, Kao L‐Y, Liu L, Wang N‐K. Diffuse chorioretinal atrophy after a single standard low‐dose intravitreal melphalan injection in a child with retinoblastoma: a case report. BMC Ophthalmol. 2016;16(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Francis JH, Marr BP, Brodie SE, Abramson DH. Anterior ocular toxicity of intravitreous melphalan for retinoblastoma. JAMA Ophthalmol. 2015;133(12):1459‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268‐1271. [DOI] [PubMed] [Google Scholar]
- 25. Ghassemi F, Amoli FA. Pathological findings in enucleated eyes after intravitreal melphalan injection. Int Ophthalmol. 2014;34(3):533‐540. [DOI] [PubMed] [Google Scholar]
- 26. Ericson L, Karlberg B, Rosengren BH. Trials of intravitreal injections of chemotherapeutic agents in rabbits. Acta Ophthalmol. 1964;42(4):721‐726. [DOI] [PubMed] [Google Scholar]
- 27. Kivela T, Eskelin S, Paloheimo M. Intravitreal methotrexate for retinoblastoma. Ophthalmology. 2011;118(8):1689,e1‐6. [DOI] [PubMed] [Google Scholar]
- 28. Lemaître S, Poyer F, Fréneaux P, et al. Low retinal toxicity of intravitreal carboplatin associated with good retinal tumour control in transgenic murine retinoblastoma. Clin Exp Ophthalmol. 2020;48(4):500‐511. [DOI] [PubMed] [Google Scholar]
- 29. Winter U, Buitrago E, Mena HA, et al. Pharmacokinetics, safety, and efficacy of intravitreal digoxin in preclinical models for retinoblastoma. Invest Ophth Vis Sci. 2015;56(8):4382‐4393. [DOI] [PubMed] [Google Scholar]
- 30. Smith SJ, Pulido JS, Salomao DR, Smith BD, Mohney B. Combined intravitreal and subconjunctival carboplatin for retinoblastoma with vitreous seeds. Br J Ophthalmol. 2012;96(8):1073‐1077. [DOI] [PubMed] [Google Scholar]
- 31. Zlioba A, Peyman GA, Nikoleit J. Retinal toxicity study of intravitreal carboplatin and iproplatin. Ann Ophthalmol. 1988;20(2):71‐72. [PubMed] [Google Scholar]
- 32. Pochop P, Darsova D, Uhlik J, et al. Retinal toxicity after repeated intravitreal carboplatin injection into rabbit eyes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(4):552‐556. [DOI] [PubMed] [Google Scholar]
- 33. Prassas I, Karagiannis GS, Batruch I, Dimitromanolakis A, Datti A, Diamandis EP. Digitoxin‐induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol Cancer Ther. 2011;10(11):2083‐2093. [DOI] [PubMed] [Google Scholar]
- 34. Felth J, Rickardson L, Rosén J, et al. Cytotoxic effects of cardiac glycosides in colon cancer cells, alone and in combination with standard chemotherapeutic drugs. J Nat Prod. 2009;72(11):1969‐1974. [DOI] [PubMed] [Google Scholar]
- 35. Hallböök H, Felth J, Eriksson A, et al. Ex vivo activity of cardiac glycosides in acute leukaemia. PLoS One. 2011;6(1):e15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Winnicka K, Bielawski K, Bielawska A, Miltyk W. Apoptosis‐mediated cytotoxicity of ouabain, digoxin and proscillaridin A in the estrogen independent MDA‐MB‐231 breast cancer cells. Arch Pharm Res. 2007;30(10):1216‐1224. [DOI] [PubMed] [Google Scholar]
- 37. Antczak C, Kloepping C, Radu C, et al. Revisiting old drugs as novel agents for retinoblastoma: in vitro and in vivo antitumor activity of cardenolides. Invest Ophth Vis Sci. 2009;50(7):3065‐3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel M, Paulus YM, Gobin YP, et al. Intra‐arterial and oral digoxin therapy for retinoblastoma. Ophthalmic Genet. 2011;32(3):147‐150. [DOI] [PubMed] [Google Scholar]
- 39. Los L. The rabbit as an animal model for post‐natal vitreous matrix differentiation and degeneration. Eye. 2008;22(10):1223‐1232. [DOI] [PubMed] [Google Scholar]
- 40. Vézina M. Comparative Ocular Anatomy in Commonly Used Laboratory Animals. Assessing Ocular Toxicology in Laboratory Animals. Totowa, NJ: Springer. 2012;1‐21. [Google Scholar]
- 41. Miller R, Dowling J. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b‐wave of the electroretinogram. J Neurophysiol. 1970;33(3):323‐341. [DOI] [PubMed] [Google Scholar]
- 42. Krill AE. The electroretinogram and electro‐oculogram: clinical applications. Invest Ophthalmol Vis Sci. 1970;9(8):600‐617. [PubMed] [Google Scholar]
- 43. Harding S, Sharman J, Faccenda E, Southan C, Pawson A, Ireland S. Nc‐Iuphar (2018). The IUPHAR/BPS guide to pharmacology in. 2018.
- 44. Alexander SP, Kelly E, Mathie A, et al. The concise guide to pharmacology 2019/20: transporters. Br J Pharmacol. 2019;176:S397‐S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Occhiutto ML, Freitas FR, Maranhao RC, Costa VP. Breakdown of the blood‐ocular barrier as a strategy for the systemic use of nanosystems. Pharmaceutics. 2012;4(2):252‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Francis JH, Abramson DH, Gobin YP, et al. Electroretinogram monitoring of dose‐dependent toxicity after ophthalmic artery chemosurgery in retinoblastoma eyes: six year review. PLoS One. 2014;9(1):e84247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


