Acute myeloid leukemia (AML) is frequently associated with hyperleukocytosis [white blood cell count (WBC) of >50 or >100 × 109/L at presentation] [1]. Hyperleukocytosis also predicts a higher risk of complications as well as early mortality; lack of intensive chemotherapy (IC) also portends inferior outcomes [1–4]. Hydroxyurea and leukapheresis are employed as cytoreductive therapies to mitigate the morbidity and mortality associated with hyperleukocytosis until intensive induction chemotherapy is administered as definitive treatment for those who are candidates. Many patients, however, are not candidates for IC [5]. Limited evidence supports the role of leukapheresis in general for patients with newly diagnosed AML presenting with hyperleukocytosis and as a result, clinical practice is inconsistent [6–10]. The clinical outcomes and benefits of leukapheresis in AML patients who do not receive IC are largely unknown. We sought to explore the clinical course among older AML patients who present with hyperleukocytosis, but do not receive intensive therapy.
Data from patients with newly diagnosed AML who presented with hyperleukocytosis, defined as WBC 50 × 109/L or greater were retrospectively collected at 12 institutions in the United States (US), Spain, Germany, and France from 1982 to 2016, and then analyzed at the coordinating center (Yale Cancer Center). We herein report on the outcomes of patients who did not receive IC. Analyses of patients who received IC, details of methods and ethical approvals were separately reported [11]. Studied metrics included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), WBC, hemoglobin (Hgb), platelet count, serum metabolic parameters, AML disease risk by cytogenetic and molecular abnormalities, presence of tumor lysis syndrome (TLS), disseminated intravascular coagulation (DIC), leukostasis, admission to an intensive care unit (ICU) at presentation, receipt of hydroxyurea, other non-intensive leukemia-directed therapy, administration of leukapheresis, and response to therapy. Kaplan–Meier analysis was used to estimate overall survival (OS) from time of presentation until death or end of follow-up.
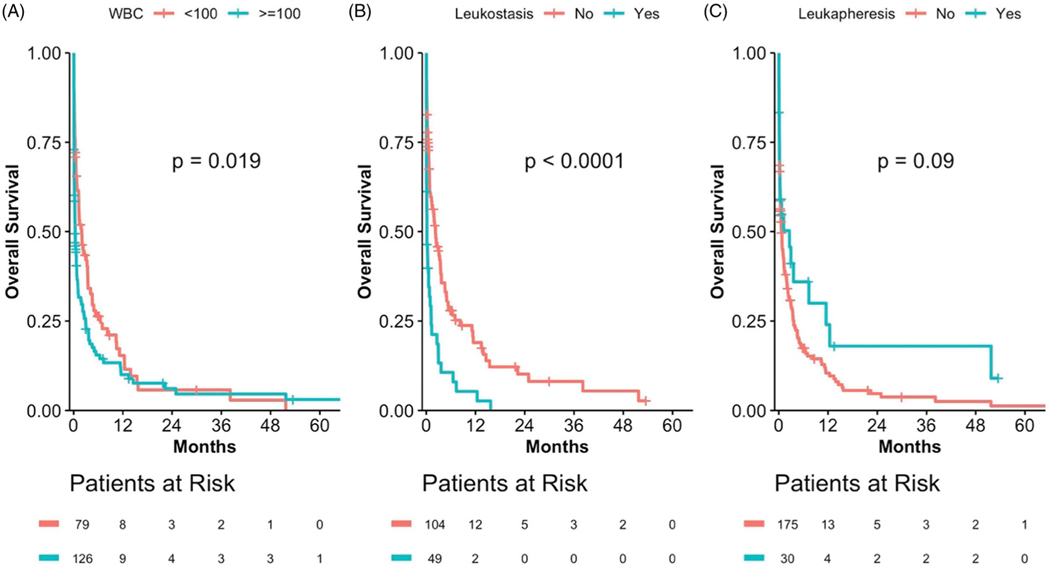
A total of 219 patients met eligibility criteria. Among these patients, the median age was 75 [interquartile range (IQR), 66–81] years, 58.0% were male, and 62.8% had an ECOG PS of two or greater (Table 1). Median WBC, Hgb, and platelet count at presentation was 131.4 × 109/L (IQR, 78–199), 8.9 g/dL (IQR, 7.7–10.6), and 34 (IQR, 11.9–62), respectively; 63.0% presented with a WBC greater than 100 × 109/L. Cytogenetically or molecularly defined poor risk AML (as per the 2017 European LeukemiaNet risk stratification) was found in 23.6% of patients [12]. TLS, DIC, or clinical leukostasis was present in 25.7, 15.8, and 34.1% of patients, respectively. Pulmonary, CNS, renal, cardiac, GI, or retinal evidence of leukostasis was present in 54.4, 16.2, 11.8, 10.3, 5.9, and 1.5%, respectively, of those with clinical leukostasis. Leukapheresis was performed in 32 (14.6%) patients. Approximately one-third (28.7%) of patients required admission to the ICU at the time of diagnosis before receipt of (non-intensive) therapy, though most patients (71.3%) required ICU admission for 48 hours or less (Table 2). For those patients undergoing leukapheresis, the reason for ICU admission (institution protocol versus medical acuity) was not recorded. The majority (72.9%) of patients received cytoreductive therapy with hydroxyurea with a median time from presentation to the administration of 12 h. Of the 43 patients for whom the specific non-intensive therapy used beyond hydroxyurea was reported, 22 patients received a hypomethylating agent (azacitidine or decitabine) and 15 received low-dose cytarabine. The 30-day mortality was 57.1% and median OS was only 22 days (95% CI: 13–38 days) (Table 2). The median OS for patients aged ≤65 and >65 years was 17 (95% CI: 4–75) and 23 (95% CI: 14–44) days, respectively. A presenting WBC of ≥100 × 109/L (Figure 1(A)) and the presence of symptoms or signs of leukostasis (Figure 1(B)) were both associated were inferior OS in univariate analyses (p = .019 and p < .0001, respectively). In univariate analysis, the use of leukapheresis had no statistically significant impact on OS (p = .09) (Figure 1(C)). The small number of patients undergoing leukapheresis and inherent selection bias limited the assessment of the impact of leukapheresis in multivariate analysis.
Table 1.
Patient characteristics for patients who did not receive intensive chemotherapya.
| Characteristics | N | All (N = 219) | Without Leukapheresis (N = 187) | Leukapheresis (N = 32) | p |
|---|---|---|---|---|---|
| Median age (IQR) years | 219 | 75 (66.5–81) | 76 (67–81) | 71 (65.2–78) | .057 |
| Female sex no (%) | 219 | 92 (42%) | 75 (40.1%) | 17 (53.1%) | .18 |
| ECOG performance status <2 no (%) | 137 | 51 (37.2%) | 46 (38.3%) | 5 (29.4%) | .596 |
| WHO type no (%) | 131 | .118 | |||
| AML with recurrent genetic abnormalities | 25 (19.1%) | 22 (19.8%) | 3 (15%) | ||
| AML with myelodysplasia-related features | 29 (22.1%) | 28 (25.2%) | 1 (5%) | ||
| AML, not otherwise specified | 71 (54.2%) | 56 (50.5%) | 15 (75%) | ||
| Therapy-related AML | 6 (4.6%) | 5 (4.5%) | 1 (5%) | ||
| Molecular characteristics no (%) | 148 | >.999 | |||
| Good/normal | 113 (76.4%) | 92 (76%) | 21 (77.8%) | ||
| Poor | 35 (23.6%) | 29 (24%) | 6 (22.2%) | ||
| Complex cytogenetics no (%) | 108 | 21 (19.4%) | 18 (20.2%) | 3 (15.8%) | >.999 |
| Monosomy karyotype no (%) | 85 | 11 (12.9%) | 10 (13.2%) | 1 (11.1%) | >.999 |
| NPM1 mutation no (%) | 82 | 27 (32.9%) | 21 (30.4%) | 6 (46.2%) | .338 |
| FLT3 mutation no (%) | 94 | 36 (38.3%) | 29 (37.7%) | 7 (41.2%) | .789 |
| Complete blood count | |||||
| Median WBC (IQR) | 219 | 131 (78–198) | 118 (75–192.6) | 177 (150.2–255.8) | <.001 |
| Median Hgb (IQR) | 216 | 8.9 (7.7–10.6) | 9.1 (8–10.9) | 8.3 (7.1–10) | .037 |
| Median Platelets (IQR) | 219 | 34 (11.9–62) | 32 (11.1–59.5) | 45 (15.8–75.2) | .079 |
| Blast % | |||||
| Median peripheral blood blast (IQR) | 206 | 76.5 (40.8–92) | 73.5 (39–90.5) | 83 (70.5–95) | .094 |
| Median bone marrow blast (IQR) | 127 | 83.5 (61–90) | 81.5 (59.8–90) | 92 (82–95) | .021 |
| Clinical presentation | |||||
| Leukostasis no (%) | 164 | 56 (34.1%) | 37 (27.6%) | 19 (63.3%) | <.001 |
| TLS no (%) | 206 | 53 (25.7%) | 46 (26.3%) | 7 (22.6%) | .824 |
| DIC no (%) | 203 | 32 (15.8%) | 25 (14.5%) | 7 (23.3%) | .275 |
| Admission on weekdays no (%) | 219 | 190 (86.8%) | 160 (85.6%) | 30 (93.8%) | .268 |
| Admission between 6 am and 6 pm no (%) | 139 | 77 (55.4%) | 70 (61.9%) | 7 (26.9%) | .002 |
| Organs affected by leukostasis no (%) | 68 | .182 | |||
| Pulmonary leukostasis | 37 (54.4%) | 22 (50%) | 15 (62.5%) | ||
| CNS leukostasis | 11 (16.2%) | 5 (11.3%) | 6 (25%) | ||
| Retinal leukostasis | 1 (1.5%) | 1 (2.2%) | 0 (0%) | ||
| Renal failure | 8 (11.8%) | 6 (13.6%) | 2 (8.3%) | ||
| Chest pain/MI | 7 (10.3%) | 7 (15.9%) | 0 (0%) | ||
| GI leukostasis | 4 (5.9%) | 3 (6.8%) | 1 (4.2%) |
AML: acute myeloid leukemia; DIC: disseminated intravascular coagulation; TLS: tumor lysis syndrome.
For continuous variables, t-test or Wilcoxon rank-sum test was used to compare the difference between treatment groups, depending on the distribution of data. For categorical variables, Fisher’s exact test was used to examine the association with treatment groups. IQR denotes interquartile range.
Table 2.
Outcomes for patients who did not receive intensive chemotherapya.
| Outcomes | N | All | Without leukapheresis | Leukapheresis | p |
|---|---|---|---|---|---|
| Death in the first 30 days n (%) | 189 | 108 (57.1%) | 95 (57.9%) | 13 (52%) | .666 |
| ICU admission n (%) | 94 | 27 (28.7%) | 15 (20.8%) | 12 (54.5%) | .006 |
| Median time in ICU (IQR) days | 25 | 1 (1–2) | 1 (1–2.8) | 2 (1–2) | .729 |
| Hemodialysis required n (%) | 95 | 9 (9.5%) | 5 (7.1%) | 4 (16%) | .236 |
| Mechanical ventilation required n (%) | 82 | 10 (12.2%) | 8 (11.9%) | 2 (13.3%) | >.999 |
| Relapse after initial response n (%) | 61 | 11 (18%) | 5 (9.6%) | 6 (66.7%) | .001 |
| Hematopoietic stem cell transplant n (%) | 141 | 6 (4.3%) | 4 (3.2%) | 2 (13.3%) | .124 |
| Median duration of CR (IQR) month | 7 | 184 (135–226) | 174 (144–191) | 243 (174–691) | .4 |
| Median overall survival (95% CI) month | 205 | 0.7 (0.4–1.3) | 0.7 (0.4–1.2) | 2.6 (0.3–12.4) | .09 |
ICU: intensive care unit; IQR: interquartile range.
For categorical variables, the comparisons between treatment groups were based on Fisher’s exact test. For continuous variables, the comparisons were based on the Wilcoxon rank-sum test. Log-rank test was used to compare the overall survival between two groups.
Figure 1.
Overall survival (OS) for patients who did not receive intensive chemotherapy (IC) based on WBC, evidence of leukostasis and receipt of leukapheresis. (A) Patients with WBC >100,000 versus <100,000. (B) Patients with evidence of leukostasis versus without evidence of leukostasis. (C) Patients who received leukapheresis versus patients who did not receive leukapheresis.
We herein report one of the largest studied cohorts of patients with newly diagnosed AML presenting with hyperleukocytosis and who did not receive IC. A quarter of patients with newly diagnosed AML do not receive any form of leukemia-directed therapy and of those that do, approximately 25% will receive non-intensive therapy [5,13]. Furthermore, a recent study revealed that, as recently as 2013, more than 40% of newly diagnosed AML patients older than 65 years in the United States do not receive any active leukemia-directed therapy [14]. The decision to proceed with nonintensive therapy is influenced by both patient- and disease-specific factors with increasing age, comorbidity burden, or a diagnosis of secondary or therapy-related AML often serving as predictors of receiving nonintensive therapy [15]. Our parallel analysis of 779 AML patients presenting with hyperleukocytosis at diagnosis, but who did receive intensive therapy revealed that leukapheresis was employed at a similar frequency (15% of cases), but had no impact on 30-day mortality or OS [11].
This study represents the first evaluation of the clinical outcomes and benefits of leukapheresis in patients not receiving IC. The median age of patients in our study was 75 years, which is older than that reported for all patients with newly diagnosed AML (∼68 years) [5]. In addition, the majority (62.8%) of patients had an ECOG PS of three or greater. Rates of TLS and DIC and disease risk were grossly similar to those historically reported for all AML patients presenting with hyperleukocytosis, including those eligible for IC [1,4,10]. Leukostasis was evident in approximately one-third of patients and was independently-associated with inferior survival. Most patients were initially cytoreduced with hydroxyurea. Only 15% of total patients underwent leukapheresis which did not significantly impact OS in univariate analysis (Figure 1(C)).
Given the results of our study, the general use of leukapheresis as a cytoreductive strategy for AML patients presenting with hyperleukocytosis and not receiving IC may be called into question. Despite a possible nonsignificant trend toward improved OS in univariate analysis for leukapheresis-treated patients, selection bias and the lack of details of specific non-intensive therapy received subsequent to leukapheresis limit conclusions. Further, the lack of impact of leukapheresis on survival among these patients needs to be weighed against its non-trivial risks. A procedure to place a stable, large-bore venous access is required. There is also the increased risk of anaphylactic reactions (given the use of donor fresh frozen plasma), and citrate-mediated toxicity such as hypocalcemia and its possible consequences (e.g. QTc prolongation and seizure) [16]. The transient net whole blood removal and volume shifts associated with leukapheresis might also heighten the risk of worsened anemia and hemodynamic instability. Leukapheresis may also delay the initiation of non-IC leukemia-directed therapy, which itself can be associated with an improved OS compared to hydroxyurea or best supportive care. Limitations of our study include the fact that the standard non-intensive therapies available during our study timeframe were the hypomethylating agents or low-dose cytarabine monotherapy. In addition, the small number of patients undergoing leukapheresis, selection bias, and lack of details of nonintensive therapies for most patients precluded multivariate analysis and definite conclusions regarding the impact of leukapheresis in this population. The clinical outcomes and management strategies in AML patients with hyperleukocytosis not receiving IC in the era of venetoclax-based combinations and FLT3/IDH inhibitors need to be studied in the future. The ultimate goal, however, is the pursuit of novel and effective therapies for this high-risk population of AML patients for whom they are urgently needed.
Acknowledgements
Amer Zeidan is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was in part supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge all the patients, and the Frederick A. DeLuca Foundation for supporting the statistical analyses.
Funding
Maximilian Stahl received funding from the MSKCC Clinical Scholars T32 Program under award number 2T32 CA009512-31.
Footnotes
Disclosure statement
Rory Michael Shallis: No relevant financial relationship(s) to disclose
Maximilian Stahl: No relevant financial relationship(s) to disclose
Wei Wei: No relevant financial relationship(s) to disclose
Pau Montesinos: Daiichi Sankyo: Consultancy and Speakers Bureau; Novartis: Research Funding and Speakers Bureau
Etienne Lengline: No relevant financial relationship(s) to disclose
Judith Neukirchen: No relevant financial relationship(s) to disclose
Vijaya R. Bhatt: Consulting fees: CSL Behring, Agios, Abbvie, Partner therapeutics and Incyte; Research funding: Jazz, Incyte, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program.
Mikkael A. Sekeres: No relevant financial relationship(s) to disclose
Amir T. Fathi: Consulting/advisory board - Celgene, Takeda, Abbvie, Forty seven, NewLink, Trovagene, Pfizer, Daiichi Sankyo, Astellas, Amphivena; Clinical trial funding - Celgene and Agios
Heiko Konig: No relevant financial relationship(s) to disclose
Selina Luger: No relevant financial relationship(s) to disclose
Irum Khan: Teva: Speakers Bureau
Gail J. Roboz: AbbVie: Consultancy; Aphivena Therapeutics: Consultancy; Argenx: Consultancy; Astex Pharmaceuticals: Consultancy; Bayer: Consultancy; Celgene Corporation: Consultancy; Celltrion: Consultancy; Daiichi Sankyo: Consultancy; Bayer: Consultancy; Eisai: Consultancy; Janssen Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Orsenix: Consultancy; Otsuka: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Sandoz: Consultancy; Cellectis: Research Fundin; Orsenix: Consultancy
Thomas Cluzeau: Celgene: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau; Menarini: Consultancy; Jazz Pharma: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau; Amgen: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau; AbbVie: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau; Sanofi: Speakers Bureau; Pfizer: Speakers Bureau
David Martínez-Cuadron: No relevant financial relationship(s) to disclose
Emmanuel Raffoux: No relevant financial relationship(s) to disclose
Ulrich Germing: Celgene: Honoraria, Research Funding and Consultancy; Novartis: Honoraria and Research Funding; Janssen: Honoraria
Jayadev Manikkam Umakanthan: No relevant financial relationship(s) to disclose
Sudipto Mukherjee: Aplastic Anemia & MDS International Foundation in Joint Partnership with Cleveland Clinic Taussig Cancer Institute: Honoraria; BioPharm Communications: Consultancy; Bristol Myers Squib: Honoraria and Speakers Bureau; LEK Consulting: Consultancy and Honoraria; Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Pfizer: Honoraria; Projects in Knowledge: Honoraria; Takeda: Membership on an entity’s Board of Directors or advisory committees
Andrew M. Brunner: Takeda: Research Funding; Celgene: Consultancy and Research Funding; Novartis: Research Funding
Adam M. Miller: No relevant financial relationship(s) to disclose
Christine M. McMahon: No relevant financial relationship(s) to disclose
Ellen K. Ritchie: Incyte: Consultancy and Speakers Bureau; Celgene: Consultancy, Other: Travel, Accommodations, Expenses and Speakers Bureau; Pfizer: Consultancy and Research Funding; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding and Speakers Bureau; ARIAD Pharmaceuticals: Speakers Bureau; Astellas Pharma: Research Funding; BristolMyers Squibb: Research Funding; NS Pharma: Research Funding
Rebeca Rodríguez Veiga: No relevant financial relationship(s) to disclose
Raphael Itzykson: No relevant financial relationship(s) to disclose
Blanca Boluda: No relevant financial relationship(s) to disclose
Florence Rabian: No relevant financial relationship(s) to disclose
Mar Tormo: No relevant financial relationship(s) to disclose
Evelyn Gloria Acuna Cruz: No relevant financial relationship(s) to disclose
Emma Rabinovich: No relevant financial relationship(s) to disclose
Brendan Yoo: No relevant financial relationship(s) to disclose
Isabel Cano: No relevant financial relationship(s) to disclose
Nikolai A. Podoltsev: Agios Pharmaceuticals: Consultancy and Honoraria; Astellas Pharma: Research Funding; Blueprint Medicines: Consultancy and Honoraria; Incyte: Consultancy and Honoraria; Novartis: Consultancy and Honoraria; Boehringer Ingelheim: Research Funding; Daiichi Snakyo: Research Funding; Sunesis Pharmaceuticals: Research Funding; Celator: Research Funding; Pfizer: Research Funding, Consultancy and Honoraria; Astex Pharmaceuticals: Research Funding; Celgene: Research Funding, Consultancy and Honoraria; Genentech: Research Funding; AI Therapeutics: Research Funding; Samus Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding; Kartos Therapeutics: Research Funding
Jan Philipp Bewersdorf: No relevant financial relationship(s) to disclose
Steven D. Gore: No relevant financial relationship(s) to disclose; Celgene: Consultancy and Research Funding
Amer M. Zeidan: A.M.Z. received research funding from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Aprea, Astex, and ADC Therapeutics. A.M.Z had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Takeda, Ionis, and Epizyme. A.M.Z received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this manuscript.
References
- [1].Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125:3246–3252. [DOI] [PubMed] [Google Scholar]
- [2].Dutcher JP, Schiffer CA, Wiernik PH. Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on remission rate and duration, and survival. JCO. 1987;5(9):1364–1372. [DOI] [PubMed] [Google Scholar]
- [3].Porcu P, Cripe LD, Ng EW, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39(1–2): 1–18. [DOI] [PubMed] [Google Scholar]
- [4].Ganzel C, Becker J, Mintz PD, Lazarus HM, et al. Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev. 2012;26(3):117–122. [DOI] [PubMed] [Google Scholar]
- [5].Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70–87. [DOI] [PubMed] [Google Scholar]
- [6].Porcu P, Danielson CF, Orazi A, et al. Therapeutic leukapheresis in hyperleucocytic leukaemias: lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol. 1997;98(2):433–436. [DOI] [PubMed] [Google Scholar]
- [7].Thiebaut A, Thomas X, Belhabri A, et al. Impact of pre-induction therapy leukapheresis on treatment outcome in adult acute myelogenous leukemia presenting with hyperleukocytosis. Ann Hematol. 2000;79:501–506. [DOI] [PubMed] [Google Scholar]
- [8].Giles FJ, Shen Y, Kantarjian HM, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long- term survival. Leuk Lymphoma. 2001;42(1–2):67–73. [DOI] [PubMed] [Google Scholar]
- [9].Nan X, Qin Q, Gentille C, et al. Leukapheresis reduces 4-week mortality in acute myeloid leukemia patients with hyperleukocytosis – a retrospective study from a tertiary center. Leuk Lymphoma. 2017;58(9):1–11. [DOI] [PubMed] [Google Scholar]
- [10].Choi MH, Choe YH, Park Y, et al. The effect of therapeutic leukapheresis on early complications and outcomes in patients with acute leukemia and hyperleukocytosis: a propensity score-matched study. Transfusion. 2018;58(1): 208–216. [DOI] [PubMed] [Google Scholar]
- [11].Stahl M, Shallis RM, Wei W, et al. Management of hyperleukocytosis and impact of leukapheresis among patients with acute myeloid leukemia (AML) on short- and long-term clinical outcomes: a large, retrospective, multi-center, international study. Leukemia. 2020. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bhatt VR, Shostrom V, Gundabolu K, et al. Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood Adv. 2018;2(11):1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zeidan AM, Podoltsev NA, Wang X, et al. Temporal patterns and predictors of receiving no active therapy among older patients with acute myeloid leukemia in the United States: a population level analysis. Cancer. 2019;125(23):4241–4251.31483484 [Google Scholar]
- [15].Shallis RM, Boddu PC, Bewersdorf JP, et al. The golden age for patients in their golden years: the progressive upheaval of age and the treatment of newly-diagnosed acute myeloid leukemia. Blood Rev. 2019:100639. DOI: 10.1016/j.blre.2019.100639 [DOI] [PubMed] [Google Scholar]
- [16].Shelat SG. Practical considerations for planning a therapeutic apheresis procedure. Am J Med. 2010;123(9):777–784. [DOI] [PubMed] [Google Scholar]