Abstract
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a long non-coding RNA that is overexpressed in various human cancers, including breast cancer. Evidence has associated the function of the α-2,8-sialyltransferase (ST8SIA) family with breast cancer. The present study aimed to investigate the potential roles of MALAT1 in breast cancer development and progression using analyses of both breast cancer tissues and cell lines. The mRNA levels of MALAT1, microRNA (miR)-26a/26b and ST8SIA4 were detected by reverse transcription-quantitative PCR (RT-qPCR) and the protein level of ST8SIA4 was assessed by western blot analysis. Cell proliferation, invasion and migration were detected by CCK-8, wound healing and Transwell assays, respectively. Interactions between MALAT1 and miR-26a/26b were assessed using fluorescence in situ hybridization, RNA immunoprecipitation and luciferase reporter assays. Herein, different levels of MALAT1 were primarily observed in human breast cancer samples and cells. Upregulated MALAT1 was a crucial predictor of poor breast cancer prognosis. Altered MALAT1 modulated cell progression in breast cancer. Moreover, miR-26a/26b was confirmed as a direct regulator of MALAT1, and ST8SIA4 was predicted as a target of miR-26a/26b. Functional analysis in human breast cancer cell lines demonstrated that MALAT1 modulated breast cancer cell tumorigenicity by acting as a competing endogenous lncRNA (ceRNA) to regulate ST8SIA4 levels by sponging miR-26a/26b. The identification of the MALAT1/miR-26a/26b/ST8SIA4 axis which contributes to breast cancer progression may constitute a potential new therapeutic target.
Keywords: metastasis-associated lung adenocarcinoma transcript 1; microRNA-26a/26b; α-2,8-sialyltransferase; breast cancer; long non-coding RNA
Introduction
Breast cancer is one of the most prevalent malignant diseases and the primary cause of cancer-related deaths in women worldwide, accounting for 23% of the total cancer cases and 14% of the cancer-related deaths (1). Intensive research efforts have revealed that this cancer is a heterogeneous and complex disease characterized by distinct morphological, clinical, and molecular features. Although great advances in diagnosis and treatment have been made, including the most comprehensive portfolio of targeted treatments for any cancer, the recurrence and metastasis rates of breast cancer remain unacceptably high. Therefore, it remains important to develop new targeted therapeutics for patients who are not aided by current treatments (2).
The cell surface is surrounded by glycans, and most proteins carry glycan modifications, which play an essential role in regulating a range of biological processes including immune regulation and cell-cell interactions. Notably, malignant transformation is often associated with aberrant glycosylation (3). Among the different forms of glycosylation, sialylation has been linked with multiple malignant characteristics, such as growth, migration, and invasion. The primary mediators of sialylation are the sialyltransferases (STs), a family of anabolic enzymes that have been closely linked to cancer progression (4,5). Specifically, increasing evidence has associated the function of the α-2,8-sialyltransferase (ST8SIA I–VI) family with several human tumors. For example, ST8SIA4 was revealed to participate in breast cancer progression by modifying the sialylation profile of breast cancer cells (6).
Long non-coding (lnc) RNAs, are a type of non-coding RNAs defined by lengths of more than 200 nucleotides. An increasing number of studies have demonstrated that lncRNAs play essential roles in both general biological processes as well as tumorigenesis where they can act either as oncogenes or tumor suppressors (7,8). For instance, the expression of the lncRNA GAS5 was revealed to be decreased in hepatocellular carcinoma and predicted a poor clinical outcome for these patients (9). The number of lncRNAs aberrantly expressed in human tumors continues to grow with extensive research efforts demonstrating that these function as signal mediators, molecular decoys and scaffolds or enhancers of transcription. In addition, a large group of lncRNAs can serve as molecular sponges that regulate functional gene expression (10).
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is one of the most well-characterized lncRNAs, that is commonly upregulated in numerous cancer types (11). For instance, Zhang et al revealed that MALAT1 was upregulated in renal cell carcinoma tissues, and knockdown of MALAT1 in renal cell carcinomas decreased their proliferation, migration and invasion (12). Sun et al verified that MALAT1 regulated human umbilical vein endothelial cell (HUVEC) proliferation through sponging of miR-320a to regulate forkhead box protein (FOX)M1 expression (13). Upregulation of MALAT1 in breast cancer was revealed to induce epithelial-mesenchymal transition (EMT) and decrease trastuzumab sensitivity in human epidermal growth factor receptor (HER)2+ breast cancer (14). However, the role of MALAT1 in breast cancer development remains controversial, and an improved understanding of the functional relevance of MALAT1 to this disease is urgently needed.
MicroRNAs (miRNAs) are a class of small nucleotide molecules with a length of 20–22 nucleotides (15). Mechanistically, miRNAs suppress mRNA transcription by directly binding to the 3′-untranslated regions (3′-UTRs) (16). Previously, miRNAs have been reported to play a multifaceted role in tumorigenesis. For example, Chen et al reported that miR-495 was downregulated in breast cancer cells (17). In gastric cancer cells, miR-92b-3p was overexpressed and accelerated cell malignant progression via upregulation of MMP-2/9 expression (18). Intriguingly, numerous lncRNAs function as competing endogenous RNAs (ceRNAs), acting as molecular sponges for miRNAs and altering their availability and regulation of their coding gene targets (REF) (19,20).
Our previous study revealed that miR-26a/26b regulated breast cancer progression by targeting ST8SIA4 (6). As a logical extension of this work, our present study sought to provide an improved definition of this mechanism. Herein, the potential associations between miR-26a/26b, ST8SIA4 and the lncRNA MALAT1 were investigated. The present study provided new insights into the underlying mechanisms regulated by MALAT1 in breast cancer and indicated that the MALAT1/miR-26a/26b/ST8SIA4 axis holds great promise as a new therapeutic target for breast cancer.
Materials and methods
Human tumor tissues
A total of 36 pairs of breast cancer and adjacent tissues were removed from the patients (age, 28–76 years; median, 45 years) with breast cancer who received surgeries at the First Affiliated Hospital of Dalian Medical University (Dalian, China) between June 2015 to August 2017. The subjects were pathologically confirmed as breast cancer, and they had hardly underwent any antitumor therapies before their tissues were obtained. All samples were stored in liquid nitrogen before analysis. The study was approved by the Ethics Committee of The First Affiliated Hospital of Dalian Medical University (approval no. YJ-KY-FB-2017-32) with all enrolled patients providing written informed consent.
Cell lines and cell culture
The breast cancer cell lines MDA-MB-231, MCF-7 and human epithelial breast cell line MCF-10A were obtained from the American Type Culture Collection (ATCC). All cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C. All media were supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and replaced every 2 days.
RNA isolation and reverse transcription-quantitative (RT-q)PCR
Total RNA from tissues and cells was extracted by TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA using SuperScript Reverse Transcriptase III (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Quantitative PCR reactions were carried out on an ABI 7500 PCR System using SYBR-Green Supermix (Applied Biosystems; Thermo Fisher Scientific, Inc.) with the following settings: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec, and melting curve analysis. Fluorescent signals were detected after each cycle. Relative mRNA expression levels were calculated with normalization to GAPDH or U6 using the 2−ΔΔCq method (21). The sequences of the primers were as follows: MALAT1 forward, 5′-CTCTCCCCTCCCTTGGTCTT-3′ and reverse, 5′-TCCCAATCCCCACATTTAAAAT-3′; ST8SIA4 forward, 5′-AGAGGTGGACGATCTGCACAATAA−3′ and reverse, 5′-TGACAAGTGACCGACTCAAAGACA−3′; GAPDH forward, 5′-CATGTTCGTCATGGGTGTGAA−3′ and reverse, 5′-GGCATGGACTGTGGTCATGAG−3′; miR-26a forward, 5′-GGGGGTTCAAGTAATCCAGGATA-3′ and reverse, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCCTA-3′; miR-26b forward, 5′-GGGGGTTCAAGTAATTCAGGAT-3′ and reverse, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACCTAT-3′; U6 forward, 5′-CGCTTCGGCAGCACATATACTA-3′ and reverse, 5′-GGAACGCTTCACGAATTTGC-3′. All experiments were performed in triplicate.
Cell transfection
The MALAT1 cDNA was subcloned into the pmirGLO vector (Promega Corporation). miR-26a/26b mimic, inhibitor, miR-NC, and small interfering (si)RNA targeting MALAT1 and scramble siRNA were synthesized by Shanghai GenePharma Co., Ltd. MALAT1 siRNA sequence was: 5′-GATCCATAATCGGTTTCAA-3′, and the sequence of the scramble siRNA was: 5′-UUCUCCGAACGUGUCACGUTT−3′. Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used for the transfection according to the manufacturer's instructions. Mock-transfected cells were used as the negative control. Cells were harvested 48 h after transfection for further studies. First, 5 µg siRNA was dissolved in 125 µl Opti-MEM culture medium with free FBS. Then, 3.75 µl Lipofectamine 3000 was dissolved in 125 µl Opti-MEM culture medium for 5 min at room temperature. Finally, the aforementioned two solutions were mixed while resting for 15 min at room temperature; then 250 µl mixed solution was added to each well in the 6-well plates, and the transfection medium was changed to cultured medium containing 10% FBS after 4–6 h at 37°C. RT-qPCR was used to determine the transfection efficiency.
Cell Counting Kit-8 (CCK-8) assay
Cell viability (1×103 cells/well) was assessed every 24 h after the indicated treatments. Briefly, 10 µl CCK-8 reagent was added and then the plates were cultured for another 1–2 h at 37°C in the dark. The density of each well was measured at 450 nm using an automated microplate reader (BioTek China). There were 3–5 multiple wells in each group. The experiment was performed in triplicate.
Colony formation assay
A total of ~300 cells were seeded into each well of 6-well plates and were further cultured for 2 weeks. When the macroscopic colonies (>50 cells) were evidently observed, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then stained with Giemsa (1X) for another 20 min at room temperature. Finally, images of the visibly stained colonies were captured and colonies were counted with the naked eye. The experiment was performed in triplicate.
Wound healing assay
Cells (2×105) were seeded into 12-well plates and cultured. Subsequently, after a 70–80% confluent culture was obtained, the cell monolayer was scraped with a 200-µl pipette tip. Then, the cells were washed and recultured with a serum-free medium. Cell wounds were observed and images were captured under an inverted microscope at 0 and 24 h and the degree of wound healing was expressed as the width change between the two-time points. At least eight fields for each condition were selected randomly in each well with a magnification of ×100. The experiment was performed in triplicate.
Cell migration and invasion assays
The detection of cell migration and invasion abilities was carried out by using a 24-well Transwell chamber (Corning, Inc.) with Matrigel (BD). The upper chamber of Transwell culture plates was coated with 0.4 mg/ml Matrigel (BD Biosciences) and incubated for 24 h at 4°C. In brief, 3×104 cells in 200 µl serum-free medium were added into the upper chambers of each Transwell insert (8-µm pore size), and 600 µl complete culture medium containing 10% FBS was added to the lower chamber. After 24 h of incubation at 37°C, cells were fixed with methanol (99.5%) for 30 min and stained for 30 min at room temperature with 0.1% crystal violet after fixation with methanol. Images of the cells on the apical chamber membrane were captured and cells selected from five random microscopic fields were counted at a magnification of ×400 with an inverted microscope.
Immunofluorescence analysis
Ki67 immunofluorescence analysis was also performed to observe cell proliferation. Cells were cultured in 24-well plates containing a sterile glass coverslip. After treatment, the cells were fixed with cold 4% paraformaldehyde for 15 min at room temperature and permeabilized in 0.2% Triton X-100 for 20 min at room temperature. Thereafter, the cells were blocked with 10% goat serum (Invitrogen; Thermo Fisher Scientific, Inc.) in phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific, Inc.) for 30 min at room temperature, before the addition of anti-Ki67 antibody (product code ab15580; 1:500; Abcam) overnight at 4°C. After washing twice with PBS, the cells were incubated with an FITC-anti-rabbit IgG (cat. no. sc-2012; 1:1,000; Santa Cruz Biotechnology, Inc.) for 2 h at room temperature and then counterstained with DAPI (0.1 µg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min in the dark at room temperature. Cell staining was visualized using fluorescence microscopy with images captured at a magnification of ×200.
Fluorescence in situ hybridization (FISH)
Specific probes targeting MALAT1 or miR-26a/26b probes were synthesized by Shanghai GenePharma Co., Ltd. The RNA FISH assay was performed using a FISH kit (Shanghai GenePharma Co., Ltd.) according to the manufacturer's protocols. Cells were fixed with 4% paraformaldehyde at room temperature and incubated with 0.5% Triton X-100 in PBS on ice for 10 min. Then, cells were pre-hybridized for 30 min at 37°C before being hybridized with 1 µM Cy3-labeled MALAT1 and 1 µM FITC-labeled miR-26a/26b probes (Shanghai GenePharma Co., Ltd.) at 37°C overnight. The cells were then counterstained with DAPI (1 µg/ml) for 10 min at room temperature before visualization using a fluorescence microscope at a magnification of ×400.
Dual-luciferase reporter assay
The pGL3-based luciferase reporter plasmids (Invitrogen; Thermo Fisher Scientific, Inc.) containing the 3′-UTR region of wild-type (WT) MALAT1 or mutant (MUT) MALAT1 mutated with altered miR-26a/26b binding sites were designed and constructed. Firefly luciferase and Renilla luciferase were used for normalization. A total of 5×104 cells were then co-transfected with pGL3-MALAT1 WT or MUT plasmid in combination with miR-26a/26b mimics using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h, the Dual-Luciferase Reporter Assay System (Promega Corporation) was used to evaluate reporter activity.
Western blotting
Cell lysates were prepared using RIPA lysis buffer (25 mM Tris•HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) by following the manufacturer's protocol. The protein concentration was measured with a BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.). Protein samples (20–40 µg) were resolved on 10% SDS-PAGE for separation and transferred onto polyvinylidene difluoride membranes, followed by incubation with blocking buffer [5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBST)] for 1 h at room temperature. Thereafter, the blots were incubated with an anti-ST8SIA4 monoclonal antibody (cat. no. AP9771C; 1:1,000; Abcepta) at 4°C overnight. Next, a secondary anti-rabbit HRP-conjugated IgG (cat. no. sc-2357; 1:1,000; Santa Cruz Biotechnology, Inc.) was added and incubated for 1 h at room temperature. After washing, the immuno-reactive bands and images were developed using an ECL kit (Amersham; Cytiva). All bands were determined and analyzed by LabWorks (TM ver4.6, UVP, BioImaging Systems). Blotting against GAPDH (cat. no. sc-47724; 1:1,000; Santa Cruz Biotechnology, Inc.) was used as the loading control and internal reference.
RNA immunoprecipitation (RIP) assay
The binding relationship between MALAT1 and miR-26a/26b was explored using the RIP assay kit (cat. no. 17-700; EMD Millipore) following the recommended guidelines from the manufacturer. Then, 2×107 cells were lysed in the presence of RNase inhibitor and mixed with magnetic beads pre-incubated with anti-argonaute (Ago)2 antibody (cat. no. 04-642-I; 1:1,000; EMD Millipore) at 4°C overnight to capture endogenous miR-26a/26b. The levels of coprecipitated MALAT1 were determined using RT-qPCR. IgG antibody (cat. no. AP124; 1:1,000; EMD Millipore) precipitates were used as the negative control.
Bioinformatics analysis
StarBase software v2.0 (http://starbase.sysu.edu.cn) was used to predict the binding sites shared by miRNAs and lncRNAs.
Statistical analysis
All analyses were performed using SPSS 17.0 statistical packages (SPSS, Inc.). The data were presented as the mean ± SD. Unpaired t-tests were used to compare two groups. Comparisons between multiple groups were performed by one-way ANOVA analysis and a post hoc pairwise analysis was performed using Tukey's post hoc test. Correlation between gene expression was performed using Spearman's correlation analysis. The Kaplan-Meier method was used to construct the survival curves and the difference was assessed by a log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MALAT1 is associated with breast cancer metastasis and overall survival
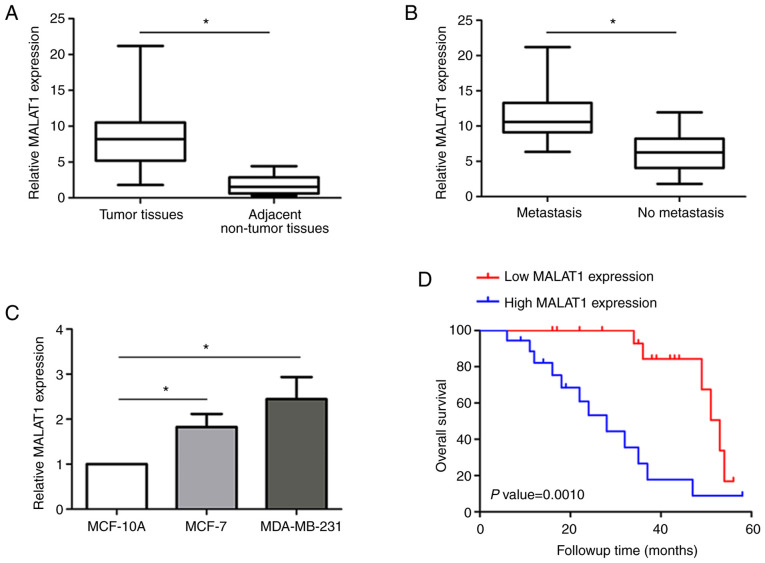
In the present study, MALAT1 expression levels in a total of 36 pairs of cancer and adjacent non-tumor tissues from breast cancer patients were detected by RT-qPCR. After normalizing against GAPDH, it was revealed that the expression of MALAT1 was significantly increased in cancer tissues compared with paired non-tumor tissues (Fig. 1A). Notably, cancer tissues from breast cancer patients with metastasis demonstrated higher MALAT1 levels than those without metastasis (Fig. 1B). MALAT1 levels were also evaluated in breast cancer cells by RT-qPCR (Fig. 1C). Breast cancer cells expressed higher MALAT1 levels than the normal MCF-10A cells, especially in MDA-MB-231 cells which displayed higher levels than MCF-7 cells. Moreover, survival analysis indicated that relatively high MALAT1 expression predicted a poor prognosis in breast cancer patients (Fig. 1D). These results indicated that MALAT1 upregulation may play a critical role in breast cancer tumorigenesis.
Figure 1.
MALAT1 is overexpressed in breast cancer tissues and cell lines. (A) Differential MALAT1 expression was detected between 36 paired breast cancer tissues and corresponding non-tumor tissues. (B) Overexpressed MALAT1 was identified in breast cancer patients with metastasis. (C) MALAT1 expression was also identified in breast cancer cell lines by reverse transcription-quantitative PCR. (D) Kaplan-Meier overall survival curves were generated based on MALAT1 expression. The data are presented as the means ± SDs of triplicate experiments. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1.
MALAT1 functions to promote breast cancer cell aggressiveness
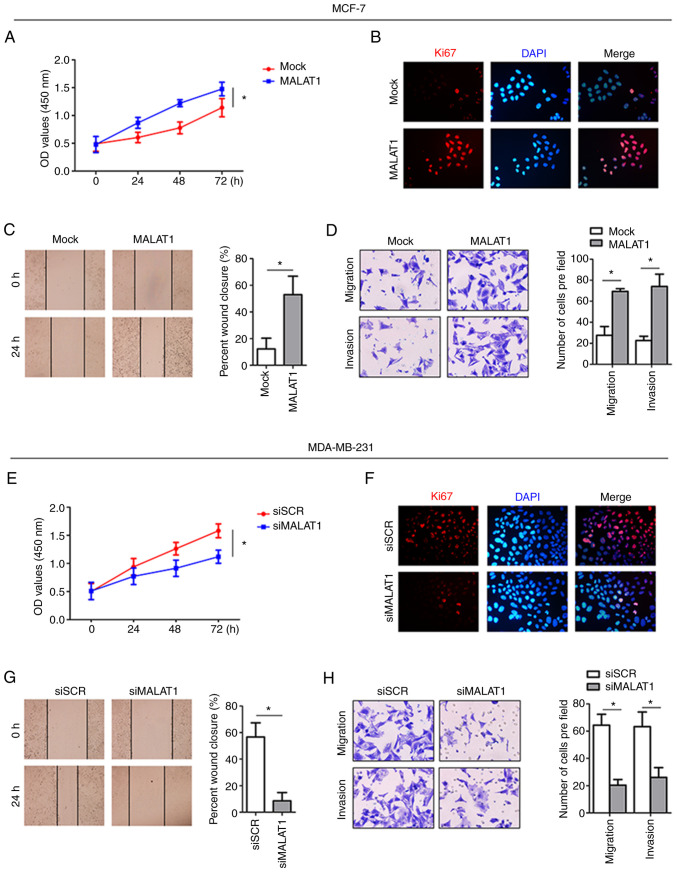
To further investigate the biological function of MALAT1 in breast cancer cells, MALAT1 was overexpressed in the MCF-7 cell line (Fig. S1A). As anticipated, MALAT1-overexpressing MCF-7 cells exhibited significantly increased cell proliferation compared with parental MCF-7 cells as determined by using CCK-8 assays (Fig. 2A). Consistently, the proportion of cells expressing Ki67 was also increased in MALAT1-overexpressing cells (Fig. 2B). Furthermore, assessment of the motility of MALAT1-overexpressing cells revealed shorter wound distances in wound healing assays relative to the negative control (mock) cells. The enhanced aggressiveness of MCF-7 cells with MALAT1 overexpression was also evident in the Transwell assays (Fig. 2D). Conversely, knockdown of MALAT1 in MDA-MB-231 cells indicated reductions in cell proliferation when assessed by CCK-8 and Ki67 immunofluorescence assays (Fig. 2E and F). Moreover, siMALAT1-transfected MDA-MB-231 cells demonstrated reduced motility in the wound healing assay relative to control cells (Fig. 2G), and more importantly, MALAT1 knockdown inhibited the migration and invasion of MDA-MB-231 cells (Fig. 2H). Thus, these results collectively indicated that MALAT1 promoted proliferation, motility and invasion in breast cancer cells.
Figure 2.
Overexpression of MALAT1 promotes the progression of MCF-7 cells, while silencing of MALAT1 inhibits the progression of MDA-MB-231 cells. (A) The growth curves based on CCK-8 assay data of MCF-7-overexpressed MALAT1 cells were compared with those of control cells. (B) Immunofluorescence staining of MCF-7 cells. Red fluorescence, MALAT1; DAPI was used to stain nuclear DNA. (C) The migration abilities of MCF-7-overexpressed MALAT1 and control cells were compared based on wound healing assays. (D) The migration and invasion abilities of transfected MCF-7 cells were determined by Transwell assays. (E) Growth curves of MDA-MB-231 siMALAT1 cells based on CCK-8 assay data were compared with those of control cells. (F) Immunofluorescence staining of MDA-MB-231 siMALAT1 cells. Red fluorescence, siMALAT1; DAPI was used to stain nuclear DNA. (G) The migration abilities of MDA-MB-231 siMALAT1 cells and control cells were compared based on wound healing assays. (H) The migration and invasion abilities of transfected MDA-MB-231 siMALAT1 cells were determined by Transwell assays. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; CCK-8, Cell Counting Kit-8; si, small interfering.
MALAT1 directly regulates miR-26a/26b
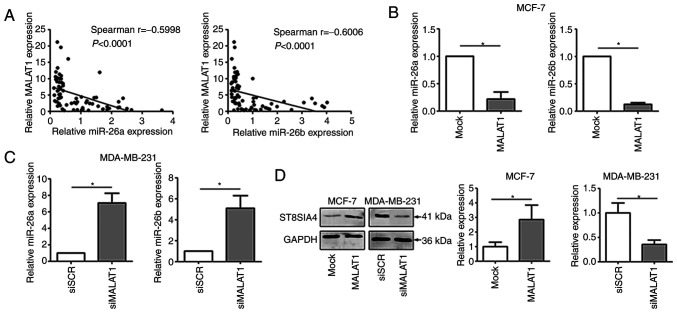
Accumulating evidence has revealed that lncRNAs can target and regulate miRNAs by ‘sponge-like’ effects, which subsequently inhibit miRNA-mediated functions (22,23). To provide improved comprehension of the molecular mechanism involving MALAT1 in breast cancer, RT-qPCR was performed to examine the expression of MALAT1 and miR-26a/26b in breast cancer tissues. The results revealed that there was a negative relationship between MALAT1 and miR-26a/26b (Fig. 3A). Moreover, significant decreases of miR-26a/26b expression were observed in MALAT1-overexpressing MCF-7 cells (Fig. 3B). Consistently, knockdown of MALAT1 increased the expression level of miR-26a/26b in MDA-MB-231 cells (Fig. 3C). Furthermore, ectopic expression of MALAT1 in MCF-7 cells significantly upregulated the expression of ST8SIA4, which is a major target of miR-26a/26b (Fig. 3D). Conversely, MALAT1 downregulation reduced the abundance of ST8SIA4 in MDA-MB-231 cells. Collectively, MALAT1 directly regulated the expression of the miR-26a/26b targeting ST8SIA4.
Figure 3.
MALAT1 interacts with miR-26a/26b to regulate ST8SIA4 expression in MCF-7 and MDA-MB-231 cells. (A) There was a negative relationship between the expression of MALAT1 and miR-26a or miR-26b expression (P<0.0001). (B) The expression of miR-26a and miR-26b was examined by RT-qPCR in the MCF-7 cells. (C) The expression of miR-26a and miR-26b was examined by RT-qPCR in the MDA-MB-231 cells. (D) ST8SIA4 protein levels were significantly increased with upregulation of MALAT1 in MCF-7 cells and decreased with knockdown of MALAT1 in MDA-MB-231 cells as revealed by western blot analysis. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; miR, microRNA; ST8SIA4, α-2,8-sialyltransferase; RT-qPCR, reverse transcription-quantitative PCR; si, small interfering; SCR, scrambled.
MALAT1 serves as a miR-26a/26b sponge in breast cancer cells
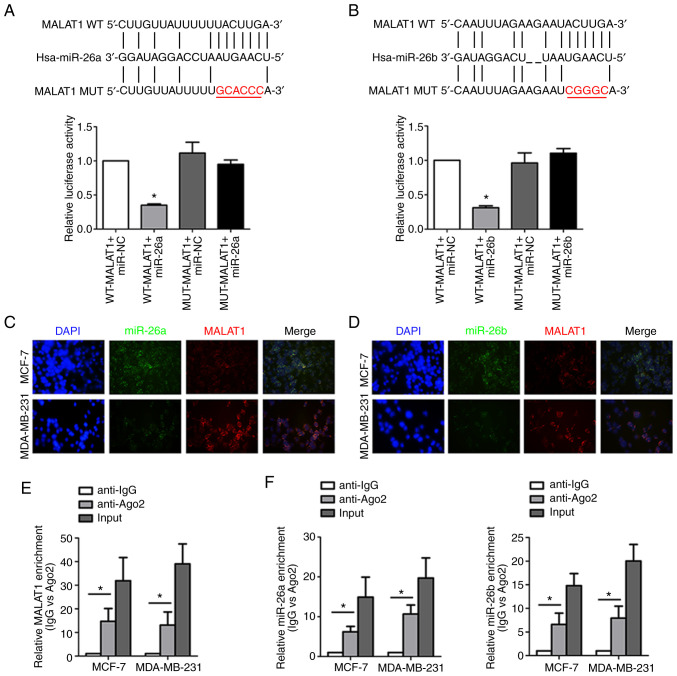
The underlying interaction mechanism by which MALAT1 and miR-26a/26b are involved in breast cancer was further investigated. Bioinformatics analysis (http://starbase.sysu.edu.cn) predicted that MALAT1 directly binds to miR-26a/26b with the complementary binding sites revealed in Fig. 4A and B. Dual-luciferase reporter assay revealed that co-transfection of miR-26a/26b mimics and WT MALAT1 led to decreased reporter activity. Instructively, there was no significant change in luciferase activity when MUT MALAT1 and miR-26a/26b mimics were cotransfected indicating a specific interaction through the identified binding sites. Consistently, the co-location of MALAT1 and miR-26a/26b in the cytoplasm of breast cancer cells was verified by the FISH assay, which indicated their functional crosstalk (Fig. 4C and D). Moreover, both MALAT1 (Fig. 4E) and miR-26a/26b (Fig. 4F) were enriched in RIP assays conducted against the Ago2 protein, indicating that Ago2 directly bound to MALAT1 and miR-26a/26b in breast cancer cells. Collectively, these data indicated that MALAT1 acted as a molecular sponge for miR-26a/26b.
Figure 4.
MALAT1 acts as a molecular sponge for miR-26a/26b. (A) The predicted binding sites between MALAT1 and miR-26a. In addition, luciferase activities were assessed to analyze the direct binding of MALAT1 and miR-26a. (B) The predicted binding sites between MALAT1 and miR-26b. Luciferase activity was also assessed to analyze the direct binding of MALAT1 and miR-26b. (C) MALAT1 and miR-26a colocalization was detected by FISH assay in MDA-MB-231 cells and MCF-7 cells. Red fluorescence, MALAT1; green fluorescence, miR-26a; DAPI was used to stain nuclear DNA. (D) MALAT1 and miR-26b colocalization were detected by FISH assay in MDA-MB-231 cells and MCF-7 cells. Red fluorescence, MALAT1; green fluorescence, miR-26b; DAPI was used to stain nuclear DNA. (E) Coprecipitated RNA was detected by RIP assay. MALAT1 levels were presented as fold enrichment in the Ago2 pellet relative to the IgG immunoprecipitate. (F) Enrichment of miR-26a/26b was revealed by the RIP assay. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; miR, microRNA; WT, wild-type; MUT, mutant; FISH, fluorescence in situ hybridization; RIP, RNA immunoprecipitation; Ago, argonaute; IgG, immunoglobulin G.
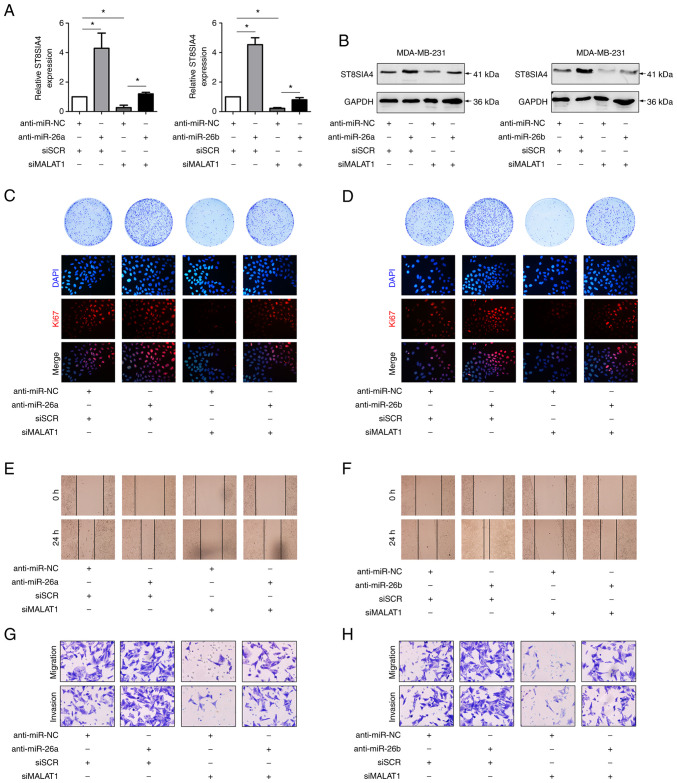
MALAT1/miR-26a/26b/ST8SIA4 axis contributes to breast cancer progression
A previous study demonstrated that miR-26a/26b suppressed breast cancer progression by downregulating the levels of ST8SIA4 (6). To confirm the effects of MALAT1 on miR-26a/26b and ST8SIA4 during breast cancer progression, further functional experiments were carried out. In fact, the modulatory effects of downregulating miR-26a/26b and MALAT1 on ST8SIA4 were increased in MDA-MB-231 cells (Figs. 5A, S1B and C). In comparison with the controls, the miR-26a/26b inhibitors resulted in increased ST8SIA4 expression, while silencing MALAT1 decreased ST8SIA4 levels. In addition, silencing of MALAT1 in miR-26a/26b-inhibited cells partially reversed the increases in ST8SIA4. Similar trends were evident in the western blot assays where knockdown of MALAT1 suppressed the increased levels of ST8SIA4 protein induced by miR-26a/26b inhibitors (Fig. 5B), consistent with a model wherein MALAT1 regulates ST8SIA4 via miR-26a/26b.
Figure 5.
MALAT1/miR-26a/26b/ST8SIA4 axis contributes to MDA-MB-231 cell viability, invasion, and migration in breast cancer. (A) The ST8SIA4 mRNA level was analyzed with miR-26a/26b mimics or siMALAT1 transfection. (B) ST8SIA4 protein levels were determined by western blotting. (C and D) Colony formation and Ki67 assays revealed the altered proliferative ability of knockdown cells. (E and F) The migration abilities of the control and modified cells were compared with wound healing assays. (G and H) The migration and invasion abilities were assessed by Transwell assays. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; miR, microRNA; ST8SIA4, α-2,8-sialyltransferase; si, small interfering; SCR, scrambled; NC, negative control.
Functional assays also revealed the likely significance of MALAT1 and miR-26a/26b during breast cancer progression. Colony formation assays along with Ki67 staining assessing the proportion of proliferating cells demonstrated that breast cancer cells transfected with miR-26a/26b mimics exhibited a higher proliferative activity than control cells, while cells transfected with siMALAT1 displayed comparatively lower proliferative activity. Interestingly, cotransfection of miR-26a/26b mimics and siMALAT1 reversed the altered proliferation which was induced by miR-26a/26b mimics alone (Fig. 5C and D). Scratch and Transwell assays also revealed that miR-26a/26b inhibition increased the ability of migration and invasion in MDA-MB-231 cells. Moreover, relative to transfection of miR-26a/26b inhibitors only, these cells exhibited further decreases in migration and invasion abilities after cotransfection of siMALAT1 with the miR-26a/26b inhibitors (Fig. 5E-H).
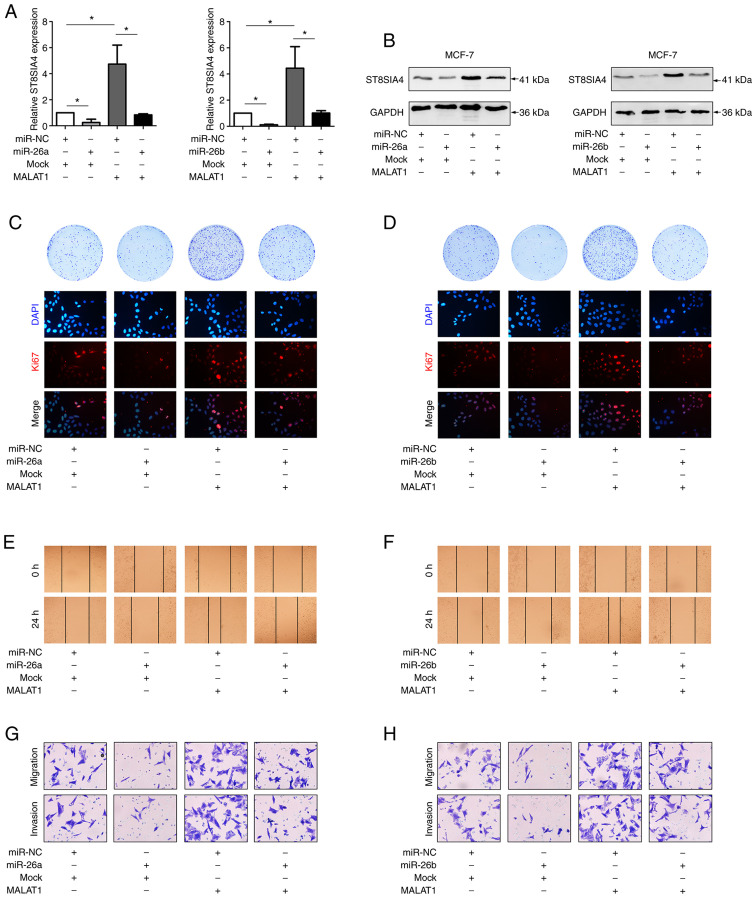
In concert with the aforementioned experiments using MDA-MB-231 cells, the effects of miR-26a/26b and MALAT1 were investigated in MCF-7 cells (Figs. 6A and B, S1B and C). Transfection of MALAT1 or miR-26a/26b into MCF-7 cells revealed that overexpression of miR-26a/26b suppressed cell viability, migration, and invasion, whereas ectopically expressed MALAT1 reversed these properties (Fig. 6C-H). Collectively, our findings indicated that MALAT1 promoted the progression of breast cancer through a miR-26a/26b/ST8SIA4 axis.
Figure 6.
MALAT1/miR-26a/26b/ST8SIA4 axis contributes to MCF-7 cell viability, invasion and migration. (A) The ST8SIA4 mRNA level was examined by reverse transcription-quantitative PCR after transfection with MALAT1 or miR-26a/26b. (B) ST8SIA4 protein levels were determined by western blot analysis. (C and D) Colony formation and Ki67 assays revealed the altered proliferative ability of transfected cells. (E and F) The migration abilities of the control and modified cells were compared with wound healing assays. (G and H) Migration and invasion were assessed by Transwell assays. *P<0.05. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; miR, microRNA; ST8SIA4, α-2,8-sialyltransferase; NC, negative control.
Discussion
Breast cancer carcinogenesis is a complex biological process involving the contribution of different genomic mutations interacting with various biological signaling networks. Mounting evidence has indicated that the genesis of breast cancer involves epigenetic modifications (e.g., DNA methylation), modulation of tumor-susceptible genes (e.g., BRCA1 and BRCA2), and the dysregulation of lncRNAs, with tumorigenesis occurring when the balance of oncogenes and anti-oncogenes are altered (24,25). Consequently, in-depth investigations of the underlying mechanisms promoting breast cancer could provide valuable insights into novel diagnostic markers as well as the identification of therapeutic targets. In the present study, the functional effects of the lncRNA MALAT1 were identified and characterized in breast cancer progression, revealing a novel mechanism by which MALAT1 regulated ST8SIA4 through miR-26a/26b.
Advances in sequencing technology along with careful mechanistic analyses have revealed that numerous lncRNAs participate in tissue homeostasis together with disease pathogenesis by exerting regulatory effects on gene expression at the transcriptional, post-transcriptional and epigenetic levels (26). Numerous lncRNAs are aberrantly expressed in tumors, and in specific cases these have been revealed to exert powerful effects on cancer initiation, progression and metastasis. For example, a study by Liu et al reported that the lncRNA HOTAIR regulated colorectal cancer malignancy by inducing c-Met sialylation and activating the JAK2/STAT3 pathway (27). Studies performed by Guan et al in breast cancer also indicated that lncRNA SNHG20 promoted cell proliferation, invasion, and migration by regulating HER2 via miR-495 (28). Moreover, Gao et al reported that lncRNA ATB/miR-200c/CDK2 signaling was responsible for intensified proliferation and restrained apoptosis of colorectal cancer cells (29). Previous studies have revealed that MALAT1 exhibited a close association with breast cancer, but its precise functions in breast cancer progression remain unclear (30,31). Our study confirmed that MALAT1 was especially overexpressed in breast cancer tissues with high MALAT1 expression being associated with metastasis and poor overall survival. Interestingly, a recent research also revealed that the MALAT1 expression in serum was increased in breast cancer patients, while its levels became downregulated after patients received breast-conserving surgery combined with neo-adjuvant chemotherapy (32). It was also confirmed that MALAT1 was overexpressed in breast cancer cell lines and moreover, provided comprehensive evidence that MALAT1 increased the migration and invasion of breast cancer cells in vitro. Therefore, consistent with the studies of Arun and Spector (33), our results indicated MALAT1 acted as an essential regulator of breast cancer progression.
Mounting evidence has indicated that lncRNAs act as competing endogenous RNAs (ceRNAs) that regulate gene expression by sponging compatible miRNAs. For example, Li et al reported that MALAT1 contributed to glioma autophagy and proliferation by sponging miR-101 (34). Functional correlations between MALAT1 and miR-30a-5p were also recorded in hepatocellular carcinoma, indicating that MALAT1 acted as an oncogenic lncRNA in HCC that promoted cell migration and invasion (35). In the present study, focus was placed on the relationship between MALAT1 and miR-26a/26b in breast cancer. miR-26a and miR-26b are members of the miR-26 family; they are transcribed from three genomic loci, miR-26a-1, miR-26a-2 and miR-26b, which reside in the introns of genes coding for the CTDSPL, CTDSP2 and CTDSP1 proteins, respectively (36). Previous studies have noted that miR-26a/26b are tumor-suppressor genes that considerably suppress the invasion process (37,38). In this study, it was revealed that the expression of MALAT1 was negatively correlated with miR-26a/26b in breast cancer tissues and that MALAT1 could inhibit miR-26a/26b expression. Furthermore, bioinformatics analysis highlighted a possible interaction between these two molecules with FISH assays demonstrating that MALAT1 and miR-26a/26b colocalized in breast cancer cells. Further substantiating this theory, luciferase reporter and RIP assays identified that MALAT1 acted as a molecular sponge for miR-26a/26b.
The malignant process of tumors is frequently accompanied by changes in the structure and expression of oligosaccharide proteins and glycolipids on the cell surface. It has been revealed that the expression of sialylated glycoconjugates are altered during cell differentiation, disease progression and oncogenic transformation (39). ST expression patterns are significantly different between cancer and normal tissues (40). It was previously reported that ST8SIA4 was upregulated in breast cancer cells and tissues and acted to promote malignant progression with evidence revealing that ST8SIA4 was a target gene of miR-26a/26b (6). Extending the aforementioned work here, a positive correlation was revealed between MALAT1 and ST8SIA4 and functional evidence for their association was provided. For example, it was demonstrated that MALAT1 silencing in miR-26a/26b-inhibited cells partially reversed the increases in ST8SIA4 levels. Therefore, a ceRNA role of MALAT1 appeared to be a reasonable hypothesis to explain these results. In fact, the mechanism whereby MALAT1 acted as a molecular sponge for miR-26a/26b, suppressing cell progression in breast cancer by targeting ST8SIA4, was analyzed. Moreover, MALAT1 overexpression partially rescued the suppression of cell proliferation, migration and invasion induced by miR-26a/26b mimics. In summary, our results demonstrated a crucial role for miR-26a/26b as a direct target of MALAT1 in regulating breast cancer progression with clear evidence of crosstalk between MALAT1, miR-26a/26b and ST8SIA4.
In conclusion, the present work revealed that MALAT1 was overexpressed in breast cancer where it functioned as an oncogene. Notably, high MALAT1 expression in breast cancer patient tissues was positively associated with tumor metastasis and poor clinical outcomes. It was also established that MALAT1 acted as a ceRNA for miR-26a/26b in breast cancer cells and the inhibition of miR-26a/26b thereby weakened its inhibitory effects on ST8SIA4 expression. The present study identified a MALAT1/miR-26a/26b/ST8SIA4 axis which crucially contributed to the cell proliferation, invasion and migration of breast cancer cells. This discovery also provided new insights into the underlying mechanisms regulated by MALAT1 in breast cancer and indicated that the MALAT1/miR-26a/26b/ST8SIA4 axis holds great promise as a new therapeutic target for breast cancer. However, lncRNA MALAT1 and miR-26a/26b could, respectively, possess targets of diverse miRNAs and mRNAs, and discovery of multiple lncRNA/miRNA/genes may be conducive to thorough suppression of breast cancer cells. This shortcoming of the present study should be improved in the future.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by grants (grant nos. 20180550476 and 20180550729) from the Natural Science Foundation of Liaoning Province (China).
Funding
The present study was supported by grants (grant nos. 20180550476 and 20180550729) from the Natural Science Foundation of Liaoning Province (China).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XM and HY conceived and supervised the study. NW and SC designed and performed the experiments. XW, LZ and NW analyzed the data. XM, NW, SC and HY wrote the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The Ethics Committee of The First Affiliated Hospital of Dalian Medical University approved the present study and written informed consents were acquired from all enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The author declare that they have no competing interests.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ruiterkamp J, Voogd AC, Tjan-Heijnen VC, Bosscha K, van der Linden YM, Rutgers EJ, Boven E, van der Sangen MJ, Ernst MF, Dutch Breast Cancer Trialists' Group (BOOG) SUBMIT: Systemic therapy with or without upfront surgery of the primary tumor in breast cancer patients with distant metastases at initial presentation. BMC Surg. 2012;12:5. doi: 10.1186/1471-2482-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kölbl AC, Andergassen U, Jeschke U. The role of glycosylation in breast cancer metastasis and cancer control. Front Oncol. 2015;5:219. doi: 10.3389/fonc.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vajaria BN, Patel KR, Begum R, Patel PS. Sialylation: An avenue to target cancer cells. Pathol Oncol Res. 2016;22:443–447. doi: 10.1007/s12253-015-0033-6. [DOI] [PubMed] [Google Scholar]
- 5.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–366. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma XL, Dong WJ, Su Z, Zhao LF, Miao Y, Li NN, Zhou HM, Li J. Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4. Cell Death Dis. 2016;7:e2561. doi: 10.1038/cddis.2016.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach DH, Lee SK. Long noncoding RNAs in cancer cells. Cancer Lett. 2018;419:152–166. doi: 10.1016/j.canlet.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: Mechanisms of action and technological advancements. Mol Cancer. 2016;15:43. doi: 10.1186/s12943-016-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu ZQ, Li RJ, Mei JZ, Li XH. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:4303–4309. [PMC free article] [PubMed] [Google Scholar]
- 10.Hombach S, Kretz M. Non-coding RNAs: Classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17. doi: 10.1007/978-3-319-42059-2_1. [DOI] [PubMed] [Google Scholar]
- 11.Li ZX, Zhu QZ, Zhang HB, Hu Y, Wang G, Zhu YS. MALAT1: A potential biomarker in cancer. Cancer Manag Res. 2018;10:6757–6768. doi: 10.2147/CMAR.S169406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Yang F, Chen SJ, Che JP, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumor Biol. 2015;36:2947–2955. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 13.Sun JY, Zhao ZW, Li WM, Yang G, Jing PY, Li P, Dang HZ, Chen Z, Zhou YA, Li XF. Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of expression. Oncotarget. 2017;8:61449–61509. doi: 10.18632/oncotarget.18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Sarkissyan M, Ogah O, Kim J, Vadgama JV. Expression of MALAT1 promotes trastuzumab resistance in HER2 overexpressing breast cancers. Cancers (Basel) 2020;12:1918. doi: 10.3390/cancers12071918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Browne G, Dragon JA, Hong D, Messier TL, Gordon JA, Farina NH, Boyd JR, VanOudenhove JJ, Perez AW, Zaidi SK, et al. MicroRNA-378-mediated suppression of Runx1 alleviates the aggressive phenotype of triple-negative MDA-MB-231 human breast cancer cells. Tumour Biol. 2016;37:8825–8839. doi: 10.1007/s13277-015-4710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Luo DL, Tian WG, Li ZR, Zhang XH. Demethylation of miR-495 inhibits cell proliferation, migration and promotes apoptosis by targeting STAT-3 in breast cancer. Oncol Rep. 2017;37:3581–3589. doi: 10.3892/or.2017.5621. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Huo B, Wang Y, Cheng C. Downregulation of microRNA-92b-3p suppresses proliferation, migration, and invasion of gastric cancer SGC-7901 cells by targeting Homeobox D10. J Cell Biochem. 2019;120:17405–17412. doi: 10.1002/jcb.29005. [DOI] [PubMed] [Google Scholar]
- 19.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19:1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhu Z, Huang S, Zhao Q, Huang C, Tang Y, Sun C, Zhang Z, Wang L, Chen H, et al. LncRNA XIST regulates proliferation and migration of hepatocellular carcinoma cells by acting as miR-497-5p molecular sponge and targeting PDCD4. Cancer Cell Int. 2019;19:198. doi: 10.1186/s12935-019-0909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Sun W, Guo Z, Zhang J, Yu H, Liu B. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020;254:116900. doi: 10.1016/j.lfs.2019.116900. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Teer JK, Tousignant RN, Levin AM, Boulware D, Chitale DA, Shaw BM, Chen Z, Zhang Y, Blakeley JO, et al. Breast cancer risk and germline genomic profiling of women with neurofibromatosis type 1 who developed breast cancer. Genes Chromosomes Cancer. 2018;57:19–27. doi: 10.1002/gcc.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amelio I, Bernassola F, Candi E. Emerging roles of long non-coding RNAs in breast cancer biology and management. Semin Cancer Biol. 2021;72:36–45. doi: 10.1016/j.semcancer.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Dimartino D, Colantoni A, Ballarino M, Martone J, Mariani D, Danner J, Bruckmann A, Meister G, Morlando M, Bozzoni I. The long non-coding RNA lnc-31 interacts with Rock1 mRNA and mediates its YB-1-dependent translation. Cell Rep. 2018;23:733–740. doi: 10.1016/j.celrep.2018.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Liu Q, Pan S, Huang Y, Qi Y, Li S, Xiao Y, Jia L. The HOTAIR/miR-214/ST6GAL1 crosstalk modulates colorectal cancer procession through mediating sialylated c-Met via JAK2/STAT3 cascade. J Exp Clin Cancer Res. 2019;38:455. doi: 10.1186/s13046-019-1468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan YX, Zhang MZ, Chen XZ, Zhang Q, Liu SZ, Zhang YL. Lnc RNA SNHG20 participated in proliferation, invasion, and migration of breast cancer cells via miR-495. J Cell Biochem. 2018;119:7971–7981. doi: 10.1002/jcb.26588. [DOI] [PubMed] [Google Scholar]
- 29.Gao Z, Zhou H, Wang Y, Chen J, Ou Y. Regulatory effects of lncRNA ATB targeting miR-200c on proliferation and apoptosis of colorectal cancer cells. J Cell Biochem. 2020;121:332–343. doi: 10.1002/jcb.29180. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Y, Pan J, Geng Q, Wang G. LncRNA MALAT1 increases the stemness of gastric cancer cells via enhancing SOX2 mRNA stability. FEBS Open Bio. 2019;9:1212–1222. doi: 10.1002/2211-5463.12649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Zuo Y, Li Y, Zhou Z, Ma M, Fu K. Long non-coding RNA MALAT1 promotes proliferation and invasion via targeting miR-129-5p in triple-negative breast cancer. Biomed Pharmacother. 2017;95:922–928. doi: 10.1016/j.biopha.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Liu J, Liu J. The expression of lncRNA-MALAT1 in breast cancer patients and its influences on prognosis. Cell Mol Biol (Noisy-le-grand) 2020;66:72–78. doi: 10.14715/cmb/2020.66.3.11. [DOI] [PubMed] [Google Scholar]
- 33.Arun G, Spector DL. MALAT1 long non-coding RNA and breast cancer. RNA Biol. 2019;16:860–863. doi: 10.1080/15476286.2019.1592072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Xu C, Ding B, Gao M, Wei X, Ji N. Long non-coding RNA MALAT1 promotes proliferation and suppresses apoptosis of glioma cells through derepressing Rap1B by sponging miR-101. J Neurooncol. 2017;134:19–28. doi: 10.1007/s11060-017-2498-5. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Tong S, Cui R, Fan J, Liu C, Lin Y, Tang J, Xie H, Lin P, Zheng T, et al. Long non-coding MALAT1 functions as a competing endogenous RNA to regulate vimentin expression by sponging miR-30a-5p in hepatocellular carcinoma. Cell Physiol Biochem. 2018;50:108–120. doi: 10.1159/000493962. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Sun Z, Liu B, Shan Y, Zhao L, Jia L. Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death Dis. 2017;8:e2892. doi: 10.1038/cddis.2017.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Y, Lu Y, Zhang Q, Liu JJ, Li TJ, Yang JR, Zeng C, Zhuang SM. MicroRNA-26a/b and their host genes cooperate to inhibit the G1/S transition by activating the pRb protein. Nucleic Acids Res. 2012;40:4615–4625. doi: 10.1093/nar/gkr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M, Goto Y, Matsushita R, Kurozumi A, Fukumoto I, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T, Seki N. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int J Oncol. 2015;47:710–718. doi: 10.3892/ijo.2015.3043. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Ding J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell. 2019;10:550–565. doi: 10.1007/s13238-018-0597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garnham R, Scott E, Livermore KE, Munkley J. ST6GAL1: A key player in cancer. Oncol Lett. 2019;18:983–989. doi: 10.3892/ol.2019.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.