Abstract
The pectoralis major fiber regions contribute uniquely to the mobility and the stability of the shoulder complex. It is unknown how age and sex influence the stiffness of these regions during volitional contractions, but this knowledge is critical to inform clinical interventions targeting the pectoralis major. The aim of the present study was to determine if the activation‐dependent stiffness of the pectoralis major fiber regions differs between the sexes and if it is altered with age. Ultrasound shear wave elastography was used to acquire shear wave velocity from the clavicular and the sternocostal fiber regions of 48 healthy participants, including 24 younger (12 males, 12 females, mean ± SD age 25 ± 4.1 years) and 24 older adults (12 males, 12 females, 55 ± 3.6 years). Participants performed vertical adduction and horizontal flexion torques in neutral and 90° externally rotated shoulder positions, and one of the two shoulder abduction positions (60° and 90°) at varying torque magnitudes (passive, 15% and 30% of maximal voluntary contraction). Separate linear mixed‐effects models were run for each fiber region and shoulder position to determine if the activation‐dependent stiffness differed between the sexes and was altered in older adults. Age‐related alterations in stiffness during volitional contractions were observed in both fiber regions and were dependent on the task. Alterations in activation‐dependent stiffness due to age were more pronounced in females than males. Additionally, females had greater stiffness than males during volitional contractions in both fiber regions. The present findings provide the first line of evidence that the activation‐dependent stiffness of the pectoralis major fiber regions is influenced by sex and changes with age.
Keywords: muscle activation, muscle stiffness, shear wave elastography, ultrasound
Age‐related alterations in the stiffness of the pectoralis major during volitional contractions were observed in both fiber regions of the muscle. These alterations in activation‐dependent stiffness due to age were more pronounced in females than males.

1. INTRODUCTION
The pectoralis major enables mobility and stability of the shoulder complex. It consists of two fiber regions, the clavicular and the sternocostal, which have divergent functions (Carlson, 2003; Corten et al., 2007; Fung et al., 2009; Haley & Zacchilli, 2014; Lee et al., 2017; Leonardis et al., 2017; Stegink‐Jansen et al., 2011; Tobin, 1985; Wolfe et al., 1992). The clavicular region primarily assists in humeral flexion and horizontal adduction (Ackland et al., 2008; Leonardis et al., 2017; Paton & Brown, 1994), while the sternocostal region assists in humeral vertical adduction, internal rotation, and extension against resistance (Ackland & Pandy, 2011; Leonardis et al., 2017; Paton & Brown, 1994; Wolfe et al., 1992). Our research group previously observed greater elasticity of the sternocostal region while at rest and reduced muscle tissue homogeneity with aging, particularly in females (Chodock et al., 2020). As muscle stiffness is influenced by tissue elasticity and muscle architecture (Cui et al., 2008), it is unknown if these findings translate to the stiffness of the pectoralis major during voluntary contractions. Addressing this knowledge gap can provide valuable information on the importance of considering sex and age in designing targeted interventions for the pectoralis major in healthy‐aging adults and individuals with neuromuscular pathologies.
Age and sex are critical factors to consider when assessing muscle properties. Aging is associated with alterations in the architectural and structural properties of the muscle, impacting functional independence and mobility (Buckwalter et al., 1993; Hill et al., 2010; McPhail et al., 2014; Palazzo et al., 2014). However, alterations in structural properties due to age do not appear to be uniform across muscles. For example, under resting conditions, muscle elasticity increases with age in the biceps brachii, the supraspinatus, and the sternocostal region of the pectoralis major (Baumer et al., 2018; Chodock et al., 2020; Eby et al., 2015) and decreases in the lateral gastrocnemius, rectus femoris, and middle trapezius (Akagi et al., 2015; Chodock et al., 2020). No changes in muscle elasticity across the lifespan are observed in the soleus, anterior deltoid, and the clavicular region of the pectoralis major (Akagi et al., 2015; Chodock et al., 2020). During voluntary contractions, muscle elasticity increases with age in the supraspinatus (Baumer et al., 2018). Moreover, differences in the structural properties between the sexes are present for some muscles, but not others. In particular, females have greater muscle stiffness while at rest than males in the biceps brachii and the soleus (Eby et al., 2015; Saeki et al., 2019), while no sex differences are observed in lateral and medial gastrocnemius and supraspinatus stiffness (Chino & Takahashi, 2016; Saeki et al., 2019).
Examining the pectoralis major fiber regions during voluntary contractions can provide invaluable insights into whether sex and age influence activation‐dependent changes in muscle stiffness. Therefore, the purpose of the present study was to determine the effect of sex and age on the stiffness of the clavicular and the sternocostal fiber regions during voluntary contractions. We utilized ultrasound shear wave elastography (SWE) to measure the shear wave velocity (SWV) from each fiber region during voluntary contractions. SWV serves as an indirect measure of muscle stiffness, as prior work indicates a direct relationship between SWV and muscle stiffness during muscular contraction (Bernabei et al., 2020). We hypothesized that the older adults would exhibit lower activation‐dependent stiffness than younger adults, independent of the fiber region. We further hypothesized females would have greater activation‐dependent stiffness than males, independent of the fiber region. If age‐ and sex‐related differences exist, this will provide new insights into the structural properties of the pectoralis major fiber regions to consider when examining pectoralis major function in healthy individuals or those with neuromuscular pathologies.
2. METHODS
2.1. Study participants
Forty‐eight healthy younger and older adults (24 females, 24 males) with no history of orthopedic or neuromuscular shoulder injuries participated in this study (Table 1). This sample size was determined using a‐priori power analysis, which calculated a minimum sample size of 24 individuals based on a conservative effect size (f) of 0.25, four groups (2 age groups × 2 sex groups), an alpha level of 0.05, and 80% power. Additional participants were recruited to enhance study power. Forty‐four of the 48 participants were right‐hand dominant. Participants were recruited through the University of Michigan's online research study portal and flyers posted across campus. The inclusion criteria were: (1) individuals between 18 to 30 years old or between 50 and 60 years old; (2) no history of orthopedic and/or neuromuscular shoulder dysfunction and injuries; and (3) recreationally active. Participants were excluded if they reported a history of neuromuscular shoulder dysfunction and upper extremity injury or surgery. Younger adult group included participants between 18 and 30 years old, while older adults group consisted of individuals between 50 and 60 years old. The University of Michigan Institutional Review Board approved all study procedures (IRB#: HUM00152691), and written informed consent was obtained from each participant before collecting any data.
TABLE 1.
General participant information (mean ± SD)
| Characteristic | Young female (n = 12) | Young male(n = 12) | Older female(n = 12) | Older male(n = 12) |
|---|---|---|---|---|
| Age (years) | 25.1 ± 4.3 | 25.0 ± 4.0 | 55.0 ± 4.4 | 55.1 ± 2.7 |
| Height (cm) | 165.4 ± 7.1 | 177.6 ± 7.2 | 156.3 ± 11.5 | 179.2 ± 5.5 |
| Weight (kg) | 59.1 ± 5.0 | 74.5 ± 6.0 | 70.4 ± 16.6 | 79.9 ± 12.4 |
| Body mass index (kg/m2) | 21.5 ± 1.9 | 23.8 ± 2.0 | 27.4 ± 5.5 | 24.6 ± 3.1 |
| Upper arm length (cm) | 29.3 ± 2.5 | 32.1 ± 3.0 | 28.7 ± 3.9 | 33.0 ± 2.3 |
| Forearm length (cm) | 26.0 ± 1.6 | 28.1 ± 1.6 | 25.9 ± 2.7 | 29.3 ± 2.3 |
| Vertical adduction strength (Nm) | 53.9 ± 15.6*** | 103.4 ± 28.2*** | 53.0 ± 10.5*** | 88.3 ± 33.2*** |
| Horizontal flexion strength (Nm) | 37.4 ± 14.7*** | 66.9 ± 17.6*** | 44.3 ± 8.9* | 65.3 ± 26.6* |
The asterisk indicates the significance level between younger females and males, and older females and males (*p < 0.05, ***p < 0.001).
2.2. Experimental protocol
The participant was seated in a custom designed Biodex chair (Biodex Medical Systems) with their dominant arm placed into a plastic, removable cast, as performed in previous studies (Chodock et al., 2020; Leonardis et al., 2017; Leonardis et al., 2019). The cast extended from the shoulder to the hand, fixing the elbow at 90° and the wrist in a neutral position. Torso movement was restricted using a seatbelt and cushioned plates along the lower, posterior back, and the left and right sides. Glenohumeral joint torques were measured using a six‐degrees‐of‐freedom load cell (45E15A4‐M63J; JR3, Inc.), which was attached to the crank arm of the motor. The motor's axis of rotation was aligned to the glenohumeral joint axis of rotation, approximated as the midpoint of a straight line connecting the acromion process to the anterior‐most portion of the axillary fold. The coordinate measurement system was defined as the motion of the humerus relative to the thorax (Y‐X‐Y order) (Wu et al., 2005). The motor was controlled in real‐time using MATLAB Simulink Real‐Time (v2016a; Mathworks, Inc).
Maximal voluntary contractions (MVC) were collected at the start of each data collection in six different torque directions: vertical adduction and abduction, horizontal adduction and abduction, internal and external rotation with the participant's arm positioned in 90° of elevation, 0° of the plane of elevation, and 0° of axial rotation. Verbal encouragement was provided during MVC performance to ensure maximal exertions were attained. The experimental protocol consisted of four arm positions, including a combination of one of two shoulder rotation positions, neutral and externally rotated 90°, and one of two shoulder abduction positions, 60° and 90°. These positions will henceforth be abbreviated as a combination of their rotation angle (N for neutral or ER for externally rotated) and frontal (i.e., coronal) plane of elevation angle (60° or 90°). The order of shoulder positions (N60, N90, ER60, ER90) was randomized first by the rotation position (i.e., N or ER) and then by the plane of elevation angle (i.e., 60° or 90°) for each participant. At each shoulder position, the participant was asked to produce and maintain isometric shoulder torques for approximately 5 s in the vertical adduction and horizontal flexion. The magnitude of torque produced in each shoulder position was scaled to 0% (e.g., at rest), 15%, and 30% of the participant's MVC. The investigators observed the performance of both maximal and submaximal trials to ensure the participant was executing each trial correctly and without the involvement of the trunk. Participants repeated torques in each direction and at each magnitude for a total of 20 trials per shoulder configuration (i.e., N60, N90, ER60, ER90). A computer monitor was placed in front of the participant to provide visual feedback of the target, which assisted with torque accuracy. An Aixplorer ultrasound elastography machine (Supersonic Imagine) connected to an SL15‐4 linear transducer array was used to collect ultrasound SWE images (Optimization: Standard, Persistence: Medium, Smoothing: 5, Frame Rate: 12 Hz) from the pectoralis major. Two ultrasound SWE images were collected per experimental condition from each fiber region, resulting in 80 images collected per participant.
Ultrasound SWE images from the clavicular and the sternocostal fiber region of the pectoralis major were collected during voluntary contractions. For the clavicular fiber region, the ultrasound transducer was initially placed ~1 cm inferior to the clavicle over the muscle's midpoint and shifted inferiorly from the clavicle until it was located mid‐belly over the clavicular fiber region. When imaging the sternocostal fiber region in male participants, the transducer was initially placed ~1 cm superior to the nipple. The transducer was then shifted superiorly until it was located mid‐belly over the sternocostal fiber region. In females, the sternocostal fiber region was imaged by placing the transducer ~4 cm inferior to the sternoclavicular joint and then moving it inferiorly until it was located mid‐belly over the sternocostal fiber region. When individual muscle fascicles were identified on the B‐mode ultrasound image, the orientation of the ultrasound transducer was considered satisfactory. An elastography color map (2.5 × 1 cm) positioned within the muscle's belly was superimposed over the B‐mode image, which provided pixel‐by‐pixel calculations of the participant's SWV. The same investigator collected all ultrasound images.
2.3. Data and statistical analyses
The SWE images were exported from the ultrasound machine and analyzed using a custom‐written MATLAB algorithm to quantify SWV and corresponding quality maps for each image (Lee et al., 2016, 2017; Leonardis et al., 2017). A region of interest encompassing the muscle belly of the fiber region of the pectoralis major was manually selected by the same investigator for each image. Adipose tissue and the aponeurosis were excluded to mitigate bias in the muscle data. The region of interest varied between the participants due to anatomical differences. The quality map for each image was used to determine the accuracy of the SWV measures at each pixel within the region of interest. Only the SWV with a quality map above 0.7 (out of 1.0) were included in analyses. An external trigger was used to obtain an elastography image and collect 2 s of torque data. Torque data were low‐pass filtered at 500 Hz with a sixth‐order analog Bessel filter. Following this, the torque data were averaged across each 2‐s trial and normalized to the MVC.
All statistical analyses were performed in MATLAB. The effect of age and sex on shoulder strength in the horizontal and vertical directions was tested using independent t‐tests. Linear mixed‐effects models were used to test our primary hypotheses that (1) females will have a greater activation‐dependent stiffness than males irrespective of the pectoralis major fiber region and that (2) older adults will have a lower activation‐dependent stiffness than younger adults. Mean SWV was treated as an outcome measure, while age (older, younger) and sex (male, female) were between‐subject factors. Raw torque was treated as a continuous variable. Separate mixed‐effects models were utilized for each region within a posture (i.e., N60, N90, ER60, and ER90). We included age by sex interaction terms within our statistical framework to determine if age‐related changes in stiffness during volitional contractions differed by sex. Random effects controlled for variability at the subject level. Bonferonni corrected post‐hoc comparisons were performed to determine if differences in SWV existed between the groups and the sexes with increases in torque. For all analyses, statistical significance was set to p < 0.05 and effect sizes for each factor were calculated as partial eta‐squared ().
3. RESULTS
Younger males were stronger than younger females in vertical adduction (t 22 = −5.31, p < 0.001; Table 1) and horizontal flexion (t 22 = −4.45, p < 0.001). Similarly, older males were stronger than older females in vertical adduction (t 22 = −3.50, p = 0.002) and horizontal flexion (t 22 = −2.58, p = 0.016). No differences in strength existed between the younger and the older males and females in vertical adduction and horizontal flexion strength (all p > 0.05).
The effect of age and sex for both pectoralis major regions during each shoulder position is visualized in SWE color maps, with representative data from younger and older males and females (Figure 1). These images show greater activation‐dependent stiffness in both pectoralis major regions in females in comparison with males across tasks. Furthermore, the color maps also show lower activation‐dependent stiffness in the clavicular region and greater activation‐dependent stiffness of the sternocostal region in older females than younger females in ER60 and N60, respectively. The influence of age and sex on activation‐dependent stiffness within each task was examined using a linear mixed‐effects model for each region (Table 2), and the results of these findings are presented below.
FIGURE 1.

Representative shear wave elastography maps for each pectoralis major fiber region. Each row shows the images from a representative young female, young male, older female, and older male participant. Each column represents the resultant color map for a given task (N60, N90, ER60, and ER90). Warmer colors indicate higher shear wave velocities, and cooler colors indicate lower shear wave velocities. Note the high shear wave velocities in females in comparison with males, irrespective of the task. These color maps indicate lower shear wave velocities for the clavicular region in older females in ER60 when compared with younger females. Higher shear wave velocities were observed in the sternocostal region in older females in N60 when compared with younger females
TABLE 2.
Parameter estimates of mean shear wave velocity (SWV) with the standard error shown for each variable within the linear mixed‐effects model for each of the pectoralis major fiber regions in each task
| Task | Measures | Mean SWV (m/s) | |
|---|---|---|---|
| Clavicular | Sternocostal | ||
| N60 | Intercept | 3.94 (0.36)*** | 2.74 (0.30)*** |
| Torque | 0.21 (0.02)*** | 0.12 (0.01)*** | |
| Age × Torque | −0.034 (0.024) | −0.024 (0.014) | |
| Sex × Torque (Reference: Female) | 0.13 (0.03)*** | 0.056 (0.02)*** | |
| Age × Sex × Torque (Reference: Female) | 0.019 (0.046) | 0.099 (0.03)*** | |
| Adjusted R 2 for model | 0.77 | 0.78 | |
| N90 | Intercept | 4.12 (0.31)*** | 3.33 (0.33)*** |
| Torque | 0.19 (0.02)*** | 0.093 (0.010)*** | |
| Age × Torque | 0.003 (0.021) | 0.009 (0.014) | |
| Sex × Torque (Reference: Female) | 0.16 (0.03)*** | 0.094 (0.021)*** | |
| Age × Sex × Torque (Reference: Female) | −0.016 (0.041) | 0.015 (0.030) | |
| Adjusted R 2 for model | 0.79 | 0.76 | |
| ER60 | Intercept | 4.71 (0.30)*** | 3.36 (0.28)*** |
| Torque | 0.17 (0.02)*** | 0.11 (0.01)*** | |
| Age × Torque | −0.009 (0.023) | −0.030 (0.014)* | |
| Sex × Torque (Reference: Female) | 0.22 (0.03)*** | 0.071 (0.019)*** | |
| Age × Sex × Torque (Reference: Female) | −0.12 (0.05)** | 0.054 (0.028) | |
| Adjusted R 2 for model | 0.75 | 0.78 | |
| ER90 | Intercept | 4.81 (0.29)*** | 4.37 (0.27)*** |
| Torque | 0.14 (0.02)*** | 0.07 (0.01)*** | |
| Age × Torque | 0.016 (0.021) | −0.021 (0.013) | |
| Sex × Torque (Reference: Female) | 0.22 (0.03)*** | 0.097 (0.019)*** | |
| Age × Sex × Torque (Reference: Female) | −0.081 (0.040)* | 0.012 (0.028) | |
| Adjusted R 2 for model | 0.78 | 0.69 | |
Raw torque was produced in the horizontal flexion direction for the clavicular fiber region and the vertical adduction direction for the sternocostal region. Significant parameters are bolded. The adjusted R 2 for each model is also shown. The asterisk indicates the significance level (*p < 0.05, **p < 0.01***p < 0.001).
3.1. The clavicular region
Females had greater SWV with increases in horizontal flexion torque than males in N60 (p < 0.001, = 0.06; Figure 2a) and N90 (p < 0.001, = 0.10; Figure 2b). Age did not influence SWV in N60 (p = 0.21) or N90 (p = 0.88). Age and sex influenced shear wave velocities in ER60 (p = 0.0065, = 0.03; Figure 2c) and ER90 (p = 0.043, = 0.01; Figure 2d). Younger females had greater shear wave velocities than younger males with increases in horizontal flexion torque in the clavicular region in ER60 (p < 0.001, = 0.29) and ER90 (p < 0.001, = 0.32). This was also observed in older adults, where older females had greater shear wave velocities than older males with increases in horizontal flexion torques in ER60 (p = 0.0015, = 0.08) and ER90 (p < 0.001, = 0.18). Aging only influenced activation‐dependent stiffness in females in ER60, where older females had lower shear wave velocities than younger females with increased horizontal flexion torques (p < 0.001, = 0.10). We did not observe such differences in ER90 (p = 0.047). Further, no differences in SWV with increased horizontal flexion torque existed between younger and older males in ER60 (p = 0.62) or ER90 (p = 0.47).
FIGURE 2.

Influence of age and sex on mean shear wave velocity in the clavicular fiber region for each task while producing horizontal flexion torques at rest, 15% and 30% maximal voluntary contractions. White filled circles represent young females; yellow filled circles represent young males; red filled squares represent older females, and orange filled squares represent older males. The resultant fit of the linear mixed effects model for each group is presented as lines of best fit with shaded regions (white solid line: young females; yellow solid line: young males; red solid line: older females; orange solid line: older males). (a) N60. Females had higher shear wave velocities than males. (b) N90. Females had higher shear wave velocities compared to males. (c) ER60. Older females had lower shear wave velocities than younger females, and females had higher shear wave velocities than males. (d) ER90. Females had higher shear wave velocities than males
3.2. The sternocostal region
Age and sex influenced shear wave velocities of the sternocostal region in N60 (Age × Sex interaction: p = 0.0011; = 0.04; Figure 3a). In terms of age effects, older females had greater SWV with increases in vertical adduction torques than younger females (p = 0.0058, = 0.06). SWV was the same in younger and older males (p = 0.102). Sex‐related differences were also observed in both older and younger adults in N60. Specifically, older females had greater shear wave velocities with increases in vertical adduction torques than older males (p < 0.001, = 0.25). Similarly, younger females had greater shear wave velocities than younger males with increases in vertical adduction torques (p = 0.0015, = 0.08). In ER60, older adults had lower shear wave velocities than younger adults (p = 0.0356, = 0.01), while females had greater shear wave velocities than males (p < 0.001, = 0.05; Figure 3c) with increases in vertical adduction torques. We also observed sex differences in N90 and ER90, where females had greater shear wave velocities than males with increases in vertical adduction torques (both p < 0.001; N90: = 0.07; ER90: = 0.09; Figure 3b,d). Shear wave velocities were not influenced by age in N90 (p = 0.52) or ER90 (p = 0.10).
FIGURE 3.
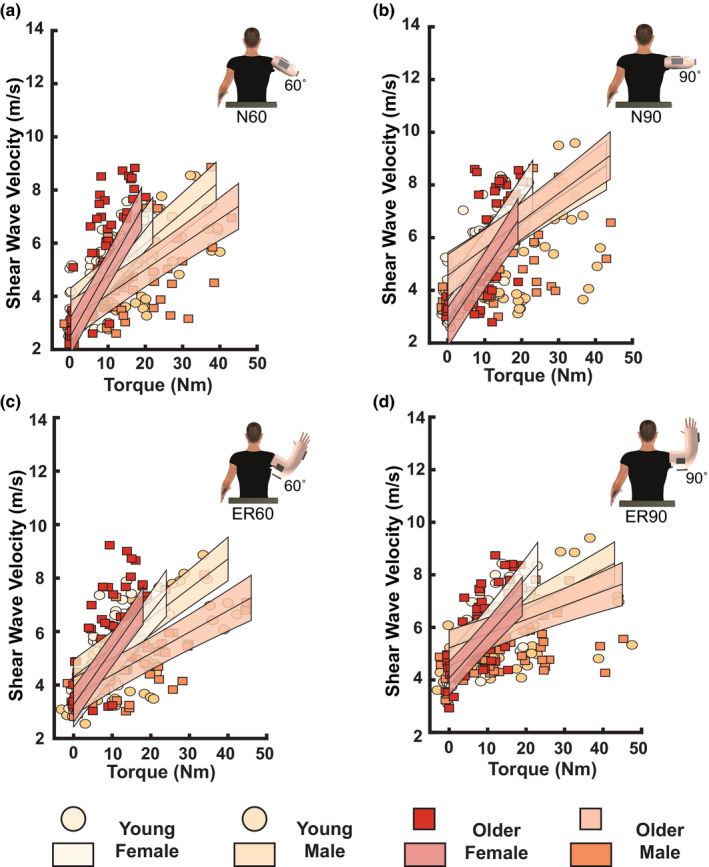
Influence of age and sex on mean shear wave velocity of the sternocostal fiber region for each task while producing vertical adduction torques at rest, 15% and 30% maximal voluntary contractions. White filled circles represent young females; yellow filled circles represent young males; red filled squares represent older females, and orange filled squares represent older males. The resultant fit of the linear mixed effects model for each group is presented as lines of best fit with shaded regions (white solid line: young females; yellow solid line: young males; red solid line: older females; orange solid line: older males). (a) N60. Note the higher shear wave velocities in older and younger females in comparison with older and younger males. Older females also had higher shear wave velocities in comparison with younger females. (b) N90. Higher shear wave velocities were observed in females than males. (c) ER60. Older adults had lower shear wave velocities than younger adults, and females had higher shear wave velocities than males. (d) ER90. Higher shear wave velocities were observed in females in comparison with the males
4. DISCUSSION
This study aimed to investigate if age and sex affect the activation‐dependent changes in the stiffness of the pectoralis major fiber regions. First, we hypothesized that the older adults would have lower activation‐dependent stiffness than younger adults across tasks, independent of the pectoralis major fiber region. By contrast, we observed reduced activation‐dependent stiffness of the clavicular region and greater activation‐dependent stiffness of the sternocostal region only in the older females in comparison with younger females in ER60 and N60, respectively. We also observed reductions in the activation‐dependent sternocostal region stiffness in older adults in ER60 compared with younger adults. Second, we hypothesized that females would have a greater stiffness than males during voluntary contractions, which was supported by our results.
The present study provides evidence that sex and age differentially affect the activation‐dependent changes to the stiffness of each fiber region of the pectoralis major. Older females exhibited greater activation‐dependent stiffness than younger females in the sternocostal region when generating vertical adduction torques in N60. By contrast, activation‐dependent stiffness was reduced in older females compared with younger females in the clavicular region when generating horizontal flexion torques in ER60. These differences cannot be attributed to potential age‐related declines in strength, as both groups exhibited similar strength in vertical and horizontal directions. The present findings may reflect task‐specific age‐related alterations in the activation of the clavicular region given SWV increases with increases in muscle activation (Chernak et al., 2013; Nordez & Hug, 2010; Yoshitake et al., 2014). While the sternocostal region contribution to vertical adduction torques is greater at higher contractions in N60, both regions equally assist in generating horizontal flexion torques in ER60 (Leonardis et al., 2017). The altered neuromuscular control of the clavicular region in older females may prompt greater utilization of the sternocostal region to generate vertical adduction torques and potentially rely on other shoulder muscles to generate horizontal flexion torques. As such, greater activation of the anterior deltoid or coracobrachialis may be observed in older females during the generation of horizontal flexion torques. We also observed reductions in the activation‐dependent stiffness of the sternocostal region in older groups as participants generated vertical adduction torques in ER60. These findings indicate position‐specific alterations in the activation‐dependent stiffness. The generation of vertical adduction torques in this position relies on the contribution of both pectoralis major fiber regions (Leonardis et al., 2017). Therefore, it may be that older adults utilize differential neuromuscular strategy to generate vertical adduction torques in this position, such as greater reliance on the clavicular fiber region or greater recruitment of other shoulder muscles.
Females had greater activation‐dependent stiffness than males irrespective of the pectoralis major fiber region in all tasks. As muscle activity modulates SWV, differences in the stiffness between these groups may be due to a neurally mediated muscle stiffening effect (Chernak et al., 2013). Sex and age‐related differences in muscle activation, architectural or neural properties are presently unknown for the pectoralis major fiber regions. Studies in other muscles reported greater surface electromyography (EMG) amplitudes in females than males in vastus medialis and rectus femoris (Krishnan & Williams, 2009), tibialis anterior (Cioni et al., 1994), and adductor pollicis (Visser & De Ruke, 1974) in submaximal tasks. Moreover, recent studies using intramuscular EMG reported higher motor unit discharge rates in females than males in tibialis anterior and vastus medialis in submaximal tasks (Inglis & Gabriel, 2020; Peng et al., 2018). Lastly, differences in architectural properties, such as muscle fiber pennation angles and muscle fiber lengths, may contribute to the observed sex‐related differences in stiffness during volitional contraction. Lower pennation angles and longer muscle fiber lengths are typically observed in females than males (Chow et al., 2000; Manal et al., 2006). Interestingly, females in the present study generated lower torques than males but required greater activation‐dependent stiffness, indicating that males require less contribution or activation of the pectoralis major fiber regions to generate greater shoulder torques. These findings further indicate that differences in neural and architectural properties may be playing a key role in the sex‐related functional utility of the pectoralis major fiber regions. The neural and architectural properties, however, were not directly measured in the present study, limiting our interpretation of the present findings.
There are limitations that accompany the current study. Our findings may not translate to elderly adults, as we did not evaluate any participants above 60 years old. There are known alterations to neuromuscular properties, fiber quantity, and strength in individuals older than 60 (Baumgartner et al., 1995; Campbell et al., 1973; Frontera et al., 2000; Fukumoto et al., 2015; Hepple & Rice, 2016; Lexell et al., 1988), and it is reasonable to suspect these changes could influence muscle stiffness. Our participants were predominantly recreationally active, and our findings may not translate to sedentary or highly trained individuals. The tasks were performed only in the frontal plane, although the pectoralis major contributes to shoulder joint torque throughout the range of motion. Further, participants generated torques only in two planes of rotation and MVCs were only obtained in a single posture (i.e., N90). Lastly, the tasks were isometric and only examined up to 30% MVC as SWE has not been validated above 60% MVC (Yoshitake et al., 2014).
These findings collectively provide the first in vivo evidence of age‐ and sex‐related differences in stiffness of the pectoralis major fiber regions during voluntary contractions. Alterations in the activation‐dependent stiffness of the clavicular fiber region are evident in older females, indicating age‐related changes in the role and utilization of the clavicular region. This may influence an older female's ability to produce horizontal flexion torques effectively. Further, females have greater activation‐dependent stiffness than males in both regions of the pectoralis major, irrespective of age, but generate substantially less torque. These differences may indicate divergent neural, architectural, and global activation properties of the pectoralis major fiber regions between the sexes. Together, these findings suggest that sex and age are important factors to consider when assessing and designing interventions targeting the function of pectoralis major fiber regions in healthy adults and individuals with neuromuscular pathologies. Moreover, differences between males and females characterized in this study may have critical implications in understanding the increased incidence of musculoskeletal injuries in females.
AUTHOR CONTRIBUTION
CAS, TL, JML, MK, DBL performed research design; CAS, JML, MK performed data acquisition; CAS, TL, DBL performed data analyses; TL, DBL performed statistical analyses; CAS, TL, DBL drafted manuscript; CAS, TL, JML, MK, DBL revised manuscript. All authors have read and approved the final submitted manuscript.
ACKNOWLEDGMENTS
This research was financially supported in part by the National Center for Medical Rehabilitation Research within the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R03HD097704.
Cheryl A. Setlock and Tea Lulic‐Kuryllo contributed equally to this work and are co‐first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ackland, D.C. , Pak, P. , Richardson, M. & Pandy, M.G. (2008) Moment arms of the muscles crossing the anatomical shoulder. Journal of Anatomy, 213(4), 383–390. 10.1111/j.1469-7580.2008.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackland, D.C. & Pandy, M.G. (2011) Moment arms of the shoulder muscles during axial rotation. Journal of Orthopaedic Research, 29(5), 658–667. 10.1002/jor.21269. [DOI] [PubMed] [Google Scholar]
- Akagi, R. , Yamashita, Y. & Ueyasu, Y. (2015) Age‐related differences in muscle shear moduli in the lower extremity. Ultrasound in Medicine and Biology, 41(11), 2906–2912. 10.1016/j.ultrasmedbio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Baumer, T.G. , Dischler, J. , Davis, L. , Labyed, Y. , Siegal, D.S. , van Holsbeeck, M. et al. (2018) Effects of age and pathology on shear wave speed of the human rotator cuff. Journal of Orthopaedic Research, 36(1), 282–288. 10.1002/jor.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner, R.N. , Stauber, P.M. , McHugh, D. , Koehler, K.M. & Garry, P.J. (1995) Cross‐sectional age differences in body composition in persons 60+ years of age. Journals of Gerontology—Series A Biological Sciences and Medical Sciences, 50A(6), M307–M316. 10.1093/gerona/50A.6.M307. [DOI] [PubMed] [Google Scholar]
- Bernabei, M. , Lee, S.S.M. , Perreault, E.J. & Sandercock, T.G. (2020) Shear wave velocity is sensitive to changes in muscle stiffness that occur independently from changes in force. Journal of Applied Physiology, 128(1), 8–16. 10.1152/japplphysiol.00112.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter, J.A. , Woo, S.L. , Goldberg, V.M. , Hadley, E.C. , Booth, F. , Oegema, T.R. & Eyre, D.R. (1993) Soft‐tissue aging and musculoskeletal function. The Journal of Bone & Joint Surgery, 75(10), 1533–1548. 10.2106/00004623-199310000-00015 [DOI] [PubMed] [Google Scholar]
- Campbell, M.J. , McComas, A.J. & Petito, F. (1973) Physiological changes in ageing muscles. Journal of Neurology Neurosurgery and Psychiatry, 36(2), 174–182. 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, E.R. (2003) Pectoralis major myocutaneous flap. Oral and Maxillofacial Surgery Clinics of North America, 15(4), 565–575. 10.1016/S1042-3699(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Chernak, L.A. , Dewall, R.J. , Lee, K.S. & Thelen, D.G. (2013) Length and activation dependent variations in muscle shear wave speed. Physiological Measurement, 34(6), 713–721. 10.1088/0967-3334/34/6/713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino, K. & Takahashi, H. (2016) Measurement of gastrocnemius muscle elasticity by shear wave elastography: association with passive ankle joint stiffness and sex differences. European Journal of Applied Physiology, 116(4), 823–830. 10.1007/s00421-016-3339-5. [DOI] [PubMed] [Google Scholar]
- Chodock, E. , Hahn, J. , Setlock, C.A. & Lipps, D.B. (2020) Identifying predictors of upper extremity muscle elasticity with healthy aging. Journal of Biomechanics, 103, 109687. 10.1016/j.jbiomech.2020.109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, R.S. , Medri, M.K. , Martin, D.C. , Leekam, R.N. , Agur, A.M. & McKee, N.H. (2000) Sonographic studies of human soleus and gastrocnemius muscle architecture: Gender variability. European Journal of Applied Physiology, 82(3), 236–244. 10.1007/s004210050677. [DOI] [PubMed] [Google Scholar]
- Cioni, R. , Giannini, F. , Paradiso, C. , Battistini, N. , Navona, C. & Starita, A. (1994) Sex differences in surface EMG interference pattern power spectrum. Journal of Applied Physiology, 77(5), 2163–2168. 10.1152/jappl.1994.77.5.2163. [DOI] [PubMed] [Google Scholar]
- Corten, E.M.L. , Schellekens, P.P.A. , Oey, P.L. , Hage, J.J. , Kerst, A. & Kon, M. (2007) Function of the clavicular part of the pectoralis major muscle after transplantation of its sternocostal part. Annals of Plastic Surgery, 58(4), 392–396. 10.1097/01.sap.0000238427.18396.ea. [DOI] [PubMed] [Google Scholar]
- Cui, L. , Perreault, E.J. , Maas, H. & Sandercock, T.G. (2008) Modeling short‐range stiffness of feline lower hindlimb muscles. Journal of Biomechanics, 41(9), 1945–1952. 10.1016/j.jbiomech.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby, S.F. , Cloud, B.A. , Brandenburg, J.E. , Giambini, H. , Song, P. , Chen, S. et al. (2015) Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clinical Biomechanics, 30(1), 22–27. 10.1016/j.clinbiomech.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera, W.R. , Hughes, V.A. , Fielding, R.A. , Fiatarone, M.A. , Evans, W.J. & Roubenoff, R. (2000) Aging of skeletal muscle: a 12‐yr longitudinal study. Journal of Applied Physiology, 88(4), 1321–1326. 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Fukumoto, Y. , Ikezoe, T. , Yamada, Y. , Tsukagoshi, R. , Nakamura, M. , Takagi, Y. et al. (2015) Age‐related ultrasound changes in muscle quantity and quality in women. Ultrasound in Medicine and Biology, 41(11), 3013–3017. 10.1016/j.ultrasmedbio.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Fung, L. , Wong, B. , Ravichandiran, K. , Agur, A. , Rindlisbacher, T. & Elmaraghy, A. (2009) Three‐dimensional study of pectoralis major muscle and tendon architecture. Clinical Anatomy, 22(4), 500–508. 10.1002/ca.20784. [DOI] [PubMed] [Google Scholar]
- Haley, C.A. & Zacchilli, M.A. (2014) Pectoralis major injuries: evaluation and treatment. Clinics in Sports Medicine, 33(4), 739–756. 10.1016/j.csm.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Hepple, R.T. & Rice, C.L. (2016) Innervation and neuromuscular control in ageing skeletal muscle. Journal of Physiology, 594(8), 1965–1978. 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C.L. , Gill, T.K. , Shanahan, E.M. & Taylor, A.W. (2010) Prevalence and correlates of shoulder pain and stiffness in a population‐based study: the North West Adelaide Health Study. International Journal of Rheumatic Diseases, 13(3), 215–222. 10.1111/j.1756-185X.2010.01475.x. [DOI] [PubMed] [Google Scholar]
- Inglis, J.G. & Gabriel, D.A. (2020) Sex differences in motor unit discharge rates at maximal and submaximal levels of force output. Applied Physiology, Nutrition, and Metabolism, 45(11), 1197–1207. 10.1139/apnm-2019-0958. [DOI] [PubMed] [Google Scholar]
- Krishnan, C. & Williams, G.N. (2009) Sex differences in quadriceps and hamstrings EMG‐moment relationships. Medicine and Science in Sports and Exercise, 41(8), 1652–1660. 10.1249/MSS.0b013e31819e8e5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.S.M. , Gaebler‐Spira, D. , Zhang, L.Q. , Rymer, W.Z. & Steele, K.M. (2016) Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clinical Biomechanics, 31, 20–28. 10.1016/j.clinbiomech.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.K. , Skalski, M.R. , White, E.A. , Tomasian, A. , Phan, D.D. , Patel, D.B. et al. (2017) US and MR imaging of pectoralis major injuries. Radiographics, 37(1), 176–189. 10.1148/rg.2017160070. [DOI] [PubMed] [Google Scholar]
- Leonardis, J.M. , Desmet, D.M. & Lipps, D.B. (2017) Quantifying differences in the material properties of the fiber regions of the pectoralis major using ultrasound shear wave elastography. Journal of Biomechanics, 63, 41–46. 10.1016/j.jbiomech.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Leonardis, J.M. , Lyons, D.A. , Giladi, A.M. , Momoh, A.O. & Lipps, D.B. (2019) Functional integrity of the shoulder joint and pectoralis major following subpectoral implant breast reconstruction. Journal of Orthopaedic Research, 37(7), 1610–1619. 10.1002/jor.24257. [DOI] [PubMed] [Google Scholar]
- Lexell, J. , Taylor, C.C. & Sjöström, M. (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. Journal of the Neurological Sciences, 84(2–3), 275–294. 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Manal, K. , Roberts, D.P. & Buchanan, T.S. (2006) Optimal pennation angle of the primary ankle plantar and dorsiflexors: variations with sex, contraction intensity, and limb. Journal of Applied Biomechanics, 22(4), 255–263. 10.1123/jab.22.4.255. [DOI] [PubMed] [Google Scholar]
- McPhail, S.M. , Schippers, M. & Marshall, A.L. (2014) Age, physical inactivity, obesity, health conditions, and health‐related quality of life among patients receiving conservative management for musculoskeletal disorders. Clinical Interventions in Aging, 9, 1069–1080. 10.2147/CIA.S61732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordez, A. & Hug, F. (2010) Muscle shear elastic modulus measured using supersonic shear imaging is highly related to muscle activity level. Journal of Applied Physiology, 108(5), 1389–1394. 10.1152/japplphysiol.01323.2009. [DOI] [PubMed] [Google Scholar]
- Palazzo, C. , Ravaud, J.F. , Papelard, A. , Ravaud, P. & Poiraudeau, S. (2014) The burden of musculoskeletal conditions. PLoS One, 9(3), 10.1371/journal.pone.0090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton, M.E. & Brown, J.M.M. (1994) An electromyographic analysis of functional differentiation in human pectoralis major muscle. Journal of Electromyography and Kinesiology, 4(3), 161–169. 10.1016/1050-6411(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Peng, Y.L. , Tenan, M.S. & Griffin, L. (2018) Hip position and sex differences in motor unit firing patterns of the vastus medialis and vastus medialis oblique in healthy individuals. Journal of Applied Physiology, 124(6), 1438–1446. 10.1152/japplphysiol.00702.2017. [DOI] [PubMed] [Google Scholar]
- Saeki, J. , Ikezoe, T. , Yoshimi, S. , Nakamura, M. & Ichihashi, N. (2019) Menstrual cycle variation and gender difference in muscle stiffness of triceps surae. Clinical Biomechanics, 61, 222–226. 10.1016/j.clinbiomech.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Stegink‐Jansen, C.W. , Buford, W.L. , Patterson, R.M. & Gould, L.J. (2011) Computer simulation of pectoralis major muscle strain to guide exercise protocols for patients after breast cancer surgery. Journal of Orthopaedic and Sports Physical Therapy, 41(6), 417–426. 10.2519/jospt.2011.3358. [DOI] [PubMed] [Google Scholar]
- Tobin, G.R. (1985) Pectoralis major segmental anatomy and segmentally split pectoralis major flaps. Plastic and Reconstructive Surgery, 75(6), 814–824. 10.1097/00006534-198506000-00009. [DOI] [PubMed] [Google Scholar]
- Visser, S.L. & De Ruke, W. (1974) Influence of sex and age on EMG contraction pattern. European Neurology, 12(4), 229–235. 10.1159/000114623. [DOI] [PubMed] [Google Scholar]
- Wolfe, S.W. , Wickiewicz, T.L. & Cavanaugh, J.T. (1992) Ruptures of the pectoralis major muscle. Surgery, 10(2), 309–312. 10.1177/036354659202000517. [DOI] [PubMed] [Google Scholar]
- Wu, G. , Van Der Helm, F.C.T. , Veeger, H.E.J. , Makhsous, M. , Van Roy, P. , Anglin, C. et al. (2005) ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—part II: Shoulder, elbow, wrist and hand. Journal of Biomechanics, 38(5), 981–992. 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Yoshitake, Y. , Takai, Y. , Kanehisa, H. & Shinohara, M. (2014) Muscle shear modulus measured with ultrasound shear‐wave elastography across a wide range of contraction intensity. Muscle and Nerve, 50(1), 103–113. 10.1002/mus.24104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


